Beta-Transducin Repeats-Containing Proteins as an Anticancer Target
Abstract
Simple Summary
Abstract
1. Introduction
2. SCFβ-TrCP Complex and Its Substrates
3. Limited Processing by β-TrCP
| Substrate (Also Known as) | Prior Phosphorylation by | Biological Functions by β-TrCP-Mediated Processing | β-TrCP Paralogue | Role in Cancer | Ref. |
|---|---|---|---|---|---|
| GLI3 | CK1/GSK3 |
| β-TrCP1 | suppressive | [78] |
| NFKB1 (NF-κB p105) | IKKβ |
| β-TrCP1/2 | promoting | [68] |
| NFKB2 (NF-κB p100) | IKKα |
| β-TrCP1 | promoting | [75,79] |
| SP1 | Cyclin A/CDK2 |
| not specified | promoting | [77] |
4. Extra Roles of β-TrCP
4.1. Role of β-TrCP in Regulation of Transcription
4.2. Stabilization of Oncogene Products by β-TrCP
5. Association of β-TrCP with Cancer
6. Regulation of β-TrCP Activity
6.1. Upstream Effectors of β-TrCP Activity
6.2. Modulation of β-TrCP Activity by Protein–Protein Interactions
6.3. Modulation of β-TrCP Activity by Viral Proteins
| Viral Effector | Virus | β-TrCP Isoform | Biological Consequences | Role in Cancer | Ref. |
|---|---|---|---|---|---|
| A49 | poxvirus | not specified |
| suppressive | [198] |
| E1A-5/E1A-12 | adenovirus | not specified |
| promoting | [199] |
| E17 | human papilloma virus 16 | not specified |
| promoting | [206] |
| EBV-miR-BART10-3p | Ebstein–Barr virus | β-TrCP1 |
| promoting | [207] |
| NS1 | influenza A virus | β-TrCP1 |
| - | [208] |
| NSP1 | rotavirus | not specified |
| promoting | [201] |
| ORF6 | simian varicella virus and varicella-zoster virus | not specified |
| - | [204] |
6.4. Modulation of β-TrCP Activity by Subcellular Localization
7. Targeting β-TrCP in Cancer
7.1. β-TrCP as a Target for Cancer Treatment
| β-TrCP Paralogue | Silencing | Cancer Cells | Effect of β-TrCP Inhibition |
|---|---|---|---|
| β-TrCP1 | siRNA | TNBC | reduces the proliferation of TNBC cells [27] |
| β-TrCP1 | shRNA | Leukemia | reverses JAK2-inhibitor-mediated β-catenin downregulation [168] |
| β-TrCP1/2 | siRNA | NSCLC | induces apoptosis through upregulation of BimEL [217] |
| β-TrCP1/2 | shRNA | Prostate cancer | inhibits prostate cancer cell growth both in vitro and in vivo by inducing the aryl hydrocarbon receptor (AhR) [215] |
7.2. Small Molecule Compounds That Modulate β-TrCP Activity
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schapira, M.; Calabrese, M.F.; Bullock, A.N.; Crews, C.M. Targeted Protein Degradation: Expanding the Toolbox. Nat. Rev. Drug Discov. 2019, 18, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, J.; Liu, Y.; Xia, J.; Li, Y.; Wang, Z.P.; Wei, W. PROTACs: A Novel Strategy for Cancer Therapy. Semin. Cancer Biol. 2020, 67, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yoon, H.; Xiong, Y.; Dixon-Clarke, S.E.; Nowak, R.P.; Fischer, E.S. Targeted Protein Degradation as a Powerful Research Tool in Basic Biology and Drug Target Discovery. Nat. Struct. Mol. Biol. 2020, 27, 605–614. [Google Scholar] [CrossRef]
