Simple Summary
The article presents the role of alpha-fetoprotein in the diagnosis and monitoring of treatment for selected genetic diseases and early childhood cancers. The authors draw attention to diagnostic pitfalls related to physiological AFP production in the first year of life, inconsistencies in laboratory tests, and result interpretation.
Abstract
Alpha-fetoprotein (AFP) is a protein commonly found during fetal development, but its role extends beyond birth. Throughout the first year of life, AFP levels can remain high, which can potentially mask various conditions from the neurological, metabolic, hematological, endocrine, and early childhood cancer groups. Although AFP reference values and clinical utility have been established in adults, evaluating AFP levels in children during the diagnostic process, treatment, and post-treatment surveillance is still associated with numerous diagnostic pitfalls. These challenges arise from the presence of physiologically elevated AFP levels, inconsistent data obtained from different laboratory tests, and the limited population of children with oncologic diseases that have been studied. To address these issues, it is essential to establish updated reference ranges for AFP in this specific age group. A population-based study involving a statistically representative group of patients could serve as a valuable solution for this purpose.
1. Introduction
The half-life of AFP in neonatal and infant populations has been determined to be 5.5 days at birth, 11 days between 14 and 30 days after birth, and 33 days up to 4 months of age. The rate at which AFP degrades depends on factors such as birth weight, feeding method, gestational age at birth, and the additional production of this fetal protein by the neonatal liver after delivery [1] (see Table 1 and Figure 1). Immediately after delivery, newborns typically exhibit alpha-fetoprotein (AFP) concentrations ranging from approximately 17,200 to 44,300 ng/mL [1,2]. However, prematurely born neonates tend to have an average concentration of 158,125 ng/mL [2]. The higher AFP level in premature neonates is attributed to their lower body weight and a less pronounced dilution effect. Over the course of the first 12 months after birth, the infant’s AFP concentration gradually decreases [1,2]. Pediatricians should bear in mind that the AFP levels can be high in first year of life and not implement hasty oncological diagnostics. Generally, the most significant decline occurs within the first 8 months postpartum, after which the AFP levels reach values typical for adults, with a maximum of 10–15 ng/mL in the serum [1,2].

Table 1.
Serum alpha-fetoprotein (AFP) levels in term neonates [2].
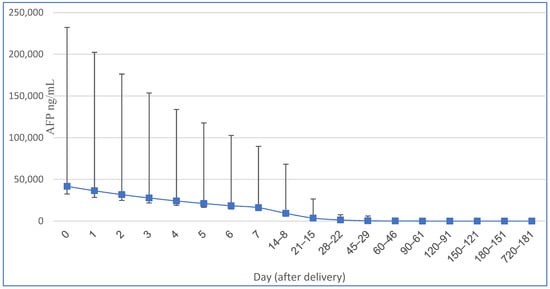
Figure 1.
Serum AFP changes (and 95.5% interval) in term neonates modified from [2].
2. AFP—Diagnostic Difficulties in Pediatrics
The precise definition of reference ranges and clinical utility of AFP in adults contrasts with the ambiguity surrounding its application in pediatric oncology. Studies conducted in pediatric oncology since the 1970s have employed varying reference standard ranges for AFP and lack stringent reference values for biological materials other than serum, such as cerebrospinal fluid (CSF) [3]. Several factors contribute to this situation, which are outlined below.
2.1. Postpartum AFP Concentrations
Differentiating pathological AFP concentrations in newborns poses significant challenges due to the ongoing high physiological production of AFP during the fetal period. The AFP level produced by a neoplasm may not exceed the physiological concentrations observed in infancy. Therefore, it becomes particularly challenging to exclude a neoplastic process, especially in children born prematurely and up to 4 months of age [3,4].
2.2. Methods of Determination and Establishment of AFP Reference Values
Current recommendations in pediatric oncology continue to rely on AFP reference ranges that are based on tests conducted over 40 years ago, utilizing RIA tests calibrated to internal standards. However, the diagnostic tests employed today yield results that are essentially incomparable to those obtained 20–40 years ago. Furthermore, due to the lack of studies employing modern tests on newborns, the veracity of the previously reported high physiological values for the neonatal period remains uncertain.
2.3. Comparing the Results of AFP Concentrations
Currently, the harmonization of diagnostic tests for AFP among laboratories continues to rely on the 1975 WHO international standard. The test results are reported in international units (IU). However, the prevailing practice is to convert these results into micrograms per liter or milliliter (µg/L or µg/mL). It is usually assumed that 1200 ng of AFP corresponds to 1000 IU [5]. However, the interpretation of this conversion can vary depending on the specific test, leading to significant variability in results. In fact, some laboratories even dispute the validity of this standard altogether [3].
2.4. Size of Study Groups
Setting reference standards for AFP in pediatric oncology is challenging due to the limited number of cases available for testing. The rarity of cancer in children makes it difficult to obtain data from a larger population, resulting in a reliance on case reports and small case series. As a consequence, the reliability of reference ranges for AFP in pediatric oncology is relatively low. It may be more practical to focus on evaluating the dynamics of changes in AFP concentrations, which tend to increase for primary lesions and gradually decrease for recurrent tumors. Additionally, assessing the half-life of AFP, which is prolonged in tumors, can provide valuable information [6]. In pediatric oncology, diagnostic imaging and histopathology should be prioritized as more reliable methods, while AFP evaluation serves a complementary role. Nonetheless, there are selected genetic syndromes and pediatric cancers in which AFP holds the potential for diagnostic, monitoring, and prognostic applications (Figure 2).
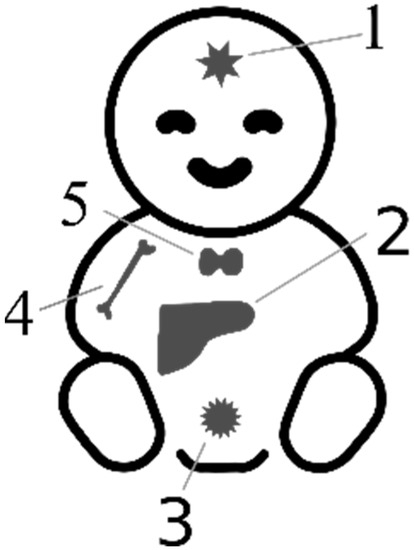
Figure 2.
Sources of elevated AFP in infancy and childhood. 1—Intracranial germ cell tumors (IC-GCTs); 2—hepatoblastoma (HB), hepatocellular carcinoma (HCC), ataxia teleangiectasia (AT), primrose syndrome, tyrosynemia type I, neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD), progressive familiar intrahepatic cholestasis (PFIC2), transaldolase deficiency (TALDO), hepatitis B (HBV); 3—malignant saccrococygeal GCT; 4—Fanconi anemia; 5—congenital hypothyroidism, van Wyk–Grumbach syndrome.
3. Liver
3.1. Ataxia Telangiectasia (AT)
The condition occurs with a frequency of 1:40,000 to 1:100,000 live births, making it the second most common autosomal recessive ataxia in children, following Friedrich ataxia. The underlying cause is a mutation in the ATM gene located on chromosome 11 at locus q22–23, which is responsible for DNA repair and regulation of the cell cycle by controlling the synthesis of the suppressor protein TP53. In 90% of patients, AFP levels are elevated, which distinguishes it from Friedrich ataxia [7,8]. This was demonstrated by Waldmann et al. in the 1970s, who conducted a study involving parents and siblings of children with AT [7,8]. AFP testing is not feasible in AT patients until they reach 2 years of age, primarily due to the high physiological levels of AFP in infants and the postnatal decline dynamics. Carrier individuals with the mutated gene typically have normal AFP levels, while AFP levels are high and tend to increase with age in AT patients [9]. There are several hypotheses regarding elevated AFP levels in certain conditions. The first hypothesis suggests that the increase in AFP is associated with progressive liver damage. The second hypothesis focuses on the role of the suppressor protein TP53, which plays a role in DNA damage repair and also acts as a repressor of the gene responsible for AFP synthesis during liver development and regeneration. When TP53 is deficient due to ATM mutations, AFP levels rise. A third hypothesis suggests that AFP synthesis increases in response to the damaged CNS’s need for building blocks for cell membranes during the process of myelination. AFP serves as a carrier protein for polyunsaturated fatty acids (PUFAs), which are essential for this purpose [9,10,11,12].
3.2. Primrose Syndrome
The condition occurs with a frequency of 1:1,000,000 births and follows an autosomal dominant inheritance pattern. It is associated with a de novo mutation in the ZBTB20 gene, which leads to a microdeletion at locus 3q13.31. The ZBTB20 gene acts as a key repressor of DNA transcription during birth and is responsible for various processes such as neurogenesis, fetal liver development, cell growth, detoxification, and glucose metabolism. The clinical presentation of the condition includes intellectual disability, macrocephaly, high postnatal growth, cataracts, deafness, auricular calcifications, and myopathy. The high levels of AFP observed in this condition are a consequence of the mutation in the ZBTB20 gene, which leads to the unblocking of AFP synthesis in the liver (normally, the gene acts as a repressor) [13].
