The Evolving Role of Cryosurgery in Breast Cancer Management: A Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
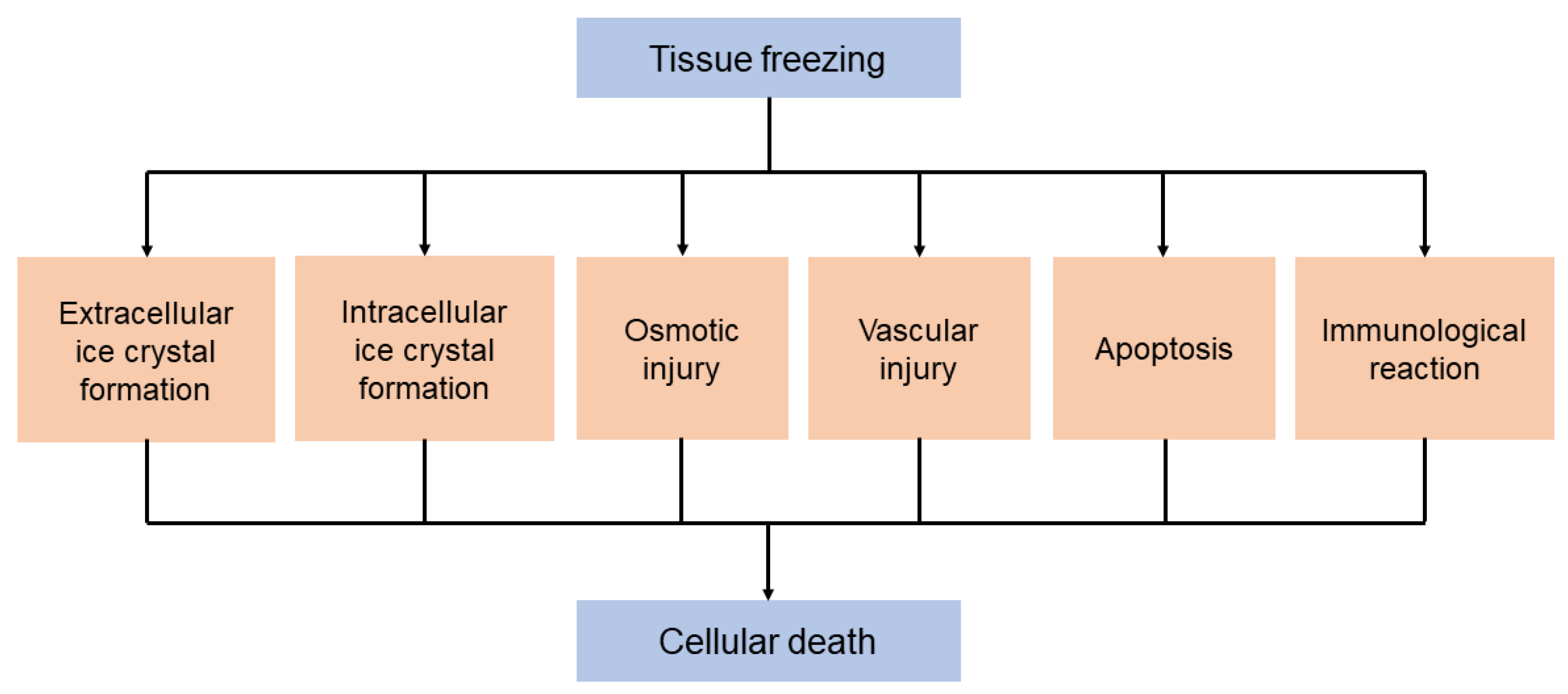
2. Cryobiology
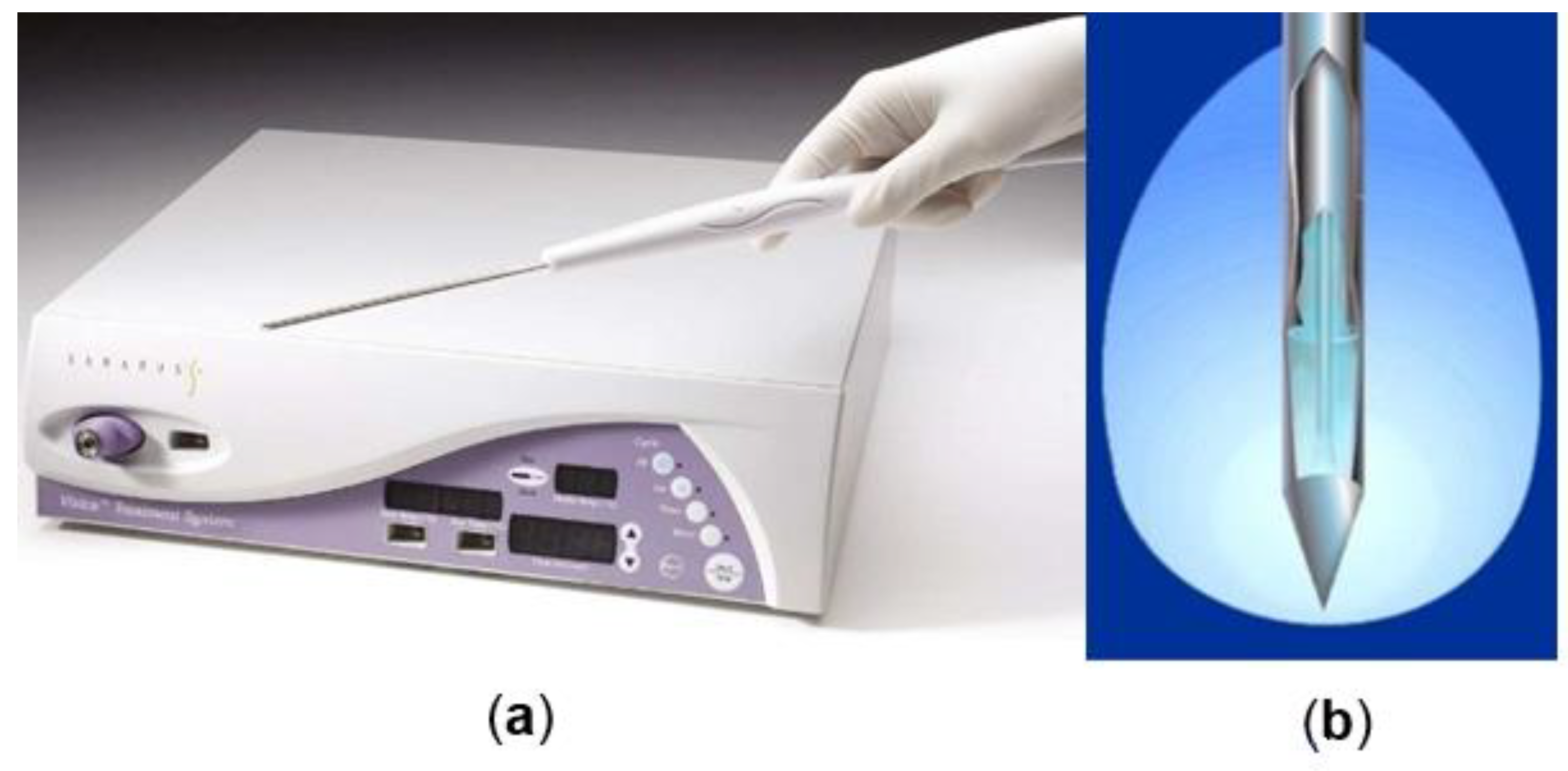
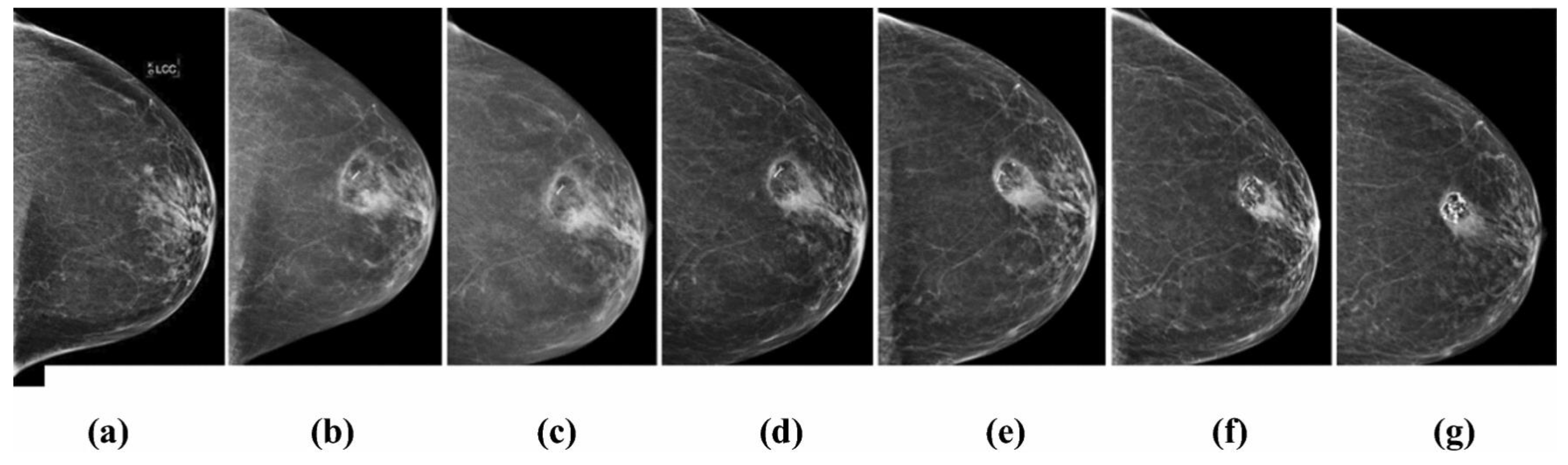
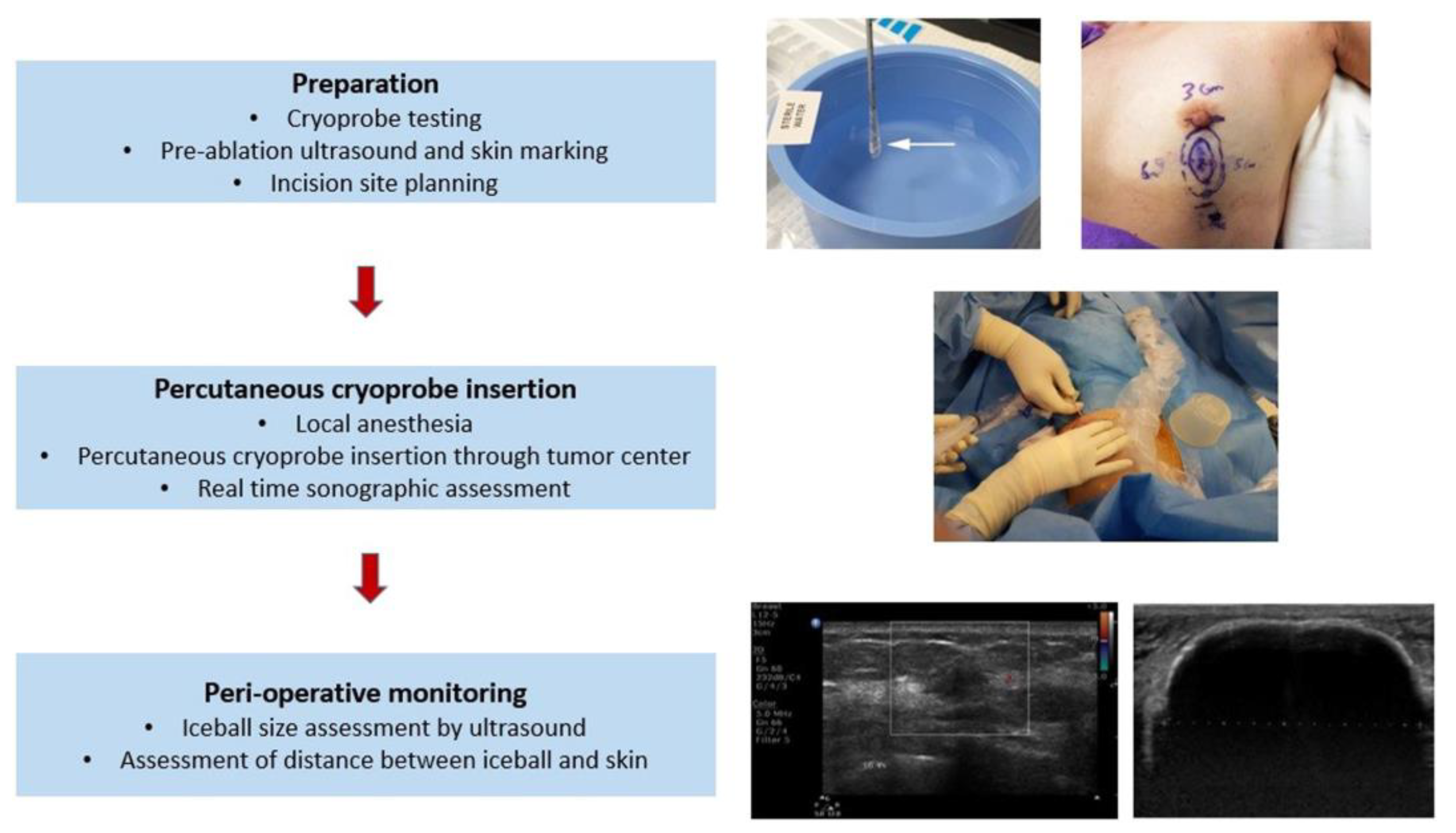
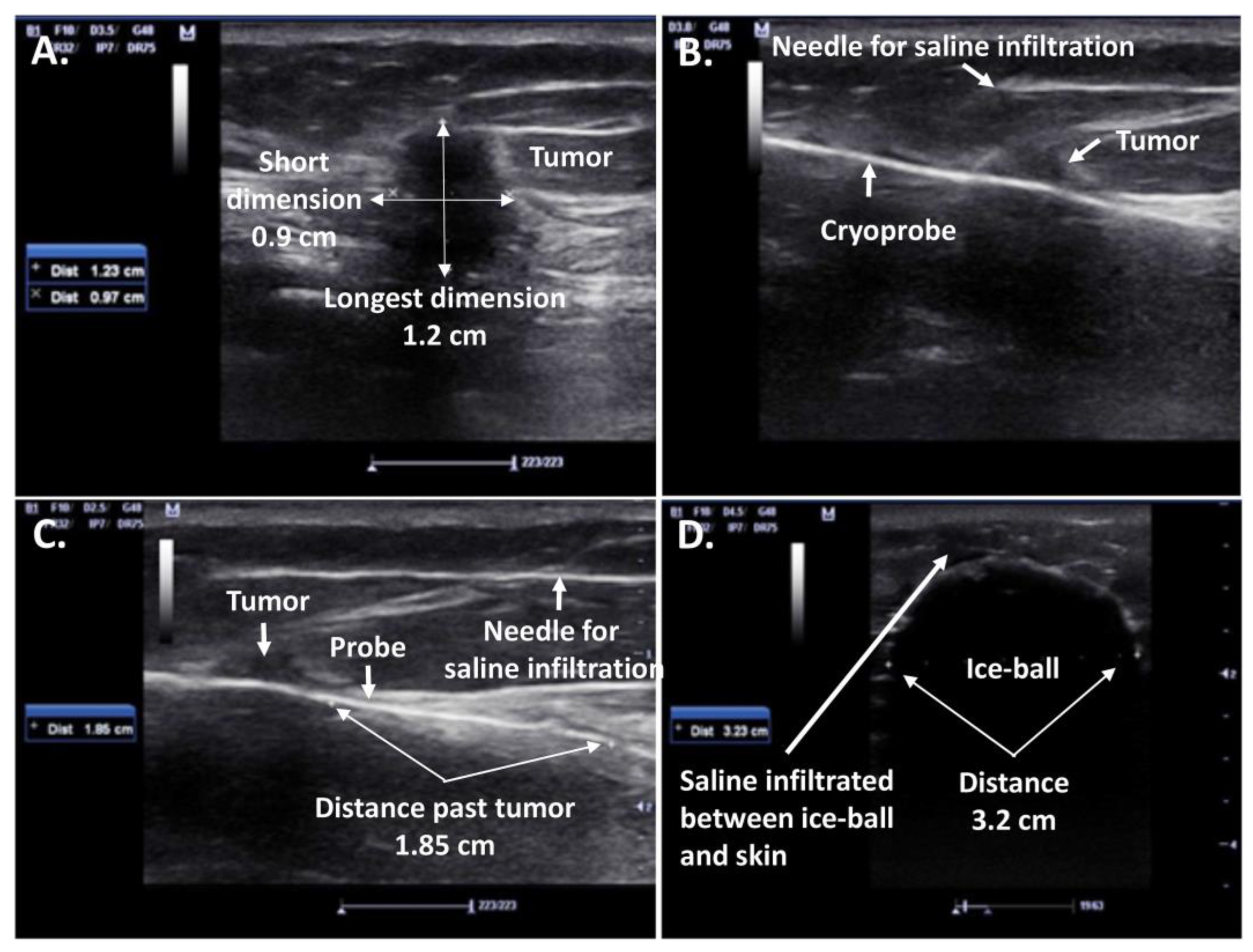
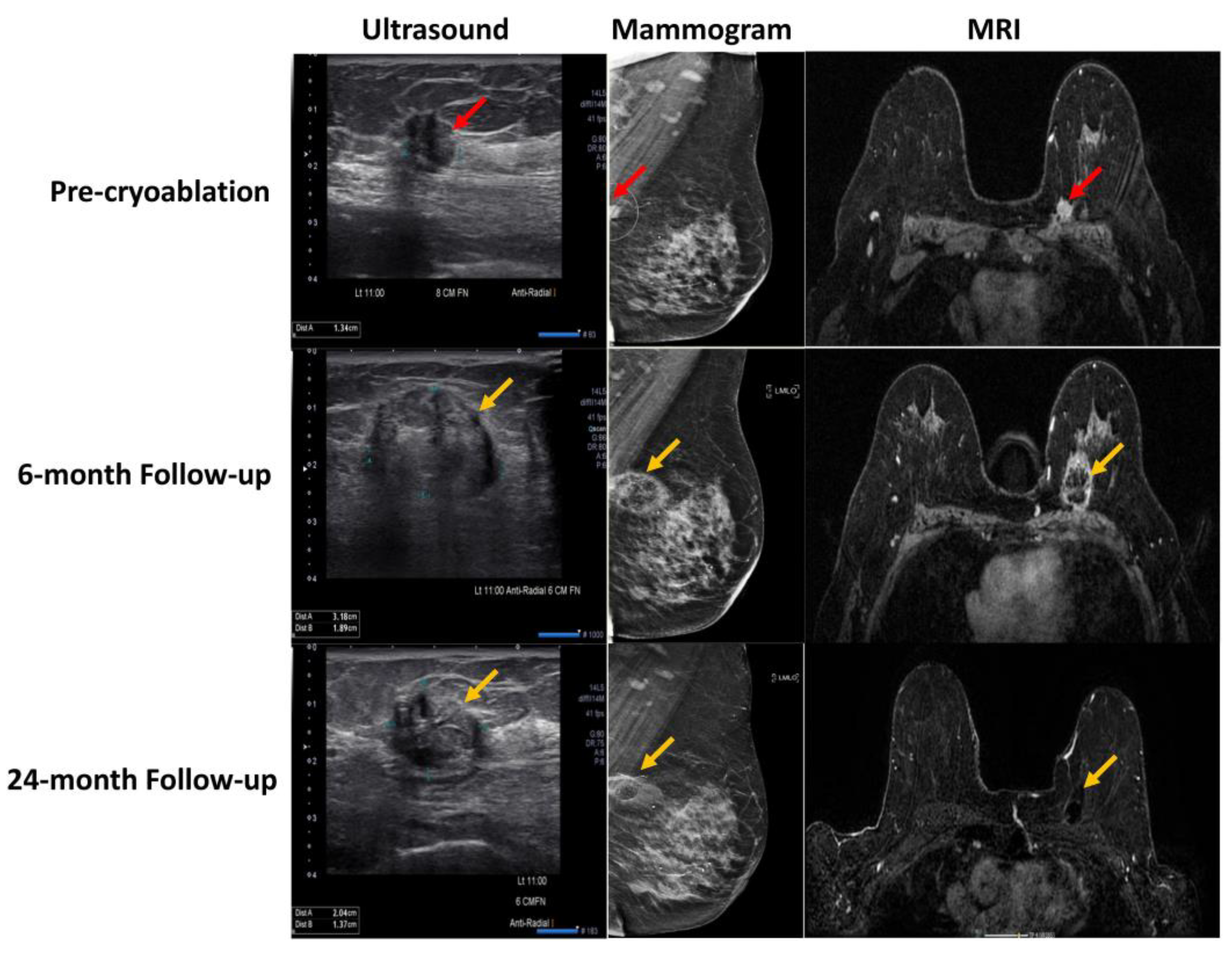
2.1. Cryosurgery and Breast Cancer
2.2. Cryosurgery Potential in Metastatic Breast Cancer
2.3. Cryosurgery and Immunotherapy
2.4. Cryosurgery and DCIS
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jouhara, H.; Chauhan, A.; Guichet, V.; Delpech, B.; Abdelkarem, M.A.; Olabi, A.G.; Trembley, J. Low-Temperature Heat Transfer Mediums for Cryogenic Applications. J. Taiwan Inst. Chem. Eng. 2023, 2023, 104709. [Google Scholar] [CrossRef]
- Ershov, B.G. Radiation-Chemical Decomposition of Seawater: The Appearance and Accumulation of Oxygen in the Earth’s Atmosphere. Radiat. Phys. Chem. 2020, 168, 108530. [Google Scholar] [CrossRef]
- Park, D.H.; Park, J.J.; Olawuyi, I.F.; Lee, W.Y. Quality of White Mushroom (Agaricus Bisporus) under Argon- and Nitrogen-Based Controlled Atmosphere Storage. Sci. Hortic. 2020, 265, 109229. [Google Scholar] [CrossRef]
- Bouganim, N.; Freiman, A. History of Cryotherapy. Dermatol. Online J. 2005, 11. [Google Scholar] [CrossRef]
- Ullrick, S.R.; Hebert, J.J.; Davis, K.W. Cryoablation in the Musculoskeletal System. Curr. Probl. Diagn. Radiol. 2008, 37, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Tafra, L.; Smith, J.S.; Woodward, J.E.; Simmons, R.M. Pilot Trial of Cryoprobe-Assisted Breast-Conserving Surgery for Small Ultrasound-Visible Cancers. Breast Dis. 2004, 15, 184–185. [Google Scholar] [CrossRef]
