New Prognostic Score (Essen Score) to Predict Postoperative Morbidity after Resection of Lung Metastases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Formation of the Study Population Exclusion/Inclusion Criteria
- –
- Patients < 18 years;
- –
- Diagnostic operations;
- –
- Metastasectomies with the intention of palliation.
2.3. Definition of Complications, Preoperative Comorbidities and Mortality
2.4. Surgical Procedure
2.5. Statistical Analysis
3. Results
3.1. Study Population
Surgical and Oncological Characteristics of the Study Population
3.2. Clinical Characteristics of the Study Population
3.3. Postoperative Morbidity and Mortality after Pulmonary Metastasectomy
3.4. Patient-Associated Risk Factors for Postoperative Morbidity after Pulmonary Metastasectomy
3.5. Surgery-Associated Risk Factors for Patients Undergoing Pulmonary Metastasectomy
3.6. Independent Risk Factors for Postoperative Morbidity after Pulmonary Metastasectomy
3.7. Development of a New Prognostic Score to Predict Postoperative Morbidity after Pulmonary Metastasectomy (Essen Score)
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfannschmidt, J.; Egerer, G.; Bischof, M.; Thomas, M.; Dienemann, H. Surgical intervention for pulmonary metastases. Dtsch. Arztebl. Int. 2012, 109, 645–651. [Google Scholar] [PubMed]
- Grapatsas, K.; Papaporfyriou, A.; Leivaditis, V.; Ehle, B.; Galanis, M. Lung Metastatectomy: Can Laser-Assisted Surgery Make a Difference? Curr. Oncol. 2022, 29, 548. [Google Scholar]
- Rodríguez-Fuster, A.; Belda-Sanchis, J.; Aguiló, R.; Embun, R.; Mojal, S.; Call, S.; Molins, L.; de Andrés, J.J.R. Morbidity and mortality in a large series of surgical patients with pulmonary metastases of colorectal carcinoma: A prospective multicentre Spanish study (GECMP-CCR-SEPAR). Eur. J. Cardiothorac. Surg. 2014, 45, 671–676. [Google Scholar] [PubMed]
- Seely, A.; Ivanovic, J.; Threader, J.; Al-Hussaini Derar Al-Shehab, A.; Ramsay, T.; Gilbert, S.; Maziak, D.E.; Shamji, F.; Sundaresan, R.S. Systematic classification of morbidity and mortality after thoracic surgery. Ann. Thorac. Surg. 2010, 90, 936–942, disscussion 942. [Google Scholar] [PubMed]
- Mirza, S.; Clay, R.D.; Koslow, M.A.; Scanlon, P.D. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin. Proc. 2018, 93, 1488–1502. [Google Scholar]
- Petrella, F.; Diotti, C.; Rimessi, A.; Spaggiari, L. Pulmonary metastasectomy: An overview. J. Thorac. Dis. 2017, 9 (Suppl. 12), S1291–S1298. [Google Scholar] [CrossRef]
- The International Registry of Lung Metastases. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J. Thorac. Cardiovasc. Surg. 1997, 113, 37–49. [Google Scholar]
- Hassan, M.; Graeter, T.; Dietrich, I.; Kemna, L.J.; Passlick, B.; Schmid, S. Surgical Morbidity and Lung Function Changes after Laser-Assisted Pulmonary Metastasectomy: A Prospective Bicentric Study. Front. Surg. 2021, 8, 646269. [Google Scholar]
