Simple Summary
Non-clear cell renal cell carcinoma (nccRCC) represents about the 20% of all RCCs but recommendations on treatment lacks evidence since the clinical trials include only clear cell RCC (ccRCC). The aim of our retrospective studies was to evaluate the efficacy of TKI and immunotherapy-based combinations in this population. We confirmed that nccRCC are heterogeneous and have a poorer prognosis as compared to ccRCC. The introduction of immunotherapy increased the efficacy of the treatments and the survival outcomes. Prognostic factors such as IMDC score or NLR are valid also for nccRCC. We highlight the importance of a pathological review and the need for prospective randomized trials designed for the different subtypes.
Abstract
Background: Non-clear cell renal cell carcinoma (nccRCC) is a heterogeneous group of cancer. Treatment recommendations are extrapolated from ccRCC and lack solid evidence. Here, we review advanced nccRCC patients treated at our institute. Patients and methods: We collected retrospective data on all advanced nccRCC pts treated at the Istituto Oncologico Veneto from January 2008. We compared overall response rate (ORR), progression free survival (PFS) and overall survival (OS) according to histological subtypes and type of systemic treatments. Kaplan-Meier method, log-rank test and Cox regression were used to estimate and compare PFS and OS. Results: Of 1370 RCC patients, 289 had a diagnosis of nccRCC and 121 were eligible for the analysis. Fifty-three pts showed papillary histology (pRCC), 15 chromophobe; 37 unclassified RCC (NOS-RCC), 16 other histologies. Pts with chromophobe and other hystologies showed poorer survival rates compared to pRCC and NOS-RCC (mOS 10.7 vs. 20.7 vs. 30.7, p = 0.34). Pts treated with combination regimens achieved a better OS (30.7 vs. 13.7, p = 0.10), PFS (12.7 vs. 6.4, p = 0.10) and ORR (42.4% vs. 13.9%, p = 0.002) than those treated with monotherapy. IMDC and Meet-URO score retained their prognostic value. Conclusion: Our retrospective real-life cohort of advanced nccRCC patients shows that immunotherapy-based combinations could improve ORR, PFS and OS compared to TKI monotherapy. Prospective trials for nccRCC patients utilizing novel therapies are ongoing and their results eagerly awaited.
1. Introduction
Renal cell carcinoma (RCC) is the most common type of kidney cancer in adults. Although RCCs are more frequently detected in the earlier stages by incidental abdominal imaging, there is still a remarkable proportion of patients with synchronous metastases and another 30–40% of patients that will develop distant metastasis after surgery [1,2].
Historically, RCC was clinically treated as two distinct entities: clear cell renal carcinoma (ccRCC) and non-clear cell renal cell carcinoma (nccRCC). According to 2016 WHO classification, nccRCC represents a highly heterogeneous group which is about 20% of RCC [3,4]. More than 15 nccRCC entities are listed including papillary renal cell carcinoma (pRCC), fumarate-hydratase deficient renal cell carcinoma (fhRCC), chromophobe renal cell carcinoma (chRCC), collecting duct carcinoma (CDC), renal medullary carcinoma (RMC), MiT family translocation renal cell carcinomas (tRCC) and oncocytoma among others. pRCC is the most frequent subtype (10–15%), followed by chRCC (5%), CDC (1%), RMC (1%), and tRCC (1%) [3].
Papillary RCCs were usually subclassified into type 1 and type 2 tumors [5], but due to diagnostic inconsistency and clinical irrelevance, this subdivision is not recommended in the 2022 WHO [6]. Molecular alterations to mesenchymal-epithelial transition (MET) including mutations and amplification of the chromosome 7 are found in 15–20% of sporadic cancers [7] in addition to rare hereditary forms characterized by MET germline mutation [8]. MET plays a significant role in the pathophysiology of pRCC and could be a therapeutic target now being investigated in clinical trials [9,10].
chRCC, like the oncocytoma, are generally characterized by an excellent prognosis but in the rare metastatic cases the outcome is very poor. It usually harbor mutations in PTEN and TP53, whole chromosome loss, and TERT gene rearrangement [11,12].
CDCs are aggressive tumors usually presenting with synchronous metastases and a very poor prognosis [13]. Cytogenetic alterations of chromosomes 1q, 8p, and 13q can be found [14].
tRCC encompasses a spectrum of fusions involving TFE3 (on Xp12), TFEB, and MITB. It has a higher incidence in pediatric patients and most of these translocations result in aggressive biology and a poor prognosis [15].
Finally, RCC with sarcomatoid differentiation (sRCC) is not a distinct morphogenetic subtype of RCC. The presence of a sarcomatoid component can be observed with any RCC histology and usually indicates a more aggressive biological behavior [16].
Since pivotal trials included only patients with ccRCC, treatments for nccRCC are mainly guided from data extrapolated from ccRCC trials or from promising case reports and retrospective series.
Data on the efficacy of single-agent target therapy in nccRCC demonstrated moderate activity in terms of response rates and limited overall survival [17]; however, NCCN and ESMO guidelines still recommend these treatments as the first-line option together with enrollment in clinical trials [18].