- Mohammed, A.; Miller, S.; Douglas-Moore, J.; Miller, M. Cryotherapy and Its Applications in the Management of Urologic Malignancies: A Review of Its Use in Prostate and Renal Cancers; Elsevier: Amsterdam, The Netherlands, 2014; Volume 31. [Google Scholar]
- Yiu, W.K.; Basco, M.T.; Aruny, J.E.; Cheng, S.W.K.; Sumpio, B.E. Cryosurgery: A Review. Int. J. Angiol. 2007, 16, 1–6. [Google Scholar] [CrossRef]
- Fine, R.E.; Gilmore, R.C.; Dietz, J.R.; Boolbol, S.K.; Berry, M.P.; Han, L.K.; Kenler, A.S.; Sabel, M.; Tomkovich, K.R.; VanderWalde, N.A.; et al. Cryoablation Without Excision for Low-Risk Early-Stage Breast Cancer: 3-Year Interim Analysis of Ipsilateral Breast Tumor Recurrence in the ICE3 Trial. Ann. Surg. Oncol. 2021, 28, 5525–5534. [Google Scholar] [CrossRef]
- Niu, L.; Mu, F.; Zhang, C.; Li, Y.; Liu, W.; Jiang, F.; Li, L.; Liu, C.; Zeng, J.; Yao, F.; et al. Cryotherapy Protocols for Metastatic Breast Cancer after Failure of Radical Surgery. Cryobiology 2013, 67, 17–22. [Google Scholar] [CrossRef]
- Kaufman, C.S.; Rewcastle, J.C. Cryosurgery for Breast Cancer. Technol. Cancer Res. Treat. 2004, 3, 165–175. [Google Scholar] [CrossRef]
- Tarkowski, R.; Rzaca, M. Cryosurgery in the Treatment of Women with Breast Cancer-a Review. Gland Surg. 2014, 3, 88–93. [Google Scholar] [CrossRef]