- Hassan, M.; Ehle, B.; Passlick, B.; Grapatsas, K. Lung Resections for Elderly Patients with Lung Metastases: A Comparative Study of the Postoperative Complications and Overall Survival. Curr. Oncol. 2022, 29, 4511–4521. [Google Scholar]
- Sponholz, S.; Schirren, M.; Oguzhan, S.; Schirren, J. Morbidity, mortality, and survival in elderly patients undergoing pulmonary metastasectomy for colorectal cancer. Int. J. Color. Dis. 2018, 33, 1401–1409. [Google Scholar]
- Ambrogi, V.; Pompeo, E.; Elia, S.; Pistolese, G.R.; Mineo, T.C. The impact of cardiovascular comorbidity on the outcome of surgery for stage I and II non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2003, 23, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Grapatsas, K.; Hassan, M.; Semmelmann, A.; Ehle, B.; Passlick, B.; Schmid, S.; Le, U.T. Should cardiovascular comorbidities be a contraindication for pulmonary metastasectomy? J. Thorac. Dis. 2022, 14, 4266–4275. [Google Scholar] [PubMed]
- Osei-Agyemang, T.; Palade, E.; Haderthauer, J.; Ploenes, T.; Yaneva, V.; Passlick, B. Pulmonary metastasectomy: An analysis of technical and oncological outcomes in 301 patients with a focus on laser resection. Zentralbl. Chir. 2013, 138 (Suppl. S1), S45–S51. [Google Scholar] [PubMed]
- Downey, R.J. Surgical treatment of pulmonary metastases. Surg. Oncol. Clin. N. Am. 1999, 8, 341. [Google Scholar]
- Treasure, T. Pulmonary metastasectomy for colorectal cancer: Weak evidence and no randomised trials. Eur. J. Cardiothorac. Surg. 2008, 33, 300–302. [Google Scholar]
- Molnar, T.F.; Gebitekin, C.; Turna, A. What are the considerations in the surgical approach in pulmonary metas-tasectomy? J. Thorac. Oncol. 2010, 5 (Suppl. S2), S140–S144. [Google Scholar]
- Rolle, A.; Pereszlenyi, A.; Koch, R.; Richard, M.; Baier, B. Is surgery for multiple lung metastases reasonable? A total of 328 consecutive patients with multiple-laser metastasectomies with a new 1318-nm Nd:YAG laser. J. Thorac. Cardiovasc. Surg. 2006, 131, 1236–1242. [Google Scholar]
- Rolle, A.; Pereszlenyi, A.; Koch, R.; Bis, B.; Baier, B. Laser resection technique and results of multiple lung metastasectomies using a new 1318 nm Nd:YAG laser system. Lasers Surg. Med. 2006, 38, 26–32. [Google Scholar] [CrossRef]
- Baier, B.; Kern, A.; Kaderali, L.; Bis, B.; Koschel, D.; Rolle, A. Retrospective survival analysis of 237 consecutive patients with multiple pulmonary metastases from advanced renal cell carcinoma exclusively resected by a 1318-nm laser. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 211–217. [Google Scholar]
- Pereszlenyi, A.; Rolle, A.; Koch, R.; Schilling, A.; Baier, B.; Bis, B. Resection of multiple lung metastases—Where are the limits? Bratisl. Lek. Listy 2005, 106, 262–265. [Google Scholar]
