Bilobar Radioembolization Carries the Risk of Radioembolization-Induced Liver Disease in the Treatment of Advanced Hepatocellular Carcinoma: Safety and Efficacy Comparison to Systemic Therapy with Atezolizumab/Bevacizumab
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Definitions
2.3. Treatments
2.4. Clinical Data and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
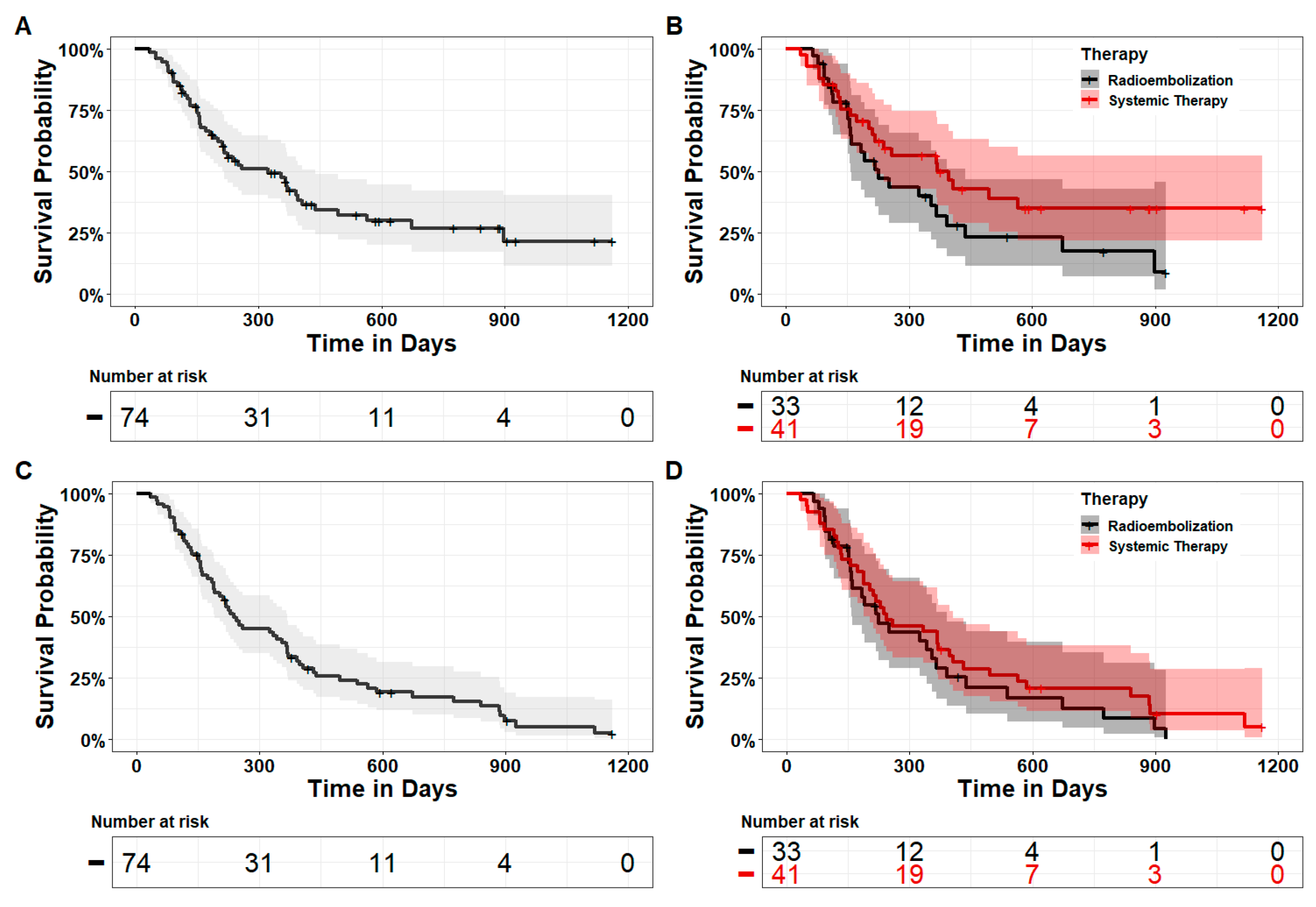
3.2. Efficacy
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Liver Factsheet. International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (accessed on 24 July 2023).