- Olagunju, A.; Forsman, T.; Ward, R.C. An Update on the Use of Cryoablation and Immunotherapy for Breast Cancer. Front. Immunol. 2022, 13, 1026475. [Google Scholar] [CrossRef] [PubMed]
- van de Voort, E.M.F.; Struik, G.M.; Birnie, E.; Moelker, A.; Verhoef, C.; Klem, T.M.A.L. Thermal Ablation as an Alternative for Surgical Resection of Small (≤2 Cm) Breast Cancers: A Meta-Analysis. Clin. Breast Cancer 2021, 21, e715–e730. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, F. Minimally-Invasive Thermal Ablation of Early-Stage Breast Cancer: A Systemic Review. Eur. J. Surg. Oncol. 2010, 36, 1149–1155. [Google Scholar] [CrossRef]
- Abdo, J.; Cornell, D.L.; Mittal, S.K.; Agrawal, D.K. Immunotherapy Plus Cryotherapy: Potential Augmented Abscopal Effect for Advanced Cancers. Front. Oncol. 2018, 8, 1. [Google Scholar] [CrossRef]
- Mehta, A.; Oklu, R.; Sheth, R.A. Thermal Ablative Therapies and Immune Checkpoint Modulation: Can Locoregional Approaches Effect a Systemic Response? Gastroenterol. Res. Pract. 2016, 2016, 9251375. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.; Co, M.; Fukuma, E. Prospective Clinical Trial on Expanding Indications for Cryosurgery for Early Breast Cancers. Clin. Breast Cancer 2023, 23, 363–368. [Google Scholar] [CrossRef]
- Roubidoux, M.A.; Sabel, M.S.; Bailey, J.E.; Kleer, C.G.; Klein, K.A.; Helvie, M.A. Small (<2.0-Cm) Breast Cancers: Mammographic and US Findings at US-Guided Cryoablation—Initial Experience1. Radiology 2004, 233, 857–867. [Google Scholar] [CrossRef]
