Effect of Local Adjuvants Following Curettage of Benign and Intermediate Tumours of Bone: A Systematic Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
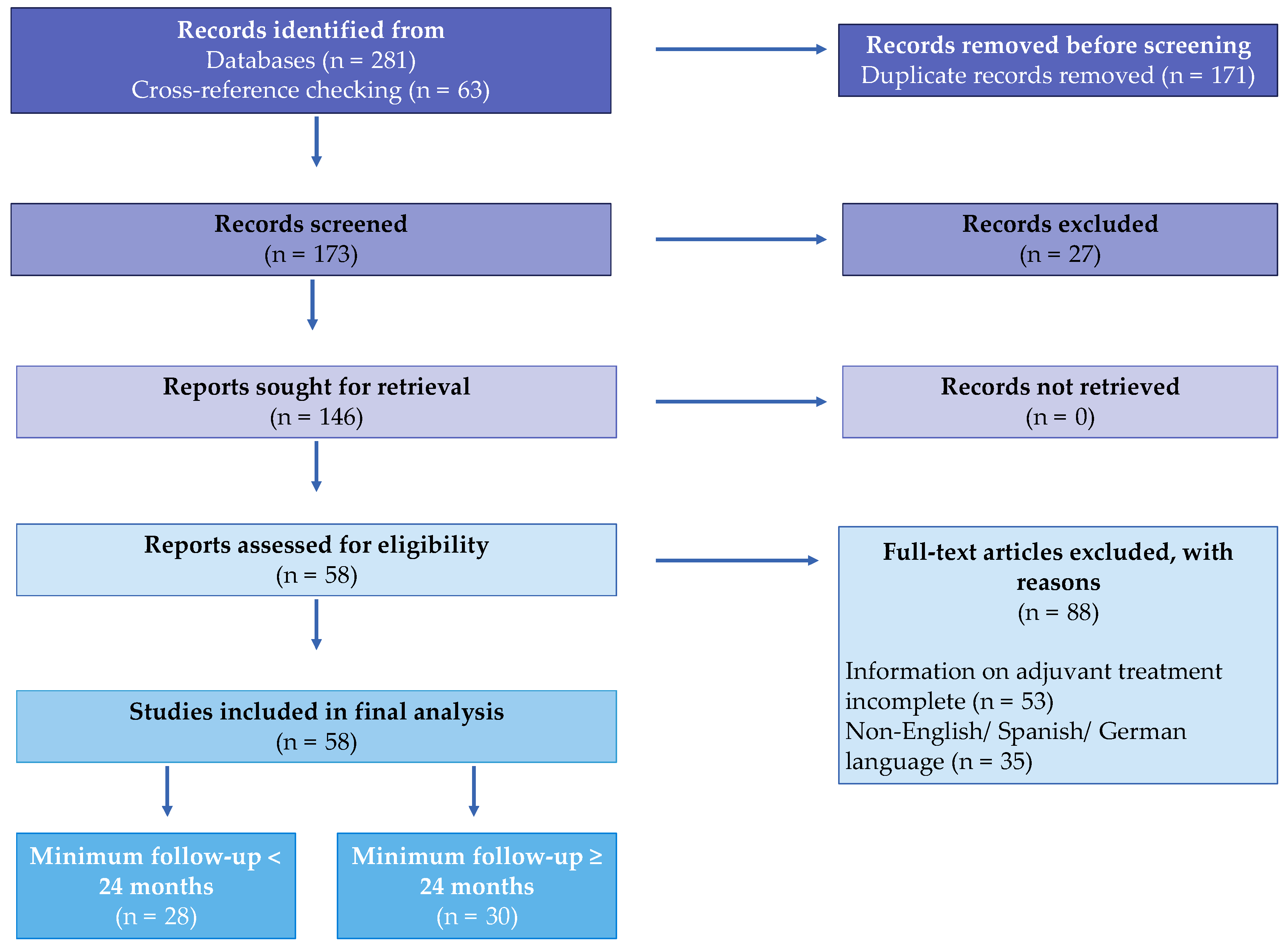
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Treatment
3.1.1. GCT
3.1.2. ACT
3.1.3. ABC
3.1.4. Chondroblastoma
3.1.5. Enchondroma
3.1.6. Rare Tumours/Tumour-like Lesions
3.2. Local Recurrences
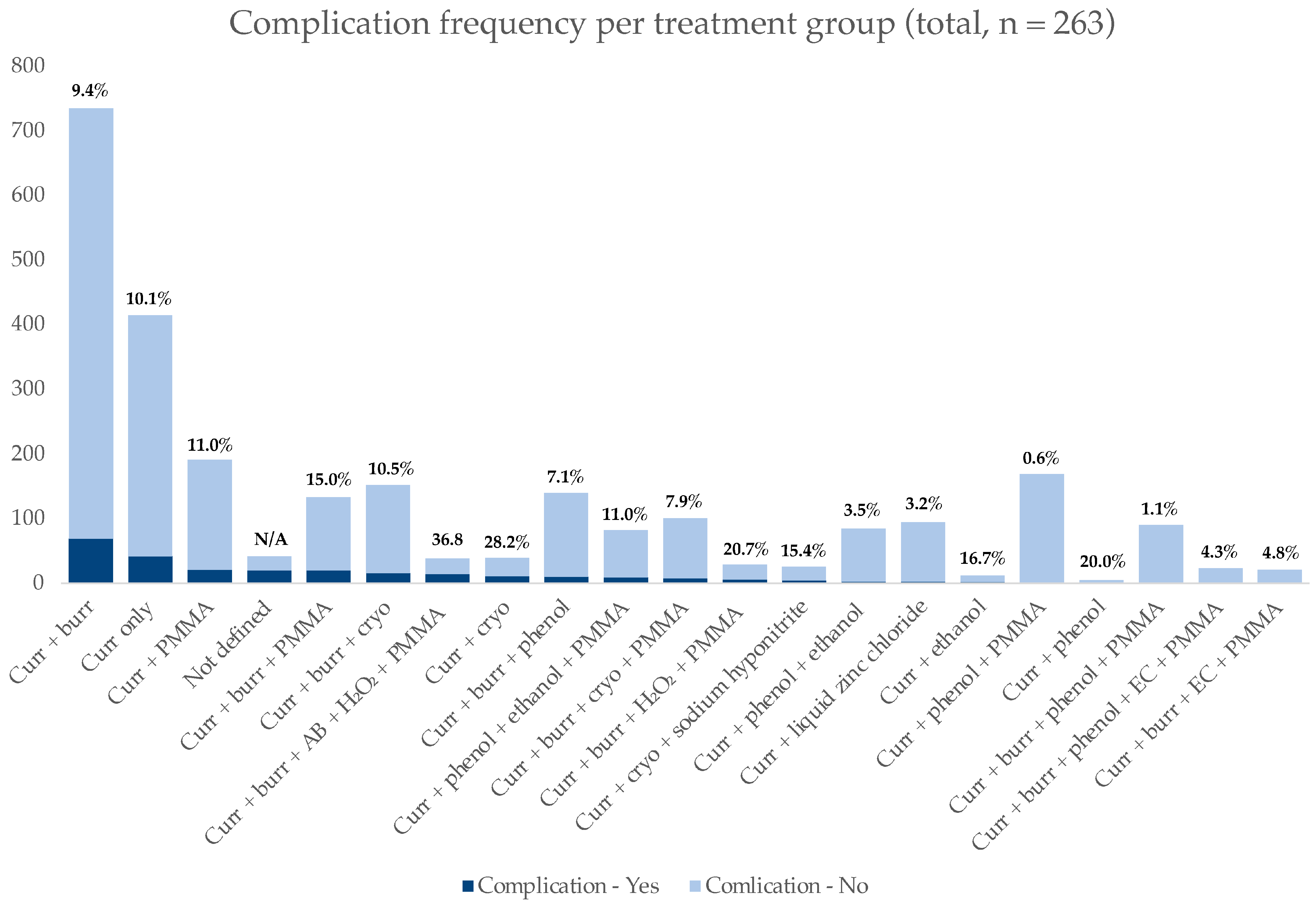
3.3. Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vlychou, M.; Athanasou, N. Radiological and pathological diagnosis of paediatric bone tumours and tumour-like lesions. Pathology 2008, 40, 196–216. [Google Scholar] [CrossRef] [PubMed]
- Chigira, M.; Watanabe, H.; Arita, S.; Noda, K.; Shimizu, T.; Shinozaki, T.; Nagase, M. Remodeling of large bone defects in the treatment of space-occupying lesions. Curettage without bone graft for treating benign bone tumors. Arch. Orthop. Trauma Surg. 1992, 111, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, C.P., Jr.; Hefele, M.C.; Peabody, T.D.; Montag, A.G.; Aithal, V.; Simon, M.A. Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr. J. Bone Jt. Surg. Am. 1999, 81, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Mittag, F.; Leichtle, C.; Kieckbusch, I.; Wolburg, H.; Rudert, M.; Kluba, T.; Leichtle, U. Cytotoxic effect and tissue penetration of phenol for adjuvant treatment of giant cell tumours. Oncol. Lett. 2013, 5, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.A.; Clemency, R.E., Jr. The closed cryosurgical treatment of giant cell tumor. Clin. Orthop. Relat. Res. 1985, 192, 149–158. [Google Scholar] [CrossRef]
- Marcove, R.C.; Weis, L.D.; Vaghaiwalla, M.R.; Pearson, R. Cryosurgery in the treatment of giant cell tumors of bone: A report of 52 consecutive cases. Clin. Orthop. Relat. Res. 1978, 134, 275–289. [Google Scholar] [CrossRef]
- Jones, K.B.; DeYoung, B.R.; Morcuende, J.A.; Buckwalter, J.A. Ethanol as a local adjuvant for giant cell tumor of bone. Iowa Orthop. J. 2006, 26, 69–76. [Google Scholar]
- Zhen, W.; Yaotian, H.; Songjian, L.; Ge, L.; Qingliang, W. Giant-cell tumour of bone. The long-term results of treatment by curettage and bone graft. J. Bone Jt. Surg. Br. 2004, 86, 212–216. [Google Scholar] [CrossRef]
- Shemesh, S.S.; Pretell-Mazzini, J.; Quartin, P.a.J.; Rutenberg, T.F.; Conway, S.A. Surgical treatment of low-grade chondrosarcoma involving the appendicular skeleton: Long-term functional and oncological outcomes. Arch. Orthop. Trauma Surg. 2019, 139, 1659–1666. [Google Scholar] [CrossRef]
- Cummings, J.E.; Smith, R.A.; Heck, R.K., Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: A preliminary study. Clin. Orthop. Relat. Res. 2010, 468, 231–237. [Google Scholar] [CrossRef]
- Bini, S.A.; Gill, K.; Johnston, J.O. Giant cell tumor of bone. Curettage and cement reconstruction. Clin. Orthop. Relat. Res. 1995, 321, 245–250. [Google Scholar]