Nowadays, combination therapies based on immune checkpoint inhibitors have markedly improved the treatment of ccRCC but data on nccRCC are still scarce, taken from small or retrospective trials [19,20] where this subgroup is often described as a single entity, while it is actually composed of numerous subtypes with distinct carcinogenesis, prognosis and probably treatment sensitivity.
The aim of this study was to review the historical series of patients treated at our institute to provide real-world data on the prognosis and efficacy of the different therapies used for the various histotypes of nccRCC. We also tried to put these data into perspective and compare them with those found in literature in order to reach some final considerations on the best possible management and therapy.
2. Materials and Methods
Patient data were examined to evaluate the real-world setting of advanced nccRCC Patients were defined advanced if they have a metastatic disease or a locally advanced disease not amenable to curative surgery or radiotherapy. All consecutive patients with nccRCC treated at the Istituto Oncologico Veneto between January 2008 and October 2022 were assessed retrospectively. The inclusion criteria included a histological diagnosis of nccRCC according to WHO 2016 and the availability of at least baseline clinical and demographic information. Patients with ccRCC with sarcomatoid differentiation were excluded. But patients with exclusive presence of sarcomatoid component in the absence of clear cell areas were considered. Clinical data were extracted from electronic patient records (EPRs), available for all the patients at the Istituto Oncologico Veneto. EPRs were used to ascertain demographic information, histology information (staging according to TNM, grading), the risk group according to the IMDC classification [21], duration of treatment, best response, progression free survival (PFS) and overall survival (OS). Tumor assessment was scheduled according to standard clinical practice, and response was measured using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [22]. For all the assessments, patients were divided into 3 groups according to histotype: pRCC, undifferentiated and not otherwise specified RCC (NOS-RCC) and others (all the other patients); and 2 groups according to the type of treatment received: combination treatment (treatment combinations based on immunotherapy) or monotherapy (TKI alone or chemotherapy). Despite the different mechanism of action we decided to group TKI alone and chemotherapy considering these as the old standard treatment to be compared with the new immune-based combination therapies. In addition only few patients had received chemotherapy as first line treatment.
The Meet-URO score was also assessed in our patient cohort: this is a 5-class score defined by a multivariable model incorporating Neutrophil-to-Lymphocytes Ratio (NLR), IMDC score and bone metastases [23].
Key metrics were summarized by means of descriptive statistics. PFS and OS were estimated using the Kaplan-Meier method. The log-rank test was used to compare patients’ PFS and OS in accordance with histotype, the IMDC risk group and Meet-URO score. The Chi-squared test was used to determine the association between each pair of variables. Results were deemed as statistically significant if their p-values were <0.05. All statistical analyses were carried out using “R” v.4.2.3
3. Results
3.1. Patients’ Characteristics
Of 1370 patients with RCC, 289 had nccRCC (21%). Of these patients, 121 were treated for advanced nccRCC in our single-centre setting between January 2008 and October 2022. The majority were male (65.3%), with a median age of 64 years old. The vast majority of patients were in good general condition (ECOG 0–1: 77.7%) at presentation. The patients’ general characteristics are summarised in Table 1.

Table 1.
The patients’ general characteristics, including the IMDC classification and ECOG PS.
The most prevalent histology was pRCC (n = 53, 43.8%). The second most common histological type was chRCC (n = 15, 12.4%), followed by a small number of CDC (n = 5, 4.1%) and other, rarer histologies such as RMC (n = 1, 0.8%), tRCC (n = 1, 0.8%), malignant epithelioid angiomyolipoma (AML) (n = 2, 1.6%) and pure sRCC (n = 7, 5.8%). Moreover, NOS-RCC was reported for 37 patients (30.6%).
Regarding the type of treatment received, of 121 patients, 13 were only deemed suitable for best supportive care (10.7%) due to an inadequate performance status. Three patients (2.4%) with a limited metastatic spreading of the disease were treated with surgery or radiotherapy of the metastatic sites. The remaining 105 (86.8%) patients were treated with first line systemic therapy. Three patients (2.9%) received platinum salts-based chemotherapy while 69 (65.7%) patients receive TKI monotherapy and 33 (31.4%) patients received (IO-IO and IO-TKI in 12 and 21 patients respectively). First-line therapies according to the histotype are reported in Table 2.

Table 2.
First-line Systemic Therapies.
Eighty-eight patients progressed to first-line therapy during the follow-up period. Twenty-eight of these patients (31.8%) did not receive a second line treatment and were considered eligible only for best supportive care, in particular 11 out of 38 pRCC patients, 8 out of 24 NOS-RCC and 9 out of 26 patients with other histotypes. On the other hand, 20 patients (22.7%) received nivolumab in second line treatment while 2 patients (2.3%) received chemotherapy and the other 38 (43.2%) a TKI (mainly Cabozantinib and Sunitinib). Furthermore, at the time of this analysis, 22 (20.9%) received three or more lines of systemic therapy.