3.3. Type I Tyrosinemia
The condition is found in approximately 1:100,000 births and follows an autosomal recessive inheritance pattern due to a mutation in the FAH gene located on chromosome 15q23–q25. This mutation results in a deficiency of fumarylacetoacetase hydrolase, leading to the accumulation of toxic tyrosine metabolites, namely fumarylacetoacetate and maleylacetoacetate, in the liver and kidneys. These metabolites have mutagenic properties and inhibit porphobilinogen synthesis, leading to porphyria-like seizures [14].
In the early form of the disease (<2 months of age), there is acute liver failure, which carries a high mortality rate. High levels of AFP, a marker of early liver regeneration that begins in fetal life, are particularly observed in this form. In the late form (occurring after 6 months of age), cirrhosis, hypophosphatemic rickets, and liver failure are typical. With time, the risk of liver cancer increases; it occurs in 37% of patients over 2 years of age, typically between 4 and 5 years of age. The most common form is hepatocellular carcinoma (HCC), but hepatoblastoma (HB), as well as mixed types, can also occur. AFP serves as an early marker of hepatic remodeling, neoplastic transformation, and porphyria seizures in this condition [15,16,17]. According to Koelink et al., not only an increase in AFP levels but also a sustained steady level with a weak downward trend can predict the onset of HCC [18]. Long-term administration of nitisinone (2-(2-nitro-4-3 trifluoro-methylbenzoyl)-1,3-cyclohexanedione—NTBC), a well-established therapy for patients with type I tyrosinemia, is recommended. NTBC reduces the risk of HCC and porphyria attacks and leads to a decrease in AFP levels [19,20]. However, Bhushan et al. observed in their patient cases that even with long-term NTBC therapy and normalization of AFP, the risk of HCC is not completely eliminated [16,21].
3.4. Progressive Familial Intrahepatic Cholestasis—PFIC2
The condition occurs with a frequency of approximately 1:50,000–1:100,000 births and follows an autosomal recessive inheritance pattern. The underlying mutation affects the ABCB11 gene, located on chromosome 2 (2q24), which is responsible for encoding the BSEP protein. BSEP is a membrane transport protein found on the surface of hepatocytes. The mutation disrupts the normal transport of bile from hepatocytes to the bile ducts, resulting in its accumulation within hepatocytes. This leads to chronic inflammation and carcinogenesis. Patients with this condition typically present with jaundice and increased pruritus either in the newborn period or early childhood. It accounts for approximately 10–15% of cases of neonatal cholestasis. The disease progresses rapidly, resulting in cirrhosis, HCC (5–15% of children with PFIC-2), biliary tract cancer, and liver transplantation. AFP is a marker of increased disease progression to neoplasia [17,22,23].
3.5. Neonatal Intrahepatic Cholestasis Caused by Citrin Deficiency—NICCD
NICCD is an autosomal recessive disorder resulting from a mutation in the SLC25A13 gene, which is responsible for encoding the citrin protein. Citrin is a mitochondrial transport protein involved in the urea cycle. A deficiency of citrin leads to serum hyperammonemia as well as abnormalities in glycolysis and beta-oxidation of fatty acids. As a result, hepatocytes are unable to utilize glucose and fatty acids as an energy source, leading to hyperlipidemia and hepatic steatosis. The condition is most commonly reported in East Asian countries such as China, Japan, and Korea, but cases have been documented worldwide. Neonatal cholestasis, accompanied by low birth weight and elevated AFP levels, is a characteristic feature. Despite these symptoms, NICCD is generally considered a benign disease, as it tends to spontaneously resolve within the first year of life with the introduction of lactose-free and/or medium-chain fatty acid (LF/MCT) nutrition. However, if left untreated, it can progress to cirrhosis, necessitating liver transplantation [24,25,26].
3.6. Transaldolase Deficiency—TALDO
The deficiency of transaldolase, caused by a mutation in the transaldolase gene located at 11p15.5–p15, is an autosomal recessive disorder that occurs with a frequency of 1:1,000,000 births. Transaldolase is an enzyme produced in the liver that plays a role in the pentose phosphate pathway. Deficiency of this enzyme results in a defect in the pentose phosphate pathway and the accumulation of polyols in the blood, urine, and CSF. Elevated AFP levels serve as a marker of liver regeneration and tumorigenesis [27]. In a study by Rodan et al., the administration of N-acetylcysteine, a precursor of glutathione, resulted in the normalization of AFP levels and a reduced risk of HCC in later life. N-acetylcysteine was shown to improve beta-catenin phosphorylation, which blocks carcinogenesis [28]. On the other hand, Lipinski et al. observed a spontaneous decrease in AFP levels with increasing age in TALDO patients without the introduction of any specific treatment [17,28,29,30].
3.7. Hepatitis B (HBV) in Children
The infection is primarily transmitted vertically. Approximately 90% of newborns from HbsAg and HbeAg-positive mothers will develop chronic infections if they do not receive postnatal immunoprophylaxis. In contrast to adults, where only 5–10% develop chronic hepatitis when infected, 25–90% of infected newborns experience chronic hepatitis. To predict the occurrence of HCC, determining the levels of AFP is useful. Elevated AFP levels typically coincide with increased aminotransferases and the presence of fibrosis, indicating necro-inflammatory changes. Elevated AFP levels can also be observed in asymptomatic chronic carriers with normal aminotransferase values. It is important to consider the possibility of HBV infection in patients with other tumors that secrete AFP to avoid unnecessary surgical interventions and chemotherapy [31]. A small percentage (0.01–0.03%) of children who are chronic HBV carriers will develop HCC before reaching adulthood. Kim et al. demonstrated that individuals with high AFP levels (>100 ng/mL) who are not diagnosed with HCC often have either HBV or HCV infections. Furthermore, persistently high AFP levels for over a year despite antiviral treatment significantly contribute to the development of HCC [32]. HBV-related HCC in children predominantly affects males, occurs later in life, and tends to be more aggressive compared to HCC caused by other factors. In countries where neonatal HBV vaccination is implemented, the incidence of hepatitis and liver cancer related to HBV has been dramatically reduced [17,33,34].
4. Hematopoietic System
Fanconi Anemia
The condition occurs with a frequency of 1–5/1,000,000 births and is inherited in an autosomal recessive manner. The genetic basis of the condition involves numerous mutations, approximately 19 in total, that affect genes responsible for DNA repair. A diagnostic feature is the instability of chromosome structure following exposure to alkylating drugs. Aslan et al., based on serial measurements of serum AFP levels in pregnant carriers, demonstrated that AFP cannot serve as a marker for amniotic fluid in prenatal diagnosis, as even pregnant women carrying affected fetuses exhibit typical serum AFP levels [35]. In this disease, AFP levels are elevated from birth, remain constant, and are independent of concurrent liver conditions and androgen treatment (originally, AFP measurement was used to detect liver adenomas resulting from androgen treatment). Studies have shown that other bone marrow disorders with a genetic basis, such as Blackfan–Diamond syndrome, Shwachman–Diamond anemia, or congenital dyskeratosis congenital, exhibit normal AFP levels [36]. Until recently, AFP was used as a simple diagnostic tool for Fanconi anemia (FA), with studies indicating varied sensitivity: 93% sensitivity and 100% specificity (Cassinat et al., 2000) [37]; 46% sensitivity (Aslan et al., 2002) [19]; and 71% sensitivity (Salem et al., 2019) [17]. However, a recent study by Alter et al. suggests a sensitivity of approximately 25%, although their study included a group of FA patients with a higher median age [36]. The primary treatment for FA is bone marrow transplantation, which does not completely normalize AFP levels, but may slightly reduce them [29,30,38]. Regarding the source of elevated AFP in patients, there is uncertainty. Studies by Salem et al. and Blanche et al. demonstrated significantly higher AFP levels in patients with FANCD1/BRCA2 mutations compared to other types of mutations [36,38]. Aslan et al. propose that impaired postnatal suppression of the AFP gene and/or a shift in production from AFP to albumin may be contributing factors [35]. It is also believed that multipotent progenitor cells in the bone marrow play a role. Two subtypes are recognized: fetal hepatic stem/progenitor cells (FHSC) and intrinsic hematopoietic stem/progenitor cells (HSPC), as well as bone marrow mesenchymal cells that can differentiate into hepatic stem cells and migrate to the liver when it is damaged. HSPCs can also migrate to the liver and serve as precursors to oval liver stem cells [37,39].