- Stefani, A.; Oricchio, F.; Cinquepalmi, A.; Aramini, B.; Morandi, U. Is laser-assisted resection preferable to lobectomy for pulmonary metastasectomy? Lasers Med. Sci. 2020, 35, 611–620. [Google Scholar] [CrossRef]
- Mineo, T.C.; Ambrogi, V.; Pompeo, E.; Nofroni, I. The value of the Nd:YAG laser for the surgery of lung metastases in a randomized trial. Chest 1998, 113, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Mineo, T.C.; Ambrogi, V.; Tonini, G.; Nofroni, I. Pulmonary metastasectomy: Might the type of resection affect survival? J. Surg. Oncol. 2001, 76, 47–52. [Google Scholar] [CrossRef]
- Porrello, C.; Gullo, R.; Vaglica, A.; Scerrino, G.; Salamone, G.; Licari, L.; Raspanti, C.; Gulotta, E.; Gulotta, G.; Cocorullo, G. Pulmonary Laser Metastasectomy by 1318-nm Neodymium-Doped Yttrium-Aluminum Garnet Laser: A Retrospective Study About Laser Metastasectomy of the Lung. Surg. Innov. 2018, 25, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, N.; Grapatsas, K.; Leivaditis, V.; Galanis, M.; Dougenis, D. Are Extensive Open Lung Resections for Elderly Patients with Lung Cancer Justified? Curr. Oncol. 2023, 30, 5470–5484. [Google Scholar] [CrossRef]
- Meyer, C.; Bartsch, D.; Mirow, N.; Kirschbaum, A. Video-Assisted Laser Resection of Lung Metastases-Feasibility of a New Surgical Technique. Thorac. Cardiovasc. Surg. 2017, 65, 382–386. [Google Scholar] [CrossRef]
- Mc Loughlin, J.; O’Sullivan, K.E.; Brown, R.H.; Eaton, D. Limax Nd:YAG laser-assisted thoracoscopic resection of pulmonary metastases; a single centre’s initial experience. Ir. J. Med. Sci. 2019, 188, 771–776. [Google Scholar] [CrossRef]
- Ojanguren, A.; Karenovics, W.; Dackam, S.; Demarchi, M.; Triponez, F. Laser pulmonary metastasectomy by video-assisted thoracic surgery. J. Vis. Surg. 2019, 5, 40. [Google Scholar] [CrossRef]
- Franzke, K.; Natanov, R.; Zinne, N.; Rajab, T.K.; Biancosino, C.; Zander, I.; Lodziewski, S.; Rickfels, M.; Kropivnitskaya, I.; Schimttio, J.D.; et al. Pulmonary metastasectomy—A retrospective comparison of surgical outcomes after laser-assisted and conventional resection. Eur. J. Surg. Oncol. 2017, 43, 1357–1364. [Google Scholar] [CrossRef]
- Abdelnour-Berchtold, E.; Perentes, J.Y.; Ris, H.B.; Beigelman, C.; Lovis, A.; Peters, S.; Krueger, T.; Gonzalez, M. Survival and Local Recurrence After Video-Assisted Thoracoscopic Lung Metastasectomy. World J. Surg. 2016, 40, 373–379. [Google Scholar]
- Hassan, M.; Ehle, B.; Le, U.T.; Titze, L.; Passlick, B.; Grapatsas, K. Outcome of Repeated Resection of Pulmonary Metastases for Renal Cell Cancer. Thorac. Cardiovasc. Surg. 2023, 71, 130–137. [Google Scholar] [CrossRef]
- Pfannschmidt, J.; Hoffmann, H.; Muley, T.; Krysa, S.; Trainer, C.; Dienemann, H. Prognostic factors for survival after pulmonary resection of metastatic renal cell carcinoma. Ann. Thorac. Surg. 2002, 74, 1653–1657. [Google Scholar] [CrossRef]