- Wada, T.; Kaya, M.; Nagoya, S.; Kawaguchi, S.; Isu, K.; Yamashita, T.; Yamawaki, S.; Ishii, S. Complications associated with bone cementing for the treatment of giant cell tumors of bone. J. Orthop. Sci. 2002, 7, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Balke, M.; Schremper, L.; Gebert, C.; Ahrens, H.; Streitbuerger, A.; Koehler, G.; Hardes, J.; Gosheger, G. Giant cell tumor of bone: Treatment and outcome of 214 cases. J. Cancer Res. Clin. Oncol. 2008, 134, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Algawahmed, H.; Turcotte, R.; Farrokhyar, F.; Ghert, M. High-Speed Burring with and without the Use of Surgical Adjuvants in the Intralesional Management of Giant Cell Tumor of Bone: A Systematic Review and Meta-Analysis. Sarcoma 2010, 2010, 586090. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Hassani, M.; Mavrogenis, A.F.; Trovarelli, G.; Romagnoli, C.; Berizzi, A.; Ruggieri, P. Chondroblastoma in adult age. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, W.A.; Hasan, B.Z.; Badr, I.T.; Mesregah, M.K. Functional and Oncological Outcome After Treatment of Chondroblastoma With Intralesional Curettage. J. Pediatr. Orthop. 2019, 39, e312–e317. [Google Scholar] [CrossRef]
- El-Moatasem, E.-H.M.; Abdel-Rahman, M.; Eid, M.A. Extended curettage and adjuvant therapy for benign tumors of the talus. Foot 2015, 25, 79–83. [Google Scholar] [CrossRef]
- Farfalli, G.L.; Albergo, J.I.; Piuzzi, N.S.; Ayerza, M.A.; Muscolo, D.L.; Ritacco, L.E.; Aponte-Tinao, L.A. Is Navigation-guided En Bloc Resection Advantageous Compared With Intralesional Curettage for Locally Aggressive Bone Tumors? Clin. Orthop. Relat. Res. 2018, 476, 511–517. [Google Scholar] [CrossRef]
- Özer, D.; Arıkan, Y.; Gür, V.; Gök, C.; Akman, Y.E. Chondroblastoma: An evaluation of the recurrences and functional outcomes following treatment. Acta Orthop. Et Traumatol. Turc. 2018, 52, 415–418. [Google Scholar] [CrossRef]
- Farouk, H.A.; Saladin, M.; Senna, W.A.; Ebeid, W. All-endoscopic management of benign bone lesions; a case series of 26 cases with minimum of 2 years follow-up. Sicot. J. 2018, 4, 50. [Google Scholar] [CrossRef]
- Lim, Y.W.; Tan, M.H. Treatment of benign giant cell tumours of bone in Singapore. Ann. Acad. Med. Singap. 2005, 34, 235–237. [Google Scholar] [PubMed]
- Omlor, G.W.; Lohnherr, V.; Hetto, P.; Gantz, S.; Fellenberg, J.; Merle, C.; Guehring, T.; Lehner, B. Surgical therapy of benign and low-grade malignant intramedullary chondroid lesions of the distal femur: Intralesional resection and bone cement filling with or without osteosynthesis. Strat. Trauma Limb Reconstr. 2018, 13, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Arbeitsgemeinschaft, K.; Becker, W.T.; Dohle, J.; Bernd, L.; Braun, A.; Cserhati, M.; Enderle, A.; Hovy, L.; Matejovsky, Z.; Szendroi, M.; et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J. Bone Jt. Surg. Am. 2008, 90, 1060–1067. [Google Scholar] [CrossRef]
- Dabak, N.; Gocer, H.; Cirakli, A. Advantages of Pressurized-Spray Cryosurgery in Giant Cell Tumors of the Bone. Balk. Med J. 2016, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-H.; Yin, J.-Q.; Xie, X.-B.; Zou, C.-Y.; Huang, G.; Wang, J.; Shen, J.-N. Local control of giant cell tumors of the long bone after aggressive curettage with and without bone cement. BMC Musculoskelet. Disord. 2014, 15, 330. [Google Scholar] [CrossRef]
- Malek, F.; Krueger, P.; Hatmi, Z.N.; Malayeri, A.A.; Faezipour, H.; O’Donnell, R.J. Local control of long bone giant cell tumour using curettage, burring and bone grafting without adjuvant therapy. Int. Orthop. 2006, 30, 495–498. [Google Scholar] [CrossRef]