5. Endocrine System
Hypothyroidism
The level of AFP during fetal life is influenced by the levels of thyroid hormones. This is because thyroid-stimulating hormone (TSH) binds with AFP in the fetal blood plasma. It is believed that triiodothyronine (T3) plays a role in the transcriptional switch from AFP to albumin early in life. In the absence of T3 (hypothyroidism), this physiological process is delayed [40]. T3 has been shown to induce the differentiation of hepatic oval cells (HOC) into hepatocytes in the rat liver. It is worth noting that only HOC in the liver produces AFP during infancy [7,41]. A laboratory study demonstrated that the level of AFP decreased in mice treated with thyroxine (T4). Conversely, in cases of congenital hypothyroidism, AFP levels increase alongside elevated TSH levels and low T4 levels. These elevated AFP levels persist after birth, whereas in healthy children, AFP levels decrease rapidly. This is attributed to the prolonged half-life of AFP, which extends to 12 days (typically 5–6 days), as a result of its impaired breakdown rate in the liver due to low T4 levels [1]. AFP is also used as a diagnostic marker for ovarian tumors in van Wyk–Grumbach syndrome, when long-term untreated hypothyroidism in children leads to precocious puberty [42,43,44].
6. Cancers
In the field of pediatric oncology, AFP is utilized as a diagnostic tool and for monitoring the effectiveness of surgical treatment and chemotherapy in cases of embryonal HB, HCC, and germ cell tumors (GCTs) [45].
6.1. Hepatoblastoma
HB is the most common malignant liver tumor in children, accounting for 67–80% of cases and occurring at a rate of 1–10/1,000,000 births. It represents approximately 1–2% of pediatric cancers [46]. The tumor is predominantly localized in the right lobe of the liver, as observed in 55–60% of cases [4]. Less than 10% of HB cases develop prenatally, and the average age of diagnosis is 18 months. HB is more frequently diagnosed in premature infants, particularly those with birth weights below 1500 g [47]. Prematurity poses challenges for diagnosis due to the typically high levels of AFP in this group of newborns compared to full-term babies. Histopathologically, HB is classified into epithelial types (including fetal, embryonal/fetal, macrotrabecular, and small cell undifferentiated) and epithelial-mesenchymal types (two subtypes). Some HB subtypes, such as fetal and undifferentiated small cells, exhibit normal AFP levels [45,48]. Only around half of HB cases show elevated AFP levels above the upper reference values, as tumor-derived AFP levels are often not significantly higher than the physiologically high levels observed during the first months of life [4]. In the past, AFP was considered to have prognostic value, with very high (>1,000,000 ng/mL) or very low (<100 ng/mL) levels indicating poor prognosis in HB [49]. However, the Children’s Hepatic Tumors International Collaboration (CHIC), after analyzing its databases and identifying SMARCB1 mutations in a group of HBs with the small cell undifferentiated subtype or HBs with low AFP levels (with survival rates of 24–37.5%), concluded that some of these HBs were rhabdoid tumors with an extremely unfavorable prognosis (3-year OS—0%). After excluding rhabdoid tumor cases from the analyzed group, it was found that the presence of the small cell undifferentiated subtype or low AFP levels no longer had poor prognostic significance [49]. After surgery, AFP levels are expected to decrease below the upper reference range, and failure to do so indicates unsuccessful tumor resection or early recurrence. Similarly, during chemotherapy, a slow decline in AFP levels indicates an unfavorable prognosis. Following the completion of treatment, an increase in AFP levels above age-specific reference values, even in the absence of clinical and imaging evidence of the tumor, suggests recurrence. Currently, there are insufficient data to determine whether there is a correlation between AFP levels at diagnosis and relapse after complete remission (CR). A study by Li et al. showed that AFP levels >1000 ng/mL at diagnosis are not an independent prognostic factor for relapse after CR, as there was no statistically significant difference in relapse-free survival (RFS) (data were inconclusive for cases <100 vs. >100) [45,48,50].
6.2. Hepatocellular Carcinoma
HCC is the second most common malignant liver tumor in children, accounting for 2–33% of cases. It occurs at a rate of 0.41/1,000,000 births. Several predisposing factors contribute to its development, including the vertical transmission of HBV, tyrosinemia, progressive familial intrahepatic cholestasis, glycogen storage diseases, Alagille syndrome, congenital portal-systemic shunts, Wilson disease, alpha-1-antitrypsin deficiency, transaldolase deficiency, Gardner’s syndrome, FA, ataxia-telangiectasia, familial adenomatous polyposis, and primary sclerosing cholangitis [51] (see Table 2). HCC primarily affects children over 5 years of age and can arise in the presence or absence of de novo cirrhosis, sometimes associated with underlying liver diseases. Histopathologically, HCC can be classified as conventional HCC, fibrolamellar HCC, or HCC with HB elements [52]. On average, approximately 50% of HCC cases exhibit elevated AFP levels [51]. In the fibrolamellar form, only 10% of cases have elevated AFP levels. High AFP levels are associated with higher mortality rates [52]. Surgical treatment, including complete tumor resection (possible in only 30% of diagnosed cases) along with additional chemotherapy or liver transplantation, is the standard approach for managing HCC [17,39,52,53].

Table 2.
Common risk factors for hepatocellular carcinoma (HCC) in infancy and childhood.
6.3. Germ Cell Tumors
GCTs account for 3.5% of cancers in children up to the age of 15 and 13.9% in the 15–19 age group [54]. They are characterized by male dominance, with the exception of SCT. GCTs encompass a group of neoplasms derived from pluripotent germ cells, including both benign and malignant tumors. They can occur within the gonads (50% of cases up to age 4) as well as outside the gonads (50% of cases up to age 4, and 10–15% after puberty) [55]. These tumors are commonly found in midline locations of the body, such as the sacrococcygeal region, mediastinum, skull (pineal region), retroperitoneal space, nasopharynx, orbit, neck, uterus, and vagina [56]. The prevailing hypothesis is that their presence in these locations results from the misplacement of primordial germ cells (PGCs) during their migration from the yolk sac to the genital ridges, from which the definitive gonads develop. Aberrant migration leads to the ectopic localization of germ cells along the midline of the body. Malignant transformation of these cells in extragonadal sites gives rise to GCTs [56]. Elevated levels of AFP in these tumors are attributed to the presence of immature or malignant tissue elements derived from the yolk follicle [55,56]. The combination of AFP and beta-HCG can detect 5–60% of GCTs, depending on the histopathological subtype, with detection rates reaching up to 85% in extracranial localizations. Only 20% of early-stage GCTs exhibit elevated AFP levels [57,58]. According to Erlich et al., AFP alone detects 10–60% of nonsquamous GCTs [59]. The prognostic value of AFP at the time of GCT diagnosis remains controversial. Frazier et al., in a summary of seven trials conducted by the Children’s Oncology Group and the Children’s Cancer and Leukemia Group, suggested that AFP levels above 10,000 ng/mL at diagnosis were associated with a worse prognosis, indicated by lower event-free survival (EFS) and overall survival (OS), although statistical significance was not achieved (p = 0.45) [5,60]. In contrast, Freseneau et al., in the TGM 95 study, reported that baseline AFP levels did not affect 5-year recurrence-free survival (5y-RFS) [61]. In adults with malignant GCTs, the decrease in AFP during treatment is an important prognostic factor [61]. However, in children, there is no consensus in the literature regarding the prognostic significance of AFP normalization. According to the French TGM 95 study conducted by Freseneau et al., the predicted time to normalization of AFP did not have significant prognostic value [61]. On the other hand, a study by Faure-Conter et al. on the TGM13-NS protocol, which aimed to achieve high cure rates with minimized chemotherapy doses in children with GCT, demonstrated that AFP normalization had a prognostic impact on EFS (HR = 1.003 [1000–1007]) [62]. O’Neill et al., analyzing data from the Children’s Oncology Group (COG) AGCT0132 protocol, showed that children who had a satisfactory decrease in AFP (with normalization of any of the first two measurements more than 7 days after starting chemotherapy or an AFP half-life of ≤7 days) had a lower cumulative 3-year recurrence rate compared to those with an unsatisfactory decrease (11 vs. 38%) [63]. AFP can serve as a useful tool for diagnosing GCT recurrences; however, it cannot be solely relied upon as a diagnostic measure. In a study conducted by Trigo et al., it was found that 68% of patients with recurrence had elevated levels of the marker (AFP or beta-HCG) at both initial diagnosis and recurrence [64]. Conversely, Keskin et al. demonstrated no significant disparity in AFP levels between patients with and without GCT recurrence, despite receiving the same treatment regimen. The only distinction observed was in the AFP half-life [65]. Nevertheless, it is important to note that not all GCTs secrete AFP, and some present challenges in terms of localization through biopsy. Therefore, a more precise alternative appears to be emerging in the form of microRNAs (miR-371a-3p/-5p—373 and miR-302/367), which offer simplicity and ease of use for diagnostic purposes. These microRNAs are short single-stranded fragments of noncoding RNA responsible for regulating gene expression by influencing translation blocking or mRNA degradation. Alterations in microRNA expression contribute to the initiation of carcinogenesis [66]. In terms of diagnosis and recurrence monitoring, miRNA demonstrates higher specificity (93.4%) and sensitivity (88.7%) compared to AFP, regardless of the histopathological type, patient age, or anatomical location of the tumor [57,67,68].
