Role of Leptin and Adiponectin in Carcinogenesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Characteristic of Leptin
2.1. Roles
2.2. Receptors
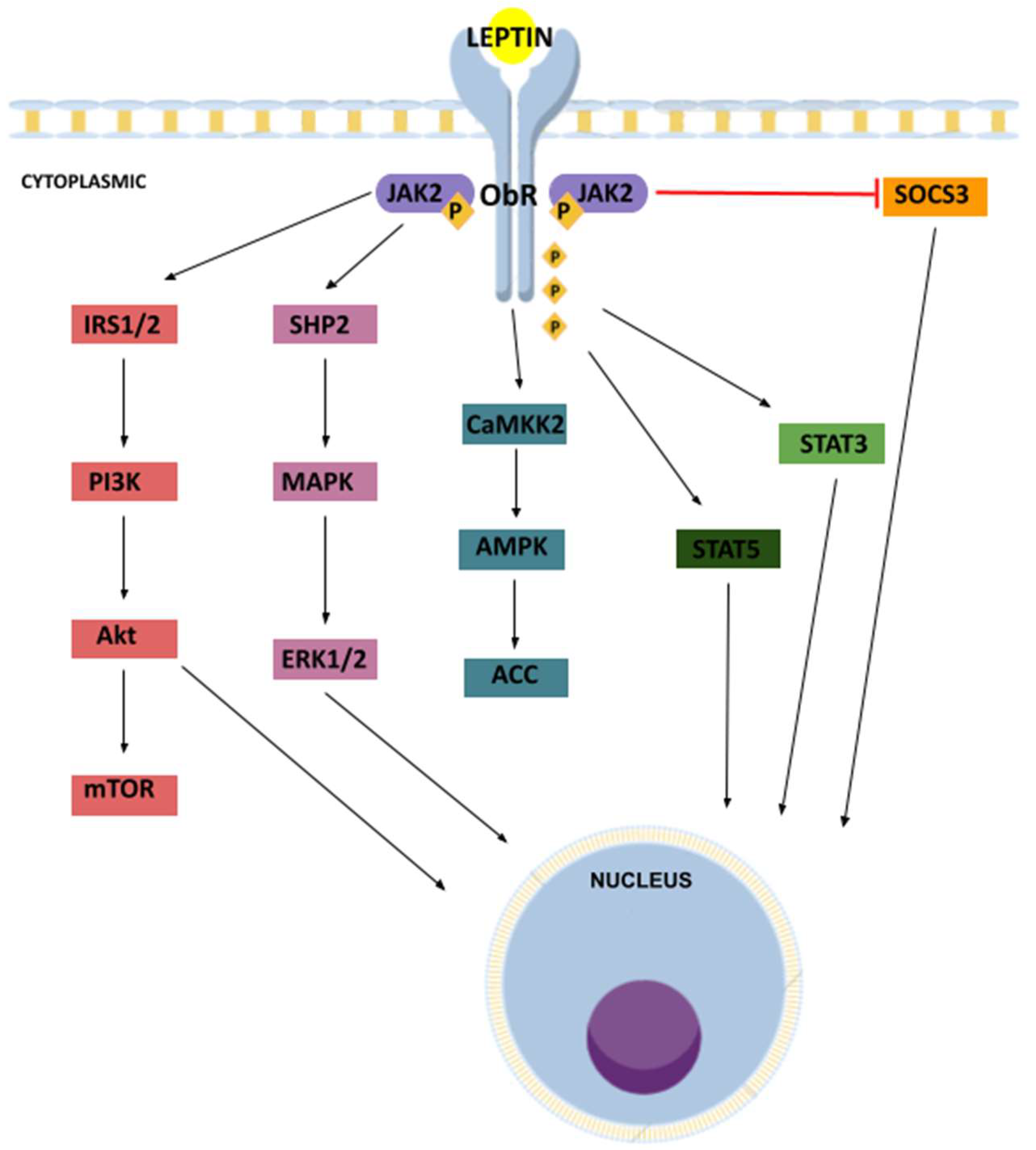
2.3. Signaling Pathways
3. Characteristic of Adiponectin
3.1. Roles
3.2. Receptors
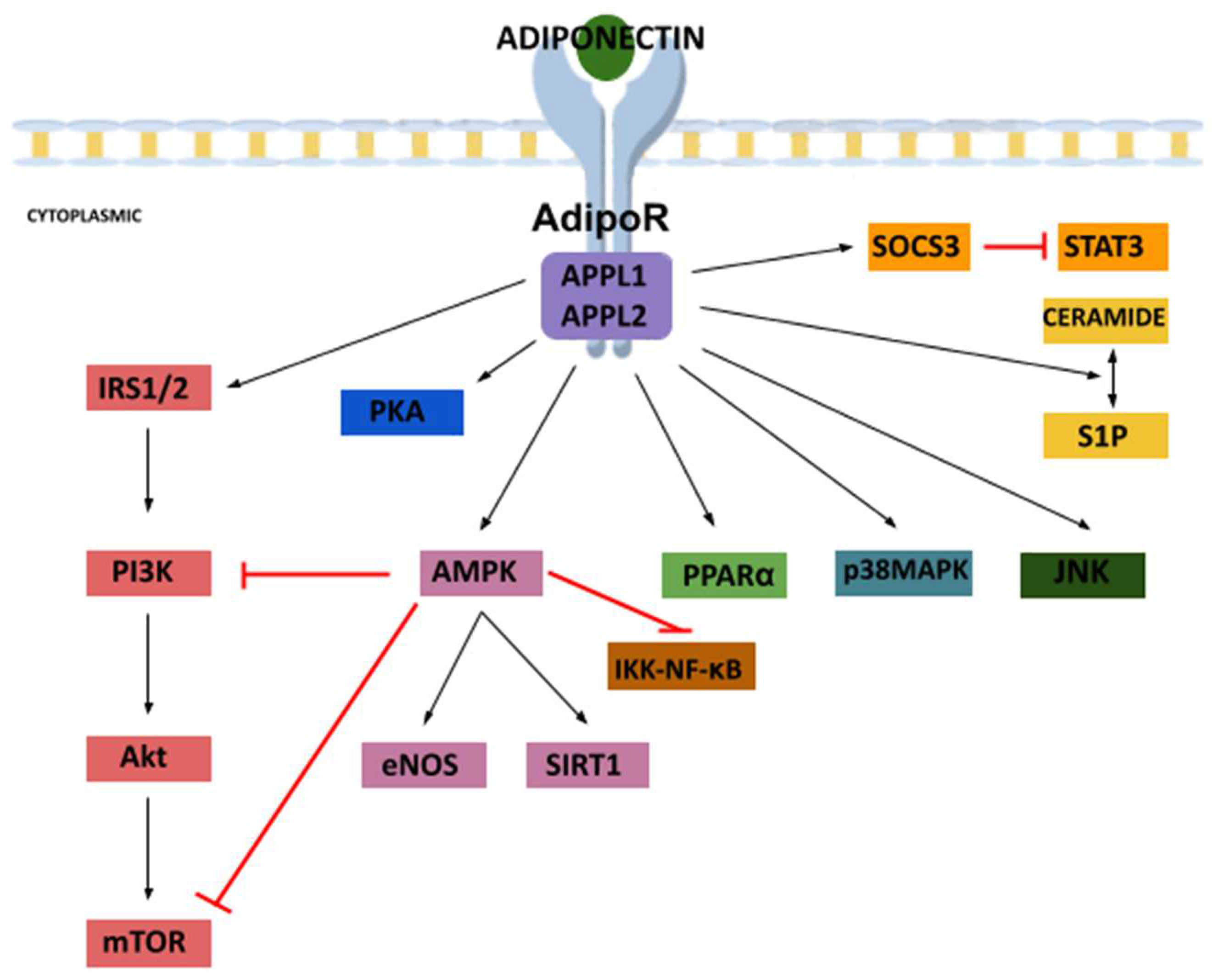
3.3. Signaling Pathways
4. Tumor Microenvironment
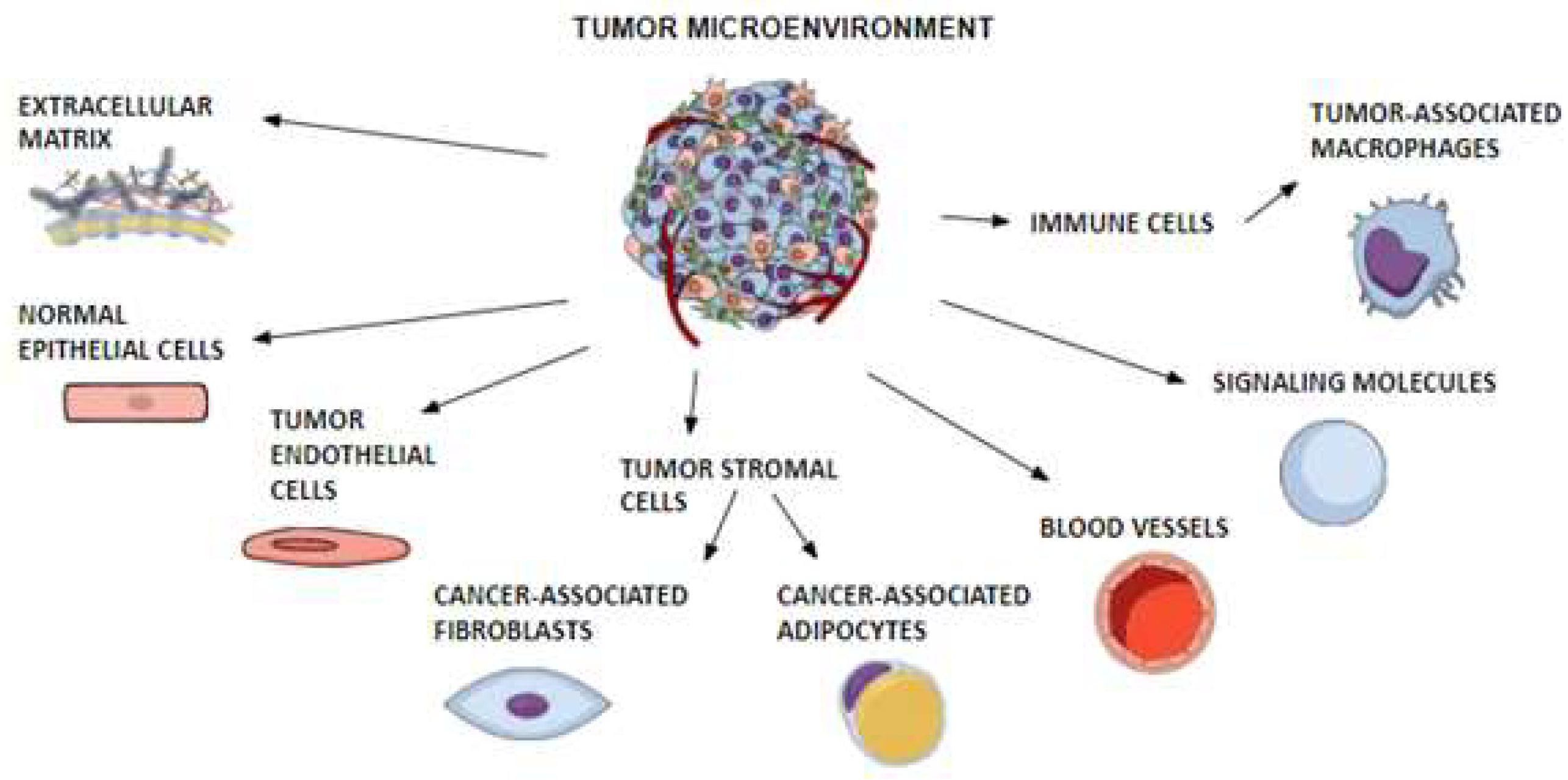
4.1. TME
4.1.1. Leptin and TME
4.1.2. Adiponectin and TME
4.2. CAFs
4.2.1. Leptin and CAFs
4.2.2. Adiponectin and CAFs
4.3. TAMs
4.3.1. Leptin and TAMs
4.3.2. Adiponectin and TAMs
5. Matrix Metalloproteinases
5.1. Leptin and MMPs
5.2. Adiponectin and MMPs
6. Epithelial–Mesenchymal Transition
6.1. Leptin and EMT
6.2. Adiponectin and EMT
7. Angiogenesis and Vasculogenic Mimicry
7.1. Angiogenesis in Obesity
7.1.1. Angiogenesis, VM and Leptin
7.1.2. Angiogenesis, VM and Adiponectin
8. Other Adipokines and Carcinogenesis
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cao, H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014, 220, 47–59. [Google Scholar] [CrossRef]
- Kompella, P.; Vasquez, K.M. Obesity and cancer: A mechanistic overview of metabolic changes in obesity that impact genetic instability. Mol. Carcinog. 2019, 58, 1531–1550. [Google Scholar] [CrossRef]
- Pham, D.V.; Park, P.H. Tumor Metabolic Reprogramming by Adipokines as a Critical Driver of Obesity-Associated Cancer Progression. Int. J. Mol. Sci. 2021, 22, 1444. [Google Scholar] [CrossRef]
- Ray, A.; Cleary, M.P. The potential role of leptin in tumor invasion and metastasis. Cytokine Growth Factor Rev. 2017, 38, 80–97. [Google Scholar] [CrossRef]
- Kadri Colakoglu, M.; Bostanci, E.B.; Ozdemir, Y.; Dalgic, T.; Aksoy, E.; Ozer, I.; Ozogul, Y.; Oter, V. Roles of adiponectin and leptin as diagnostic markers in pancreatic cancer. Bratisl. Lek. Listy. 2017, 118, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Chen, D. Targeting Adipokines in Obesity-Related Tumors. Front. Oncol. 2021, 11, 685923. [Google Scholar] [CrossRef]
- Vansaun, M.N. Molecular pathways: Adiponectin and leptin signaling in cancer. Clin. Cancer Res. 2013, 19, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Barone, I.; Vircillo, V.; Panza, S.; Malivindi, R.; Gelsomino, L.; Pellegrino, M.; Rago, V.; Mauro, L.; Lanzino, M.; et al. Activated FXR Inhibits Leptin Signaling and Counteracts Tumor-promoting Activities of Cancer-Associated Fibroblasts in Breast Malignancy. Sci. Rep. 2016, 6, 21782. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cortegana, C.; López-Saavedra, A.; Sánchez-Jiménez, F.; Pérez-Pérez, A.; Castiñeiras, J.; Virizuela-Echaburu, J.A.; de la Cruz-Merino, L.; Sánchez-Margalet, V. Leptin, Both Bad and Good Actor in Cancer. Biomolecules 2021, 11, 913. [Google Scholar] [CrossRef]
- Matafome, P.; Santos-Silva, D.; Sena, C.M.; Seiça, R. Common mechanisms of dysfunctional adipose tissue and obesity-related cancers. Diabetes Metab. Res. Rev. 2013, 29, 285–295. [Google Scholar] [CrossRef]
- Olea-Flores, M.; Juárez-Cruz, J.C.; Zuñiga-Eulogio, M.D.; Acosta, E.; García-Rodríguez, E.; Zacapala-Gomez, A.E.; Mendoza-Catalán, M.A.; Ortiz-Ortiz, J.; Ortuño-Pineda, C.; Navarro-Tito, N. New Actors Driving the Epithelial-Mesenchymal Transition in Cancer: The Role of Leptin. Biomolecules 2020, 12, 1676. [Google Scholar] [CrossRef] [PubMed]
- Ayed, K.; Nabi, L.; Akrout, R.; Mrizak, H.; Gorrab, A.; Bacha, D.; Boussen, H.; Gati, A. Obesity and cancer: Focus on leptin. Mol. Biol. Rep. 2023, 50, 6177–6189. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Tsang, J.Y.; Ho, D.H.; Zhang, R.; Xiao, H.; Li, D.; Zhu, J.; Wang, F.; Bian, Z.; Lui, V.C.; et al. Modulatory effects of adiponectin on the polarization of tumor-associated macrophages. Int. J. Cancer 2015, 137, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Piperigkou, Z.; Karamanos, N.K.; Zolota, V. Altered Adipokine Expression in Tumor Microenvironment Promotes Development of Triple Negative Breast Cancer. Cancers 2022, 17, 4139. [Google Scholar] [CrossRef] [PubMed]
- O’Rahilly, S. 20 years of leptin: What we know and what the future holds. J. Endocrinol. 2014, 223, E1–E3. [Google Scholar] [CrossRef]
- Jasińska, A.; Pietruczuk, M. Adipocytokines—Proteins of multidirectional function. J. Mol. Diagn. 2010, 46, 331–338. [Google Scholar]
- Nowak, A.; Kobierzycki, C.; Dzięgiel, P. The role of leptin in pathogenesis of obesity-related cancers. Adv. Cell Biol. 2015, 42, 309–328. [Google Scholar]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, 77–99. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Magkos, F.; Brinkoetter, M.; Sienkiewicz, E.; Dardeno, T.A.; Kim, S.Y.; Hamnvik, O.P.; Koniaris, A. Leptin in human physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2011, 301, 567–584. [Google Scholar] [CrossRef]
- Murawska-Ciałowicz, E. Adipose tissue—Morphological and biochemical characteristic of different depots. Postępy Hig. Med. Dosw. 2017, 71, 466–484. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Maymó, J.; Dueñas, J.L.; Varone, C.; Sánchez-Margalet, V. Role of leptin in female reproduction. Clin. Chem. Lab. Med. 2015, 53, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Naylor, C.; Petri, W.A., Jr. Leptin Regulation of Immune Responses. Trends Mol. Med. 2016, 22, 88–98. [Google Scholar] [CrossRef]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Carbone, F.; La Rocca, C.; Formisano, L.; Matarese, G. Role of adipokines signaling in the modulation of T cells function. Front. Immunol. 2013, 18, 332. [Google Scholar] [CrossRef]
- Trinh, T.; Broxmeyer, H.E. Role for Leptin and Leptin Receptors in Stem Cells During Health and Diseases. Stem Cell Rev. Rep. 2021, 17, 511–522. [Google Scholar] [CrossRef]
- Li, X.; Shi, S.; Chen, J.; Zhong, G.; Li, X.; Liu, Z. Leptin differentially regulates endochondral ossification in tibial and vertebral epiphyseal plates. Cell Biol. Int. 2018, 42, 169–179. [Google Scholar] [CrossRef]
- Gorska, E.; Popko, K.; Stelmaszczyk-Emmel, A.; Ciepiela, O.; Kucharska, A.; Wasik, M. Leptin receptors. Eur. J. Med. Res. 2010, 15 (Suppl. S2), 50. [Google Scholar] [CrossRef]
- Zhou, Y.; Rui, L. Leptin signaling and leptin resistance. Front. Med. 2013, 7, 207–222. [Google Scholar] [CrossRef]
- St-Pierre, J.; Tremblay, M.L. Modulation of leptin resistance by protein tyrosine phosphatases. Cell Metab. 2012, 15, 292–297. [Google Scholar] [CrossRef][Green Version]
- Greer, K.B.; Falk, G.W.; Bednarchik, B.; Li, L.; Chak, A. Associations of Serum Adiponectin and Leptin with Barrett’s Esophagus. Clin. Gastroenterol. Hepatol. 2015, 13, 2265–2272. [Google Scholar] [CrossRef]
- Ye, R.; Scherer, P.E. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol. Metab. 2013, 2, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 2, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, F. Regulation of adiponectin multimerization, signaling and function. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 25–31. [Google Scholar] [CrossRef]
- Sayeed, M.; Gautam, S.; Verma, D.P.; Afshan, T.; Kumari, T.; Srivastava, A.K.; Ghosh, J.K. A collagen domain-derived short adiponectin peptide activates APPL1 and AMPK signaling pathways and improves glucose and fatty acid metabolisms. J. Biol. Chem. 2018, 293, 13509–13523. [Google Scholar] [CrossRef] [PubMed]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Graziano, M.; Sciacca, L.; Baratta, R.; Frittitta, L. Adipose Tissue, Obesity and Adiponectin: Role in Endocrine Cancer Risk. Int. J. Mol. Sci. 2019, 20, 2863. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Polito, R.; Bartollino, S.; Nigro, E.; Porcile, C.; Bianco, A.; Daniele, A.; Moncharmont, B. Adiponectin as Link Factor between Adipose Tissue and Cancer. Int. J. Mol. Sci. 2019, 20, 839. [Google Scholar] [CrossRef]
- Warakomski, J.; Siemińska, L. The role of adipose tissue with particular emphasis on cytokines in the pathogenesis of neoplastic diseases. Wiad. Lek. 2019, 72, 1551–1558. [Google Scholar] [CrossRef]
- Choubey, M.; Bora, P. Emerging Role of Adiponectin/AdipoRs Signaling in Choroidal Neovascularization, Age-Related Macular Degeneration, and Diabetic Retinopathy. Biomolecules 2023, 13, 982. [Google Scholar] [CrossRef]