- Meimarakis, G.; Angele, M.; Staehler, M.; Clevert, D.-A.; Crispin, A.; Rüttinger, D.; Löhe, F.; Preissler, G.; Hatz, R.; Winter, H. Evaluation of a new prognostic score (Munich score) to predict long-term survival after resection of pulmonary renal cell car-cinoma metastases. Am. J. Surg. 2011, 202, 158–167. [Google Scholar] [CrossRef]
- Margaryan, R.; Moscarelli, M.; Gasbarri, T.; Bianchi, G.; Kallushi, E.; Cerillo, A.G.; Farneti, P.; Solinas, M. EuroSCORE Perfor-mance in Minimally Invasive Cardiac Surgery: Discrimination Ability and External Calibration. Innovations 2017, 12, 282–286. [Google Scholar] [CrossRef]
- Amar, D.; Munoz, D.; Shi, W.; Zhang, H.; Thaler, H.T. A clinical prediction rule for pulmonary complications after thoracic surgery for primary lung cancer. Anesth. Analg. 2010, 110, 1343–1348. [Google Scholar] [CrossRef]
- Yepes-Temiño, M.J.; Monedero, P.; Pérez-Valdivieso, J.R.; Grupo Español de Anestesia Toracica. Risk prediction model for respiratory complications after lung resection: An observational multicentre study. Eur. J. Anaesthesiol. 2016, 33, 326–333. [Google Scholar] [CrossRef]
| Oncological Characteristics | |
|---|---|
| Clinical Factor | n Patients (%) |
| Primary tumor -Bone or soft tissue and other types of sarcoma -Colorectal cancer -Renal cell cancer -Head and neck cancer -Malignant melanoma -Breast cancer -Endometrial and ovarial carcinoma -Germ cell tumors -Urothelial carcinoma -Cholangiocellular carcinoma -Pancreatic cancer -Hepatocellular carcinoma -Gastric cancer -Esophageal cancer -Other | 459 (35.3%) 194 (14.9%) 87 (6.7%) 78 (6.0%) 74 (5.7%) 70 (5.4%) 40 (3.1%) 31 (2.4%) 27 (2.1%) 15 (1.2%) 14 (1.1%) 14 (1.1%) 12 (0.9%) 8 (0.6%) 161 (12.4%) |
| Gender -Male -Female -Unknown | 722 (56.3%) 560 (43.7%) 2 (-) |
| Preoperative therapy -Chemotherapy -Checkpoint inhibitor -Immunotherapy + chemotherapy -Chemotherapy + radiotherapy -Radiotherapy -Unknown | 336 (26.3%%) 1 (0.1%) 2 (0.2%) 263 (20.2%) 0 (0.0%) 117 (9%) |
| Surgical characteristics of the pulmonary metastasectomy | |
| Surgical approach -Open thoracotomy -VATS | 729 (56%) 554 (42.5%) |
| Surgical resection -Repeated pulmonary metastasectomy | 120 (9.2%) |
| Extent of the lung resection -Bilobectomy -Lobectomy -Pneumonectomy -Segmentectomy -Wedge resection | 3 (0.3%) 94 (8.5%) 10 (0.9%) 65 (5.9%) 936 (84.5%) |
| Lymphadenectomy -Not performed -Lymphadenectomy | 802 (61.6%) 482 (37.0%) |
| Number of wedge resection/operation -Single wedge resection -Multiple wedge resections | 419 (45.0%) 513 (55.0%) |
| Clinical Characteristics | n Patients (%) |
|---|---|
| Elderly patients (age > 70 years) | 261 (20%) |
| Obesity (BMI > 30) | 237 (18.2%) |
| ASA Score -ASA 3 -ASA 4 | 438 (33.6%) 34 (2.8%) |
| Hypertension | 445 (34.2%) |
| Cardiovascular comorbidity | 157 (12.1%) |