3.2. Treatment Outcomes
Median follow up of the entire population was 47.3 months (95% CI, 34.7–96.8). In particular, median follow up was 78.5 months (95% CI, 39.0–NA) for patients that received monotherapy treatment and 21.9 months (95% CI, 19.1–NA) for them that received immune-based combination treatment.
Best response to first-line therapy was evaluable in 103 patients. Of these 103 patients, 38 (36.9%) showed progressive disease at the first radiological evaluation. On the other hand, the best response was stable disease in 41 (39.8%) and partial response in 21 (20.4%) patients. Three (2.9%) complete responses were detected in our single-centre setting. One with pRCC, one with NOS-RCC and one with sRCC; two of them received a combination treatment but all were characterized by a single site (lung or lymph nodes) disease. ORR was superior for combination treatment than monotherapy in the entire population (42.4% vs. 13.9%, p = 0.002).
The first-line median PFS was 7.7 months (95% CI, 6.0–11.5), while the median OS was 18.5 months (95% CI, 13.3–33.2) for the 105 patients treated with systemic therapy. Comparing tumor histology, pRCC had an mPFS at the first-line treatment of 9.7 months (95% CI, 6.1–15.1), NOS-RCC had a mPFS of 9.2 (95% CI, 6.5–18.9) while other RCCs had a mPFS of 3.0 months (95% CI, 2.4–12.7) (p = 0.03). However, the mOS did not significantly differ between the three groups: mOS 20.7 months (95% CI, 13.7–42.4) vs. 30.7 (95% CI, 10.9–NR) vs. 10.7 (95% CI, 4.0–38.0) respectively (p = 0.34) (Figure 1).
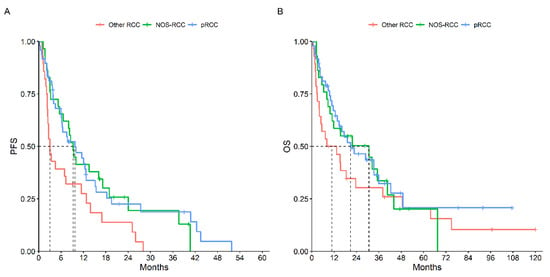
Figure 1.
PFS (A) and OS (B) According to Histotype.
On the other hand, comparing the type of treatment received, patients treated with first-line immune-based combination therapy compared to those treated with first-line monotherapy had a slightly better mPFS (12.7 months, 95% CI: 9.2–27.7 vs. 6.4 months, 95% CI: 3.8–10.0, p = 0.10) and mOS (30.7 months, 95% CI: 8–33 vs. 13.7 months, 95% CI: 9.8–28.9, p = 0.10) (Figure 2).
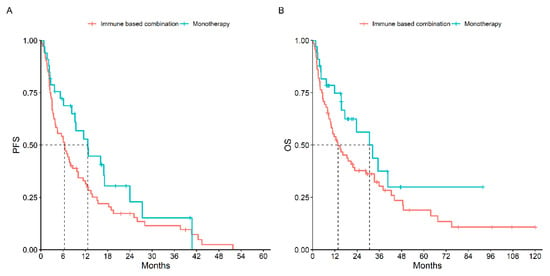
Figure 2.
PFS (A) and OS (B) According to Type of Treatment Received.
A total of 53 patients received first- or second-line immunotherapy. The mOS of patients receiving immunotherapy during their clinical history was significantly higher than those who had never received it: 32.4 months (95% CI, 19.2–48.5) vs. 9.8 months (95% CI, 7.2–21.7), p = 0.01 (Figure 3). Results of ORR, mPFS and mOS according to histotype and type of treatment are reported in Table 3.
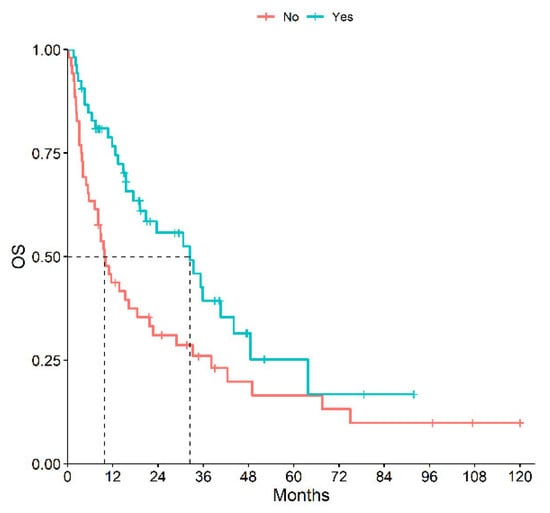
Figure 3.
OS of Patients Receiving or Not Immunotherapy during Their History.

Table 3.
Treatment Outcomes According to Histotype and Treatment Type.
In our cohort, 79 patients were metastatic at diagnosis but only 67 of these patients were treated. The PFS and OS were better for the 35 patients who underwent to a cytoreductive nephrectomy before the treatment compared to the 32 patients who received systemic treatment immediately (mPFS 7.2 months, 98% CI: 4.5–26.0 vs. 3.0 months, 95% CI: 2.3–8.5, respectively, p < 0.01; mOS 19.2 months, 95% CI: 13.3–44.1 vs. 8.4 months, 95% CI: 4.0–32.4, respectively, p = 0.05). In comparison to these results, the 38 remaining patients with metachronous disease who received systemic treatment at least 6 months after the nephrectomy (1 year for the majority) had a significantly longer PFS and OS (mPFS 12.0 months, 95% CI: 8.0–18.3; p = 0.002 and mOS 38.1 months, 95% CI: 20.7–NA; p = 0.003).