- Yarrow, J.F.; Beggs, L.A.; Conover, C.F.; McCoy, S.C.; Beck, D.T.; Borst, S.E. Influence of androgens on circulating adiponectin in male and female rodents. PLoS ONE 2012, 10, 47315. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Rajitha, B.; Aliya, S.; Kotipatruni, R.P.; Madanraj, A.S.; Hammond, A.; Park, D.; Chigurupati, S.; Alam, A.; Pattnaik, S. The role of adiponectin in obesity-associated female-specific carcinogenesis. Cytokine Growth Factor Rev. 2016, 31, 37–48. [Google Scholar] [CrossRef]
- Pascolutti, R.; Erlandson, S.C.; Burri, D.J.; Zheng, S.; Kruse, A.C. Mapping and engineering the interaction between adiponectin and T-cadherin. J. Biol. Chem. 2020, 295, 2749–2759. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Luo, Z. Effect of Adiponectin Variant on Lipid Profile and Plasma Adiponectin Levels: A Multicenter Systematic Review and Meta-Analysis. Cardiovasc. Ther. 2022, 2022, 4395266. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhuang, R.; Ma, Y.; Zhang, C.; Tang, K.; Yi, H.; Jin, B. Adiponectin’s globular domain inhibits T cell activation by interacting with LAIR-1. Biochem. Biophys. Res. Commun. 2021, 573, 117–124. [Google Scholar] [CrossRef]
- Ramos-Ramírez, P.; Malmhäll, C.; Tliba, O.; Rådinger, M.; Bossios, A. Adiponectin/AdipoR1 Axis Promotes IL-10 Release by Human Regulatory T Cells. Front. Immunol. 2021, 12, 677550. [Google Scholar] [CrossRef]
- Barbe, A.; Bongrani, A.; Mellouk, N.; Estienne, A.; Kurowska, P.; Grandhaye, J.; Elfassy, Y.; Levy, R.; Rak, A.; Froment, P.; et al. Mechanisms of Adiponectin Action in Fertility: An Overview from Gametogenesis to Gestation in Humans and Animal Models in Normal and Pathological Conditions. Int. J. Mol. Sci. 2019, 20, 1526. [Google Scholar] [CrossRef]
- Michalakis, K.G.; Segars, J.H. The role of adiponectin in reproduction: From polycystic ovary syndrome to assisted reproduction. Fertil. Steril. 2010, 94, 1949–1957. [Google Scholar] [CrossRef]
- Turer, A.T.; Scherer, P.E. Adiponectin: Mechanistic insights and clinical implications. Diabetologia 2012, 55, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.L.; Yang, Z.; Yang, S.S. Roles of Adipokines in Digestive Diseases: Markers of Inflammation, Metabolic Alteration and Disease Progression. Int. J. Mol. Sci. 2020, 21, 8308. [Google Scholar] [CrossRef]
- Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Kadowaki, T. Adiponectin receptors: A review of their structure, function and how they work. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.J.; Cohen, P. Adipose crosstalk with other cell types in health and disease. Exp. Cell Res. 2017, 360, 6–11. [Google Scholar] [CrossRef]
- Roy, B.; Palaniyandi, S.S. Tissue-specific role and associated downstream signaling pathways of adiponectin. Cell Biosci. 2021, 11, 77. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Frost, A.R.; Hurst, D.R.; Shevde, L.A.; Samant, R.S. The influence of the cancer microenvironment on the process of metastasis. Int. J. Breast Cancer 2012, 2012, 756257. [Google Scholar] [CrossRef]
- Ribeiro, R.J.; Monteiro, C.P.; Cunha, V.F.; Azevedo, A.S.; Oliveira, M.J.; Monteiro, R.; Fraga, A.M.; Príncipe, P.; Lobato, C.; Lobo, F.; et al. Tumor cell-educated periprostatic adipose tissue acquires an aggressive cancer-promoting secretory profile. Cell Physiol. Biochem. 2012, 29, 233–240. [Google Scholar] [CrossRef]
- Akrida, I.; Papadaki, H. Adipokines and epithelial-mesenchymal transition (EMT) in cancer. Mol. Cell Biochem. 2023, 30. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Gelsomino, L.; Panza, S.; Accattatis, F.M.; Naimo, G.D.; Barone, I.; Giordano, C.; Catalano, S.; Andò, S. Leptin: A Heavyweight Player in Obesity-Related Cancers. Biomolecules 2023, 13, 1084. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Ramaiyer, M.; Begum, U.A.M.; Borahay, M.A. Adipocyte and Adipokines Promote a Uterine Leiomyoma Friendly Microenvironment. Nutrients 2023, 15, 715. [Google Scholar] [CrossRef]
- Hosney, M.; Sabet, S.; El-Shinawi, M.; Gaafar, K.M.; Mohamed, M.M. Leptin is overexpressed in the tumor microenvironment of obese patients with estrogen receptor positive breast cancer. Exp. Ther. Med. 2017, 13, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Verras, G.-I.; Tchabashvili, L.; Chlorogiannis, D.-D.; Mulita, F.; Argentou, M.-I. Updated Clinical Evidence on the Role of Adipokines and Breast Cancer: A Review. Cancers 2023, 15, 1572. [Google Scholar] [CrossRef]
- Le Guennec, D.; Hatte, V.; Farges, M.C.; Rougé, S.; Goepp, M.; Caldefie-Chezet, F.; Vasson, M.P.; Rossary, A. Modulation of inter-organ signalling in obese mice by spontaneous physical activity during mammary cancer development. Sci. Rep. 2020, 10, 8794. [Google Scholar] [CrossRef] [PubMed]
- Bouche, C.; Quail, D.F. Fueling the Tumor Microenvironment with Cancer-Associated Adipocytes. Cancer Res. 2023, 83, 1170–1172. [Google Scholar] [CrossRef]
- Chakraborty, D.; Jin, W.; Wang, J. The bifurcated role of adiponectin in colorectal cancer. Life Sci. 2021, 278, 119524. [Google Scholar] [CrossRef]
- Cancel, M.; Pouillot, W.; Mahéo, K.; Fontaine, A.; Crottès, D.; Fromont, G. Interplay between Prostate Cancer and Adipose Microenvironment: A Complex and Flexible Scenario. Int. J. Mol. Sci. 2022, 23, 10762. [Google Scholar] [CrossRef]
- Naimo, G.D.; Paolì, A.; Giordano, F.; Forestiero, M.; Panno, M.L.; Andò, S.; Mauro, L. Unraveling the Role of Adiponectin Receptors in Obesity-Related Breast Cancer. Int. J. Mol. Sci. 2023, 24, 8907. [Google Scholar] [CrossRef]
- Llanos, A.A.M.; Yao, S.; Singh, A.; Aremu, J.B.; Khiabanian, H.; Lin, Y.; Omene, C.; Omilian, A.R.; Khoury, T.; Hong, C.C.; et al. Gene expression of adipokines and adipokine receptors in the tumor microenvironment: Associations of lower expression with more aggressive breast tumor features. Breast Cancer Res. Treat. 2021, 158, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Barone, I.; Catalano, S.; Gelsomino, L.; Marsico, S.; Giordano, C.; Panza, S.; Bonofiglio, D.; Bossi, G.; Covington, K.R.; Fuqua, S.A.; et al. Leptin mediates tumor-stromal interactions that promote the invasive growth of breast cancer cells. Cancer Res. 2012, 72, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, S.; Guo, T.; Li, J.; Gu, C. The Nutritional Cytokine Leptin Promotes NSCLC by Activating the PI3K/AKT and MAPK/ERK Pathways in NSCLC Cells in a Paracrine Manner. Biomed. Res. Int. 2019, 18, 2585743. [Google Scholar] [CrossRef]
- Goicoechea, S.M.; García-Mata, R.; Staub, J.; Valdivia, A.; Sharek, L.; McCulloch, C.G.; Hwang, R.F.; Urrutia, R.; Yeh, J.J.; Kim, H.J.; et al. Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene 2014, 33, 1265–1273. [Google Scholar] [CrossRef]
- Cai, L.; Xu, S.; Piao, C.; Qiu, S.; Li, H.; Du, J. Adiponectin induces CXCL1 secretion from cancer cells and promotes tumor angiogenesis by inducing stromal fibroblast senescence. Mol. Carcinog. 2016, 55, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Chen, D.; Cai, J.; Yuan, Z.; Huang, B.; Li, Y.; Wang, H.; Luo, Q.; Kuang, Y.; Liang, W.; et al. Enhancing cancer-associated fibroblast fatty acid catabolism within a metabolically challenging tumor microenvironment drives colon cancer peritoneal metastasis. Mol. Oncol. 2021, 15, 1391–1411. [Google Scholar] [CrossRef]
- Gelsomino, L.; Naimo, G.D.; Malivindi, R.; Augimeri, G.; Panza, S.; Giordano, C.; Barone, I.; Bonofiglio, D.; Mauro, L.; Catalano, S.; et al. Knockdown of Leptin Receptor Affects Macrophage Phenotype in the Tumor Microenvironment Inhibiting Breast Cancer Growth and Progression. Cancers 2020, 12, 2078. [Google Scholar] [CrossRef]
- Cao, H.; Huang, Y.; Wang, L.; Wang, H.; Pang, X.; Li, K.; Dang, W.; Tang, H.; Wei, L.; Su, M.; et al. Leptin promotes migration and invasion of breast cancer cells by stimulating IL-8 production in M2 macrophages. Oncotarget 2016, 40, 65441–65453. [Google Scholar] [CrossRef]