6.4. Intracranial Germ Cell Tumors (IC-GCTs)
IC-GCTs account for 0.3–3.4% of childhood CNS tumors in North America and Europe, but the incidence rises to 15% in East Asia [69]. IC-GCTs can be categorized into two main types: germinomas (GER) and nongerminoma GCTs (NG-GCT), which include YST, EC, choriocarcinomas, teratomas, and mixed GCTs [70]. Intracranial teratomas are the most common, followed by immature teratomas. They can present in various forms, ranging from large tumors causing mass effects and spreading into the nasopharynx and orbit to smaller lesions causing hydrocephalus. The most common sites of origin are around the pineal gland, the Turkish saddle, and the third ventricle. In approximately one-third of cases, the starting point cannot be determined due to the mass effect. To avoid the need for a biopsy, determination of AFP and b-HCG levels in CSF and serum can be performed. If AFP is confirmed to be >25 ng/mL and b-HCG is confirmed to be >50 IU/L in at least one sample of serum or fluid, a biopsy can be avoided. According to the International Society of Pediatric Oncology (SIOP) study, serum and/or CSF AFP levels ≥25 ng/mL and/or b-HCG levels greater than or equal to 50 IU/L indicate a diagnosis of NG-GCT. The Children’s Oncology Group (COG) suggests cutoff points of 10 ng/mL for AFP and 100 IU/L for b-HCG [71,72]. In a study by Sathisamitphong et al. involving 63 IC-GCT cases, there was an 84.3% concordance between serum AFP and CSF levels [73]. Frappaz et al. reported that AFP levels are higher in serum compared to CSF, whereas b-HCG levels are comparable [71]. Legault et al. demonstrated that AFP levels are highest in serum, followed by CSF obtained through a lumbar puncture, and finally, CSF obtained through a ventricular puncture [74]. Takami et al. showed that the sensitivity of individual markers in detecting NG-GCT CNS is as follows: b-HCG (>100 IU/L) at 61.5%, AFP (>10 ng/mL) at 83.3%, and both markers together at 94.7% [75]. If these two markers are negative, a biopsy is required to differentiate between teratoma and germ cell carcinoma, as the treatment regimens differ [70]. In cases where there is no admixture of YST within the tumor mass, high levels of AFP are attributed to the presence of immature elements from glandular epithelium characteristic of the gastrointestinal tract and/or ependyma (a type of glial tissue) lining the ventricular system, and occasionally structures resembling liver tissue. Hong et al. demonstrated that only the determination of AFP (≥10 ng/mL) along with beta-HCG (≥50 IU/mL) has prognostic value for NG-GCT or malignant GCT, indicating worse EFS and OS compared to the determination of a single marker alone [76]. In the SIOP-CNS-GCT-96 trial, patients with serum AFP >1000 ng/mL and/or CSF post-treatment with the established residual disease had worse progression-free survival [72]. Among patients with recurrence, those who had AFP in serum or CSF ≤25 ng/mL had a better prognosis [56,77,78].
6.5. Malignant Saccrococygeal Germ Cell Tumor
The occurrence of SCT has a frequency of 1 in every 35,000–40,000 births. The most common type is mature teratoma; however, 11–35% of cases involve a mixture of a malignant component, most commonly YST or EC [60]. Unlike other GCTs, this condition is more prevalent among girls. It presents as the most common tumor in newborns, typically as an outward-growing mass. It can also manifest after infancy, typically before the age of 3, with an inward-growing growth pattern characterized by buttock asymmetry and gastrointestinal and/or urinary tract issues, as well as lower limb dysfunction. The risk of malignant transformation increases with the child’s age, from 11 to 35% at birth to over 70% in cases diagnosed after >1 year of age. The primary treatment is surgical intervention, with the addition of chemotherapy for malignant cases. The use of AFP for detecting prenatal lesions is not reliable, as it does not consistently elevate in maternal serum in most cases, regardless of whether the tumor is mature or immature or whether it is covered by skin [56]. However, AFP is valuable as an early marker for assessing the completeness of tumor resection as well as for monitoring malignant recurrence after initial surgery (75% of recurrences show elevated AFP) and chemotherapy (monitoring for up to 3–5 years post-treatment) [79]. This is because teratomas prone to malignancy often contain a mixture of YST (present in 22–56% of recurrences). It has been demonstrated that AFP has a prolonged half-life in SCT tumors with a tendency for recurrence (with YST admixture) and in immature teratomas (without YST admixture). AFP levels have prognostic significance [56,80,81,82].
6.6. Special Histopathological Cases of GCT Connected with High AFP Levels
6.6.1. Yolk Sac Tumor (YST = Endodermal Sinus Tumor)
YST is the most common malignant germ cell tumor in children and is histologically composed of yolk sac mesenchymal cells. Approximately 70–90% of YST cases secrete AFP [83,84], but in some instances, metastatic lesions or treatment remnants may lose the ability to produce AFP [85]. It primarily occurs in the male and female gonads [55,56]. In boys, it is the leading cause of testicular cancer, while in girls, it is a rare tumor of the ovary [55]. YST can occur independently in about 60% of cases, mainly in the pediatric population, or as a component of other GCTs, most commonly teratomas or dysgerminomas, accounting for 40% of cases in the postpubertal age group [56,86]. Approximately 15% of YST lesions can occur extragonadal in midline organs of the body, such as the CNS, paranasal sinuses, bladder, vagina, prostate, and retroperitoneal space [83] (known as extragonadal germ cell tumors—EGGCT). It can also manifest in the liver, where it must be differentiated from HB, as both tumors can exhibit high levels of AFP. Another extramedian site of occurrence is the kidneys, which can sometimes be mistaken for a Wilms tumor [87]. Except for AFP, no other feature of the preoperative clinical examination differentiates these two tumors [88]. However, AFP is not produced in the kidneys during prenatal or childhood stages, and thus AFP has never been a marker for Wilms tumor. There are rare situations in which Wilms tumors consist of tissue resembling nephroblastoma as well as tissue morphologically corresponding to a teratoma, leading to AFP production [89,90,91]. In giant mixed GCTs, small foci of YST, the source of AFP, may be accidentally overlooked during the examination of tumor samples [56,92]. AFP is used for monitoring and evaluating the effectiveness of treatment but is not helpful for prognosis [54]. A meta-analysis by Guo et al. on the prognostic value of AFP in ovarian YST demonstrated that only postoperative AFP values are useful. High postoperative AFP levels were associated with worse OS (OR = 0.16, 95% CI: 0.05–0.48) and RFS (OR = 0.18, 95% CI: 0.08–0.43) compared to low postoperative AFP levels in ovarian yolk sac tumor (OYST) patients [93]. De la Motte Rouge et al. showed that an early decline in AFP levels during chemotherapy for OYST predicts better OS (100%) compared to an unfavorable decline (OS 49%, 95% CI: 26–72%) [94]. Currently, a more sensitive marker, ZBTB16 (Zinc finger and BTB ((Broad/complex/Tramtrack/Bric a Brac) domain-containing 16)), is used, which can also detect extragonadal and metastatic YST lesions with a sensitivity of 91.6% [85].
6.6.2. Embryonal Carcinoma (EC)
Histopathologically, germinoma is the most primitive form of germ cell tumor, capable of differentiating into YST or immature teratoma. It consists of cells that produce AFP (from YST) and b-HCG (from choriocarcinoma) [56]. Germinoma is a common component of mixed germ cell tumors (MGCT). According to a study by Ataikiru et al. involving Romanian children with MGCT, 87.5% of cases before puberty and 64.7% after puberty had an EC component [57]. It primarily affects males and is extremely rare in females [95]. Germinoma can occur in both the gonads and the CNS. In the CNS, it typically arises in the region of the third ventricle and pineal gland. The diagnosis can be improved by measuring B-HCG and AFP levels in both serum and CSF, as mentioned earlier [96].