- Mashhour, M.A.; Abdel Rahman, M. Lower recurrence rate in chondroblastoma using extended curettage and cryosurgery. Int. Orthop. 2014, 38, 1019–1024. [Google Scholar] [CrossRef]
- Mohaidat, Z.M.; Al-Jamal, H.Z.; Bany-Khalaf, A.M.; Radaideh, A.M.; Audat, Z.A. Giant cell tumor of bone: Unusual features of a rare tumor. Rare Tumors 2019, 11, 2036361319878894. [Google Scholar] [CrossRef]
- Mohler, D.G.; Chiu, R.; McCall, D.A.; Avedian, R.S. Curettage and cryosurgery for low-grade cartilage tumors is associated with low recurrence and high function. Clin. Orthop. Relat. Res. 2010, 468, 2765–2773. [Google Scholar] [CrossRef]
- Sasaki, H.; Nagano, S.; Shimada, H.; Yokouchi, M.; Setoguchi, T.; Ishidou, Y.; Kunigou, O.; Maehara, K.; Komiya, S. Diagnosing and discriminating between primary and secondary aneurysmal bone cysts. Oncol. Lett. 2017, 13, 2290–2296. [Google Scholar] [CrossRef]
- Schreuder, H.B.; Pruszczynski, M.; Veth, R.P.; Lemmens, J.A.M. Treatment of benign and low-grade malignant intramedullary chondroid tumours with curettage and cryosurgery. Eur. J. Surg. Oncol. (EJSO) 1998, 24, 120–126. [Google Scholar] [CrossRef]
- Solooki, S.; Keikha, Y.; Vosoughi, A.R. Can ethanol be used as an adjuvant to extended curettage in order to reduce the recurrence rate of aneurysmal bone cyst? Rev. Bras. Ortop. 2017, 52, 349–353. [Google Scholar] [CrossRef] [PubMed]
- van der Geest, I.C.M.; van Noort, M.P.; Schreuder, H.W.B.; Pruszczynski, M.; de Rooy, J.W.J.; Veth, R.P.H. The cryosurgical treatment of chondroblastoma of bone: Long-term oncologic and functional results. J. Surg. Oncol. 2007, 96, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Hirn, M.; de Silva, U.; Sidharthan, S.; Grimer, R.J.; Abudu, A.; Tillman, R.M.; Carter, S.R. Bone defects following curettage do not necessarily need augmentation. Acta Orthop. 2009, 80, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Klenke, F.M.; Wenger, D.E.; Inwards, C.Y.; Rose, P.S.; Sim, F.H. Giant cell tumor of bone: Risk factors for recurrence. Clin. Orthop. Relat. Res. 2011, 469, 591–599. [Google Scholar] [CrossRef]
- Masui, F.; Ushigome, S.; Kamitani, K.; Asanuma, K.; Fujii, K. Chondroblastoma: A study of 11 cases. Eur. J. Surg. Oncol. (EJSO) 2002, 28, 869–874. [Google Scholar] [CrossRef]
- Rahman, M.A.; El Masry, A.M.; Azmy, S.I. Review of 16 cases of aneurysmal bone cyst in the proximal femur treated by extended curettage and cryosurgery with reconstruction using autogenous nonvascularized fibula graft. J. Orthop. Surg. 2018, 26, 2309499018783905. [Google Scholar] [CrossRef]
- Tunn, P.U.; Schlag, P.M. Giant cell tumor of bone. An evaluation of 87 patients. Z Orthop Ihre Grenzgeb 2003, 141, 690–698. [Google Scholar] [CrossRef]
- Brown, M.T.; Gikas, P.D.; Bhamra, J.S.; Skinner, J.A.; Aston, W.J.S.; Pollock, R.C.; Saifuddin, A.; Briggs, T.W.R. How safe is curettage of low-grade cartilaginous neoplasms diagnosed by imaging with or without pre-operative needle biopsy? Bone Jt. J. 2014, 96-B, 1098–1105. [Google Scholar] [CrossRef]
- Dierselhuis, E.F.; Gerbers, J.G.; Ploegmakers, J.J.; Stevens, M.; Suurmeijer, A.J.; Jutte, P.C. Local Treatment with Adjuvant Therapy for Central Atypical Cartilaginous Tumors in the Long Bones: Analysis of Outcome and Complications in One Hundred and Eight Patients with a Minimum Follow-up of Two Years. J. Bone Jt. Surg. 2016, 98, 303–313. [Google Scholar] [CrossRef]