| Chronic kidney disease | 46 (3.5%) |
| Gastroesophageal reflux/gastric ulcer | 37 (2.8%) |
| COPD | 47 (3.6%) |
| Active smoker | 124 (9.5%) |
| Insulin-dependent diabetes | 31 (2.4%) |
| Liver disease | 49 (3.8%) |
| Myasthenia gravis | 3 (0.2%) |
| Other comorbidities | 589 (45.2%) |
| Postoperative Complication | n Patients (%) |
|---|---|
| At least one complication per patient | 145 (11.29%) |
| Major complications per patient | 32 (2.49%) |
| Major cardiopulmonary complications per patient Prolonged mechanical ventilation with the need for a tracheostomy ARDS Postoperative atelectasis requiring intervention Pneumonia Pleural empyema Bronchopleural fistula Myocardial infarct Postoperative atrial arrythmia Ventricular arrhythmia | 21 (1.6%) 1 (0.1%) 1 (0.1%) 5 (0.4%) 4 (0.3%) 3 (0.2%) 1 (0.1%) 1 (0.1%) 6 (0.5%) 3 (0.2%) |
| Minor complications per patient | 123 (9.57%) |
| Air leak > 5 days | 50 (3.8%) |
| Re-operation for bleeding | 10 (0.8%) |
| Cerebro-vascular complications | 2 (0.2%) |
| Chylothorax | 7 (0.5%) |
| Deep vene thrombosis | 1 (0.1%) |
| O2-need after hospital discharge | 2 (0.2%) |
| Chest wall hematoma | 4 (0.3%) |
| Renal failure | 2 (0.2%) |
| Wound infection | 5 (0.4%) |
| Mortality In-hospital mortality 30-day mortality 90-day mortality | 1 (0.07%) 11 (0.856%) 38 (3.0%) |
| Clinical Factor | No Complication Postoperative (n Patients, %) | Complication Postoperative (n Patients, %) | p-Value |
|---|---|---|---|
| Patients with at least one comorbidity preoperatively | 1120 (98.8%) | 145 (98.6%) | 0.89 |
| Elderly patients (age > 70 years) | 229 (20.1%) | 32 (21.8%) | 0.64 |
| Obesity (BMI > 30) | 226 (20.3%) | 11 (7.6%) | <0.01 |
| ASA score ASA score 3 ASA Score 4 | 387 (34%) 30 (2.6%) | 51 (34.7%) 4 (2.7%) | 0.98 |
| ECOG ECOG 2 ECOG 3 | 29 (2.6%) 8 (0.7%) | 4 (2.7%) 1 (0.7%) | 0.96 |
| Histology of the primary tumor -Bone or soft tissue and other types of sarcoma -Colorectal cancer | 408 (35.9%) 171 (15.0%) | 51 (34.7%) 23 (15.6%) | 0.47 |
| Hypertension | 395 (34.7%) | 50 (34.0%) | 0.86 |
| Cardiovascular comorbidity | 130 (11.4%) | 27 (18.4%) | 0.01 |
| Chronic kidney disease | 39 (3.4%) | 7 (4.8%) | 0.41 |
| Gastroesophageal reflux/gastric ulcer | 33 (2.9%) | 4 (2.7%) | 0.90 |
| COPD | 38 (3.3%) | 9 (6.1%) | 0.09 |
| Smoking status -Smoker (one month before the operation) -Past smoker | 110 (9.7%) 295 (25.9%) | 14 (9.5%) 50 (34.0%) | 0.17 |
| Insulin-dependent diabetes | 28 (2.5%) | 3 (2.0%) | 0.75 |
| Liver disease | 44 (3.9%) | 5 (3.4%) | 0.78 |
| Myasthenia gravis | 2 (0.2%) | 1 (0.7%) | 0.23 |
| Other preoperative comorbidities | 522 (45.9%) | 67 (45.6%) | 0.93 |
| Surgical Characteristics | No Complication Postoperative (n Patients, %) | Complication Postoperative (n Patients, %) | p-Value |
|---|---|---|---|