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv238–iv255. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sala, M.; Llovet, J.M. Chemoembolization for hepatocellular carcinoma. Gastroenterology 2004, 127 (Suppl. S1), S179–S188. [Google Scholar] [CrossRef]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef]
- Dhondt, E.; Lambert, B.; Hermie, L.; Huyck, L.; Vanlangenhove, P.; Geerts, A.; Verhelst, X.; Aerts, M.; Vanlander, A.; Berrevoet, F.; et al. (90)Y Radioembolization versus Drug-eluting Bead Chemoembolization for Unresectable Hepatocellular Carcinoma: Results from the TRACE Phase II Randomized Controlled Trial. Radiology 2022, 303, 699–710. [Google Scholar] [CrossRef]
- Facciorusso, A.; Serviddio, G.; Muscatiello, N. Transarterial radioembolization vs chemoembolization for hepatocarcinoma patients: A systematic review and meta-analysis. World J. Hepatol. 2016, 8, 770–778. [Google Scholar] [CrossRef]
- Sangro, B.; Inarrairaegui, M.; Bilbao, J.I. Radioembolization for hepatocellular carcinoma. J. Hepatol. 2012, 56, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Hilgard, P.; Hamami, M.; Fouly, A.E.; Scherag, A.; Muller, S.; Ertle, J.; Heusner, T.; Cicinnati, V.R.; Paul, A.; Bockisch, A.; et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010, 52, 1741–1749. [Google Scholar] [CrossRef]
- Inarrairaegui, M.; Martinez-Cuesta, A.; Rodriguez, M.; Bilbao, J.I.; Arbizu, J.; Benito, A.; Alegre, F.; D’Avola, D.; Herrero, J.I.; Quiroga, J.; et al. Analysis of prognostic factors after yttrium-90 radioembolization of advanced hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Chow, P.K.H.; Gandhi, M.; Tan, S.B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef]
- Braat, M.N.; van Erpecum, K.J.; Zonnenberg, B.A.; van den Bosch, M.A.; Lam, M.G. Radioembolization-induced liver disease: A systematic review. Eur. J. Gastroenterol. Hepatol. 2017, 29, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Gil-Alzugaray, B.; Rodriguez, J.; Sola, I.; Martinez-Cuesta, A.; Viudez, A.; Chopitea, A.; Inarrairaegui, M.; Arbizu, J.; Bilbao, J.I. Liver disease induced by radioembolization of liver tumors: Description and possible risk factors. Cancer 2008, 112, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Gil-Alzugaray, B.; Chopitea, A.; Inarrairaegui, M.; Bilbao, J.I.; Rodriguez-Fraile, M.; Rodriguez, J.; Benito, A.; Dominguez, I.; D’Avola, D.; Herrero, J.I.; et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology 2013, 57, 1078–1087. [Google Scholar] [CrossRef]
- de Castro, T.; Jochheim, L.S.; Bathon, M.; Welland, S.; Scheiner, B.; Shmanko, K.; Roessler, D.; Ben Khaled, N.; Jeschke, M.; Ludwig, J.M.; et al. Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: A real-world experience. Ther. Adv. Med. Oncol. 2022, 14, 17588359221080298. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Thurston, K.G. Radioembolization with 90Yttrium microspheres: A state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J. Vasc. Interv. Radiol. 2006, 17, 1251–1278. [Google Scholar] [CrossRef] [PubMed]
- Agirrezabal, I.; Brennan, V.K.; Colaone, F.; Shergill, S.; Pereira, H.; Chatellier, G.; Vilgrain, V. Transarterial Radioembolization Versus Atezolizumab-Bevacizumab in Unresectable Hepatocellular Carcinoma: A Matching-Adjusted Indirect Comparison of Time to Deterioration in Quality of Life. Adv. Ther. 2022, 39, 2035–2051. [Google Scholar] [CrossRef]