3.3. Prognostic Factors
We evaluated prognostic factors such as International Metastatic RCC Database Consortium (IMDC) score, neutrophil-to-lymphocyte ratio (NLR) and Meet-URO score as well as the number of metastatic sites. The IMDC score confirmed its prognostic role even in this setting: the mOS for patients classified as having a good, intermediate or poor risk was 48.5, 32.4 and 3.8 months, respectively (p < 0.01) while the mPFS was 13.5, 8.6 and 2.5 months respectively (p < 0.01).
The NLR was evaluable for 77 treated patients; using a cut-off of 3, it demonstrated significant differences between the two groups: patients with a NLR < 3 had a significantly better PFS and OS than patients with an NLR ≥ 3 (mOS 20.7 months, 95% CI: 12.6–40.5 vs. 5.4 months, 95% CI: 3.9–22.7, respectively, p < 0.01; mPFS 9.7 months, 95% CI: 6.5–15.4 vs. 3.8 months, 95% CI: 2.7–11.4, respectively, p = 0.03).
The Meet-URO score was available for all the patients. Ten out of 33 patients (30.3%) in class 4 and 5 did not received any treatment. These data confirm the poor prognosis of these patients and, in part due to the low number of treated patients in these classes, allow us to combine class 4 and 5 for the survival analysis. So, taking into consideration 4 categories of the Meet-URO score the mPFS was 11.5, 12, 6.8 and 2.4 months respectively (p < 0.01) and the mOS was 63.7, 33.2, 23.1 and 3.7 months respectively (p < 0.01).
Finally, we assessed the prognostic value of the number of metastatic sites. We found that there was a significant trend towards decreased OS rising from 1 to 2 to ≥3 sites involved (mOS 23.6 vs. 15.5 vs. 11.6 months respectively, p = 0.07).
4. Discussion
Despite examining all the cases of advanced/metastatic nccRCC at a high-volume institute, patient numbers were relatively low. Probably, a smaller proportion of nccRCC patients than those with ccRCC will develop metastatic disease and will be referred to an Oncology Department after complete surgery of the primary cancer. In fact, it is well-known that the most common non-clear histotype, pRCC and chRCC, usually has a better prognosis and a lower risk of cancer-associated death than ccRCC when diagnosed in a localized stage [12,24]. Furthermore, given that no adjuvant therapies are available for this subgroup of patients, the follow up could be performed without an oncological referral.
However, when locally advanced or metastatic, nccRCC seems to have a worse prognosis, compared to ccRCC [24]. In our case series, in fact, mPFS and mOS are about 8 and 18 months respectively without considering patients untreated because suitable only for best paliative care. In addition, mPFS and mOS were even shorter for patients with histotypes other than pRCC or NOS-RCC. This difference highlights the importance of the new WHO classification where, for example, the division between type 1 and 2 in pRCC is no longer recommended and the Fumarate Hydratase deficient RCC is now considered a unique entity [6]. Furthermore, considering all the prognostic and therapeutical implications, this emphasizes the importance of an expert pathological review. In our retrospective cohort, the incidence of different nccRCC histologic subtypes was comparable to published data, with a higher ratio of NOS-RCC because many cases were not reviewed [25]. This group of patients had a mPFS and mOS similar to the pRCC (where type 1 and 2 were included indistinctly) but it is probably composed of many different subtypes with likely very different sensitivities to the treatments and certainly very different outcomes.
The majority of patients (65.3%) in our single-centre study were metastatic at diagnosis and about half of them (46.8%) underwent cytoreductive nephrectomy. The efficacy of this conventional practice has been questioned by the results of the CARMENA trial [26]; however, it was standard practice in the period in which our patients were diagnosed. It should be noted that only ccRCC patients were included in the CARMENA trial, but some retrospective evidence, in line with our results, suggests a role for cytoreductive surgery in selected patients with metastatic nccRCC [27].
As expected, most of the patients who received systemic first-line therapy were treated with only a tyrosine kinase inhibitor (TKI), predominantly Sunitinib. PFS and OS in these patients were similar to those reported in literature [9,10,28,29,30,31,32,33,34]. Significant TKI data are being accumulated in these trials with nccRCC patients: in the ESPN trial, Sunitinib performed slightly better (median OS 16.2 vs. 13 months) [30], while Cabozantinib showed promising results in the SWOG 1500 trial [10]. In our historical cohort, however, only 13 patients received Cabozantinib and the group size is too small for any statistical inferences. Finally, the MET inhibitors Savolitinib and Foretinib demonstrated promising data in nccRCC in phase II trials [10,35,36]. However, despite the fact that the phase III trial SAVOIR confirmed the encouraging efficacy of Savolitinib, small patient numbers and short follow-up were unsuitable to reaching robust conclusions [33]. In summary, as indicated by the literature [37], the response rate is poor and the majority of the patients treated with a TKI only progressed at their first or second assessment.