7. Ovarian Sertoli-Leydig Cell Tumor (SLCT)
A rare, unilateral mixed-sex cord-stromal tumor. It is mainly found in young women (75% of cases), but the youngest known case was 9 months old [97]. Approximately 40–50% produce androgens (virilization symptoms), less often estrogens, and least often both types of sex hormones simultaneously [56,97]. The tumor is composed of Sertoli cells, Leydig cells, fibroblasts, and stromal cells in varying proportions, but may also have a component of glandular intestinal cells producing mature or immature hepatocytes, which are responsible for AFP production [98]. Another hypothesis is that Leydig cells and hepatocytes are morphologically similar. Still, other authors suggest that the source of AFP is the presence of cells similar to Leydig cells, but without the presence of typical crystals on histopathological examination [99]. Another AFP-producing component may be poorly differentiated tissue fragments of the endodermal sinus difficult to identify on histopathological examination (YST-like) [99,100,101].
8. Conclusions
Careful consideration should be given to using AFP levels as a basis for clinical decisions in neonatology since they are physiologically elevated from birth until the first year of life.
In young children, elevated AFP levels can mask the presence of certain genetic diseases, liver regeneration in chronic diseases, and tumorigenesis processes.
In pediatric cases, AFP remains a valuable marker for liver tumors and GCTs that involve tissue elements derived from the yolk follicle. Monitoring AFP levels in children with various chronic liver diseases can help predict the early onset of HCC.
It is necessary to develop new pediatric reference ranges for modern AFP diagnostic tests based on current laboratory techniques.
Author Contributions
Conceptualization, J.G.-C. and R.K.; methodology, J.G.-C., M.S. and R.K.; validation, A.K., R.R. and R.K.; formal analysis, R.R., C.S.v.K. and R.K.; investigation, J.G.-C. and M.S.; resources, J.G.-C., C.S.v.K. and R.K.; data curation, J.G.-C. and A.K.; writing—original draft preparation, J.G.-C. and R.K.; writing—review and editing, M.S., A.K. and R.K.; visualization, R.R. and R.K.; supervision, R.R. and C.S.v.K.; project administration, R.K.; funding acquisition, A.K., R.R., C.S.v.K. and R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a New Med Medical Center grant (JGCPHD20202024) and the University of Zielona Gora.
Acknowledgments
The authors would like to express our special gratitude to Jakub Ciemny, for his technical support and preparation of all original figures and tables.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AFP | alpha-fetoprotein |
| AT | ataxia-telangiectasia |
| AOA | ataxia with oculomotor apraxia |
| BRCA2 | BReast CAncer gene 2 |
| CR | complete remission |
| CSF | cerebrospinal fluid |
| EC | embryonal carcinoma |
| EGGCT | extragonadal germ cell tumors |
| EFS | event-free survival |
| FA | Fanconi anemia |
| FHSC | fetal hepatic stem/progenitor cells |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| HB | hepatoblastoma |
| HOC | hepatic oval cell |
| HSPC | hematopoietic stem/progenitor cells |
| GCTs | germ cell tumors |
| IC-GCT | intracranial germ cell tumor |
| LF/MCT | medium-chain triglyceride |
| NICCD | neonatal intrahepatic cholestasis caused by citrin deficiency |
| NTBC | 2-(2-nitro-4-3 trifluoro-methylbenzoyl)-1,3-cyclohexanedione |
| MGCT | mixed germ cell tumor |
| miRNA | micro ribonucleic acid |
| OS | overall survival |
| OYST | ovarian yolk sac tumor |
| PGC | primordial germ cell |
| PFIC-2 | progressive familial intrahepatic cholestasis type 2 |
| PUFA | polyunsaturated fatty acid |
| RIA | radioimmunoassay |
| RFS | relapse-free survival |
| SCT | sacrococcygeal teratoma |
| SLCT | Sertoli–Leydig cell tumor |
| T3 | triiodothyronine |
| T4 | thyroxine |
| TALDO | transaldolase deficiency |
| VATER/VACTERL | VATER/VACTERL association |
| YST | yolk sac tumor |
References
- Mizejewski, G.J. Levels of alpha-fetoprotein during pregnancy and early infancy in normal and disease states. Obstet. Gynecol. Surv. 2003, 58, 804–826. [Google Scholar] [CrossRef]
- Blohm, M.E.; Vesterling-Hörner, D.; Calaminus, G.; Göbel, U. Alpha 1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr. Hematol. Oncol. 1998, 15, 135–142. [Google Scholar] [CrossRef]
- Ferraro, S.; Panzeri, A.; Braga, F.; Panteghini, M. Serum α-fetoprotein in pediatric oncology: Not a children’s tale. Clin. Chem. Lab. Med. 2019, 57, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Dall’Igna, P.; Brugieres, L.; Christin, A.S.; Maibach, R.; Casanova, M.; Alaggio, R.; de Goyet, J.V.; Zsiros, J.; Morland, B.; Czauderna, P.; et al. Hepatoblastoma in children aged less than six months at diagnosis: A report from the SIOPEL group. Pediatr. Blood Cancer 2018, 65, e26791. [Google Scholar] [CrossRef]
- Frazier, A.L.; Hale, J.P.; Rodriguez-Galindo, C.; Dang, H.; Olson, T.; Murray, M.J.; Amatruda, J.F.; Thornton, C.; Arul, G.S.; Billmire, D.; et al. Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J. Clin. Oncol. 2015, 33, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Cho, M.J.; Kim, D.Y.; Kim, S.C. Half-life of alpha-fetoprotein in neonatal sacrococcygeal teratoma. J. Pediatr. Surg. 2018, 53, 2470–2474. [Google Scholar] [CrossRef] [PubMed]
- McGrath-Morrow, S.A.; Rothblum-Oviatt, C.C.; Wright, J.; Schlechter, H.; Lefton-Greif, M.A.; Natale, V.A.; Crawford, T.O.; Lederman, H.M. Multidisciplinary Management of Ataxia Telangiectasia: Current Perspectives. J. Multidiscip. Healthc. 2021, 14, 1637–1644. [Google Scholar] [CrossRef]
- Schieving, J.H.; de Vries, M.; van Vugt, J.M.; Weemaes, C.; van Deuren, M.; Nicolai, J.; Wevers, R.A.; Willemsen, M.A. Alpha-fetoprotein, a fascinating protein and biomarker in neurology. Eur. J. Paediatr. Neurol. 2014, 18, 243–248. [Google Scholar] [CrossRef]
- Renaud, M.; Tranchant, C.; Koenig, M.; Anheim, M. Autosomal Recessive Cerebellar Ataxias With Elevated Alpha-Fetoprotein: Uncommon Diseases, Common Biomarker. Mov. Disord. 2020, 35, 2139–2149. [Google Scholar] [CrossRef]
- Devaney, R.; Pasalodos, S.; Suri, M.; Bush, A.; Bhatt, J.M. Ataxia telangiectasia: Presentation and diagnostic delay. Arch. Dis. Child. 2017, 102, 328–330. [Google Scholar] [CrossRef]
- Stray-Pedersen, A.; Borresen-Dale, A.L.; Paus, E.; Lindman, C.R.; Burgers, T.; Abrahamsen, T.G. Alpha fetoprotein is increasing with age in ataxia-telangiectasia. Eur. J. Paediatr. Neurol. 2007, 11, 375–380. [Google Scholar] [CrossRef]
- Lynch, D.R.; McCormick, A.; Schadt, K.; Kichula, E. Pediatric Ataxia: Focus on Chronic Disorders. Semin. Pediatr. Neurol. 2018, 25, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Melis, D.; Carvalho, D.; Barbaro-Dieber, T.; Espay, A.J.; Gambello, M.J.; Gener, B.; Gerkes, E.; Hitzert, M.M.; Hove, H.B.; Jansen, S.; et al. Primrose syndrome: Characterization of the phenotype in 42 patients. Clin. Genet. 2020, 97, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.J.; Netzel, B.C.; Singh, R.H.; Pino, G.B.; Gavrilov, D.K.; Oglesbee, D.; Raymond, K.M.; Rinaldo, P.; Tortorelli, S.; Smith, W.E.; et al. Laboratory monitoring of patients with hereditary tyrosinemia type I. Mol. Genet. Metab. 2020, 130, 247–254. [Google Scholar] [CrossRef] [PubMed]