- Li, K.; Wei, L.; Huang, Y.; Wu, Y.; Su, M.; Pang, X.; Wang, N.; Ji, F.; Zhong, C.; Chen, T. Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. Int. J. Oncol. 2016, 48, 2479–2487. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Li, Z.; Zhu, B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics 2021, 11, 1016–1030. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Y.; He, L.; Li, Y.; Lin, K.; Kang, Q.; Liu, L.; Zou, H. Gallbladder Cancer Cell-Derived Exosome-Mediated Transfer of Leptin Promotes Cell Invasion and Migration by Modulating STAT3-Mediated M2 Macrophage Polarization. Anal. Cell Pathol. 2022, 24, 9994906. [Google Scholar] [CrossRef] [PubMed]
- Scheurlen, K.M.; Snook, D.L.; Walter, M.N.; Cook, C.N.; Fiechter, C.R.; Pan, J.; Beal, R.J.; Galandiuk, S. Itaconate and leptin affecting PPARγ in M2 macrophages: A potential link to early-onset colorectal cancer. Surgery 2022, 171, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lodish, H.F. Adiponectin deficiency promotes tumor growth in mice by reducing macrophage infiltration. PLoS ONE 2010, 8, 11987. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Huang, H.; Huan, Q.; Liao, C.; Guo, Z.; Hu, D.; Shen, X.; Xiao, H. Adiponectin Deficiency Enhances Anti-Tumor Immunity of CD8+ T Cells in Rhabdomyosarcoma Through Inhibiting STAT3 Activation. Front. Oncol. 2022, 12, 847088. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, X.; Du, L.; Yang, Y.; Cheng, H.; Zhang, X.; Li, Z.; Wang, L.; Li, J.; Liu, H.; et al. Leptin-mediated regulation of MT1-MMP localization is KIF1B dependent and enhances gastric cancer cell invasion. Carcinogenesis 2013, 34, 974–983. [Google Scholar] [CrossRef]
- Strong, A.L.; Ohlstein, J.F.; Biagas, B.A.; Rhodes, L.V.; Pei, D.T.; Tucker, H.A.; Llamas, C.; Bowles, A.C.; Dutreil, M.F.; Zhang, S.; et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015, 17, 112. [Google Scholar] [CrossRef]
- Olea-Flores, M.; Zuñiga-Eulogio, M.; Tacuba-Saavedra, A.; Bueno-Salgado, M.; Sánchez-Carvajal, A.; Vargas-Santiago, Y.; Mendoza-Catalán, M.A.; Pérez Salazar, E.; García-Hernández, A.; Padilla-Benavides, T.; et al. Leptin Promotes Expression of EMT-Related Transcription Factors and Invasion in a Src and FAK-Dependent Pathway in MCF10A Mammary Epithelial Cells. Cells 2019, 8, 1133. [Google Scholar] [CrossRef]
- Beales, I.L.P.; Garcia-Morales, C.; Ogunwobi, O.O.; Mutungi, G. Adiponectin inhibits leptin-induced oncogenic signalling in oesophageal cancer cells by activation of PTP1B. Mol. Cell Endocrinol. 2014, 382, 150–158. [Google Scholar] [CrossRef]
- Zou, H.; Liu, Y.; Wei, D.; Wang, T.; Wang, K.; Huang, S.; Liu, L.; Li, Y.; Ge, J.; Li, X.; et al. Leptin promotes proliferation and metastasis of human gallbladder cancer through OB-Rb leptin receptor. Int. J. Oncol. 2016, 49, 197–206. [Google Scholar] [CrossRef]
- Ghasemi, A.; Hashemy, S.I.; Aghaei, M.; Panjehpour, M. Leptin induces matrix metalloproteinase 7 expression to promote ovarian cancer cell invasion by activating ERK and JNK pathways. J. Cell Biochem. 2018, 119, 2333–2344. [Google Scholar] [CrossRef]
- Lin, M.C.; Wang, F.Y.; Kuo, Y.H.; Tang, F.Y. Cancer chemopreventive effects of lycopene: Suppression of MMP-7 expression and cell invasion in human colon cancer cells. J. Agric. Food Chem. 2011, 59, 11304–11318. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Gan, Y.; Shen, Y.; Cai, X.; Song, Y.; Zhao, F.; Yao, M.; Gu, J.; Tu, H. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget 2015, 6, 16120–16134. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.R.; Liu, P.L.; Chen, Y.H.; Chou, S.H.; Cheng, Y.J.; Hwang, J.J.; Chong, I.W. Curcumin Inhibits Non-Small Cell Lung Cancer Cells Metastasis through the Adiponectin/NF-κb/MMPs Signaling Pathway. PLoS ONE 2015, 10, e0144462. [Google Scholar] [CrossRef]
- Kleinmann, N.; Duivenvoorden, W.C.; Hopmans, S.N.; Beatty, L.K.; Qiao, S.; Gallino, D.; Lhotak, S.; Daya, D.; Paschos, A.; Austin, R.C.; et al. Underactivation of the adiponectin-adiponectin receptor 1 axis in clear cell renal cell carcinoma: Implications for progression. Clin. Exp. Metastasis 2014, 31, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Ng, K.T.; Xu, A.; Cheng, Q.; Lo, C.M.; Xiao, J.W.; Sun, B.S.; Lim, Z.X.; Cheung, J.S.; Wu, E.X.; et al. Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of Rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin. Cancer Res. 2010, 16, 967–977. [Google Scholar] [CrossRef]
- Liao, Z.; Tan, Z.W.; Zhu, P.; Tan, N.S. Cancer-associated fibroblasts in tumor microenvironment—Accomplices in tumor malignancy. Cell Immunol. 2019, 343, 103729. [Google Scholar] [CrossRef]
- Hamabe-Horiike, T.; Harada, S.I.; Yoshida, K.; Kinoshita, J.; Yamaguchi, T.; Fushida, S. Adipocytes contribute to tumor progression and invasion of peritoneal metastasis by interacting with gastric cancer cells as cancer associated fibroblasts. Cancer Rep. 2023, 6, e1647. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Donohoe, C.L.; Pidgeon, G.P.; Lysaght, J.; Reynolds, J.V. Obesity and gastrointestinal cancer. Br. J. Surg. 2010, 97, 628–642. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef]
- De Palma, M.; Lewis, C.E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013, 23, 277–286. [Google Scholar] [CrossRef]
- Li, M.; He, L.; Zhu, J.; Zhang, P.; Liang, S. Targeting tumor-associated macrophages for cancer treatment. Cell Biosci. 2022, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Pratt, B.T.; Barnes, M.; McMullen, M.R.; Nagy, L.E. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: Link between the metabolic and innate immune activity of full-length adiponectin. J. Biol. Chem. 2011, 286, 13460–13469. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A.; et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Quan, A.; Szmitko, P.E.; Singh, K.K.; Shukla, P.C.; Gupta, M.; Chan, L.; Al-Omran, M.; Teoh, H.; et al. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H656–H663. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, M. Adiponectin: A versatile player of innate immunity. J. Mol. Cell Biol. 2016, 8, 120–128. [Google Scholar] [CrossRef]
- Xuan, D.; Han, Q.; Tu, Q.; Zhang, L.; Yu, L.; Murry, D.; Tu, T.; Tang, Y.; Lian, J.B.; Stein, G.S.; et al. Epigenetic Modulation in Periodontitis: Interaction of Adiponectin and JMJD3-IRF4 Axis in Macrophages. J. Cell Physiol. 2016, 231, 1090–1096. [Google Scholar] [CrossRef]
- Blicharz-Dorniak, J.; Kos-Kudła, B.; Foltyn, W.; Kajdaniuk, D.; Marek, B.; Zemczak, A.; Strzelczyk, J. Is determination of matrix metalloproteinases and their tissue inhibitors serum concentrations useful in patients with gastroenteropancreatic and bronchopulmonary neuroendocrine neoplasms? Endokrynol. Pol. 2012, 63, 470–476. [Google Scholar]
- Hadler-Olsen, E.; Winberg, J.O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013, 34, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Bauters, D.; Scroyen, I.; Van Hul, M.; Lijnen, H.R. Gelatinase A (MMP-2) promotes murine adipogenesis. Biochim. Biophys. Acta 2015, 1850, 1449–1456. [Google Scholar] [CrossRef]
- Van Hul, M.; Lijnen, H.R. Matrix metalloproteinase inhibition impairs murine adipose tissue development independently of leptin. Endocr. J. 2011, 58, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Meissburger, B.; Stachorski, L.; Röder, E.; Rudofsky, G.; Wolfrum, C. Tissue inhibitor of matrix metalloproteinase 1 (TIMP1) controls adipogenesis in obesity in mice and in humans. Diabetologia 2011, 54, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Matulewicz, N.; Stefanowicz, M.; Nikolajuk, A.; Karczewska-Kupczewska, M. Markers of Adipogenesis, but Not Inflammation, in Adipose Tissue Are Independently Related to Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2017, 102, 3040–3049. [Google Scholar] [CrossRef] [PubMed]
- Szczęsny, W.; Kuligowska-Prusińska, M.; Dąbrowiecki, S.; Szmytkowski, J.; Reśliński, A.; Słupski, M. Activity of metalloproteinases and adiponectin in obese patients-a possible factor of incisional hernias after bariatric procedures. J. Zhejiang Univ. Sci. B. 2018, 19, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.F.; Tang, P.; Li, Q.; Yu, Z.T. Obesity, adipokines and hepatocellular carcinoma. Int. J. Cancer 2013, 133, 1776–1783. [Google Scholar] [CrossRef]
- Stępień, S.; Olczyk, P.; Gola, J.; Komosińska-Vassev, K.; Mielczarek-Palacz, A. The Role of Selected Adipocytokines in Ovarian Cancer and Endometrial Cancer. Cells 2023, 12, 1118. [Google Scholar] [CrossRef]