- Li, X.; Song, Y. Proteolysis-Targeting Chimera (PROTAC) for Targeted Protein Degradation and Cancer Therapy. J. Hematol. Oncol. 2020, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Alabi, S.; Crews, C. Major Advances in Targeted Protein Degradation: PROTACs, LYTACs, and MADTACs. J. Biol. Chem. 2021, 296, 100647. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. The PROTAC Gold Rush. Nat. Biotechnol. 2021, 40, 12–16. [Google Scholar] [CrossRef]
- Buckley, D.L.; Raina, K.; Darricarrere, N.; Hines, J.; Gustafson, J.L.; Smith, I.E.; Miah, A.H.; Harling, J.D.; Crews, C.M. HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins. Acs Chem. Biol. 2015, 10, 1831–1837. [Google Scholar] [CrossRef]
- Ghidini, A.; Cléry, A.; Halloy, F.; Allain, F.H.T.; Hall, J. RNA-PROTACs: Degraders of RNA-Binding Proteins. Angew. Chem. Int. Ed. 2021, 60, 3163–3169. [Google Scholar] [CrossRef]
- Samarasinghe, K.T.G.; Jaime-Figueroa, S.; Burgess, M.; Nalawansha, D.A.; Dai, K.; Hu, Z.; Bebenek, A.; Holley, S.A.; Crews, C.M. Targeted Degradation of Transcription Factors by TRAFTACs: TRAnscription Factor TArgeting Chimeras. Cell Chem. Biol. 2021, 28, 648–661.e5. [Google Scholar] [CrossRef]
- Bondeson, D.P.; Mares, A.; Smith, I.E.D.; Ko, E.; Campos, S.; Miah, A.H.; Mulholland, K.E.; Routly, N.; Buckley, D.L.; Gustafson, J.L.; et al. Catalytic in Vivo Protein Knockdown by Small-Molecule PROTACs. Nat. Chem. Biol. 2015, 11, 611–617. [Google Scholar] [CrossRef]
- Galdeano, C. Drugging the Undruggable: Targeting Challenging E3 Ligases for Personalized Medicine. Future Med. Chem. 2017, 9, 347–350. [Google Scholar] [CrossRef]
- Huang, X.; Dixit, V.M. Drugging the Undruggables: Exploring the Ubiquitin System for Drug Development. Cell Res. 2016, 26, 484–498. [Google Scholar] [CrossRef]
- Bhaduri, U.; Merla, G. Ubiquitination, Biotech Startups, and the Future of TRIM Family Proteins: A TRIM-Endous Opportunity. Cells 2021, 10, 1015. [Google Scholar] [CrossRef]
- Winston, J.T.; Koepp, D.M.; Zhu, C.; Elledge, S.J.; Harper, J.W. A Family of Mammalian F-Box Proteins. Curr. Biol. 1999, 9, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
- Frescas, D.; Pagano, M. Deregulated Proteolysis by the F-Box Proteins SKP2 and β-TrCP: Tipping the Scales of Cancer. Nat. Rev. Cancer 2008, 8, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Tekcham, D.S.; Chen, D.; Liu, Y.; Ling, T.; Zhang, Y.; Chen, H.; Wang, W.; Otkur, W.; Qi, H.; Xia, T.; et al. F-Box Proteins and Cancer: An Update from Functional and Regulatory Mechanism to Therapeutic Clinical Prospects. Theranostics 2020, 10, 4150–4167. [Google Scholar] [CrossRef]
- Fuchs, S.Y.; Spiegelman, V.S.; Kumar, K.G.S. The Many Faces of β-TrCP E3 Ubiquitin Ligases: Reflections in the Magic Mirror of Cancer. Oncogene 2004, 23, 2028–2036. [Google Scholar] [CrossRef]
- Guardavaccaro, D.; Frescas, D.; Dorrello, N.V.; Peschiaroli, A.; Multani, A.S.; Cardozo, T.; Lasorella, A.; Iavarone, A.; Chang, S.; Hernando, E.; et al. Control of Chromosome Stability by the β-TrCP–REST–Mad2 Axis. Nature 2008, 452, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and Structure-Based Prediction of Eukaryotic Protein Phosphorylation Sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef]