- Peeters, S.; Van der Geest, I.; de Rooy, J.; Veth, R.; Schreuder, H. Aneurysmal bone cyst: The role of cryosurgery as local adjuvant treatment. J. Surg. Oncol. 2009, 100, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.-S.; Kim, S.-S.; Moon, J.-L.; Kim, S.-S.; Moon, H. Treating Giant Cell Tumours with Curettage, Electrocautery, Burring, Phenol Irrigation, and Cementation. J. Orthop. Surg. 2013, 21, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Wu, P.-K.; Chen, C.-F.; Chen, W.-M. Intralesional curettage of central low-grade chondrosarcoma: A midterm follow-up study. J. Chin. Med. Assoc. 2017, 80, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Dürr, H.R.; Maier, M.; Jansson, V.; Baur, A.; Refior, H.J. Phenol as an adjuvant for local control in the treatment ofgiant cell tumour of the bone. Eur. J. Surg. Oncol. (EJSO) 1999, 25, 610–618. [Google Scholar] [CrossRef]
- Gaston, C.L.; Bhumbra, R.; Watanuki, M.; Abudu, A.T.; Carter, S.R.; Jeys, L.M.; Tillman, R.M.; Grimer, R.J. Does the addition of cement improve the rate of local recurrence after curettage of giant cell tumours in bone? J. Bone Jt. Surg. 2011, 93-B, 1665–1669. [Google Scholar] [CrossRef]
- Horstmann, P.F.; Hettwer, W.H.; Petersen, M.M. Treatment of benign and borderline bone tumors with combined curettage and bone defect reconstruction. J. Orthop. Surg. 2018, 26, 2309499018774929. [Google Scholar] [CrossRef]
- Lausten, G.S.; Jensen, P.K.; Schiødt, T.; Lund, B. Local recurrences in giant cell tumour of bone. Int. Orthop. 1996, 20, 172–176. [Google Scholar] [CrossRef]
- Lin, W.-H.; Lan, T.-Y.; Chen, C.-Y.; Wu, K.; Yang, R.-S. Similar Local Control between Phenol- and Ethanol-treated Giant Cell Tumors of Bone. Clin. Orthop. Relat. Res. 2011, 469, 3200–3208. [Google Scholar] [CrossRef]
- Morii, T.; Mochizuki, K.; Tajima, T.; Satomi, K. Treatment outcome of enchondroma by simple curettage without augmentation. J. Orthop. Sci. 2010, 15, 112–117. [Google Scholar] [CrossRef]
- Suneja, R.; Grimer, R.J.; Belthur, M.; Jeys, L.; Carter, S.R.; Tillman, R.M.; Davies, A.M. Chondroblastoma of bone. J. Bone Jt. Surg. 2005, 87, 974–978. [Google Scholar] [CrossRef]
- Sheth, D.S.; Healey, J.H.; Sobel, M.; Lane, J.M.; Marcove, R.C. Giant cell tumor of the distal radius. J. Hand Surg. 1995, 20, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-N.; Hsu, R.W.-W.; Sim, F.H. Excision Curettage and Allografting of Giant Cell Tumor. World J. Surg. 1998, 22, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-P.; Chen, W.-M.; Chen, T.-H. Giant-cell tumors of bone: An analysis of 87 cases. Int. Orthop. 2004, 28, 239–243. [Google Scholar] [CrossRef]
- Trieb, K.; Bitzan, P.; Lang, S.; Dominkus, M.; Kotz, R. Recurrence of curetted and bone-grafted giant-cell tumours with and without adjuvant phenol therapy. Eur. J. Surg. Oncol. (EJSO) 2001, 27, 200–202. [Google Scholar] [CrossRef]
- van der Heijden, L.; van der Geest, I.C.M.; Schreuder, H.W.B.; van de Sande, M.A.J.; Dijkstra, P.D.S. Liquid Nitrogen or Phenolization for Giant Cell Tumor of Bone?: A Comparative Cohort Study of Various Standard Treatments at Two Tertiary Referral Centers. JBJS 2014, 96, e35. [Google Scholar] [CrossRef] [PubMed]