- Ahn, J.H.; Choi, Y.S.; Choi, J.H. Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol. Hum. Reprod. 2015, 21, 792–802. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, H.; Shao, Q.; Dong, Z.; Xie, Q.; Zhao, L.; Wang, Q.; Kong, B.; Qu, X. Leptin-promoted human extravillous trophoblast invasion is MMP14 dependent and requires the cross talk between Notch1 and PI3K/Akt signaling. Biol. Reprod. 2014, 90, 78. [Google Scholar] [CrossRef]
- Jo, Y.S.; Lee, G.S.; Nam, S.Y.; Kim, S.J. Progesterone Inhibits Leptin-Induced Invasiveness of BeWo Cells. Int. J. Med. Sci. 2015, 12, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, X.; Jiang, L.; Huang, X.; Zhang, Y.; Wei, X.; Zhao, X.; Du, Y. Advances in understanding the role of adiponectin in renal fibrosis. Nephrology 2021, 26, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, J.; Liu, H.; Jian, X.; Zou, Q.; Zhao, Q.; Le, Q.; Chen, H.; Gao, X.; He, C. Adiponectin Is Involved in Connective Tissue Growth Factor-Induced Proliferation, Migration and Overproduction of the Extracellular Matrix in Keloid Fibroblasts. Int. J. Mol. Sci. 2017, 18, 1044. [Google Scholar] [CrossRef] [PubMed]
- Dadson, K.; Turdi, S.; Boo, S.; Hinz, B.; Sweeney, G. Temporal and Molecular Analyses of Cardiac Extracellular Matrix Remodeling following Pressure Overload in Adiponectin Deficient Mice. PLoS ONE 2015, 10, e0121049. [Google Scholar] [CrossRef] [PubMed]
- Adya, R.; Tan, B.K.; Chen, J.; Randeva, H.S. Protective actions of globular and full-length adiponectin on human endothelial cells: Novel insights into adiponectin-induced angiogenesis. J. Vasc. Res. 2012, 49, 534–543. [Google Scholar] [CrossRef]
- Jenke, A.; Schur, R.; Röger, C.; Karadeniz, Z.; Grüger, M.; Holzhauser, L.; Savvatis, K.; Poller, W.; Schultheiss, H.P.; Landmesser, U.; et al. Adiponectin attenuates profibrotic extracellular matrix remodeling following cardiac injury by up-regulating matrix metalloproteinase 9 expression in mice. Physiol. Rep. 2017, 5, e13523. [Google Scholar] [CrossRef]
- Harasymowicz, N.S.; Azfer, A.; Burnett, R.; Simpson, H.; Salter, D.M. Chondrocytes from osteoarthritic cartilage of obese patients show altered adiponectin receptors expression and response to adiponectin. J. Orthop. Res. 2021, 39, 2333–2339. [Google Scholar] [CrossRef]
- Ruan, G.; Xu, J.; Wang, K.; Wu, J.; Zhu, Q.; Ren, J.; Bian, F.; Chang, B.; Bai, X.; Han, W.; et al. Associations between knee structural measures, circulating inflammatory factors and MMP13 in patients with knee osteoarthritis. Osteoarthr. Cartil. 2018, 26, 1063–1069. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Wang, B.J.; Chu, G.; Cao, Q.; Sun, B.G.; Dai, Q.Y. Inhibition of leptin-induced vascular extracellular matrix remodelling by adiponectin. J. Mol. Endocrinol. 2014, 53, 145–154. [Google Scholar] [CrossRef]
- Suzuki, M.; Mihara, M. Adiponectin induces CCL20 expression synergistically with IL-6 and TNF-α in THP-1 macrophages. Cytokine 2012, 58, 344–350. [Google Scholar] [CrossRef]
- Handy, J.A.; Saxena, N.K.; Fu, P.; Lin, S.; Mells, J.E.; Gupta, N.A.; Anania, F.A. Adiponectin activation of AMPK disrupts leptin-mediated hepatic fibrosis via suppressors of cytokine signaling (SOCS-3). J. Cell Biochem. 2010, 110, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Handy, J.A.; Fu, P.P.; Kumar, P.; Mells, J.E.; Sharma, S.; Saxena, N.K.; Anania, F.A. Adiponectin inhibits leptin signalling via multiple mechanisms to exert protective effects against hepatic fibrosis. Biochem. J. 2011, 440, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Leggett, S.E.; Hruska, A.M.; Guo, M.; Wong, I.Y. The epithelial-mesenchymal transition and the cytoskeleton in bioengineered systems. Cell Commun. Signal. 2021, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Nigro, E.; Schettino, P.; Polito, R.; Scudiero, O.; Monaco, M.L.; De Palma, G.D.; Daniele, A. Adiponectin and colon cancer: Evidence for inhibitory effects on viability and migration of human colorectal cell lines. Mol. Cell Biochem. 2018, 448, 125–135. [Google Scholar] [CrossRef]
- Lai, X.; Li, Q.; Wu, F.; Lin, J.; Chen, J.; Zheng, H.; Guo, L. Epithelial-Mesenchymal Transition and Metabolic Switching in Cancer: Lessons from Somatic Cell Reprogramming. Front. Cell Dev. Biol. 2020, 8, 760. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. EMT International Association (TEMTIA). Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Derynck, R.; Muthusamy, B.P.; Saeteurn, K.Y. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2014, 31, 56–66. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Peixoto, P.; Etcheverry, A.; Aubry, M.; Missey, A.; Lachat, C.; Perrard, J.; Hendrick, E.; Delage-Mourroux, R.; Mosser, J.; Borg, C.; et al. EMT is associated with an epigenetic signature of ECM remodeling genes. Cell Death Dis. 2019, 10, 205. [Google Scholar] [CrossRef]
- Chaffer, C.L.; San Juan, B.P.; Lim, E.; Weinberg, R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016, 35, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Parish, C.R.; Wong, M.L.; Licinio, J.; Blackburn, A.C. Leptin signals via TGFB1 to promote metastatic potential and stemness in breast cancer. PLoS ONE 2017, 12, e0178454. [Google Scholar] [CrossRef] [PubMed]
- Manfioletti, G.; Fedele, M. Epithelial–Mesenchymal Transition (EMT). Int. J. Mol. Sci. 2023, 24, 11386. [Google Scholar] [CrossRef]
- Williams, E.D.; Gao, D.; Redfern, A.; Thompson, E.W. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat. Rev. Cancer 2019, 19, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Z.; Horta, C.A.; Yang, J. Regulation of epithelial-mesenchymal transition by tumor microenvironmental signals and its implication in cancer therapeutics. Semin. Cancer Biol. 2023, 88, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Barone, I.; Giordano, C.; Bonofiglio, D.; Andò, S.; Catalano, S. The weight of obesity in breast cancer progression and metastasis: Clinical and molecular perspectives. Semin. Cancer Biol. 2020, 60, 274–284. [Google Scholar] [CrossRef]
- Juárez-Cruz, J.C.; Okoniewski, M.; Ramírez, M.; Ortuño-Pineda, C.; Navarro-Tito, N.; Castañeda-Saucedo, E. Chronic Leptin Treatment Induces Epithelial-Mesenchymal Transition in MCF10A Mammary Epithelial Cells. J. Mammary Gland Biol. Neoplasia. 2022, 27, 19–36. [Google Scholar] [CrossRef]
- Bowers, L.W.; Rossi, E.L.; McDonell, S.B.; Doerstling, S.S.; Khatib, S.A.; Lineberger, C.G.; Albright, J.E.; Tang, X.; de Graffenried, L.A.; Hursting, S.D. Leptin Signaling Mediates Obesity-Associated CSC Enrichment and EMT in Preclinical TNBC Models. Mol Cancer Res. 2018, 16, 869–879. [Google Scholar] [CrossRef]
- Al Moustafa, A.E. Epithelial-mesenchymal transition and its regulators are major targets of triple-negative breast cancer. Cell Adh. Migr. 2013, 7, 424–425. [Google Scholar] [CrossRef]
- Yan, D.; Avtanski, D.; Saxena, N.K.; Sharma, D. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J. Biol. Chem. 2012, 28, 8598–8612. [Google Scholar] [CrossRef]
- Wang, L.; Tang, C.; Cao, H.; Li, K.; Pang, X.; Zhong, L.; Dang, W.; Tang, H.; Huang, Y.; Wei, L.; et al. Activation of IL-8 via PI3K/Akt-dependent pathway is involved in leptin-mediated epithelial-mesenchymal transition in human breast cancer cells. Cancer Biol. Ther. 2015, 16, 1220–1230. [Google Scholar] [CrossRef]
- Wei, L.; Li, K.; Pang, X.; Guo, B.; Su, M.; Huang, Y.; Wang, N.; Ji, F.; Zhong, C.; Yang, J.; et al. Leptin promotes epithelial-mesenchymal transition of breast cancer via the upregulation of pyruvate kinase M2. J. Exp. Clin. Cancer Res. 2016, 35, 166. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Lu, Y.; Xie, W.; Nong, L.; Jia, Y.; Tan, A.; Liu, Y. Leptin promotes bone metastasis of breast cancer by activating the SDF-1/CXCR4 axis. Aging 2020, 12, 16172–16182. [Google Scholar] [CrossRef]
- Trevellin, E.; Scarpa, M.; Carraro, A.; Lunardi, F.; Kotsafti, A.; Porzionato, A.; Saadeh, L.; Cagol, M.; Alfieri, R.; Tedeschi, U.; et al. Esophageal adenocarcinoma and obesity: Peritumoral adipose tissue plays a role in lymph node invasion. Oncotarget 2015, 6, 11203–11215. [Google Scholar] [CrossRef]