- Rabin, Y.; Julian, T.B.; Olson, P.; Taylor, M.J.; Wolmark, N. Long-Term Follow-Up Post-Cryosurgery in a Sheep Breast Model. Cryobiology 1999, 39, 29–46. [Google Scholar] [CrossRef]
- Ward, R.C.; Lourenco, A.P.; Mainiero, M.B. Ultrasound-Guided Breast Cancer Cryoablation. Am. J. Roentgenol. 2019, 213, 716–722. [Google Scholar] [CrossRef]
- Habrawi, Z.; Melkus, M.W.; Khan, S.; Henderson, J.; Brandi, L.; Chu, V.; Layeequr Rahman, R. Cryoablation: A Promising Non-Operative Therapy for Low-Risk Breast Cancer. Am. J. Surg. 2021, 221, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sabel, M.S.; Kaufman, C.S.; Whitworth, P.; Chang, H.; Stocks, L.H.; Simmons, R.; Schultz, M. Cryoablation of Early-Stage Breast Cancer: Work-in-Progress Report of a Multi-Institutional Trial. Ann. Surg. Oncol. 2004, 11, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.M.; Ballman, K.V.; Cox, C.; Carp, N.; Sabol, J.; Hwang, R.F.; Attai, D.; Sabel, M.; Nathanson, D.; Kenler, A.; et al. A Phase II Trial Exploring the Success of Cryoablation Therapy in the Treatment of Invasive Breast Carcinoma: Results from ACOSOG (Alliance) Z1072. Ann. Surg. Oncol. 2016, 23, 2438. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Machida, Y.; Fukuma, E.; Tateishi, U. Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Findings after Percutaneous Cryoablation of Early Breast Cancer. Cancer Imaging 2020, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Littrup, P.J.; Jallad, B.; Chandiwala-Mody, P.; D’Agostini, M.; Adam, B.A.; Bouwman, D. Cryotherapy for Breast Cancer: A Feasibility Study without Excision. J. Vasc. Interv. Radiol. 2009, 20, 1329–1341. [Google Scholar] [CrossRef]