- Park, H.C.; Seong, J.; Han, K.H.; Chon, C.Y.; Moon, Y.M.; Suh, C.O. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 150–155. [Google Scholar] [CrossRef]
- Reincke, M.; Schultheiss, M.; Doppler, M.; Verloh, N.; Uller, W.; Sturm, L.; Thimme, R.; Goetz, C.; Bettinger, D. Hepatic decompensation after transarterial radioembolization: A retrospective analysis of risk factors and outcome in patients with hepatocellular carcinoma. Hepatol. Commun. 2022, 6, 3223–3233. [Google Scholar] [CrossRef]
- Garin, E.; Lenoir, L.; Rolland, Y.; Edeline, J.; Mesbah, H.; Laffont, S.; Poree, P.; Clement, B.; Raoul, J.L.; Boucher, E. Dosimetry based on 99mTc-macroaggregated albumin SPECT/CT accurately predicts tumor response and survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microspheres: Preliminary results. J. Nucl. Med. 2012, 53, 255–263. [Google Scholar] [CrossRef]
- Garin, E.; Rolland, Y.; Edeline, J.; Icard, N.; Lenoir, L.; Laffont, S.; Mesbah, H.; Breton, M.; Sulpice, L.; Boudjema, K.; et al. Personalized dosimetry with intensification using 90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J. Nucl. Med. 2015, 56, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Rolland, Y.; Edeline, J. (90)Y-Loaded Microsphere SIRT of HCC Patients With Portal Vein Thrombosis: High Clinical Impact of 99mTc-MAA SPECT/CT-Based Dosimetry. Semin. Nucl. Med. 2019, 49, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Rimassa, L.; et al. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: A large real-life worldwide population. Eur. J. Cancer 2023, 180, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Goin, J.E.; Salem, R.; Carr, B.I.; Dancey, J.E.; Soulen, M.C.; Geschwind, J.F.; Goin, K.; Van Buskirk, M.; Thurston, K. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: Factors associated with liver toxicities. J. Vasc. Interv. Radiol. 2005, 16, 205–213. [Google Scholar] [CrossRef]
- Antkowiak, M.; Gabr, A.; Das, A.; Ali, R.; Kulik, L.; Ganger, D.; Moore, C.; Abecassis, M.; Katariya, N.; Mouli, S.; et al. Prognostic Role of Albumin, Bilirubin, and ALBI Scores: Analysis of 1000 Patients with Hepatocellular Carcinoma Undergoing Radioembolization. Cancers 2019, 11, 879. [Google Scholar] [CrossRef]
- Hickey, R.; Mouli, S.; Kulik, L.; Desai, K.; Thornburg, B.; Ganger, D.; Baker, T.; Abecassis, M.; Ralph Kallini, J.; Gabr, A.; et al. Independent Analysis of Albumin-Bilirubin Grade in a 765-Patient Cohort Treated with Transarterial Locoregional Therapy for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2016, 27, 795–802. [Google Scholar] [CrossRef]
- Lescure, C.; Estrade, F.; Pedrono, M.; Campillo-Gimenez, B.; Le Sourd, S.; Pracht, M.; Palard, X.; Bourien, H.; Muzellec, L.; Uguen, T.; et al. ALBI Score Is a Strong Predictor of Toxicity Following SIRT for Hepatocellular Carcinoma. Cancers 2021, 13, 3794. [Google Scholar] [CrossRef]
- De la Garza-Ramos, C.; Overfield, C.J.; Montazeri, S.A.; Liou, H.; Paz-Fumagalli, R.; Frey, G.T.; McKinney, J.M.; Ritchie, C.A.; Devcic, Z.; Lewis, A.R.; et al. Biochemical Safety of Ablative Yttrium-90 Radioembolization for Hepatocellular Carcinoma as a Function of Percent Liver Treated. J. Hepatocell. Carcinoma 2021, 8, 861–870. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Network, N.C.C. Hepatobiliary Cancers. Version 5. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 24 July 2023).