- Park, K.B.; Kim, E.Y.; Chin, H.; Yoon, D.J.; Jun, K.H. Leptin stimulates migration and invasion and maintains cancer stem-like properties in gastric cancer cells. Oncol. Rep. 2022, 48, 162. [Google Scholar] [CrossRef]
- Peng, C.; Sun, Z.; Li, O.; Guo, C.; Yi, W.; Tan, Z.; Jiang, B. Leptin stimulates the epithelial-mesenchymal transition and pro-angiogenic capability of cholangiocarcinoma cells through the miR-122/PKM2 axis. Int. J. Oncol. 2019, 55, 298–308. [Google Scholar] [CrossRef]
- Choi, S.S.; Syn, W.K.; Karaca, G.F.; Omenetti, A.; Moylan, C.A.; Witek, R.P.; Agboola, K.M.; Jung, Y.; Michelotti, G.A.; Diehl, A.M. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J. Biol. Chem. 2010, 285, 36551–36560. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.S.; Zhou, B.H.; Li, C.L.; Zhang, F.; Wang, X.F.; Zhang, G.; Bu, X.Z.; Cai, S.H.; Du, J. Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS ONE 2013, 8, e56664. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Liu, Q.; Zhang, N.; Zheng, L.; Sang, M.; Feng, J.; Zhang, J.; Wu, X.; Shan, B. Leptin promotes metastasis by inducing an epithelial-mesenchymal transition in A549 lung cancer cells. Oncol. Res. 2013, 21, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Cao, F.L.; Li, N.; Gao, X.; Su, X.; Jiang, X. Leptin induces epithelial-to-mesenchymal transition via activation of the ERK signaling pathway in lung cancer cells. Oncol. Lett. 2018, 16, 4782–4788. [Google Scholar] [CrossRef] [PubMed]
- Gorrab, A.; Pagano, A.; Ayed, K.; Chebil, M.; Derouiche, A.; Kovacic, H.; Gati, A. Leptin Promotes Prostate Cancer Proliferation and Migration by Stimulating STAT3 Pathway. Nutr. Cancer 2021, 73, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, X.; Feng, J.; Deng, L.L.; Liu, Y.; Li, B.; Zhu, M.; Lu, C.; Zhou, L. MT2-MMP induces proteolysis and leads to EMT in carcinomas. Oncotarget 2016, 7, 48193–48205. [Google Scholar] [CrossRef] [PubMed]
- Conlon, G.A.; Murray, G.I. Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2019, 247, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Motallebnezhad, M.; Aghebati-Maleki, L.; Jadidi-Niaragh, F.; Nickho, H.; Samadi-Kafil, H.; Shamsasenjan, K.; Yousefi, M. The insulin-like growth factor-I receptor (IGF-IR) in breast cancer: Biology and treatment strategies. Tumour Biol. 2016, 37, 11711–11721. [Google Scholar] [CrossRef]
- Hwang, M.S.; Yu, N.; Stinson, S.Y.; Yue, P.; Newman, R.J.; Allan, B.B.; Dornan, D. miR-221/222 targets adiponectin receptor 1 to promote the epithelial-to-mesenchymal transition in breast cancer. PLoS ONE 2013, 8, e66502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, J.; Xu, Q.; Xing, C.; Li, Y.; Zhou, S.; Zhao, Z.; Mu, Y.; Zhao, Z.; Cao, S.; et al. Adiponectin Suppresses Metastasis of Nasopharyngeal Carcinoma through Blocking the Activation of NF-κB and STAT3 Signaling. Int. J. Mol. Sci. 2022, 23, 12729. [Google Scholar] [CrossRef]
- Cui, E.; Guo, H.; Shen, M.; Yu, H.; Gu, D.; Mao, W.; Wang, X. Adiponectin inhibits migration and invasion by reversing epithelial-mesenchymal transition in non-small cell lung carcinoma. Oncol. Rep. 2018, 40, 1330–1338. [Google Scholar] [CrossRef]
- Tae, C.H.; Kim, S.E.; Jung, S.A.; Joo, Y.H.; Shim, K.N.; Jung, H.K.; Kim, T.H.; Cho, M.S.; Kim, K.H.; Kim, J.S. Involvement of adiponectin in early stage of colorectal carcinogenesis. BMC Cancer 2014, 14, 811. [Google Scholar] [CrossRef]
- Tan, W.; Wang, L.; Ma, Q.; Qi, M.; Lu, N.; Zhang, L.; Han, B. Adiponectin as a potential tumor suppressor inhibiting epithelial-to-mesenchymal transition but frequently silenced in prostate cancer by promoter methylation. Prostate 2015, 75, 1197–1205. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Liu, Z.; Zhu, S.; Shen, P.; Zhang, H.; Zhang, M.; Chen, N.; Zhao, J.; Chen, J.; et al. The Adiponectin-AdipoR1 Axis Mediates Tumor Progression and Tyrosine Kinase Inhibitor Resistance in Metastatic Renal Cell Carcinoma. Neoplasia 2019, 21, 921–931. [Google Scholar] [CrossRef]
- Sternberg, J.; Wankell, M.; Subramaniam, V.N.; Hebbard, L.W. The functional roles of T-cadherin in mammalian biology. AIMS Mol. Sci. 2017, 4, 62–81. [Google Scholar] [CrossRef]
- Ren, J.Z.; Huo, J.R. Correlation between T-cadherin gene expression and aberrant methylation of T-cadherin promoter in human colon carcinoma cells. Med. Oncol. 2012, 29, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, M.R.; Stein, S.; Heida, N.M.; Leifheit-Nestler, M.; Cheng, I.F.; Gogiraju, R.; Christiansen, H.; Maier, L.S.; Shah, A.M.; Hasenfuss, G.; et al. Leptin promotes the mobilization of vascular progenitor cells and neovascularization by NOX2-mediated activation of MMP9. Cardiovasc. Res. 2012, 93, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, J.; Zhao, W.; Peng, Z.; Liu, X.; Li, B.; Zhang, H.; Shan, B.; Zhang, C.; Duan, C. Vasculogenic mimicry in carcinogenesis and clinical applications. J. Hematol. Oncol. 2020, 13, 19. [Google Scholar] [CrossRef]
- Delgado-Bellido, D.; Oliver, F.J.; Vargas Padilla, M.V.; Lobo-Selma, L.; Chacón-Barrado, A.; Díaz-Martin, J.; de Álava, E. VE-Cadherin in Cancer-Associated Angiogenesis: A Deceptive Strategy of Blood Vessel Formation. Int. J. Mol. Sci. 2023, 24, 9343. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T.; d’Amati, A.; Ingravallo, G.; Specchia, G. Vascular Growth in Lymphomas: Angiogenesis and Alternative Ways. Cancers 2023, 15, 3262. [Google Scholar] [CrossRef]
- Fernández-Cortés, M.; Delgado-Bellido, D.; Oliver, F.J. Vasculogenic Mimicry: Become an Endothelial Cell “But Not So Much”. Front. Oncol. 2019, 9, 803. [Google Scholar] [CrossRef]
- Knopik-Skrocka, A.B.; Krȩplewska, P.; Jarmołowska-Jurczyszyn, D. Tumor blood vessels and vasculogenic mimicry—Current knowledge and searching for new cellular/molecular targets of anti-angiogenic therapy. Adv. Cell Biol. 2017, 5, 50–71. [Google Scholar] [CrossRef]
- Kim, H.S.; Won, Y.J.; Shim, J.H.; Kim, H.J.; Kim, J.; Hong, H.N.; Kim, B.S. Morphological characteristics of vasculogenic mimicry and its correlation with EphA2 expression in gastric adenocarcinoma. Sci. Rep. 2019, 9, 3414. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guadarrama, G.; García-Becerra, R.; Méndez-Pérez, E.A.; García-Quiroz, J.; Avila, E.; Díaz, L. Vasculogenic Mimicry in Breast Cancer: Clinical Relevance and Drivers. Cells 2021, 10, 1758. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J Clin Investig. 2017, 127, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Bibi, A.; Valoti, M.; Fusi, F. Perivascular Adipose Tissue and Vascular Smooth Muscle Tone: Friends or Foes? Cells 2023, 12, 1196. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Xia, N. The Interplay Between Adipose Tissue and Vasculature: Role of Oxidative Stress in Obesity. Front. Cardiovasc. Med. 2021, 8, 650214. [Google Scholar] [CrossRef]
- Di Somma, M.; Vliora, M.; Grillo, E.; Castro, B.; Dakou, E.; Schaafsma, W.; Vanparijs, J.; Corsini, M.; Ravelli, C.; Sakellariou, E.; et al. Role of VEGFs in metabolic disorders. Angiogenesis 2020, 2, 119–130. [Google Scholar] [CrossRef]
- Sung, H.K.; Doh, K.O.; Son, J.E.; Park, J.G.; Bae, Y.; Choi, S.; Nelson, S.M.; Cowling, R.; Nagy, K.; Michael, I.P.; et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013, 17, 61–72. [Google Scholar] [CrossRef]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef]
- Adya, R.; Tan, B.K.; Randeva, H.S. Differential effects of leptin and adiponectin in endothelial angiogenesis. J. Diabetes Res. 2015, 2015, 648239. [Google Scholar] [CrossRef]
- Dubois, V.; Delort, L.; Billard, H.; Vasson, M.P.; Caldefie-Chezet, F. Breast cancer and obesity: In vitro interferences between adipokines and proangiogenic features and/or antitumor therapies? PLoS ONE 2013, 8, e58541. [Google Scholar] [CrossRef]
- Liu, H.; Wan, D.; Pan, Z.; Cao, L.; Wu, X.; Lu, Z.; Kang, T. Expression and biological significance of leptin, leptin receptor, VEGF, and CD34 in colorectal carcinoma. Cell Biochem. Biophys. 2011, 60, 241–244. [Google Scholar] [CrossRef]