- Pfleiderer, S.O.R.; Freesmeyer, M.G.; Marx, C.; Kühne-Heid, R.; Schneider, A.; Kaiser, W.A. Cryotherapy of Breast Cancer under Ultrasound Guidance: Initial Results and Limitations. Eur. Radiol. 2002, 12, 3009–3014. [Google Scholar] [CrossRef] [PubMed]
- Pfleiderer, S.O.R.; Marx, C.; Camara, O.; Gajda, M.; Kaiser, W.A. Ultrasound-Guided, Percutaneous Cryotherapy of Small (<15 Mm) Breast Cancers. Invest. Radiol. 2005, 40, 472–477. [Google Scholar] [CrossRef]
- Machida, Y.; Shimauchi, A.; Igarashi, T.; Fukuma, E. MRI Findings After Cryoablation of Primary Breast Cancer Without Surgical Resection. Acad. Radiol. 2019, 26, 744–751. [Google Scholar] [CrossRef]
- Whitworth, P.W.; Rewcastle, J.C. Cryoablation and Cryolocalization in the Management of Breast Disease. J. Surg. Oncol. 2005, 90, 1–9. [Google Scholar] [CrossRef]
- Tafra, L.; Smith, S.J.; Woodward, J.E.; Fernandez, K.L.; Sawyer, K.T.; Grenko, R.T. Pilot Trial of Cryoprobe-Assisted Breast-Conserving Surgery for Small Ultrasound-Visible Cancers. Ann. Surg. Oncol. 2003, 10, 1018–1024. [Google Scholar] [CrossRef]
- Manenti, G.; Scarano, A.L.; Pistolese, C.A.; Perretta, T.; Bonanno, E.; Orlandi, A.; Simonetti, G. Subclinical Breast Cancer: Minimally Invasive Approaches. Our Experience with Percutaneous Radiofrequency Ablation vs. Cryotherapy. Breast Care 2013, 8, 356. [Google Scholar] [CrossRef]