Therefore, there has been an increase in data on immunotherapy and immuno-based combination therapy in recent years [20]. When used as single agents, Nivolumab [38] and Pembrolizumab [39] demonstrated short PFS (2.2–4.2 months) but promising ORR (13.6 and 26.7% respectively). Due to these data, IO-based combinations like Ipilimumab-Nivolumab [40], Atezolizumab-Cabozantinib [41], Nivolumab-Cabozantinib [42], Durvalumab-Savolitinib [43] and Pembrolizumab-Lenvatinib [44] were tested in prospective trials. The results of these trails are very promising, with PFS varying from 3.7 to 12.5 months and ORR ranging froffm 19.6% to 49%.
In our findings, patients who were treated with a first-line immunotherapy combination had a PFS of 12.7 months and an ORR of 42.4%. These results are in line with those reported in the prospective trials described above but, more importantly, are similar to those recently reported in two real-world experiences [25,45]. Furthermore, we can also report that patients who received immunotherapy in their history achieved longer OS than those who did not. This result confirms that immunotherapy could be an effective option for nccRCC patients and combination treatment in first line is a promising strategy in this subgroup of patients. Nevertheless, there are different outcomes based on the histologic subtypes, which require further investigation. It is important, in fact, that future randomized trials take into consideration the differences between the various histotypes, limiting the rate of NOS-RCC, in order to understand the individual sensitivities to treatment and implement biology-tailored management.
Fortunately, prognostic scores appear to maintain their ability to stratify patients even in nccRCC. In our cohort, we found that the IMDC score confirmed its strong prognostic role as expected. In fact, between 5% and 13% of nccRCC patients were included in both the first IMDC score report [21] and in the external validation group [46]. Notably, non-clear cell histology was associated with poor OS. Nevertheless, the score is still significant in nccRCC patients, comparable to data previously published by Kroeger et al. [47]. We also tested in our cohort the Meet-URO score, which was implemented in collaboration with our research group [23] and validated in patients treated with either Nivolumab or Cabozantinib in second-line treatment [48]. Even in an unselected cohort of nccRCC patients, the Meet-URO score retained its prognostic value and was able to stratify patients accurately according to their prognosis.
Finally, the NLR score also proved valuable in our cohort of nccRCC patients. The negative prognostic value of the NLR, in fact, is largely known in ccRCC but only few trials include and consider nccRCC patients [49,50]. These trials, in line with our report, verify that the NLR retains its value in this population but also confirm that patients with nccRCC have a higher NLR compared to patients with ccRCC [49].
Our trial has several limitations, including the retrospective collection of data with the possible risk of selection bias, the short follow-up, especially for the combination treatments, the small number of patients treated and the lack of a central pathological review. Nevertheless, this trial, like that reported by Izarn et al. [25], reflects what really happens in a referral center and all the challenges that an oncologist has to face when dealing with an nccRCC patient. These limitations, in fact, underline the significance of the pathological classification and the need to perform an expert review in order to reach a correct diagnosis and take an appropriate treatment decision. In addition, this work emphasizes the importance of immunotherapy and, overall, the importance of prospective randomized clinical trials, which confirm these results, to improving the standard of care and the survival outcomes in this heterogeneous and poor prognosis population.
5. Conclusions
NccRCCs are uncommon but account for about 20% of all RCCs. Our real-world data are comparable to those published in the literature for nccRCC and demonstrated that immunotherapy has led to an improvement in the prognosis, especially when used in first-line combination treatments.
Nonetheless, advancements in histopathology and molecular genetics are necessary in order to open new horizons for therapeutic options; while the results of the ongoing prospective selective trials for nccRCC utilizing IO and new targeted agents are urgently required to further improve patient outcome. In future, we recommend studies tailored to individual histologies because the nccRCC is group is too heterogeneous and, to date, this “umbrella” definition is limiting and should already be viewed as outdated.
Author Contributions
All authors made substantial contributions to the conception or design of the work; the acquisition, analysis and interpretation of data; drafting the work or its substantive revision; and approving the submitted version. All authors agrees to being personally accountable for their own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even in which the author was not personally involved, are appropriately investigated, resolved and documented in the literature. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Istituto Oncologico Veneto-IRCCS on 17 February 2020.
Informed Consent Statement
At the time of their first visit to our institute, all patients gave their written consent for the use of their clinical data for scientific purposes.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical reasons.