- van Ginkel, W.G.; Pennings, J.P.; van Spronsen, F.J. Liver Cancer in Tyrosinemia Type 1. Adv. Exp. Med. Biol. 2017, 959, 101–109. [Google Scholar] [CrossRef]
- Bhushan, S.; Noble, C.; Balouch, F.; Lewindon, P.; Lampe, G.; Hodgkinson, P.; McGill, J.; Ee, L. Hepatocellular carcinoma requiring liver transplantation in hereditary tyrosinemia type 1 despite nitisinone therapy and α1-fetoprotein normalization. Pediatr. Transplant. 2022, 26, e14334. [Google Scholar] [CrossRef]
- Khanna, R.; Verma, S.K. Pediatric hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 3980–3999. [Google Scholar] [CrossRef]
- Koelink, C.J.; van Hasselt, P.; van der Ploeg, A.; van den Heuvel-Eibrink, M.M.; Wijburg, F.A.; Bijleveld, C.M.; van Spronsen, F.J. Tyrosinemia type I treated by NTBC: How does AFP predict liver cancer? Mol. Genet. Metab. 2006, 89, 310–315. [Google Scholar] [CrossRef]
- El-Karaksy, H.; Abdullatif, H.M.; Ghobrial, C.M.; Mogahed, E.A.; Yasin, N.A.; Talal, N.; Rashed, M. Clinical experience with hepatorenal tyrosinemia from a single Egyptian center. PLoS ONE 2022, 17, e0268017. [Google Scholar] [CrossRef]
- Fuenzalida, K.; Leal-Witt, M.J.; Guerrero, P.; Hamilton, V.; Salazar, M.F.; Peñaloza, F.; Arias, C.; Cornejo, V. NTBC Treatment Monitoring in Chilean Patients with Tyrosinemia Type 1 and Its Association with Biochemical Parameters and Liver Biomarkers. J. Clin. Med. 2021, 10, 5832. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, S.; Salo, M.K.; Kuusela, P.; Holmberg, C.; Simell, O.; Heikinheimo, M. Serum levels of oncofetal markers CA 125, CA 19-9, and alpha-fetoprotein in children with hereditary tyrosinemia type I. Pediatr. Res. 1994, 35, 205–208. [Google Scholar] [CrossRef][Green Version]
- Srivastava, A. Progressive familial intrahepatic cholestasis. J. Clin. Exp. Hepatol. 2014, 4, 25–36. [Google Scholar] [CrossRef]
- Vinayagamoorthy, V.; Srivastava, A.; Sarma, M.S. Newer variants of progressive familial intrahepatic cholestasis. World J. Hepatol. 2021, 13, 2024–2038. [Google Scholar] [CrossRef] [PubMed]
- Saheki, T.; Song, Y.Z. Citrin Deficiency. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2021. [Google Scholar]
- Lipiński, P.; Jurkiewicz, D.; Ciara, E.; Płoski, R.; Więcek, S.; Bogdańska, A.; Stradomska, T.; Socha, P.; Rokicki, D.; Tylki-Szymańska, A.; et al. Neonatal cholestasis due to citrin deficiency: Diagnostic pitfalls. Acta Biochim. Pol. 2020, 67, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, K. Metabolic basis and treatment of citrin deficiency. J. Inherit. Metab. Dis. 2021, 44, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Valayannopoulos, V.; Altassan, R.; Chung, W.K.; Heijboer, A.C.; Keng, W.T.; Lapatto, R.; McClean, P.; Mulder, M.F.; Tylki-Szymańska, A.; et al. Clinical, biochemical, and molecular overview of transaldolase deficiency and evaluation of the endocrine function: Update of 34 patients. J. Inherit. Metab. Dis. 2019, 42, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Rodan, L.H.; Berry, G.T. N-Acetylcysteine Therapy in an Infant with Transaldolase Deficiency Is Well Tolerated and Associated with Normalization of Alpha Fetoprotein Levels. JIMD Rep. 2017, 31, 73–77. [Google Scholar] [CrossRef]
- Lipiński, P.; Stradomska, T.; Tylki-Szymańska, A. Transaldolase deficiency-clinical outcome, pathogenesis, diagnostic process. Dev. Period. Med. 2018, 22, 187–196. (In Polish) [Google Scholar] [CrossRef]
- Grammatikopoulos, T.; Hadzic, N.; Foskett, P.; Strautnieks, S.; Samyn, M.; Vara, R.; Dhawan, A.; Hertecant, J.; Al Jasmi, F.; Rahman, O.; et al. Liver Disease and Risk of Hepatocellular Carcinoma in Children With Mutations in TALDO1. Hepatol. Commun. 2022, 6, 473–479. [Google Scholar] [CrossRef]
- Zong, X.; Yang, J.X.; Zhang, Y. Persistently elevated alpha-fetoprotein associated with chronic hepatitis B during chemotherapy for malignant ovarian germ cell tumors: A case series and a review of the literature. J. Ovarian Res. 2019, 12, 124. [Google Scholar] [CrossRef]
- Kim, C.Y.; Kim, B.R.; Lee, S.S.; Jeon, D.H.; Lee, C.M.; Kim, W.S.; Cho, H.C.; Kim, J.J.; Lee, J.M.; Kim, H.J.; et al. Clinical features of hepatitis B and C virus infections, with high α-fetoprotein levels but not hepatocellular carcinoma. Medicine 2017, 96, e5844. [Google Scholar] [CrossRef] [PubMed]
- Kerkar, N. Hepatitis B in children: Complexities in management. Pediatr. Transplant. 2005, 9, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.P.Y.; Soh, S.Y.; Cheng, F.W.C.; Pang, H.H.; Luk, C.W.; Li, C.H.; Ho, K.K.H.; Chan, E.K.W.; Chan, A.C.Y.; Chung, P.H.Y.; et al. Hepatitis B Virus Seropositivity Is a Poor Prognostic Factor of Pediatric Hepatocellular Carcinoma: A Population-Based Study in Hong Kong and Singapore. Front. Oncol. 2020, 10, 570479. [Google Scholar] [CrossRef] [PubMed]
- Aslan, D.; Karabacak, R.O.; Aslan, O.D. Maternal serum alpha-fetoprotein levels are normal in Fanconi anemia: Can it be a lack of postnatal inhibition of AFP gene resulting in the elevation? Pediatr. Blood Cancer 2017, 64, e26297. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.P.; Giri, N. Serum alpha fetoprotein levels in Fanconi anaemia. Br. J. Haematol. 2019, 184, 1074–1076. [Google Scholar] [CrossRef]
- Cassinat, B.; Guardiola, P.; Chevret, S.; Schlageter, M.H.; Toubert, M.E.; Rain, J.D.; Gluckman, E. Constitutive elevation of serum alpha-fetoprotein in Fanconi anemia. Blood 2000, 96, 859–863. [Google Scholar] [CrossRef]
- Salem, B.; Mitchell, R.; DeFor, T.E.; Tryon, R.; Wagner, J.E.; MacMillan, M.L. Elevations in serum alpha fetoprotein levels in patients with Fanconi anaemia. Br. J. Haematol. 2019, 184, 1032–1035. [Google Scholar] [CrossRef]
- Lakhi, N.A.; Mizejewski, G.J. Alpha-fetoprotein and Fanconi Anemia: Relevance to DNA Repair and Breast Cancer Susceptibility. Fetal Pediatr. Pathol. 2017, 36, 49–61. [Google Scholar] [CrossRef]
- Anteby, E.; Shpan, P.; Dushnik, M.; Zvang, A.; Zer, T.; Ben-Neriah, Z.; Yagel, S. The regulatory role of tri-iodothyronine on the production of alpha-fetoprotein and albumin by mouse fetal liver cells. Hum. Reprod. 1993, 8, 1576–1578. [Google Scholar] [CrossRef]
- László, V.; Dezso, K.; Baghy, K.; Papp, V.; Kovalszky, I.; Sáfrány, G.; Thorgeirsson, S.S.; Nagy, P.; Paku, S. Triiodothyronine accelerates differentiation of rat liver progenitor cells into hepatocytes. Histochem. Cell Biol. 2008, 130, 1005–1014. [Google Scholar] [CrossRef]
- Patni, N.; Cervantes, L.F.; Diaz, A. Elevated alpha-fetoprotein levels in Van Wyk-Grumbach syndrome: A case report and review of literature. J. Pediatr. Endocrinol. Metab. 2012, 25, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Lin-Su, K. Van Wyk-Grumbach syndrome in a female pediatric patient with trisomy 21: A case report. Int. J. Pediatr. Endocrinol. 2020, 2020, 2. [Google Scholar] [CrossRef]
- Baranowski, E.; Högler, W. An unusual presentation of acquired hypothyroidism: The Van Wyk-Grumbach syndrome. Eur. J. Endocrinol. 2012, 166, 537–542. [Google Scholar] [CrossRef]
- Thompson, P.A.; Chintagumpala, M. Renal and hepatic tumors in the neonatal period. Semin. Fetal Neonatal Med. 2012, 17, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R.L. Tumors of the liver in children. Surg. Oncol. 2007, 16, 195–203. [Google Scholar] [CrossRef]