- Gera, R.; Chehade, H.E.L.H.; Wazir, U.; Tayeh, S.; Kasem, A.; Mokbel, K. Locoregional therapy of the primary tumour in de novo stage IV breast cancer in 216,066 patients: A meta-analysis. Sci. Rep. 2020, 10, 2952. [Google Scholar] [CrossRef] [PubMed]
- Patrick, J.; Khan, S.A. Surgical Management of de Novo Stage IV Breast Cancer. JNCCN J. Natl. Compr. Cancer Netw. 2015, 13, 487–493. [Google Scholar] [CrossRef][Green Version]
- Kolben, T.; Kolben, T.M.; Himsl, I.; Degenhardt, T.; Engel, J.; Wuerstlein, R.; Mahner, S.; Harbeck, N.; Kahlert, S. Local Resection of Primary Tumor in Upfront Stage IV Breast Cancer. Breast Care 2016, 11, 411–417. [Google Scholar] [CrossRef]
- Lang, J.E.; Tereffe, W.; Mitchell, M.P.; Rao, R.; Feng, L.; Meric-Bernstam, F.; Bedrosian, I.; Kuerer, H.M.; Hunt, K.K.; Hortobagyi, G.N.; et al. Primary Tumor Extirpation in Breast Cancer Patients Who Present with Stage IV Disease Is Associated with Improved Survival. Ann. Surg. Oncol. 2013, 20, 1893–1899. [Google Scholar] [CrossRef]
- Ruiterkamp, J.; Ernst, M.F.; van de Poll-Franse, L.V.; Bosscha, K.; Tjan-Heijnen, V.C.G.; Voogd, A.C. Surgical Resection of the Primary Tumour Is Associated with Improved Survival in Patients with Distant Metastatic Breast Cancer at Diagnosis. Eur. J. Surg. Oncol. 2009, 35, 1146–1151. [Google Scholar] [CrossRef][Green Version]
- Pusceddu, C.; Sotgia, B.; Amucano, G.; Fele, R.M.; Pilleri, S.; Meloni, G.B.; Melis, L. Breast Cryoablation in Patients with Bone Metastatic Breast Cancer. J. Vasc. Interv. Radiol. 2014, 25, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, C.; Melis, L.; Ballicu, N.; Meloni, P.; Sanna, V.; Porcu, A.; Fancellu, A. Cryoablation of Primary Breast Cancer in Patients with Metastatic Disease: Considerations Arising from a Single-Centre Data Analysis. BioMed Res. Int. 2017, 2017, 3839012. [Google Scholar] [CrossRef] [PubMed]