- Kurtovic, S.; Ng, T.T.; Gupta, A.; Arumugaswami, V.; Chaiboonma, K.L.; Aminzadeh, M.A.; Makkar, R.; Dafoe, D.C.; Talavera-Adame, D. Leptin enhances endothelial cell differentiation and angiogenesis in murine embryonic stem cells. Microvasc. Res. 2015, 97, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ferla, R.; Bonomi, M.; Otvos, L., Jr.; Surmacz, E. Glioblastoma-derived leptin induces tube formation and growth of endothelial cells: Comparison with VEGF effects. BMC Cancer 2011, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Jiménez, F.; Pérez-Pérez, A.; de la Cruz-Merino, L.; Sánchez-Margalet, V. Obesity and Breast Cancer: Role of Leptin. Front. Oncol. 2019, 9, 596. [Google Scholar] [CrossRef]
- Huang, F.; Xiong, X.; Wang, H.; You, S.; Zeng, H. Leptin-induced vascular smooth muscle cell proliferation via regulating cell cycle, activating ERK1/2 and NF-kappaB. Acta Biochim. Biophys. Sin. 2010, 42, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, R.R.; Xu, Y.; Guo, S.; Watters, A.; Zhou, W.; Leibovich, S.J. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell Signal. 2010, 22, 1350–1362. [Google Scholar] [CrossRef]
- Yu, F.; Fu, R.l.; Liu, L.; Wang, X.; Wu, T.; Shen, W.; Gui, Z.; Mo, X.; Fang, B.; Xia, L. Leptin-Induced Angiogenesis of EA.Hy926 Endothelial Cells via the Akt and Wnt Signaling Pathways In Vitro and In Vivo. Front. Pharmacol. 2019, 10, 1275. [Google Scholar] [CrossRef]
- Heida, N.M.; Leifheit-Nestler, M.; Schroeter, M.R.; Müller, J.P.; Cheng, I.F.; Henkel, S.; Limbourg, A.; Limbourg, F.P.; Alves, F.; Quigley, J.P.; et al. Leptin enhances the potency of circulating angiogenic cells via src kinase and integrin (alpha)vbeta5: Implications for angiogenesis in human obesity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 200–206. [Google Scholar] [CrossRef]
- Parker-Duffen, J.L.; Nakamura, K.; Silver, M.; Kikuchi, R.; Tigges, U.; Yoshida, S.; Denzel, M.S.; Ranscht, B.; Walsh, K. T-cadherin is essential for adiponectin-mediated revascularization. J. Biol. Chem. 2013, 288, 24886–24897. [Google Scholar] [CrossRef]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Lavoie, V.; Kernaleguen, A.E.; Charron, G.; Farhat, N.; Cossette, M.; Mamarbachi, A.M.; Allen, B.G.; Rhéaume, E.; Tardif, J.C. Functional effects of adiponectin on endothelial progenitor cells. Obesity 2011, 19, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, K.; Nareshkumar, R.N.; Sivagurunathan, S.; Raman, R.; Sulochana, K.N.; Chidambaram, S. Anti-angiogenic effect of adiponectin in human primary microvascular and macrovascular endothelial cells. Microvasc. Res. 2019, 122, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Hebbard, L.; Ranscht, B. Multifaceted roles of adiponectin in cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 59–69. [Google Scholar] [CrossRef]
- Li, F.Y.; Cheng, K.K.; Lam, K.S.; Vanhoutte, P.M.; Xu, A. Cross-talk between adipose tissue and vasculature: Role of adiponectin. Acta Physiol. 2011, 203, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Ueta, T.; Jiang, S.; Lin, H.; Wang, Y.; Vavvas, D.; Wen, R.; Chen, Y.G.; Luo, Z. Metformin inhibits ALK1-mediated angiogenesis via activation of AMPK. Oncotarget 2017, 8, 32794–32806. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef]
- Yabushita, H.; Iwasaki, K.; Obayashi, Y.; Wakatsuki, A. Clinicopathological roles of adiponectin and leptin receptors in endometrial carcinoma. Oncol. Lett. 2014, 4, 1109–1117. [Google Scholar] [CrossRef][Green Version]
- Nagaraju, G.P.; Aliya, S.; Alese, O.B. Role of adiponectin in obesity related gastrointestinal carcinogenesis. Cytokine Growth Factor Rev. 2015, 26, 83–93. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, Q.F.; Liu, X.Q.; Guo, Z.J.; Li, C.Y.; Sun, G. MiR-122 targets VEGFC in bladder cancer to inhibit tumor growth and angiogenesis. Am. J. Transl. Res. 2016, 8, 3056–3066. [Google Scholar]
- Li, L.; Zhang, Y.; Qiao, J.; Yang, J.J.; Liu, Z.R. Pyruvate kinase M2 in blood circulation facilitates tumor growth by promoting angiogenesis. J. Biol. Chem. 2014, 289, 25812–25821. [Google Scholar] [CrossRef]
- Manjunathan, R.; Devarajan, N.; Ragunathan, M. Possible Mechanism of Human Recombinant Leptin-Induced VEGF A Synthesis via PI3K/Akt/mTOR/S6 Kinase Signaling Pathway while Inducing Angiogenesis: An Analysis Using Chicken Chorioallantoic Membrane Model. J. Vasc. Res. 2021, 6, 343–360. [Google Scholar] [CrossRef]
- Yang, W.H.; Chen, J.C.; Hsu, K.H.; Lin, C.Y.; Wang, S.W.; Wang, S.J.; Chang, Y.S.; Tang, C.H. Leptin increases VEGF expression and enhances angiogenesis in human chondrosarcoma cells. Biochim. Biophys. Acta. 2014, 1840, 3483–3493. [Google Scholar] [CrossRef] [PubMed]
- Aleffi, S.; Navari, N.; Delogu, W.; Galastri, S.; Novo, E.; Rombouts, K.; Pinzani, M.; Parola, M.; Marra, F. Mammalian target of rapamycin mediates the angiogenic effects of leptin in human hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G210–G219. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Mallardo, M.; Polito, R.; Scialò, F.; Bianco, A.; Daniele, A. Adiponectin and Leptin Exert Antagonizing Effects on HUVEC Tube Formation and Migration Modulating the Expression of CXCL1, VEGF, MMP-2 and MMP-9. Int. J. Mol. Sci. 2021, 22, 7516. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Liu, X.; Nagel, J.M.; Chamberland, J.P.; Diakopoulos, K.N.; Brinkoetter, M.T.; Hatziapostolou, M.; Wu, Y.; Robson, S.C.; Iliopoulos, D.; et al. Salutary effects of adiponectin on colon cancer: In vivo and in vitro studies in mice. Gut. 2013, 62, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Lin, C.Y.; Shih, J.S.; Fong, Y.C.; Wang, S.W.; Li, T.M.; Tang, C.H. Adiponectin promotes VEGF-A-dependent angiogenesis in human chondrosarcoma through PI3K, Akt, mTOR, and HIF-α pathway. Oncotarget 2015, 6, 36746–36761. [Google Scholar] [CrossRef]
- Gao, Q.; Zheng, J.; Yao, X.; Peng, B. Adiponectin inhibits VEGF-A in prostate cancer cells. Tumour Biol. 2015, 36, 4287–4292. [Google Scholar] [CrossRef]
- Gao, Q.; Yao, X.; Zheng, J. MiR-323 Inhibits Prostate Cancer Vascularization Through Adiponectin Receptor. Cell Physiol. Biochem. 2015, 36, 1491–1498. [Google Scholar] [CrossRef]
- Conde, J.; Scotece, M.; López, V.; Gómez, R.; Lago, F.; Pino, J.; Gómez-Reino, J.J.; Gualillo, O. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLoS ONE 2012, 7, e52533. [Google Scholar] [CrossRef]
- Addabbo, F.; Nacci, C.; De Benedictis, L.; Leo, V.; Tarquinio, M.; Quon, M.J.; Montagnani, M. Globular adiponectin counteracts VCAM-1-mediated monocyte adhesion via AdipoR1/NF-κB/COX-2 signaling in human aortic endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1143–E1154. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, S.; Gonzalez-Perez, R.R. Leptin pro-angiogenic signature in breast cancer is linked to IL-1 signalling. Br. J. Cancer 2011, 104, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Gonzalez-Perez, R.R. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS ONE 2011, 6, e21467. [Google Scholar] [CrossRef] [PubMed]
- Colbert, L.S.; Wilson, K.; Kim, S.; Liu, Y.; Oprea-Ilies, G.; Gillespie, C.; Dickson, T.; Newman, G.; Gonzalez-Perez, R.R. NILCO biomarkers in breast cancer from Chinese patients. BMC Cancer 2014, 14, 249. [Google Scholar] [CrossRef] [PubMed]
- Ouh, Y.T.; Cho, H.W.; Lee, J.K.; Choi, S.H.; Choi, H.J.; Hong, J.H. CXC chemokine ligand 1 mediates adiponectin-induced angiogenesis in ovarian cancer. Tumour Biol. 2019, 42, 1010428319842699. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Kim, H.G.; Jin, S.W.; Kim, Y.A.; Khanal, T.; Lee, G.H.; Kim, S.J.; Rhee, S.D.; Chung, Y.C.; Hwang, Y.J.; Jeong, T.C.; et al. Leptin induces CREB-dependent aromatase activation through COX-2 expression in breast cancer cells. Food Chem. Toxicol. 2017, 106, 232–241. [Google Scholar] [CrossRef]
- Garonna, E.; Botham, K.M.; Birdsey, G.M.; Randi, A.M.; Gonzalez-Perez, R.R.; Wheeler-Jones, C.P. Vascular endothelial growth factor receptor-2 couples cyclo-oxygenase-2 with pro-angiogenic actions of leptin on human endothelial cells. PLoS ONE 2011, 6, e18823. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.P.; Jen, C.Y.; Chang, C.C.; Chou, Y.; Lin, H.; Chou, C.M.; Juan, S.H. Mechanisms of adiponectin-mediated COX-2 induction and protection against iron injury in mouse hepatocytes. J. Cell Physiol. 2010, 224, 837–847. [Google Scholar] [CrossRef]
- Napoleone, E.; Cutrone, A.; Cugino, D.; Latella, M.C.; Zurlo, F.; Iacoviello, L.; de Gaetano, G.; Donati, M.B.; Lorenzet, R. Leptin upregulates tissue factor expression in human breast cancer MCF-7 cells. Thromb. Res. 2012, 129, 641–647. [Google Scholar] [CrossRef]