- Vouche, M.; Habib, A.; Ward, T.J.; Kim, E.; Kulik, L.; Ganger, D.; Mulcahy, M.; Baker, T.; Abecassis, M.; Sato, K.T.; et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: Multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology 2014, 60, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, R.J.; Gabr, A.; Abouchaleh, N.; Ali, R.; Al Asadi, A.; Mora, R.A.; Kulik, L.; Ganger, D.; Desai, K.; Thornburg, B.; et al. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology 2018, 287, 1050–1058. [Google Scholar] [CrossRef]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef]
- Meserve, J.; Facciorusso, A.; Holmer, A.K.; Annese, V.; Sandborn, W.J.; Singh, S. Systematic review with meta-analysis: Safety and tolerability of immune checkpoint inhibitors in patients with pre-existing inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2021, 53, 374–382. [Google Scholar] [CrossRef]
- Golden, E.B.; Apetoh, L. Radiotherapy and immunogenic cell death. Semin. Radiat. Oncol. 2015, 25, 11–17. [Google Scholar] [CrossRef]
- Procureur, A.; Simonaggio, A.; Bibault, J.E.; Oudard, S.; Vano, Y.A. Enhance the Immune Checkpoint Inhibitors Efficacy with Radiotherapy Induced Immunogenic Cell Death: A Comprehensive Review and Latest Developments. Cancers 2021, 13, 678. [Google Scholar] [CrossRef]

| All Patients (n = 74) | Patients Undergoing Radioembolization (n = 33) | Patients Receiving Systemic Therapy (n = 41) | p-Value (RE vs. ST) | |
|---|---|---|---|---|
| Age (years), mean ± SD | 69.5 (10.6) | 71.1 (9.7) | 68.1 (11.2) | 0.23 |
| Male gender, N (%) | 53 (71.6) | 22 (66.6) | 31 (75.6) | 0.44 |
| ECOG Performance Score | ||||
| 0, N (%) | 20 (27.0) | 6 (18.1) | 14 (34.1) | 0.32 |
| 1, N (%) | 49 (66.2) | 24 (72.7) | 25 (61.0) | |
| 2, N (%) | 5 (6.8) | 3 (9.1) | 2 (4.9) | |
| Liver Cirrhosis, N (%) | 54 (73.0) | 27 (81.8) | 27 (65.9) | 0.19 |
| Underlying Liver Disease | ||||
| ASH, N (%) | 17 (23.0) | 7 (21.2) | 10 (24.4) | 0.45 |
| NASH, N (%) | 23 (31.1) | 13 (39.4) | 10 (24.4) | |
| HCV, N (%) | 16 (21.6) | 5 (15.2) | 11 (26.8) | |
| HBV, N (%) | 3 (4.1) | 2 (6.1) | 1 (2.4) | |
| Autoimmune, N (%) | 2 (2.7) | 2 (6.0) | 0 (0) | |
| Other, N (%) | 11 (14.9) | 3 (9.1) | 8 (19.5) | |
| Child–Pugh Score | ||||
| A, N (%) | 67 (90.5) | 32 (97.0) | 35 (85.4) | 0.12 |
| B, N (%) | 7 (9.5) | 1 (3.0) | 6 (14.6) | |
| C, N (%) | 0 (0) | 0 (0) | 0 (0) | |
| ALBI grade | ||||
| 1, N (%) | 54 (73.0) | 27 (81.8) | 27 (65.9) | 0.11 |
| 2, N (%) | 8 (10.8) | 4 (12.1) | 4 (9.8) | |
| 3, N (%) | 12 (16.2) | 2 (6.1) | 10 (24.4) | |
| Complications of Cirrhosis | ||||
| Ascites, N (%) | 13 (17.6) | 4 (12.1) | 9 (22.0) | 0.36 |
| Refractory ascites, N (%) | 3 (4.1) | 0 (0) | 3 (7.3) | 0.25 |