- McArthur, H.L.; Diab, A.; Page, D.B.; Yuan, J.; Solomon, S.B.; Sacchini, V.; Comstock, C.; Durack, J.C.; Maybody, M.; Sung, J.; et al. A Pilot Study of Preoperative Single-Dose Ipilimumab and/or Cryoablation in Women with Early-Stage Breast Cancer with Comprehensive Immune Profiling. Clin. Cancer Res. 2016, 22, 5729–5737. [Google Scholar] [CrossRef]
- Page, D.B.; Yuan, J.; Redmond, D.; Wen, Y.H.; Durack, J.C.; Emerson, R.; Solomon, S.; Dong, Z.; Wong, P.; Comstock, C.; et al. Deep Sequencing of T-Cell Receptor DNA as a Biomarker of Clonally Expanded TILs in Breast Cancer after Immunotherapy. Cancer Immunol. Res. 2016, 4, 835–844. [Google Scholar] [CrossRef]
- Comen, E. A Study of Pre-Operative Treatment with Cryoablation and Immune Therapy in Early Stage Breast Cancer June 2016–June 2023. Identifier NCT02833233. Available online: https://clinicaltrials.gov/ct2/show/NCT02833233 (accessed on 26 June 2023).
- McArthur, H. Peri-Operative Ipilimumab+Nivolumab and Cryoablation in Women with Triple-Negative Breast Cancer 12 November 2019–June 2026. Identifier NCT03546686. Available online: https://clinicaltrials.gov/ct2/show/NCT03546686 (accessed on 26 June 2023).
- Gabriel, E.M. Cryoablation, Atezolizumab/Nab-Paclitaxel for Locally Advanced or Metastatic Triple Negative Breast Cancer 23 January 2020–17 November 2021. Identifier NCT04249167. Available online: https://clinicaltrials.gov/ct2/show/NCT04249167 (accessed on 26 June 2023).