Conflicts of Interests
The authors have no financial relationships or conflicts of interest to declare.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Ravi, P.; Abdollah, F.; Abd-El-Barr, A.E.; Becker, A.; Popa, I.; Briganti, A.; Karakiewicz, P.I.; Trinh, Q.D.; Jewett, M.A.; et al. Contemporary incidence and mortality rates of kidney cancer in the United States. Can. Urol. Assoc. J. 2014, 8, 247–252. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Ahrens, M.; Scheich, S.; Hartmann, A.; Bergmann, L.; IAG-N Interdisciplinary Working Group Kidney Cancer of the German Cancer Society. Non-Clear Cell Renal Cell Carcinoma—Pathology and Treatment. Oncol. Res. Treat. 2019, 42, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Barthélémy, P.; Rioux-Leclercq, N.; Thibault, C.; Saldana, C.; Borchiellini, D.; Chevreau, C.; Desmoulins, I.; Gobert, A.; Hilgers, W.; Khalil, A.; et al. Non-clear cell renal carcinomas: Review of new molecular insights and recent clinical data. Cancer Treat. Rev. 2021, 97, 102191. [Google Scholar] [CrossRef] [PubMed]
- Alaghehbandan, R.; Siadat, F.; Trpkov, K. What’s new in the WHO 2022 classification of kidney tumours? Pathologica 2022, 115, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Durinck, S.; Stawiski, E.W.; Pavía-Jiménez, A.; Modrusan, Z.; Kapur, P.; Jaiswal, B.S.; Zhang, N.; Toffessi-Tcheuyap, V.; Nguyen, T.T.; Pahuja, K.B.; et al. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat. Genet. 2015, 47, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Duh, F.M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M.; et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef]
- Schöffski, P.; Wozniak, A.; Escudier, B.; Rutkowski, P.; Anthoney, A.; Bauer, S.; Sufliarsky, J.; van Herpen, C.; Lindner, L.H.; Grünwald, V.; et al. Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type. Eur. J. Cancer 2017, 87, 147–163. [Google Scholar] [CrossRef]
- Pal, S.K.; Tangen, C.; Thompson, I.M.; Balzer-Haas, N.; George, D.J.; Heng, D.Y.C.; Shuch, B.; Stein, M.; Tretiakova, M.; Humphrey, P.; et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial. Lancet 2021, 397, 695–703. [Google Scholar] [CrossRef]
- Davis, C.F.; Ricketts, C.J.; Wang, M.; Yang, L.; Cherniack, A.D.; Shen, H.; Buhay, C.; Kang, H.; Kim, S.C.; Fahey, C.C.; et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 2014, 26, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Novara, G.; Antonelli, A.; Bertini, R.; Billia, M.; Carmignani, G.; Cunico, S.C.; Longo, N.; Martignoni, G.; Minervini, A.; et al. Chromophobe renal cell carcinoma (RCC): Oncological outcomes and prognostic factors in a large multicentre series. BJU Int. 2012, 110, 76–83. [Google Scholar] [CrossRef]
- Wright, J.L.; Risk, M.C.; Hotaling, J.; Lin, D.W. Effect of collecting duct histology on renal cell cancer outcome. J. Urol. 2009, 182, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Choueiri, T.K.; Wang, K.; Khaira, D.; Karam, J.A.; Van Allen, E.; Palma, N.A.; Stein, M.N.; Johnson, A.; Squillace, R.; et al. Characterization of Clinical Cases of Collecting Duct Carcinoma of the Kidney Assessed by Comprehensive Genomic Profiling. Eur. Urol. 2016, 70, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Furuya, M.; Baba, M.; Kameda, Y.; Yasuda, M.; Nishimoto, K.; Oyama, M.; Yamasaki, T.; Ogawa, O.; Niino, H.; et al. RBM10-TFE3 renal cell carcinoma characterised by paracentric inversion with consistent closely split signals in break-apart fluorescence in-situ hybridisation: Study of 10 cases and a literature review. Histopathology 2019, 75, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Pichler, R.; Compérat, E.; Klatte, T.; Pichler, M.; Loidl, W.; Lusuardi, L.; Schmidinger, M. Renal Cell Carcinoma with Sarcomatoid Features: Finally New Therapeutic Hope? Cancers 2019, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, C.; Iacovelli, R.; Brunelli, M.; Massari, F.; Bimbatti, D.; Fantinel, E.; De Marco, V.; Porcaro, A.B.; Martignoni, G.; Artibani, W.; et al. Addressing the best treatment for non-clear cell renal cell carcinoma: A meta-analysis of randomised clinical trials comparing VEGFR-TKis versus mTORi-targeted therapies. Eur. J. Cancer 2017, 83, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A.; ESMO Guidelines Committee. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef]
- Gulati, S.; Philip, E.; Salgia, S.; Pal, S.K. Evolving treatment paradigm in metastatic non clear cell renal cell carcinoma. Cancer Treat. Res. Commun. 2020, 23, 100172. [Google Scholar] [CrossRef]