- Denzel, M.S.; Scimia, M.C.; Zumstein, P.M.; Walsh, K.; Ruiz-Lozano, P.; Ranscht, B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J. Clin. Investig. 2010, 120, 4342–4352. [Google Scholar] [CrossRef]
- Mahbouli, S.; Der Vartanian, A.; Ortega, S.; Rougé, S.; Vasson, M.P.; Rossary, A. Leptin induces ROS via NOX5 in healthy and neoplastic mammary epithelial cells. Oncol. Rep. 2017, 38, 3254–3264. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wei, J.; Tang, Y.; Wang, B.; Zhang, Y.; Shi, L.; Guo, J.; Hu, F.; Li, X. Leptin-induced migration and angiogenesis in rheumatoid arthritis is mediated by reactive oxygen species. FEBS Open Bio. 2017, 12, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Inácio Pinto, N.; Carnier, J.; Oyama, L.M.; Otoch, J.P.; Alcântara, P.S.; Tokeshi, F.; Nascimento, C.M. Cancer as a Proinflammatory Environment: Metastasis and Cachexia. Mediat. Inflamm. 2015, 2015, 791060. [Google Scholar] [CrossRef] [PubMed]
- Sobrinho Santos, E.M.; Guimarães, T.A.; Santos, H.O.; Cangussu, L.M.B.; de Jesus, S.F.; Fraga, C.A.C.; Cardoso, C.M.; Santos, S.H.S.; de Paula, A.M.B.; Gomez, R.S.; et al. Leptin acts on neoplastic behavior and expression levels of genes related to hypoxia, angiogenesis, and invasiveness in oral squamous cell carcinoma. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef]
- Choi, E.; Byeon, S.J.; Kim, S.H.; Lee, H.J.; Kwon, H.J.; Ahn, H.; Kim, D.H.; Chang, M.S. Implication of Leptin-Signaling Proteins and Epstein-Barr Virus in Gastric Carcinomas. PLoS ONE 2015, 10, e0130839. [Google Scholar] [CrossRef]
- Ren, H.; Jia, L.; Zhao, T.; Zhang, H.; Chen, J.; Yang, S.; Liu, J.; Yu, M.; Hao, J. Hypoxia inducible factor (HIF)-1α directly activates leptin receptor (Ob-R) in pancreatic cancer cells. Cancer Lett. 2014, 354, 172–180. [Google Scholar] [CrossRef]
- Calgani, A.; Delle Monache, S.; Cesare, P.; Vicentini, C.; Bologna, M.; Angelucci, A. Leptin contributes to long-term stabilization of HIF-1α in cancer cells subjected to oxygen limiting conditions. Cancer Lett. 2016, 379, 1–9. [Google Scholar] [CrossRef]
- Piao, L.; Yu, C.; Xu, W.; Inoue, A.; Shibata, R.; Li, X.; Nan, Y.; Zhao, G.; Wang, H.; Meng, X.; et al. Adiponectin/AdiopR1 signal inactivation contributes to impaired angiogenesis in mice of advanced age. Int. J. Cardiol. 2018, 267, 150–155. [Google Scholar] [CrossRef]
- Tahergorabi, Z.; Khazaei, M. Leptin and its cardiovascular effects: Focus on angiogenesis. Adv. Biomed. Res. 2015, 4, 79. [Google Scholar] [CrossRef]
- Liu, Q.; Qiao, L.; Liang, N.; Xie, J.; Zhang, J.; Deng, G.; Luo, H.; Zhang, J. The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. J. Cell Mol. Med. 2016, 20, 1761–1769. [Google Scholar] [CrossRef]
- Casado, M.E.; Collado-Pérez, R.; Frago, L.M.; Barrios, V. Recent Advances in the Knowledge of the Mechanisms of Leptin Physiology and Actions in Neurological and Metabolic Pathologies. Int. J. Mol. Sci. 2023, 24, 1422. [Google Scholar] [CrossRef]
- Aronis, K.N.; Diakopoulos, K.N.; Fiorenza, C.G.; Chamberland, J.P.; Mantzoros, C.S. Leptin administered in physiological or pharmacological doses does not regulate circulating angiogenesis factors in humans. Diabetologia 2011, 54, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vargas, A.K.; García-Rodríguez, E.; Olea-Flores, M.; Mendoza-Catalán, M.A.; Flores-Alfaro, E.; Navarro-Tito, N. Pro-angiogenic activity and vasculogenic mimicry in the tumor microenvironment by leptin in cancer. Cytokine Growth Factor Rev. 2021, 62, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Geng, Z.; Wang, S.; Yu, Z.; Liu, T.; Guan, S.; Du, S.; Zhu, C. The driving mechanism and targeting value of mimicry between vascular endothelial cells and tumor cells in tumor progression. Biomed. Pharmacother. 2023, 165, 115029. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, J.; Wang, R.; Wang, K.; Xu, Y.; Song, G.; Wu, C.; Yin, Y. Leptin and HER-2 are associated with gastric cancer progression and prognosis of patients. Biomed. Pharmacother. 2012, 66, 419–424. [Google Scholar] [CrossRef]
- Skrypnik, D.; Skrypnik, K.; Suliburska, J.; Bogdański, P. Leptin-VEGF crosstalk in excess body mass and related disorders: A systematic review. Obes. Rev. 2023, 34, e13575. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, Y.; Tian, N.; Yang, J.; Ya, D.; Xiang, W.; Zhou, Z.; Jiang, Y.; Deng, J.; Yang, B.; et al. Leptin Promotes Angiogenesis via Pericyte STAT3 Pathway upon Intracerebral Hemorrhage. Cells 2022, 11, 2755. [Google Scholar] [CrossRef]
- Azoitei, N.; Becher, A.; Steinestel, K.; Rouhi, A.; Diepold, K.; Genze, F.; Simmet, T.; Seufferlein, T. PKM2 promotes tumor angiogenesis by regulating HIF-1α through NF-κB activation. Mol. Cancer 2016, 15, 3. [Google Scholar] [CrossRef]
- Eales, K.L.; Hollinshead, K.E.; Tennant, D.A. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Ding, Y.; Wan, M.; Xu, M. The Role of Adipokines in Pancreatic Cancer. Front. Oncol. 2022, 8, 926230. [Google Scholar] [CrossRef]
- Newman, G.; Gonzalez-Perez, R.R. Leptin-cytokine crosstalk in breast cancer. Mol. Cell Endocrinol. 2014, 382, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Mazor, R.; Alsaigh, T.; Shaked, H.; Altshuler, A.E.; Pocock, E.S.; Kistler, E.B.; Karin, M.; Schmid-Schönbein, G.W. Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. J. Biol. Chem. 2013, 288, 598–607. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, R.R.; Lanier, V.; Newman, G. Leptin’s Pro-Angiogenic Signature in Breast Cancer. Cancers 2013, 5, 1140–1162. [Google Scholar] [CrossRef]
- Guo, S.; Liu, M.; Wang, G.; Torroella-Kouri, M.; Gonzalez-Perez, R.R. Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochim. Biophys. Acta. 2012, 1825, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Nakatsu, M.N.; Chou, W.; Gershon, P.D.; Hughes, C.C. The requirement for fibroblasts in angiogenesis: Fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol. Biol. Cell 2011, 20, 3791–3800. [Google Scholar] [CrossRef]
- Wang, F.T.; Sun, W.; Zhang, J.T.; Fan, Y.Z. Cancer-associated fibroblast regulation of tumor neo-angiogenesis as a therapeutic target in cancer. Oncol. Lett. 2019, 17, 3055–3065. [Google Scholar] [CrossRef]
- Han, G.; Li, Y.; Cao, Y.; Yue, Z.; Zhang, Y.; Wang, L.; Liu, J. Overexpression of leptin receptor in human glioblastoma: Correlation with vasculogenic mimicry and poor prognosis. Oncotarget 2017, 8, 58163–58171. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Yang, H.; Ren, Y.; Yang, Z.; Huang, J.; Li, C.; Xiong, Y.; Yu, B. Distinct roles of ADIPOR1 and ADIPOR2: A pan-cancer analysis. Front. Endocrinol. 2023, 14, 1119534. [Google Scholar] [CrossRef]
- Kiefer, F.; Siekmann, A.F. The role of chemokines and their receptors in angiogenesis. Cell Mol. Life Sci. 2011, 68, 2811–2830. [Google Scholar] [CrossRef] [PubMed]
- Argraves, K.M.; Wilkerson, B.A.; Argraves, W.S. Sphingosine-1-phosphate signaling in vasculogenesis and angiogenesis. World J. Biol. Chem. 2010, 1, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, Y.; Du, W.; Qi, X.; Okamoto, Y.; Takuwa, N.; Yoshioka, K. Roles of sphingosine-1-phosphate signaling in angiogenesis. World J. Biol. Chem. 2010, 10, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; El Buri, A.; Adams, D.R.; Pyne, S. Sphingosine 1-phosphate and cancer. Adv. Biol. Regul. 2018, 68, 97–106. [Google Scholar] [CrossRef]
- Kong, D.-H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef]
- Hu, H.; Han, T.; Zhuo, M.; Wu, L.L.; Yuan, C.; Wu, L.; Lei, W.; Jiao, F.; Wang, L.W. Elevated COX-2 Expression Promotes Angiogenesis Through EGFR/p38-MAPK/Sp1-Dependent Signalling in Pancreatic Cancer. Sci. Rep. 2017, 7, 470. [Google Scholar] [CrossRef]
- Xu, L.; Stevens, J.; Hilton, M.B.; Seaman, S.; Conrads, T.P.; Veenstra, T.D.; Logsdon, D.; Morris, H.; Swing, D.A.; Patel, N.L.; et al. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci. Transl. Med. 2014, 6, 242. [Google Scholar] [CrossRef]