- Tanimura, M.; Matsui, I.; Abe, J.; Ikeda, H.; Kobayashi, N.; Ohira, M.; Yokoyama, M.; Kaneko, M. Increased risk of hepatoblastoma among immature children with a lower birth weight. Cancer Res. 1998, 58, 3032–3035. [Google Scholar] [PubMed]
- Isaacs, H., Jr. Fetal and neonatal hepatic tumors. J. Pediatr. Surg. 2007, 42, 1797–1803. [Google Scholar] [CrossRef]
- Trobaugh-Lotrario, A.D.; Maibach, R.; Aronson, D.C.; Rangaswami, A.; Häberle, B.; O’Neill, A.F.; Schmid, I.; Ansari, M.; Hishiki, T.; Ranganathan, S.; et al. Outcomes of Patients Treated for Hepatoblastoma with Low Alpha-Fetoprotein and/or Small Cell Undifferentiated Histology: A Report from the Children’s Hepatic Tumors International Collaboration (CHIC). Cancers 2023, 15, 467. [Google Scholar] [CrossRef]
- Li, F.; Zhang, W.; Hu, H.; Zhu, X.; Zhang, Y.; Huang, D. Factors influencing recurrence after complete remission in children with hepatoblastoma: A 14-year retrospective study in China. PLoS ONE 2021, 16, e0259503. [Google Scholar] [CrossRef]
- Sintusek, P.; Phewplung, T.; Sanpavat, A.; Poovorawan, Y. Liver tumors in children with chronic liver diseases. World J. Gastrointest. Oncol. 2021, 13, 1680–1695. [Google Scholar] [CrossRef]
- Short, S.S.; Kastenberg, Z.J.; Wei, G.; Bondoc, A.; Dasgupta, R.; Tiao, G.M.; Watters, E.; Heaton, T.E.; Lotakis, D.; La Quaglia, M.P.; et al. Histologic type predicts disparate outcomes in pediatric hepatocellular neoplasms: A Pediatric Surgical Oncology Research Collaborative study. Cancer 2022, 128, 2786–2795. [Google Scholar] [CrossRef] [PubMed]
- Weeda, V.B.; Aronson, D.C.; Verheij, J.; Lamers, W.H. Is hepatocellular carcinoma the same disease in children and adults? Comparison of histology, molecular background, and treatment in pediatric and adult patients. Pediatr. Blood Cancer 2019, 66, e27475. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Frazier, A.L.; Shaikh, F. Germ Cell Tumors in Adolescents and Young Adults. J. Oncol. Pract. 2019, 15, 433–441. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.K.; Heerema-McKenney, A.; Rouse, R.V. Extragonadal germ cell tumors: A review with emphasis on pathologic features, clinical prognostic variables, and differential diagnostic considerations. Adv. Anat. Pathol. 2007, 14, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, H., Jr. Perinatal (fetal and neonatal) germ cell tumors. J. Pediatr. Surg. 2004, 39, 1003–1013. [Google Scholar] [CrossRef]
- Ataikiru, U.O.; Iacob, E.R.; Miron, I.; Popoiu, C.M.; Boia, E.S. A 10-year retrospective single-center study of alpha-fetoprotein and beta-human chorionic gonadotropin in Romanian children with (para)gonadal tumors and cysts. J. Pediatr. Endocrinol. Metab. 2021, 35, 363–371. [Google Scholar] [CrossRef]
- Perkins, G.L.; Slater, E.D.; Sanders, G.K.; Prichard, J.G. Serum tumor markers. Am. Fam. Physician 2003, 68, 1075–1082. [Google Scholar]
- Ehrlich, Y.; Beck, S.D.; Foster, R.S.; Bihrle, R.; Einhorn, L.H. Serum tumor markers in testicular cancer. Urol. Oncol. 2013, 31, 17–23. [Google Scholar] [CrossRef]
- D’Angelo, P.; De Pasquale, M.D.; Barretta, F.; Affinita, M.C.; Conte, M.; Dall’Igna, P.; Di Cataldo, A.; Inserra, A.; Provenzi, M.; Quaglietta, L.; et al. Malignant sacrococcygeal germ cell tumors in childhood: The Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) experience. Pediatr. Blood Cancer 2021, 68, e28812. [Google Scholar] [CrossRef]
- Fresneau, B.; Orbach, D.; Faure-Conter, C.; Sudour-Bonnange, H.; Vérité, C.; Gandemer, V.; Pasquet, M.; Fasola, S.; Rome, A.; Raimbault, S.; et al. Is alpha-fetoprotein decline a prognostic factor of childhood non-seminomatous germ cell tumours? Results of the French TGM95 study. Eur. J. Cancer 2018, 95, 11–19. [Google Scholar] [CrossRef]
- Faure-Conter, C.; Orbach, D.; Sudour-Bonnange, H.; Verité, C.; Mansuy, L.; Rome, A.; Dumesnil, C.; Thebaud, E.; Renard, M.; Hameury, F.; et al. Extracranial germ cell tumours in children and adolescents: Results from the French TGM13 protocol. Pediatr. Blood Cancer 2023, 70, e30117. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.F.; Xia, C.; Krailo, M.D.; Shaikh, F.; Pashankar, F.D.; Billmire, D.F.; Olson, T.A.; Amatruda, J.F.; Villaluna, D.; Huang, L.; et al. α-Fetoprotein as a predictor of outcome for children with germ cell tumors: A report from the Malignant Germ Cell International Consortium. Cancer 2019, 125, 3649–3656. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.M.; Tabernero, J.M.; Paz-Ares, L.; García-Llano, J.L.; Mora, J.; Lianes, P.; Esteban, E.; Salazar, R.; López-López, J.J.; Cortés-Funes, H. Tumor markers at the time of recurrence in patients with germ cell tumors. Cancer 2000, 88, 162–168. [Google Scholar] [CrossRef]
- Keskin, S.; Ekenel, M.; Başaran, M.; Bavbek, S. Predictive value of marker half-life in relapsed and nonrelapsed nonseminomatous germ cell testicular tumor patients undergoing chemotherapy. Am. J. Clin. Oncol. 2012, 35, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, M.; Gawrychowska, A.; Stefanowicz, J. Diagnostic, Prognostic and Predictive Markers in Pediatric Germ Cell Tumors-Past, Present and Future. Diagnostics 2022, 12, 278. [Google Scholar] [CrossRef]
- Saliyeva, S.; Boranbayeva, R.; Konoplya, N.; Bulegenova, M.; Blau, O.; Belousov, V.; Granica, J.; Mukushkina, D.; Altynbayeva, G. Pediatric Extracranial Germ Cell Tumors: Expression of microRNA. J. Pediatr. Hematol. Oncol. 2023, 45, e174–e179. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Bell, E.; Raby, K.L.; Rijlaarsdam, M.A.; Gillis, A.J.; Looijenga, L.H.; Brown, H.; Destenaves, B.; Nicholson, J.C.; Coleman, N. A pipeline to quantify serum and cerebrospinal fluid microRNAs for diagnosis and detection of relapse in paediatric malignant germ-cell tumours. Br. J. Cancer 2016, 114, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, R.; DaSilva, N.S.; Cappellano, A.; Belessiotis, C.; Diez, B.; Gardner, S.; Allen, J.; Weinblatt, M.; Gottardo, N.; Dhall, G.; et al. Relapse and outcome patterns of patients with central nervous system mixed malignant germ cell tumors treated without irradiation: Findings from the third international central nervous system (CNS) germ cell tumor (GCT) study. Pediatr. Blood Cancer 2015, 62, 1920–1924. [Google Scholar] [CrossRef]
- PDQ Pediatric Treatment Editorial Board. Childhood Central Nervous System Germ Cell Tumors Treatment (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Frappaz, D.; Dhall, G.; Murray, M.J.; Goldman, S.; Faure Conter, C.; Allen, J.; Kortmann, R.D.; Haas-Kogen, D.; Morana, G.; Finlay, J.; et al. EANO, SNO and Euracan consensus review on the current management and future development of intracranial germ cell tumors in adolescents and young adults. Neuro Oncol. 2022, 24, 516–527. [Google Scholar] [CrossRef]
- Calaminus, G.; Frappaz, D.; Kortmann, R.D.; Krefeld, B.; Saran, F.; Pietsch, T.; Vasiljevic, A.; Garre, M.L.; Ricardi, U.; Mann, J.R.; et al. Outcome of patients with intracranial non-germinomatous germ cell tumors-lessons from the SIOP-CNS-GCT-96 trial. Neuro Oncol. 2017, 19, 1661–1672. [Google Scholar] [CrossRef]
- Sathitsamitphong, L.; Monsereenusorn, C.; Techavichit, P.; Shotelersuk, K.; Suwanpakdee, P.; Rujkijyanont, P.; Charoenkwan, P. Clinical Outcomes and Diagnostic Consistency of Serum and CSF Tumor Markers in Pediatric Intracranial Germ Cell Tumors in Thailand: A Multicenter Study. Glob. Pediatr. Health 2022, 9, 2333794X221141765. [Google Scholar] [CrossRef]