- Verdegaal, S.H.M.; Brouwers, H.F.G.; van Zwet, E.W.; Hogendoorn, P.C.W.; Taminiau, A.H.M. Low-Grade Chondrosarcoma of Long Bones Treated with Intralesional Curettage Followed by Application of Phenol, Ethanol, and Bone-Grafting. JBJS 2012, 94, 1201–1207. [Google Scholar] [CrossRef]
- Wallace, M.T.; Henshaw, R.M. Results of cement versus bone graft reconstruction after intralesional curettage of bone tumors in the skeletally immature patient. J. Pediatr. Orthop. 2014, 34, 92–100. [Google Scholar] [CrossRef]
- Wang, E.H.M.; Marfori, M.L.; Serrano, M.V.T.; Rubio, D.A. Is curettage and high-speed burring sufficient treatment for aneurysmal bone cysts? Clin. Orthop. Relat. Res. 2014, 472, 3483–3488. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, B.; Keshav, P.; Chen, X.; Gao, W.; Yan, H. The management and surgical intervention timing of enchondromas: A 10-year experience. Medicine 2017, 96, e6678. [Google Scholar] [CrossRef]
- Benevenia, J.; Rivero, S.M.; Moore, J.; Ippolito, J.A.; Siegerman, D.A.; Beebe, K.S.; Patterson, F.R. Supplemental Bone Grafting in Giant Cell Tumor of the Extremity Reduces Nononcologic Complications. Clin. Orthop. Relat. Res. 2017, 475, 776–783. [Google Scholar] [CrossRef]
- Blackley, H.R.; Wunder, J.S.; Davis, A.M.; White, L.M.; Kandel, R.; Bell, R.S. Treatment of Giant-Cell Tumors of Long Bones with Curettage and Bone-Grafting*. J. Bone Jt. Surg. 1999, 81, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Kivioja, A.H.; Blomqvist, C.; Hietaniemi, K.; Trovik, C.; Walloe, A.; Bauer, H.C.F.; Jorgensen, P.H.; Bergh, P.; Follerås, G. Cement is recommended in intralesional surgery of giant cell tumors: A Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008, 79, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Lackman, R.D.; Hosalkar, H.S.; Ogilvie, C.M.; Torbert, J.T.; Fox, E.J. Intralesional Curettage for Grades II and III Giant Cell Tumors of Bone. Clin. Orthop. Relat. Res. 2005, 438, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Mermerkaya, M.U.; Bekmez, S.; Karaaslan, F.; Danisman, M.; Kosemehmetoglu, K.; Gedikoglu, G.; Ayvaz, M.; Tokgozoglu, A.M. Intralesional curettage and cementation for low-grade chondrosarcoma of long bones: Retrospective study and literature review. World J. Surg. Oncol. 2014, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Prosser, G.H.; Baloch, K.G.; Tillman, R.M.; Carter, S.R.; Grimer, R.J. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin. Orthop. Relat. Res. 2005, 211–218. [Google Scholar] [CrossRef]
- Omlor, G.W.; Lange, J.; Streit, M.; Gantz, S.; Merle, C.; Germann, T.; Mechtersheimer, G.; Fellenberg, J.; Lehner, B. Retrospective analysis of 51 intralesionally treated cases with progressed giant cell tumor of the bone: Local adjuvant use of hydrogen peroxide reduces the risk for tumor recurrence. World J. Surg. Oncol. 2019, 17, 73. [Google Scholar] [CrossRef]
- Schreuder, H.W.B.; Veth, R.P.H.; Pruszczynski, M.; Lemmens, J.A.M.; Koops, H.S.; Molenaar, W.M. Aneurysmal Bone Cysts Treated By Curettage, Cryotherapy And Bone Grafting. J. Bone Jt. Surg. 1997, 79, 20–25. [Google Scholar] [CrossRef]