- Kamińska, M.S.; Lubkowska, A.; Panczyk, M.; Walaszek, I.; Grochans, S.; Grochans, E.; Cybulska, A.M. Relationships of Body Mass Index, Relative Fat Mass Index, and Waist Circumference with Serum Concentrations of Parameters of Chronic Inflammation. Nutrients 2023, 15, 2789. [Google Scholar] [CrossRef]
- Akimoto, M.; Maruyama, R.; Kawabata, Y.; Tajima, Y.; Takenaga, K. Antidiabetic adiponectin receptor agonist AdipoRon suppresses tumour growth of pancreatic cancer by inducing RIPK1/ERK-dependent necroptosis. Cell Death Dis. 2018, 9, 804. [Google Scholar] [CrossRef]
- Laria, A.E.; Messineo, S.; Arcidiacono, B.; Varano, M.; Chiefari, E.; Semple, R.K.; Rocha, N.; Russo, D.; Cuda, G.; Gaspari, M.; et al. Secretome Analysis of Hypoxia-Induced 3T3-L1 Adipocytes Uncovers Novel Proteins Potentially Involved in Obesity. Proteomics 2018, 7, e1700260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, J.; Shi, H.; Wang, S.; Yan, Y.; Xu, Q.; Zhou, S.; Zhao, Z.; Mu, Y.; Qian, C.; et al. Adiponectin suppresses tumor growth of nasopharyngeal carcinoma through activating AMPK signaling pathway. J. Transl. Med. 2022, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Richard, S.; Peixoto, E.; Yin, J.; Seelig, D.M.; Carotenuto, P.; Salati, M.; Franco, B.; Roberts, L.R.; Gradilone, S.A. The NAMPT Inhibitor FK866 in Combination with Cisplatin Reduces Cholangiocarcinoma Cells Growth. Cells 2023, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yi, X.; Lu, C.; Wang, Y.; Xiao, Q.; Zhang, L.; Pang, Y.; Guan, X. Study Progression of Apelin/APJ Signaling and Apela in Different Types of Cancer. Front. Oncol. 2021, 11, 658253. [Google Scholar] [CrossRef] [PubMed]
- Siemińska, L.; Borowski, A.; Marek, B.; Nowak, M.; Kajdaniuk, D.; Warakomski, J.; Kos-Kudła, B. Serum concentrations of adipokines in men with prostate cancer and benign prostate hyperplasia. Endokrynol. Pol. 2018, 69, 120–127. [Google Scholar] [CrossRef]
- Xu, C.H.; Yang, Y.; Wang, Y.C.; Yan, J.; Qian, L.H. Prognostic significance of serum chemerin levels in patients with non-small cell lung cancer. Oncotarget 2017, 8, 22483–22489. [Google Scholar] [CrossRef]
- Mulita, F.; Lampropoulos, C.; Kehagias, D.; Verras, G.I.; Tchabashvili, L.; Kaplanis, C.; Liolis, E.; Iliopoulos, F.; Perdikaris, I.; Kehagias, I. Long-term nutritional deficiencies following sleeve gastrectomy: A 6-year single-centre retrospective study. Prz. Menopauzalny 2021, 20, 170–176. [Google Scholar] [CrossRef]
- Wilson, R.B.; Lathigara, D.; Kaushal, D. Systematic Review and Meta-Analysis of the Impact of Bariatric Surgery on Future Cancer Risk. Int. J. Mol. Sci. 2023, 24, 6192. [Google Scholar] [CrossRef]
- Brown, K.A.; Scherer, P.E. Update on Adipose Tissue and Cancer. Endocr. Rev. 2023, bnad015. [Google Scholar] [CrossRef]
- Abdul-Ghafar, J.; Oh, S.S.; Park, S.M.; Wairagu, P.; Lee, S.N.; Jeong, Y.; Eom, M.; Yong, S.J.; Jung, S.H. Expression of adiponectin receptor 1 is indicative of favorable prognosis in non-small cell lung carcinoma. Tohoku J. Exp. Med. 2013, 229, 153–162. [Google Scholar] [CrossRef]
- Giordano, C.; Chemi, F.; Panza, S.; Barone, I.; Bonofiglio, D.; Lanzino, M.; Cordella, A.; Campana, A.; Hashim, A.; Rizza, P.; et al. Leptin as a mediator of tumor-stromal interactions promotes breast cancer stem cell activity. Oncotarget 2016, 7, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
| Component | Adipokine | Cancer Types | Effect | Mechanisms | References |
|---|---|---|---|---|---|
| TME | ↑ Leptin | [64] | |||
| ↓ Adiponectin | [69] | ||||
| CAFs | Leptin | Breast cancer | ↑ Proliferation ↑ Migration ↑ Invasion | [9,72] | |
| NSCLC | ↑ Malignancy | [73] | |||
| Pancreatic cancer | ↑ Invasion | [74] | |||
| Adiponectin | Colon cancer | ↑ Angiogenesis ↑ Tumor growth ↑ Proliferation ↑ Migration ↑ Invasion | [75] [76] | ||
| TAMs | Leptin | Breast cancer | ↑ Malignancy ↑ Tumor growth ↑ Progression | [77,78,79] | |
| Melanoma | ↑ Metastasis | [80] | |||
| Gallbladder cancer | ↑ Invasion ↑ Migration | [81] | |||
| Colorectal cancer | ↑ Tumor growth | [82] | |||
| Adiponectin | Melanoma | ↓ Tumor growth | [83] | ||
| Lung cancer | ↓ Tumor growth | [83] | |||
| Rhabdomyosarcoma | ↓ Tumor growth | [84] | |||
| MMPs | Leptin | Gastric cancer | ↑ Invasion ↑ Metastasis | MMP-1 | [85] |
| Breast cancer | ↑ Progression | MMP-2 MMP-9 | [64,86,87] | ||
| Oesophageal cancer | ↑ Invasion | MMP-2 MMP-9 | [88] | ||
| Gallbladder cancer | ↑ Metastasis | MMP-3 MMP-9 | [89] | ||
| Ovarian cancer | ↑ Migration ↑ Invasion | MMP-7 | [90] | ||
| Colon cancer | ↑ Progression | MMP-7 | [91] | ||
| Pancreatic cancer | ↑ Migration ↑ Invasion | MMP-13 | [92] | ||
| Adiponectin | NSCLC | ↑ Invasion | MMP-1 MMP-2 MMP-9 MMP-14 | [93] | |
| RCC | ↓ Tumor growth ↓ Metastasis ↓ Angiogenesis | MMP-2 MMP-9 | [94] | ||
| Liver cancer | ↓ Tumor growth ↓ Metastasis | MMP-9 | [95] | ||
| Oesophageal cancer | ↓ Invasion | MMP-2 MMP-9 | [88] | ||
| Angiogenic Process or Factor | Adipokine | Mechanisms or Involved Intracellular Signaling Pathways | Reference |
|---|---|---|---|
| Proliferation, migration and differentiation of endothelial cells | Leptin | JAK2/STAT3 JAK2/STAT3/SOCS3 NF-κB ERK1/2 ERK2 Akt Wnt PI3K COX-2 CACs, Src kinase and integrin αvβ5 | [4,6,12,190,191,192,193] [194] [195,196] [195] [12] [193,197] [197] [12] [12] [198] |
| Adiponectin | STAT3 AMPK Akt Ras/ERK1/2 MAPK eNOS/NO PI3K The cascade activation of caspase-8, -9 and -3 | [11,154,190,199,200,201,202] [36] [54,59,75,125,189,203,204,205,206] [8,75,125,189,203] [203] [189,203] [8,189,203,204] [75] [54,207,208] | |
| VEGF | Leptin | MAPK, PI3K JNK, p38 MAPK, PKC PI3K/AKT/mTOR/S6 kinase MAPK (p38, ERK and JNK) miR-122/PKM2 Akt and Wnt mTOR JAK2/PI3K/ERK/mTOR | [6,10,190,191,192,197,209,210] [196] [196] [211] [212] [156] [197] [213] [12] |
| Adiponectin | AMPK-Akt ROCK/IP10/angiopoietin 1/MMP-9/VEGF PI3K/Akt/m-TOR/HIF-1α AMPK/TSC2 miR-323 | [75,214,215] [94,125,190] [95] [216] [217] [218] | |
| FGF | Leptin | [10,191,192] | |
| Adiponectin | [6] | ||
| PDGF | Leptin | Akt and Wnt | [197] |
| LIF | Leptin | [12] | |
| VCAM | Leptin | [219] | |
| Adiponectin | NF-kB/COX-2 | [219,220] | |
| IL-1 | Leptin | JAK2/STAT3 MAPK/ERK1/2 PI3K/Akt1 PKC p38 JNK NF-kB | [221,222,223] [221,222,223] [221,222,223] [221] [221,222,223] [221,222,223] [221,222,223] |
| IL-1β | Leptin | JAK2/PI3K/ERK/mTOR | [12] |
| IL-6 | Leptin | [10] | |
| Adiponectin | NF-κB/cAMP | [11,189] | |
| IL-12 | Adiponectin | [215] | |
| CD31 | Leptin | Akt and Wnt | [154] [197] |
| Adiponectin | [215] | ||
| CD144 | Leptin | Akt and Wnt | [197] |
| CXCL1 | Adiponectin | [75,214,224] | |
| CCR2 | Adiponectin | [214] | |
| CXCR4 | Adiponectin | [214] | |
| S1P | Adiponectin | Ceramidase activity | [54,225] |
| COX-2 | Leptin | p38MAPK/Akt/COX-2 | [226] [227] |
| Adiponectin | SphK-1/COX-2 NF-kB/COX-2 PPARα/COX-2 | [56] [219,220] [228] | |
| TF | Leptin | [229] | |
| T-cadherin | Adiponectin | [36,54,199,203,214,230] | |
| ROS | Leptin | [231,232] | |
| Hypoxia | Leptin | HIF-1α | [233,234,235,236,237] [156,210] |
| Adiponectin | mTOR/HIF-1 PPARγ/PGC-1α | [54,214] [94] [238] | |
| MMP-1 | Leptin | [85] | |
| Adiponectin | [93,127] | ||
| MMP-2 | Leptin | [64,86,87,88,173,239] | |
| Adiponectin | [93,94,125,127,214] | ||
| MMP-7 | Leptin | [90,91] | |
| MMP-9 | Leptin | [64,86,87,88,173,239] | |
| Adiponectin | ROCK/IP10/angiopoietin | [75,93,94,125,126,127,214] [95] | |
| MMP-14 | Leptin | [120] | |
| Adiponectin | [93] | ||
| TIMP1 | Adiponectin | [42,129] | |
| EMT | Leptin | [240] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocian-Jastrzębska, A.; Malczewska-Herman, A.; Kos-Kudła, B. Role of Leptin and Adiponectin in Carcinogenesis. Cancers 2023, 15, 4250. https://doi.org/10.3390/cancers15174250
Bocian-Jastrzębska A, Malczewska-Herman A, Kos-Kudła B. Role of Leptin and Adiponectin in Carcinogenesis. Cancers. 2023; 15(17):4250. https://doi.org/10.3390/cancers15174250
Chicago/Turabian StyleBocian-Jastrzębska, Agnes, Anna Malczewska-Herman, and Beata Kos-Kudła. 2023. "Role of Leptin and Adiponectin in Carcinogenesis" Cancers 15, no. 17: 4250. https://doi.org/10.3390/cancers15174250
APA StyleBocian-Jastrzębska, A., Malczewska-Herman, A., & Kos-Kudła, B. (2023). Role of Leptin and Adiponectin in Carcinogenesis. Cancers, 15(17), 4250. https://doi.org/10.3390/cancers15174250