| Number of PM -Only one PM -RPM | 1028 (89.2%) 98 (81.7%) | 125 (10.8%) 22 (183%) | 0.01 |
| Lymphadenectomy -Not performed -Performed | 716 (89.3%) 421 (87.3%) | 86 (10.7%) 61 (12.7%) | 0.23 |
| Neoadjuvant therapy -Chemotherapy -Immunotherapy -Immunotherapy + chemotherapy -Chemotherapy + radiotherapy | 301 (26.6%) 1 (0.1%) 2 (0.2%) 234 (20.7%) | 35 (23.8%) 0 (0%) 0 (0%) 29 (19.7%) | 0.63 |
| Extent of the resection -Minor resection -Major resection | 893 (89.2%) 79 (73.8%) | 108 (10.8%) 28 (26.2%) | <0.01 |
| Surgical approach -Open thoracotomy -VATS | 625 (85.7%) 511 (92.2%) | 104 (14.3%) 43 (7.8%) | <0.01 |
| Number of wedge resections -Single -Multiple | 380 (90.7%) 455 (88.7%) | 39 (9.3%) 58 (11.3%) | 0.32 |
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| Preoperative cardiovascular comorbidities | 2.299 | 1.412–3.744 | 0.01 |
| Major lung resection | 2.727 | 1.678–4.431 | <0.01 |
| Repeated pulmonary metastasectomy | 1.759 | 1.040–2.976 | 0.03 |
| Open Thoracotomy | 0.621 | 0.415–0.930 | 0.02 |
| Essen Score | Patients in the Study Population Group (n Patients, %) | Complication Rate (%) | p-Value |
|---|---|---|---|
| I (THT) | 729 (56%) | 14.3% | - |
| II (THT + RPM) | 82 (6.3%) | 24.4% | 0.006 |
| IIA (THT + RPM + CVC) | 10 (0.77%) | 30% | 0.65 |
| III (THT + maj. resection) | 14 (1.09%) | 85.7% | <0.01 |
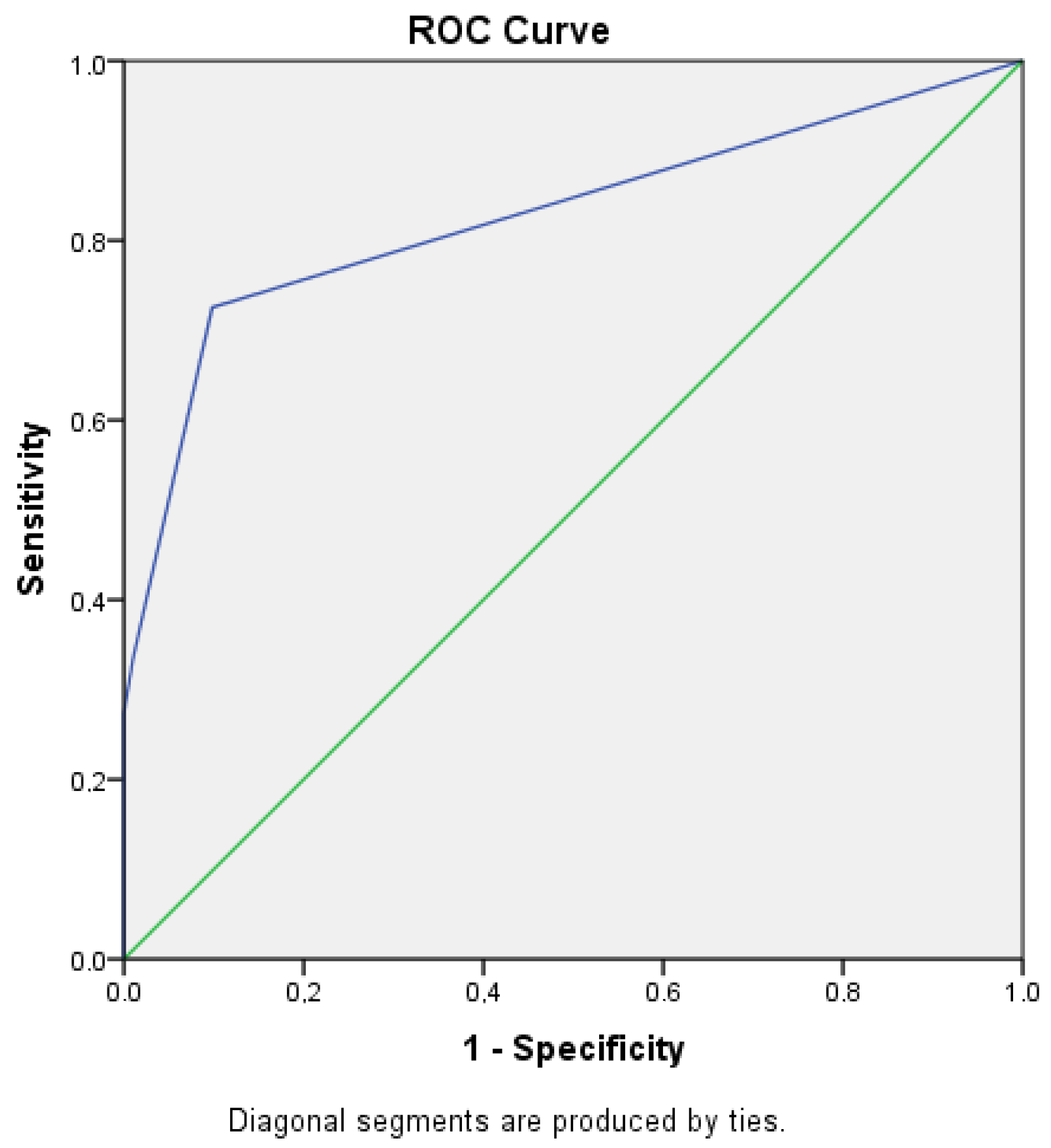
| OCC | ROC-Analysis | |
|---|---|---|
| Essen Score | 94.6% | 0.828 95% CI: 0.795–0.903 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grapatsas, K.; Dörr, F.; Menghesha, H.; Schuler, M.; Grünwald, V.; Bauer, S.; Schmidt, H.H.-J.; Lang, S.; Kimmig, R.; Kasper, S.; et al. New Prognostic Score (Essen Score) to Predict Postoperative Morbidity after Resection of Lung Metastases. Cancers 2023, 15, 4355. https://doi.org/10.3390/cancers15174355
Grapatsas K, Dörr F, Menghesha H, Schuler M, Grünwald V, Bauer S, Schmidt HH-J, Lang S, Kimmig R, Kasper S, et al. New Prognostic Score (Essen Score) to Predict Postoperative Morbidity after Resection of Lung Metastases. Cancers. 2023; 15(17):4355. https://doi.org/10.3390/cancers15174355
Chicago/Turabian StyleGrapatsas, Konstantinos, Fabian Dörr, Hruy Menghesha, Martin Schuler, Viktor Grünwald, Sebastian Bauer, Hartmut H. -J. Schmidt, Stephan Lang, Rainer Kimmig, Stefan Kasper, and et al. 2023. "New Prognostic Score (Essen Score) to Predict Postoperative Morbidity after Resection of Lung Metastases" Cancers 15, no. 17: 4355. https://doi.org/10.3390/cancers15174355
APA StyleGrapatsas, K., Dörr, F., Menghesha, H., Schuler, M., Grünwald, V., Bauer, S., Schmidt, H. H.-J., Lang, S., Kimmig, R., Kasper, S., Baldes, N., & Bölükbas, S. (2023). New Prognostic Score (Essen Score) to Predict Postoperative Morbidity after Resection of Lung Metastases. Cancers, 15(17), 4355. https://doi.org/10.3390/cancers15174355