- Blom, N.; Sicheritz-Pontén, T.; Gupta, R.; Gammeltoft, S.; Brunak, S. Prediction of Post-translational Glycosylation and Phosphorylation of Proteins from the Amino Acid Sequence. Proteomics 2004, 4, 1633–1649. [Google Scholar] [CrossRef]
- Chiaur, D.S.; Murthy, S.; Cenciarelli, C.; Parks, W.; Loda, M.; Inghirami, G.; Demetrick, D.; Pagano, M. Five Human Genes Encoding F-Box Proteins: Chromosome Mapping and Analysis in Human Tumors. Cytogenet. Genome Res. 2000, 88, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, A.V.; Almeida, T.A.; Zhao, G.; Chess, E.; Shih, I.; Buhler, K.; Pienta, K.; Rubin, M.A.; Vessella, R.; Papadopoulos, N. APC/CTNNB1 (β-catenin) Pathway Alterations in Human Prostate Cancers. Genes Chromosomes Cancer 2002, 34, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, J.; Wiemann, S.; Scheurlen, W.; Korn, B.; Hayashi, Y.; Wilgenbus, K.K.; von Deimling, A.; Poustka, A. DMBT1, a New Member of the SRCR Superfamily, on Chromosome 10q25.3–26.1 Is Deleted in Malignant Brain Tumours. Nat. Genet. 1997, 17, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Michalkiewicz, E.; Boyett, J.M.; Sublett, J.E.; Entrekin, R.E.; Ragsdale, S.T.; Valentine, M.B.; Behm, F.G.; Li, H.; Heideman, R.L.; et al. Extensive Genomic Abnormalities in Childhood Medulloblastoma by Comparative Genomic Hybridization. Cancer Res. 1997, 57, 4042–4047. [Google Scholar]
- Koike, J.; Sagara, N.; Kirikoshi, H.; Takagi, A.; Miwa, T.; Hirai, M.; Katoh, M. Molecular Cloning and Genomic Structure of the ΒTRCP2 Gene on Chromosome 5q35.1. Biochem. Biophys. Res. Commun. 2000, 269, 103–109. [Google Scholar] [CrossRef]
- Bi, Y.; Cui, D.; Xiong, X.; Zhao, Y. The Characteristics and Roles of β-TrCP1/2 in Carcinogenesis. FEBS J. 2020, 288, 3351–3374. [Google Scholar] [CrossRef]
- Yi, Y.W.; Kang, H.J.; Bae, E.J.; Oh, S.; Seong, Y.-S.; Bae, I. β-TrCP1 Degradation Is a Novel Action Mechanism of PI3K/MTOR Inhibitors in Triple-Negative Breast Cancer Cells. Exp. Mol. Med. 2015, 47, e143. [Google Scholar] [CrossRef]
- Suzuki, H.; Chiba, T.; Suzuki, T.; Fujita, T.; Ikenoue, T.; Omata, M.; Furuichi, K.; Shikama, H.; Tanaka, K. Homodimer of Two F-Box Proteins ΒTrCP1 or ΒTrCP2 Binds to IκBα for Signal-Dependent Ubiquitination*. J. Biol. Chem. 2000, 275, 2877–2884. [Google Scholar] [CrossRef]
- Yaron, A.; Hatzubai, A.; Davis, M.; Lavon, I.; Amit, S.; Manning, A.M.; Andersen, J.S.; Mann, M.; Mercurio, F.; Ben-Neriah, Y. Identification of the Receptor Component of the IκBα–Ubiquitin Ligase. Nature 1998, 396, 590–594. [Google Scholar] [CrossRef]
- Suzuki, H.; Chiba, T.; Kobayashi, M.; Takeuchi, M.; Suzuki, T.; Ichiyama, A.; Ikenoue, T.; Omata, M.; Furuichi, K.; Tanaka, K. IκBα Ubiquitination Is Catalyzed by an SCF-like Complex Containing Skp1, Cullin-1, and Two F-Box/WD40-Repeat Proteins, ΒTrCP1 and ΒTrCP2. Biochem. Biophys. Res. Commun. 1999, 256, 127–132. [Google Scholar] [CrossRef]