- Climent, C.; Soriano, S.; Bonfill, T.; Lopez, N.; Rodriguez, M.; Sierra, M.; Andreu, P.; Fragio, M.; Busquets, M.; Carrasco, A.; et al. The role of immunotherapy in non-clear cell renal cell carcinoma. Front. Oncol. 2023, 13, 941835. [Google Scholar] [CrossRef]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Rebuzzi, S.E.; Signori, A.; Banna, G.L.; Maruzzo, M.; De Giorgi, U.; Pedrazzoli, P.; Sbrana, A.; Zucali, P.A.; Masini, C.; Naglieri, E.; et al. Inflammatory indices and clinical factors in metastatic renal cell carcinoma patients treated with nivolumab: The development of a novel prognostic score (Meet-URO 15 study). Ther. Adv. Med. Oncol. 2021, 13, 17588359211019642. [Google Scholar] [CrossRef] [PubMed]
- Steffens, S.; Janssen, M.; Roos, F.C.; Becker, F.; Schumacher, S.; Seidel, C.; Wegener, G.; Thüroff, J.W.; Hofmann, R.; Stöckle, M.; et al. Incidence and long-term prognosis of papillary compared to clear cell renal cell carcinoma—A multicentre study. Eur. J. Cancer 2012, 48, 2347–2352. [Google Scholar] [CrossRef] [PubMed]
- Izarn, F.; Allignet, B.; Gille, R.; Boyle, H.; Neidhardt, E.M.; Négrier, S.; Fléchon, A. Real World Data of Diagnosis, Survival, and Treatment Outcomes in Patients with Metastatic Non-Clear Cell Renal Cell Carcinoma. Clin. Genitourin. Cancer 2023, 21, e35–e43. [Google Scholar] [CrossRef] [PubMed]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Colas, S.; Beauval, J.B.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Aizer, A.A.; Urun, Y.; McKay, R.R.; Kibel, A.S.; Nguyen, P.L.; Choueiri, T.K. Cytoreductive nephrectomy in patients with metastatic non-clear-cell renal cell carcinoma (RCC). BJU Int. 2014, 113, E67-74. [Google Scholar] [CrossRef]
- Paglino, C.; Imarisio, I.; Ganini, C.; Morbini, P.; Vercelli, A.; Bregant, C.; Porta, C. Sunitinib in advanced metastatic non-clear cell renal cell carcinoma: A single institution retrospective study. Future Oncol. 2012, 8, 1605–1612. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Halabi, S.; Eisen, T.; Broderick, S.; Stadler, W.M.; Jones, R.J.; Garcia, J.A.; Vaishampayan, U.N.; Picus, J.; Hawkins, R.E.; et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016, 17, 378–388. [Google Scholar] [CrossRef]
- Tannir, N.M.; Jonasch, E.; Albiges, L.; Altinmakas, E.; Ng, C.S.; Matin, S.F.; Wang, X.; Qiao, W.; Dubauskas Lim, Z.; Tamboli, P.; et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur. Urol. 2016, 69, 866–874. [Google Scholar] [CrossRef]
- Negrier, S.; Rioux-Leclercq, N.; Ferlay, C.; Gross-Goupil, M.; Gravis, G.; Geoffrois, L.; Chevreau, C.; Boyle, H.; Rolland, F.; Blanc, E.; et al. Axitinib in first-line for patients with metmetastatic papillary renal cell carcinoma: Results of the multicentre, open-label, single-arm, phase II AXIPAP trial. Eur. J. Cancer 2020, 129, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Ravaud, A.; Oudard, S.; De Fromont, M.; Chevreau, C.; Gravis, G.; Zanetta, S.; Theodore, C.; Jimenez, M.; Sevin, E.; Laguerre, B.; et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: A phase II study (SUPAP) by the French Genitourinary Group. (GETUG). Ann. Oncol. 2015, 26, 1123–1128. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Heng, D.Y.C.; Lee, J.L.; Cancel, M.; Verheijen, R.B.; Mellemgaard, A.; Ottesen, L.H.; Frigault, M.M.; L’Hernault, A.; Szijgyarto, Z.; et al. Efficacy of Savolitinib vs. Sunitinib in Patients With MET-Driven Papillary Renal Cell Carcinoma: The SAVOIR Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1247–1255. [Google Scholar] [CrossRef]
- Buti, S.; Bersanelli, M.; Massari, F.; De Giorgi, U.; Caffo, O.; Aurilio, G.; Basso, U.; Carteni, G.; Caserta, C.; Galli, L.; et al. First-line pazopanib in patients with advanced non-clear cell renal carcinoma: An Italian case series. World J. Clin. Oncol. 2021, 12, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Vaishampayan, U.; Rosenberg, J.E.; Logan, T.F.; Harzstark, A.L.; Bukowski, R.M.; Rini, B.I.; Srinivas, S.; Stein, M.N. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J. Clin. Oncol. 2013, 31, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Plimack, E.; Arkenau, H.T.; Jonasch, E.; Heng, D.Y.C.; Powles, T.; Frigault, M.M.; Clark, E.A.; Handzel, A.A.; Gardner, H.; et al. Biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer. J. Clin. Oncol. 2017, 35, 2993–3001. [Google Scholar] [CrossRef]