- Verdegaal, S.H.; Hartigh, J.D.; Hogendoorn, P.C.; Brouwers, H.F.; Taminiau, A.H. Phenol levels during intralesional curettage and local adjuvant treatment of benign and low-grade malignant bone tumours. Clin. Sarcoma Res. 2012, 2, 10. [Google Scholar] [CrossRef]
- Park, H.Y.; Yang, S.K.; Sheppard, W.L.; Hegde, V.; Zoller, S.D.; Nelson, S.D.; Federman, N.; Bernthal, N.M. Current management of aneurysmal bone cysts. Curr. Rev. Musculoskelet. Med. 2016, 9, 435–444. [Google Scholar] [CrossRef]
- Gava, N.F.; Engel, E.E. Treatment alternatives and clinical outcomes of bone filling after benign tumour curettage. A systematic review. Orthop. Traumatol. Surg. Res. 2022, 108, 102966. [Google Scholar] [CrossRef]
- Andreou, D.; Gilg, M.M.; Gosheger, G.; Werner, M.; Hardes, J.; Pink, D.; Leithner, A.; Tunn, P.-U.; Streitbürger, A. Metastatic Potential of Grade I Chondrosarcoma of Bone: Results of a Multi-institutional Study. Ann. Surg. Oncol. 2015, 23, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Santacreu, E.M.; Ortiz-Cruz, E.J.; Díaz-Almirón, M.; Kreilinger, J.J.P. Enchondroma versus Chondrosarcoma in Long Bones of Appendicular Skeleton: Clinical and Radiological Criteria—A Follow-Up. J. Oncol. 2016, 2016, 8262079. [Google Scholar] [CrossRef] [PubMed]
- Crim, J.; Schmidt, R.; Layfield, L.; Hanrahan, C.; Manaster, B.J. Can imaging criteria distinguish enchondroma from grade 1 chondrosarcoma? Eur. J. Radiol. 2015, 84, 2222–2230. [Google Scholar] [CrossRef]
- Ye, Y.; Pringle, L.M.; Lau, A.W.; Riquelme, D.N.; Wang, H.; Jiang, T.; Lev, D.; Welman, A.; Blobel, G.A.; Oliveira, A.M.; et al. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-κB. Oncogene 2010, 29, 3619–3629. [Google Scholar] [CrossRef]
- Varshney, M.K.; Rastogi, S.; Khan, S.A.; Trikha, V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin. Orthop. Relat. Res 2010, 468, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Bentur, Y.; Shoshani, O.; Tabak, A.; Binnun, A.; Ramon, Y.; Ulman, Y.; Berger, Y.; Nachlieli, T.; Peled, Y.J. Prolonged Elimination Half-Life of Phenol After Dermal Exposure. J. Toxicol. Clin. Toxicol. 1998, 36, 707–711. [Google Scholar] [CrossRef]
- Veth, R.; Schreuder, B.; van Beem, H.; Pruszczynski, M.; de Rooy, J. Cryosurgery in aggressive, benign, and low-grade malignant bone tumours. Lancet Oncol. 2005, 6, 25–34. [Google Scholar] [CrossRef]
- Malawer, M.M.; Bickels, J.; Meller, I.; Buch, R.G.; Henshaw, R.M.; Kollender, Y. Cryosurgery in the treatment of giant cell tumor. A long-term followup study. Clin. Orthop. Relat. Res. 1999, 359, 176–188. [Google Scholar] [CrossRef]
| Tumour Entity | Total Case Number (n = 3316) | Minimum Follow-Up ≥ 24 Months (n = 1483) | Minimum Follow-Up < 24 Months (n = 1833) |
|---|---|---|---|
| GCT | 2235 | 861 | 1374 |
| Chondroblastoma | 219 | 201 | 18 |
| ABC | 262 | 165 | 97 |
| ACT | 333 | 167 | 166 |
| EC | 235 | 57 | 178 |
| Osteoblastoma | 7 | 7 | 0 |
| Fibrous dysplasia | 14 | 14 | 0 |
| Non-ossifying fibroma | 5 | 5 | 0 |
| Osteoid osteoma | 5 | 5 | 0 |
| Chondromyxoid fibroma | 1 | 1 | 0 |
| Giant Cell Tumour (GCT) of Bone | ||||
| Total | No LR | LR | p-value | |
| Curettage + burr | 263 | 219 | 44 | N/A |
| Curettage | 12 | 8 | 4 | 0.233 * |
| Curettage + adjuvant | 95 | 83 | 12 | 0.346 |
| Curettage + adjuvant + PMMA | 83 | 59 | 24 | 0.015 |
| Curettage + burr + adjuvant | 164 | 133 | 31 | 0.602 |