- Legault, G.; Allen, J.C. Potential role of ventricular tumor markers in CNS germ cell tumors. Pediatr. Blood Cancer 2013, 60, 1647–1650. [Google Scholar] [CrossRef]
- Takami, H.; Graffeo, C.S.; Perry, A.; Giannini, C.; Nakazato, Y.; Saito, N.; Matsutani, M.; Nishikawa, R.; Ichimura, K.; Daniels, D.J. Roles of Tumor Markers in Central Nervous System Germ Cell Tumors Revisited with Histopathology-Proven Cases in a Large international cohort. Cancers 2022, 14, 979. [Google Scholar] [CrossRef]
- Hong, K.T.; Han, J.W.; Fuji, H.; Byun, H.K.; Koh, K.N.; Wong, R.X.; Lee, H.L.; Yoon, H.I.; Lee, J.H.; Phi, J.H.; et al. Outcomes of intracranial non-germinomatous germ cell tumors: A retrospective Asian multinational study on treatment strategies and prognostic factors. J. Neurooncol. 2022, 160, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Abu-Arja, M.H.; Osorio, D.S.; Lassaletta, A.; Graham, R.T.; Coven, S.L.; Stanek, J.R.; Bouffet, E.; Finlay, J.L.; Abdelbaki, M.S. Prognostic factors for patients with relapsed central nervous system nongerminomatous germ cell tumors. Pediatr. Blood Cancer 2022, 69, e29365. [Google Scholar] [CrossRef]
- Cheng, C.M.; Chiang, Y.H.; Nieh, S. Pineal region teratoma with high serum and CSF alpha-fetoprotein levels. J. Clin. Neurosci. 2006, 13, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Niramis, R.; Anuntkosol, M.; Buranakitjaroen, V.; Tongsin, A.; Mahatharadol, V.; Poocharoen, W.; La-Orwong, S.; Tiansri, K. Long-Term Outcomes of Sacrococcygeal Germ Cell Tumors in Infancy and Childhood. Surg. Res. Pract. 2015, 2015, 398549. [Google Scholar] [CrossRef]
- Chen, S.H.; Du, C.J.; Lai, J.Y.; Chang, T.Y.; Yang, C.P.; Hung, I.J.; Jaing, T.H.; Ming, Y.C.; Hsueh, C. Malignant sacrococcygeal germ cell tumors in children in Taiwan: A retrospective single-center case series. Medicine 2021, 100, e24323. [Google Scholar] [CrossRef]
- Phi, J.H. Sacrococcygeal Teratoma: A Tumor at the Center of Embryogenesis. J. Korean Neurosurg. Soc. 2021, 64, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Kirkinen, P.; Heinonen, S.; Vanamo, K.; Ryynänen, M. Maternal serum alpha-fetoprotein and epithelial tumour marker concentrations are not increased by fetal sacrococcygeal teratoma. Prenat. Diagn. 1997, 17, 47–50. [Google Scholar] [CrossRef]
- Nogales, F.F.; Preda, O.; Nicolae, A. Yolk sac tumours revisited. A review of their many faces and names. Histopathology. 2012, 60, 1023–1033. [Google Scholar] [CrossRef]
- Shah, J.P.; Kumar, S.; Bryant, C.S.; Ali-Fehmi, R.; Malone, J.M., Jr.; Deppe, G.; Morris, R.T. A population-based analysis of 788 cases of yolk sac tumors: A comparison of males and females. Int. J. Cancer 2008, 123, 2671–2675. [Google Scholar] [CrossRef]
- Ma, X.; Cao, D.; Peng, P.; Xiao, Y.; Yang, J.; Huang, H.; Zhang, Y.; Yu, M.; Wang, J.; Zhou, H.; et al. Preservation of sexual and reproductive function in the treatment of extragonadal yolk sac tumors in the female genital tract. Front. Pediatr. 2022, 10, 1004501. [Google Scholar] [CrossRef]
- Xiao, G.Q.; Priemer, D.S.; Wei, C.; Aron, M.; Yang, Q.; Idrees, M.T. ZBTB16 is a sensitive and specific marker in detection of metastatic and extragonadal yolk sac tumour. Histopathology 2017, 71, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Littooij, A.S.; McHugh, K.; McCarville, M.B.; Sebire, N.J.; Bahrami, A.; Roebuck, D.J. Yolk sac tumour: A rare cause of raised serum alpha-foetoprotein in a young child with a large liver mass. Pediatr. Radiol. 2014, 44, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Qayoom, S.; Shabbir, N.; Singhai, A.; Verma, N.; Rawat, S. Primary Pure Intrarenal Yolk Sac Tumor in 1.5-Year-Old Boy-A Rare Case Report. Int. J. Surg. Pathol. 2023, 10668969221149131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Xia, C.; Yang, J.; Liu, Z.; Zhao, X.; Li, Y.; Liu, B.; Yang, Y.; She, Y. Renal Yolk Sac Tumor Clinically Misdiagnosed as Nephroblastoma: A Case Report. Fetal Pediatr. Pathol. 2023, 42, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, C.; Orazi, A.; Massimino, M.; Luksch, R. A cystic partially differentiated nephroblastoma producing alpha-fetoprotein. Am. J. Pediatr. Hematol. Oncol. 1992, 14, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Crocoli, A.; Madafferi, S.; Jenkner, A.; Zaccara, A.; Inserra, A. Elevated serum alpha-fetoprotein in Wilms tumor may follow the same pattern of other fetal neoplasms after treatment: Evidence from three cases. Pediatr. Surg. Int. 2008, 24, 499–502. [Google Scholar] [CrossRef]
- Yang, A.; Patterson, A.; Pavlock, T.; Chen, K.S.; Gagan, J.; Hatley, M.E.; Frazier, A.L.; Amatruda, J.F.; Laetsch, T.W.; Rakheja, D. Pitfalls in the diagnosis of yolk sac tumor: Lessons from a clinical trial. Pediatr. Blood Cancer 2022, 69, e29451. [Google Scholar] [CrossRef]
- Guo, Y.L.; Zhang, Y.L.; Zhu, J.Q. Prognostic value of serum α-fetoprotein in ovarian yolk sac tumors: A systematic review and meta-analysis. Mol. Clin. Oncol. 2015, 3, 125–132. [Google Scholar] [CrossRef] [PubMed]
- de la Motte Rouge, T.; Pautier, P.; Genestie, C.; Rey, A.; Gouy, S.; Leary, A.; Haie-Meder, C.; Kerbrat, P.; Culine, S.; Fizazi, K.; et al. Prognostic significance of an early decline in serum alpha-fetoprotein during chemotherapy for ovarian yolk sac tumors. Gynecol. Oncol. 2016, 142, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Paramita, P.; Preeti, A.; Mili, J.; Ridhi, J.; Mala, S.; Mm, G. Spectrum of Germ Cell Tumor (GCT): 5 Years’ Experience in a Tertiary Care Center and Utility of OCT4 as a Diagnostic Adjunct. Indian J. Surg. Oncol. 2022, 13, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Raynald; Yang, H.; Wang, J.; Du, J.; Zhang, W.; Shao, Q.; Li, C. Primary intracranial embryonal carcinoma in children: Report of two cases with review of the literature. Int. J. Clin. Exp. Pathol. 2017, 10, 10700–10710. [Google Scholar]
- Shu, H.; Yang, X.H.; Gao, A.F. Ovarian Sertoli-Leydig cell tumor in a 9-month-old infant with special histologic pattern. Fetal Pediatr. Pathol. 2012, 31, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Mooney, E.E.; Nogales, F.F.; Tavassoli, F.A. Hepatocytic differentiation in retiform Sertoli-Leydig cell tumors: Distinguishing a heterologous element from Leydig cells. Hum. Pathol. 1999, 30, 611–617. [Google Scholar] [CrossRef]
- Al-Hussaini, M.; Al-Othman, Y.; Hijazi, E.; McCluggage, W.G. A Report of Ovarian Sertoli-Leydig Cell Tumors With Heterologous Intestinal-type Glands and Alpha Fetoprotein Elevation and Review of the Literature. Int. J. Gynecol. Pathol. 2018, 37, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Strus, M.; Rajtar-Ciosek, A.; Jach, R.; Hankus, J.; Szczepański, W. Ovarian Sertoli-Leydig cell tumour with α-fetoprotein-producing intestinal glandular cells. Clinical case and short review of basic literature. Pol. J. Pathol. 2019, 70, 226–231. [Google Scholar] [CrossRef]
- Serife, K.; Karampelas, S.; Hottat, N.; Devalck, C.; Vanden Houte, K. 15-Year-Old Patient with an Unusual Alpha-Fetoprotein-Producing Sertoli-Leydig Cell Tumor of Ovary. Case Rep. Obstet. Gynecol. 2022, 2022, 4759826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).