- Fernández-Pello, S.; Hofmann, F.; Tahbaz, R.; Marconi, L.; Lam, T.B.; Albiges, L.; Bensalah, K.; Canfield, S.E.; Dabestani, S.; Giles, R.H.; et al. A Systematic Review and Meta-analysis Comparing the Effectiveness and Adverse Effects of Different Systemic Treatments for Non-clear Cell Renal Cell Carcinoma. Eur. Urol. 2017, 71, 426–436. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, J.J.; Kochenderfer, M.D.; Olsen, M.R.; Bauer, T.M.; Molina, A.; Hauke, R.J.; Reeves, J.A.; Babu, S.; Van Veldhuizen, P.; Somer, B.; et al. Safety and Efficacy of Nivolumab in Patients with Advanced Non-Clear Cell Renal Cell Carcinoma: Results From the Phase IIIb/IV CheckMate 374 Study. Clin. Genitourin. Cancer 2020, 18, 469–476. [Google Scholar] [CrossRef]
- McDermott, D.F.; Lee, J.L.; Ziobro, M.; Suarez, C.; Langiewicz, P.; Matveev, V.B.; Wiechno, P.; Gafanov, R.A.; Tomczak, P.; Pouliot, F.; et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients with Advanced Non-Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2021, 39, 1029–1039. [Google Scholar] [CrossRef]
- Tykodi, S.S.; Gordan, L.N.; Alter, R.S.; Arrowsmith, E.; Harrison, M.R.; Percent, I.; Singal, R.; Van Veldhuizen, P.; George, D.J.; Hutson, T.; et al. Safety and efficacy of nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma: Results from the phase 3b/4 CheckMate 920 trial. J. Immunother. Cancer 2022, 10, e003844. [Google Scholar] [CrossRef]
- Pal, S.K.; McGregor, B.; Suárez, C.; Tsao, C.K.; Kelly, W.; Vaishampayan, U.; Pagliaro, L.; Maughan, B.L.; Loriot, Y.; Castellano, D.; et al. Cabozantinib in Combination with Atezolizumab for Advanced Renal Cell Carcinoma: Results From the COSMIC-021 Study. J. Clin. Oncol. 2021, 39, 3725–3736. [Google Scholar] [CrossRef]
- Lee, C.H.; Voss, M.H.; Carlo, M.I.; Chen, Y.B.; Zucker, M.; Knezevic, A.; Lefkowitz, R.A.; Shapnik, N.; Dadoun, C.; Reznik, E.; et al. Phase II Trial of Cabozantinib Plus Nivolumab in Patients with Non-Clear-Cell Renal Cell Carcinoma and Genomic Correlates. J. Clin. Oncol. 2022, 40, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Suárez, C.; Larkin, J.M.G.; Patel, P.; Valderrama, B.P.; Rodriguez-Vida, A.; Glen, H.; Thistlethwaite, F.; Ralph, C.; Srinivasan, G.; Mendez-Vidal, M.J.; et al. Phase II Study Investigating the Safety and Efficacy of Savolitinib and Durvalumab in Metastatic Papillary Renal Cancer (CALYPSO). J. Clin. Oncol. 2023, 41, 2493–2502. [Google Scholar] [CrossRef]
- Albiges, L.; Gurney, H.P.; Atduev, V.; Suárez, C.; Climent Duran, M.A.; Pook, D.; Tomczak, P.; Barthelemy, P.; Lee, J.; Nalbandian, T.; et al. Phase II KEYNOTE-B61 study of pembrolizumab (Pembro) + lenvatinib (Lenva) as first-line treatment for non-clear cell renal cell carcinoma (nccRCC). Ann. Oncol. 2022, 33, S660–S680. [Google Scholar] [CrossRef]
- Stellato, M.; Buti, S.; Maruzzo, M.; Bersanelli, M.; Pierantoni, F.; De Giorgi, U.; Di Napoli, M.; Iacovelli, R.; Vitale, M.G.; Ermacora, P.; et al. Pembrolizumab Plus Axitinib for Metastatic Papillary and Chromophobe Renal Cell Carcinoma: NEMESIA (Non Clear MEtaStatic Renal Cell Carcinoma Pembrolizumab Axitinib) Study, a Subgroup Analysis of I-RARE Observational Study (Meet-URO 23a). Int. J. Mol. Sci. 2023, 24, 1096. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, N.; Xie, W.; Lee, J.L.; Bjarnason, G.A.; Knox, J.J.; Mackenzie, M.J.; Wood, L.; Srinivas, S.; Vaishamayan, U.N.; Rha, S.Y.; et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: Characterization of survival outcome and application of the International mRCC Database Consortium. criteria. Cancer 2013, 119, 2999–3006. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Cerbone, L.; Signori, A.; Santoni, M.; Murianni, V.; De Giorgi, U.; Procopio, G.; Porta, C.; Milella, M.; Basso, U.; et al. Application of the Meet-URO score to metastatic renal cell carcinoma patients treated with second- and third-line cabozantinib. Ther. Adv. Med. Oncol. 2022, 14, 17588359221079580. [Google Scholar] [CrossRef]
- Chen, X.; Meng, F.; Jiang, R. Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Patients with Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 746976. [Google Scholar] [CrossRef]
- Lalani, A.A.; Xie, W.; Martini, D.J.; Steinharter, J.A.; Norton, C.K.; Krajewski, K.M.; Duquette, A.; Bossé, D.; Bellmunt, J.; Van Allen, E.M.; et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J. Immunother. Cancer 2018, 6, 5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).