| Curettage + burr + adjuvant + PMMA | 223 | 198 | 25 | 0.082 |
| Curettage + burr + PMMA | 22 | 18 | 4 | 0.773 * |
| Curettage + PMMA | 1 | 1 | 0 | n.c. |
| Aneurysmal Bone Cyst (ABC) | ||||
| Total | No LR | LR | p-value | |
| Curettage + burr | 36 | 34 | 2 | N/A |
| Curettage | 20 | 19 | 1 | 1.000 * |
| Curettage + burr + adjuvant | 101 | 91 | 10 | 0.428 |
| Curettage + burr + adjuvant + PMMA | 2 | 2 | 0 | 1.000 * |
| Curettage + PMMA | 6 | 5 | 1 | 0.378 * |
| Atypical Cartilaginous Tumour (ACT) | ||||
| Total | No LR | LR | p-value | |
| Curettage + burr | 5 | 0 | 0 | N/A |
| Curettage | 7 | 6 | 1 | 1.000 * |
| Curettage + adjuvant | 23 | 23 | 0 | n.c. |
| Curettage + adjuvant + PMMA | 93 | 0 | 0 | n.c. |
| Curettage + burr + adjuvant | 16 | 15 | 1 | 1.000 * |
| Curettage + burr + adjuvant + PMMA | 21 | 21 | 0 | n.c. |
| Curettage + burr + PMMA | 1 | 1 | 0 | n.c. |
| Curettage + PMMA | 1 | 0 | 0 | n.c. |
| Chondroblastoma | ||||
| Total | No LR | LR | p-value | |
| Curettage + burr | 97 | 89 | 8 | N/A |
| Curettage | 34 | 30 | 4 | 0.508 * |
| Curettage + adjuvant + PMMA | 2 | 0 | 2 | 0.009 * |
| Curettage + burr + adjuvant | 34 | 32 | 2 | 1.000 * |
| Curettage + burr + PMMA | 28 | 28 | 0 | 0.116 |
| Curettage + PMMA | 6 | 4 | 2 | 0.103 * |
| Type of Complication | Count (n; % of 263) | Affected Treatment Group |
|---|---|---|
| Postoperative fracture | 68 (25.9%) | Curettage + burr (n = 24) Curettage + burr + argon beam + H2O2 + PMMA (n = 6) Curettage only (n = 5) Curettage + burr + PMMA (n = 5) Curettage + burr + H2O2 + PMMA (n = 4) Curettage + burr + cryotherapy + PMMA (n = 3) Curettage + burr + cryotherapy (n = 2) Curettage + burr + phenol (n = 2) Curettage + phenol + ethanol (n = 2) Curettage + cryotherapy + sodium hyponitrite (n = 2) Curettage + cryotherapy (n = 1) Curettage + ethanol (n = 1) Not defined (n = 11) |
| Osteoarthritis of adjacent joint | 62 (23.6%) | Curettage + burr (n = 19) Curettage + burr + PMMA (n = 12) Curettage + burr + argon beam + H2O2 + PMMA (n = 8) Curettage + phenol + ethanol + PMMA (n = 6) Curettage + PMMA (n = 4) Curettage + burr + cryotherapy + PMMA (n = 4) Curettage + burr + cryotherapy (n = 3) Curettage only (n = 2) Curettage + liquid zinc chloride (n = 2) Curettage + burr + phenol + PMMA (n = 1) Curettage + burr + phenol + electrocauterization + PMMA (n = 1) |
| Persisting pain | 30 (11.4%) | Curettage only (n = 21) |
| Curettage + PMMA (n = 7) | ||
| Curettage + burr (n = 2) | ||
| Deep wound infection | 16 (6.1%) | Curettage + burr + cryotherapy (n = 3) Curettage only (n = 2) Curettage + cryotherapy (n = 2) Curettage + burr + PMMA (n = 2) Curettage + burr (n = 1) Curettage + liquid zinc chloride (n = 1) Curettage + burr + phenol (n = 1) Curettage + cryotherapy + sodium hyponitrite (n = 1) Curettage + phenol + ethanol + PMMA (n = 1) Curettage + burr + cryotherapy + PMMA (n = 1) Not defined (n = 1) |
| Nerve injury | 14 (5.3%) | Curettage + cryotherapy (n = 4) |
| Curettage + burr + cryotherapy (n = 4) | ||
| Curettage only (n = 2) | ||
| Curettage + burr (n = 1) | ||
| Curettage + PMMA (n = 1) | ||
| Curettage + burr + PMMA (n = 1) | ||
| Curettage + burr + phenol (n = 1) | ||
| Superficial wound infection | 12 (4.6%) | Curettage + burr (n = 3) |
| Curettage only (n = 2) | ||
| Curettage + ethanol (n = 1) | ||