- Strack, P.; Caligiuri, M.; Pelletier, M.; Boisclair, M.; Theodoras, A.; Beer-Romero, P.; Glass, S.; Parsons, T.; Copeland, R.A.; Auger, K.R.; et al. SCFβ-TRCP and Phosphorylation Dependent Ubiquitination of IκBα Catalyzed by Ubc3 and Ubc4. Oncogene 2000, 19, 3529–3536. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Fuchs, S.Y.; Chen, A.; Tan, P.; Gomez, C.; Ronai, Z.; Pan, Z.-Q. The SCFHOS/β-TRCP-ROC1 E3 Ubiquitin Ligase Utilizes Two Distinct Domains within CUL1 for Substrate Targeting and Ubiquitin Ligation. Mol. Cell. Biol. 2000, 20, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, D.; Craig, K.L.; Tyers, M.; Elledge, S.J.; Harper, J.W. F-Box Proteins Are Receptors That Recruit Phosphorylated Substrates to the SCF Ubiquitin-Ligase Complex. Cell 1997, 91, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.M.R.; Correll, C.C.; Kaplan, K.B.; Deshaies, R.J. A Complex of Cdc4p, Skp1p, and Cdc53p/Cullin Catalyzes Ubiquitination of the Phosphorylated CDK Inhibitor Sic1p. Cell 1997, 91, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Fuchs, S.Y.; Chen, A.; Wu, K.; Gomez, C.; Ronai, Z.; Pan, Z.-Q. Recruitment of a ROC1–CUL1 Ubiquitin Ligase by Skp1 and HOS to Catalyze the Ubiquitination of IκBα. Mol. Cell 1999, 3, 527–533. [Google Scholar] [CrossRef]
- Ohta, T.; Michel, J.J.; Schottelius, A.J.; Xiong, Y. ROC1, a Homolog of APC11, Represents a Family of Cullin Partners with an Associated Ubiquitin Ligase Activity. Mol. Cell 1999, 3, 535–541. [Google Scholar] [CrossRef]
- Furukawa, M.; Zhang, Y.; McCarville, J.; Ohta, T.; Xiong, Y. The CUL1 C-Terminal Sequence and ROC1 Are Required for Efficient Nuclear Accumulation, NEDD8 Modification, and Ubiquitin Ligase Activity of CUL1. Mol. Cell. Biol. 2000, 20, 8185–8197. [Google Scholar] [CrossRef]
- Duda, D.M.; Olszewski, J.L.; Tron, A.E.; Hammel, M.; Lambert, L.J.; Waddell, M.B.; Mittag, T.; DeCaprio, J.A.; Schulman, B.A. Structure of a Glomulin-RBX1-CUL1 Complex: Inhibition of a RING E3 Ligase through Masking of Its E2-Binding Surface. Mol. Cell 2012, 47, 371–382. [Google Scholar] [CrossRef]
- Kitagawa, M.; Hatakeyama, S.; Shirane, M.; Matsumoto, M.; Ishida, N.; Hattori, K.; Nakamichi, I.; Kikuchi, A.; Nakayama, K.; Nakayama, K. An F-box Protein, FWD1, Mediates Ubiquitin-dependent Proteolysis of β-catenin. EMBO J. 1999, 18, 2401–2410. [Google Scholar] [CrossRef]
- Shirane, M.; Hatakeyama, S.; Hattori, K.; Nakayama, K.; Nakayama, K. Common Pathway for the Ubiquitination of IκBα, IκBβ, and IκBε Mediated by the F-Box Protein FWD1*. J. Biol. Chem. 1999, 274, 28169–28174. [Google Scholar] [CrossRef]
- Amir, R.E.; Haecker, H.; Karin, M.; Ciechanover, A. Mechanism of Processing of the NF-Kappa B2 P100 Precursor: Identification of the Specific Polyubiquitin Chain-Anchoring Lysine Residue and Analysis of the Role of NEDD8-Modification on the SCF(Beta-TrCP) Ubiquitin Ligase. Oncogene 2004, 23, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Busino, L.; Donzelli, M.; Chiesa, M.; Guardavaccaro, D.; Ganoth, D.; Dorrello, N.V.; Hershko, A.; Pagano, M.; Draetta, G.F. Degradation of Cdc25A by β-TrCP during S Phase and in Response to DNA Damage. Nature 2003, 426, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Shirogane, T.; Xu, L.; Nalepa, G.; Qin, J.; Elledge, S.J.; Harper, J.W. SCFβ-TRCP Links Chk1 Signaling to Degradation of the Cdc25A Protein Phosphatase. Genes Dev. 2003, 17, 3062–3074. [Google Scholar] [CrossRef] [PubMed]