| Tumor Stage | ||||
| BCLC A, N (%) | 2 (2.7) | 2 (6.1) | 0 (0) | 0.34 |
| BCLC B, N (%) | 11 (14.9) | 5 (15.2) | 6 (14.6) | |
| BCLC C, N (%) | 61 (82.4) | 26 (78.8) | 35 (85.4) | |
| Presence of macrovascular invasion, extrahepatic spread, or both, N (%) | 36 (48.6) | 6 (18.2) | 30 (73.2) | <0.001 |
| Extrahepatic Spread, N (%) | 29 (39.2) | 0 (0) | 29 (70.7) | <0.001 |
| Macrovascular Invasion, N (%) | 18 (24.3) | 6 (18.2) | 12 (29.3) | 0.29 |
| Tumor burden > 25%, N (%) | 22 (29.7) | 10 (30.3) | 12 (29.3) | 1.0 |
| AFP (IU/mL), median (min–max) | 42.1 (1.1–256,801.9) | 12.9 (1.1–2374.2) | 195.4 (1.2–256,801.9) | 0.16 |
| Prior therapy | ||||
| Resection, N(%) | 16 (21.6) | 3 (9.1) | 13 (31.7) | 0.024 |
| TACE, N(%) | 17 (23.0) | 11 (33.3) | 6 (14.6) | 0.09 |
| RE, N(%) | 8 (10.8) | 2 (6.1) | 6 (14.6) | 0.29 |
| Metachronous tumors, N (%) | 3 (4.1) | 0 (0) | 3 (7.3) | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeschke, M.; Ludwig, J.M.; Leyh, C.; Pabst, K.M.; Weber, M.; Theysohn, J.M.; Lange, C.M.; Herrmann, K.; Schmidt, H.H.-J.; Jochheim, L.S. Bilobar Radioembolization Carries the Risk of Radioembolization-Induced Liver Disease in the Treatment of Advanced Hepatocellular Carcinoma: Safety and Efficacy Comparison to Systemic Therapy with Atezolizumab/Bevacizumab. Cancers 2023, 15, 4274. https://doi.org/10.3390/cancers15174274
Jeschke M, Ludwig JM, Leyh C, Pabst KM, Weber M, Theysohn JM, Lange CM, Herrmann K, Schmidt HH-J, Jochheim LS. Bilobar Radioembolization Carries the Risk of Radioembolization-Induced Liver Disease in the Treatment of Advanced Hepatocellular Carcinoma: Safety and Efficacy Comparison to Systemic Therapy with Atezolizumab/Bevacizumab. Cancers. 2023; 15(17):4274. https://doi.org/10.3390/cancers15174274
Chicago/Turabian StyleJeschke, Matthias, Johannes M. Ludwig, Catherine Leyh, Kim M. Pabst, Manuel Weber, Jens M. Theysohn, Christian M. Lange, Ken Herrmann, Hartmut H. -J. Schmidt, and Leonie S. Jochheim. 2023. "Bilobar Radioembolization Carries the Risk of Radioembolization-Induced Liver Disease in the Treatment of Advanced Hepatocellular Carcinoma: Safety and Efficacy Comparison to Systemic Therapy with Atezolizumab/Bevacizumab" Cancers 15, no. 17: 4274. https://doi.org/10.3390/cancers15174274
APA StyleJeschke, M., Ludwig, J. M., Leyh, C., Pabst, K. M., Weber, M., Theysohn, J. M., Lange, C. M., Herrmann, K., Schmidt, H. H.-J., & Jochheim, L. S. (2023). Bilobar Radioembolization Carries the Risk of Radioembolization-Induced Liver Disease in the Treatment of Advanced Hepatocellular Carcinoma: Safety and Efficacy Comparison to Systemic Therapy with Atezolizumab/Bevacizumab. Cancers, 15(17), 4274. https://doi.org/10.3390/cancers15174274