| Curettage + burr + cryotherapy (n = 1) | ||
| Curettage + burr + phenol (n = 1) | ||
| Curettage + phenol + ethanol (n = 1) | ||
| Curettage + burr + electrocauterization + PMMA (n = 1) | ||
| Not defined (n = 2) | ||
| Restricted mobility | 10 (3.8%) | Curettage + burr (n = 5) |
| Curettage + PMMA (n = 2) | ||
| Curettage only (n = 1) | ||
| Curettage + burr + phenol (n = 1) | ||
| Not defined (n = 1) | ||
| Physeal arrest | 9 (3.4%) | Curettage only (n = 2) |
| Curettage + burr (n = 2) | ||
| Curettage + burr + phenol (n = 2) | ||
| Curettage + PMMA (n = 1) | ||
| Curettage + burr + cryotherapy (n = 1) | ||
| Not defined (n = 1) | ||
| Joint collapse | 6 (2.3%) | Curettage + burr (n = 3) |
| Curettage + cryotherapy (n = 3) | ||
| Limb deformity | 5 (1.9%) | Curettage + burr (n = 2) |
| Curettage + PMMA (n = 2) | ||
| Not defined (n = 1) | ||
| Non-union | 5 (1.9%) | Curettage + burr (n = 3) |
| Curettage + phenol + ethanol + PMMA (n = 2) | ||
| Implant irritation | 4 (1.5%) | Curettage + burr (n = 2) |
| Curettage + burr + H2O2 + PMMA (n = 2) | ||
| Skin necrosis | 3 (1.1%) | Curettage only (n = 1) |
| Curettage + cryotherapy (n = 1) | ||
| Not defined (n = 1) | ||
| Crack of affected bone | 2 (0.8%) | Not defined (n = 2) |
| Delayed wound healing | 2 (0.8%) | Curettage only (n = 2) |
| Abnormal banded signal around PMMA on MRI | 2 (0.8%) | Curettage + PMMA (n = 2) |
| Intraoperative fracture | 2 (0.8%) | Curettage + burr (n = 1) |
| Curettage + phenol (n = 1) | ||
| PMMA excavation | 2 (0.8%) | Curettage + PMMA (n = 1) |
| Curettage + phenol + PMMA (n = 1) | ||
| Cartilage defect | 1 (<0.5%) | Curettage + burr + cryotherapy (n = 1) |
| Deep vein thrombosis | 1 (<0.5%) | Curettage only (n = 1) |
| Lung embolism | 1 (<0.5%) | Curettage only (n = 1) |
| Unknown | 1 (<0.5%) | Curettage + burr + phenol (n = 1) |
| Periarticular ossification | 1 (<0.5%) | Curettage + burr (n = 1) |
| Bone graft reabsorption | 1 (<0.5%) | Curettage + burr + phenol (n = 1) |
| Secondary sarcoma | 1 (<0.5%) | Curettage + PMMA (n = 1) |
| Skin blisters | 1 (<0.5%) | Curettage + burr + cryotherapy (n = 1) |
| Venous gas embolism | 1 (<0.5%) | Curettage + cryotherapy + sodium hyponitrite (n = 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolle, M.A.; Roessl, V.; Leithner, A. Effect of Local Adjuvants Following Curettage of Benign and Intermediate Tumours of Bone: A Systematic Review of the Literature. Cancers 2023, 15, 4258. https://doi.org/10.3390/cancers15174258
Smolle MA, Roessl V, Leithner A. Effect of Local Adjuvants Following Curettage of Benign and Intermediate Tumours of Bone: A Systematic Review of the Literature. Cancers. 2023; 15(17):4258. https://doi.org/10.3390/cancers15174258
Chicago/Turabian StyleSmolle, Maria Anna, Veronika Roessl, and Andreas Leithner. 2023. "Effect of Local Adjuvants Following Curettage of Benign and Intermediate Tumours of Bone: A Systematic Review of the Literature" Cancers 15, no. 17: 4258. https://doi.org/10.3390/cancers15174258
APA StyleSmolle, M. A., Roessl, V., & Leithner, A. (2023). Effect of Local Adjuvants Following Curettage of Benign and Intermediate Tumours of Bone: A Systematic Review of the Literature. Cancers, 15(17), 4258. https://doi.org/10.3390/cancers15174258