- Lassot, I.; Ségéral, E.; Berlioz-Torrent, C.; Durand, H.; Groussin, L.; Hai, T.; Benarous, R.; Margottin-Goguet, F. ATF4 Degradation Relies on a Phosphorylation-Dependent Interaction with the SCFβTrCPUbiquitin Ligase. Mol. Cell. Biol. 2001, 21, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Lang, V.; Janzen, J.; Fischer, G.Z.; Soneji, Y.; Beinke, S.; Salmeron, A.; Allen, H.; Hay, R.T.; Ben-Neriah, Y.; Ley, S.C. ΒTrCP-Mediated Proteolysis of NF-ΚB1 P105 Requires Phosphorylation of P105 Serines 927 and 932. Mol. Cell. Biol. 2003, 23, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Kroll, M.; Margottin, F.; Kohl, A.; Renard, P.; Durand, H.; Concordet, J.-P.; Bachelerie, F.; Arenzana-Seisdedos, F.; Benarous, R. Inducible Degradation of IκBα by the Proteasome Requires Interaction with the F-Box Protein h-ΒTrCP*. J. Biol. Chem. 1999, 274, 7941–7945. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.; Jiang, J.; Chen, Z.J. Signal-Induced Ubiquitination of IκBα by the F-Box Protein Slimb/β-TrCP. Genes Dev. 1999, 13, 284–294. [Google Scholar] [CrossRef]
- Winston, J.T.; Strack, P.; Beer-Romero, P.; Chu, C.Y.; Elledge, S.J.; Harper, J.W. The SCFβ-TRCP–Ubiquitin Ligase Complex Associates Specifically with Phosphorylated Destruction Motifs in IκBα and β-Catenin and Stimulates IκBα Ubiquitination in Vitro. Genes Dev. 1999, 13, 270–283. [Google Scholar] [CrossRef]
- Fukuchi, M.; Imamura, T.; Chiba, T.; Ebisawa, T.; Kawabata, M.; Tanaka, K.; Miyazono, K. Ligand-Dependent Degradation of Smad3 by a Ubiquitin Ligase Complex of ROC1 and Associated Proteins. Mol. Biol. Cell 2001, 12, 1431–1443. [Google Scholar] [CrossRef]
- Lo, R.S.; Massagué, J. Ubiquitin-Dependent Degradation of TGF-β-Activated Smad2. Nat. Cell Biol. 1999, 1, 472–478. [Google Scholar] [CrossRef]
- Kanemori, Y.; Uto, K.; Sagata, N. β-TrCP Recognizes a Previously Undescribed Nonphosphorylated Destruction Motif in Cdc25A and Cdc25B Phosphatases. Proc. Natl. Acad. Sci. USA 2005, 102, 6279–6284. [Google Scholar] [CrossRef] [PubMed]
- Margottin, F.; Bour, S.P.; Durand, H.; Selig, L.; Benichou, S.; Richard, V.; Thomas, D.; Strebel, K.; Benarous, R. A Novel Human WD Protein, h-ΒTrCP, That Interacts with HIV-1 Vpu Connects CD4 to the ER Degradation Pathway through an F-Box Motif. Mol. Cell 1998, 1, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Bour, S.; Perrin, C.; Akari, H.; Strebel, K. The Human Immunodeficiency Virus Type 1 Vpu Protein Inhibits NF-κB Activation by Interfering with βTrCP-Mediated Degradation of IκB. J. Biol. Chem. 2001, 276, 15920–15928. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U.; Henklein, P.; Boldyreff, B.; Wingender, E.; Strebel, K.; Porstmann, T. The Human Immunodeficiency Virus Type 1 Encoded Vpu Protein Is Phosphorylated by Casein Kinase-2 (CK-2) at Positions Ser52 and Ser56 within a Predicted Alpha-Helix-Turn-Alpha-Helix-Motif. J. Mol. Biol. 1994, 236, 16–25. [Google Scholar] [CrossRef]