- Pilewskie, M.; Olcese, C.; Patil, S.; Van Zee, K.J. Women with Low-Risk DCIS Eligible for the LORIS Trial After Complete Surgical Excision: How Low Is Their Risk After Standard Therapy? Ann. Surg. Oncol. 2016, 23, 4253–4261. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, K.; Escobar, P.F.; Matsunaga, T. Mammary ductoscopy: Current status and future prospects. Eur. J. Surg. Oncol. 2005, 31, 3–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokbel, K.; Kodresko, A.; Ghazal, H.; Mokbel, R.; Trembley, J.; Jouhara, H. The Evolving Role of Cryosurgery in Breast Cancer Management: A Comprehensive Review. Cancers 2023, 15, 4272. https://doi.org/10.3390/cancers15174272
Mokbel K, Kodresko A, Ghazal H, Mokbel R, Trembley J, Jouhara H. The Evolving Role of Cryosurgery in Breast Cancer Management: A Comprehensive Review. Cancers. 2023; 15(17):4272. https://doi.org/10.3390/cancers15174272
Chicago/Turabian StyleMokbel, Kefah, Alevtina Kodresko, Heba Ghazal, Ramia Mokbel, Jon Trembley, and Hussam Jouhara. 2023. "The Evolving Role of Cryosurgery in Breast Cancer Management: A Comprehensive Review" Cancers 15, no. 17: 4272. https://doi.org/10.3390/cancers15174272
APA StyleMokbel, K., Kodresko, A., Ghazal, H., Mokbel, R., Trembley, J., & Jouhara, H. (2023). The Evolving Role of Cryosurgery in Breast Cancer Management: A Comprehensive Review. Cancers, 15(17), 4272. https://doi.org/10.3390/cancers15174272