- Belaïdouni, N.; Marchal, C.; Benarous, R.; Besnard-Guérin, C. Involvement of the ΒTrCP in the Ubiquitination and Stability of the HIV-1 Vpu Protein. Biochem. Biophys. Res. Commun. 2007, 357, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.; Concordet, J.-P.; Lassot, I.; Albert, I.; del los Santos, R.; Durand, H.; Perret, C.; Rubinfeld, B.; Margottin, F.; Benarous, R.; et al. The F-Box Protein β-TrCP Associates with Phosphorylated β-Catenin and Regulates Its Activity in the Cell. Curr. Biol. 1999, 9, 207–211. [Google Scholar] [CrossRef]
- Fuchs, S.Y.; Chen, A.; Xiong, Y.; Pan, Z.-Q.; Ronai, Z. HOS, a Human Homolog of Slimb, Forms an SCF Complex with Skp1 and Cullin1 and Targets the Phosphorylation-Dependent Degradation of IκB and β-Catenin. Oncogene 1999, 18, 2039–2046. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation Meets Ubiquitination: The Control of NF-ΚB Activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Mercurio, F.; DiDonato, J.A.; Rosette, C.; Karin, M. P105 and P98 Precursor Proteins Play an Active Role in NF-Kappa B-Mediated Signal Transduction. Genes Dev. 1993, 7, 705–718. [Google Scholar] [CrossRef]
- Rice, N.R.; MacKichan, M.L.; Israël, A. The Precursor of NF-Kappa B P50 Has I Kappa B-like Functions. Cell 1992, 71, 243–253. [Google Scholar] [CrossRef]
- Salmerón, A.; Janzen, J.; Soneji, Y.; Bump, N.; Kamens, J.; Allen, H.; Ley, S.C. Direct Phosphorylation of NF-ΚB1 P105 by the IκB Kinase Complex on Serine 927 Is Essential for Signal-Induced P105 Proteolysis*. J. Biol. Chem. 2001, 276, 22215–22222. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.G.S.; Tang, W.; Ravindranath, A.K.; Clark, W.A.; Croze, E.; Fuchs, S.Y. SCFHOS Ubiquitin Ligase Mediates the Ligand-induced Down-regulation of the Interferon-α Receptor. EMBO J. 2003, 22, 5480–5490. [Google Scholar] [CrossRef] [PubMed]
- van Kerkhof, P.; Putters, J.; Strous, G.J. The Ubiquitin Ligase SCF(ΒTrCP) Regulates the Degradation of the Growth Hormone Receptor*. J. Biol. Chem. 2007, 282, 20475–20483. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Pavlish, O.A.; Spiegelman, V.S.; Parkhitko, A.A.; Fuchs, S.Y. Interaction of Epstein-Barr Virus Latent Membrane Protein 1 with SCFHOS/β-TrCP E3 Ubiquitin Ligase Regulates Extent of NF-ΚB Activation. J. Biol. Chem. 2003, 278, 48942–48949. [Google Scholar] [CrossRef] [PubMed]
- Besnard-Guerin, C.; Belaïdouni, N.; Lassot, I.; Segeral, E.; Jobart, A.; Marchal, C.; Benarous, R. HIV-1 Vpu Sequesters β-Transducin Repeat-Containing Protein (ΒTrCP) in the Cytoplasm and Provokes the Accumulation of β-Catenin and Other SCFβTrCP Substrates*. J. Biol. Chem. 2004, 279, 788–795. [Google Scholar] [CrossRef]
- Noubissi, F.K.; Elcheva, I.; Bhatia, N.; Shakoori, A.; Ougolkov, A.; Liu, J.; Minamoto, T.; Ross, J.; Fuchs, S.Y.; Spiegelman, V.S. CRD-BP Mediates Stabilization of ΒTrCP1 and c-Myc MRNA in Response to β-Catenin Signalling. Nature 2006, 441, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Qian, G.; Zhang, S.; Zheng, H.; Fan, S.; Lesinski, G.B.; Owonikoko, T.K.; Ramalingam, S.S.; Sun, S.-Y. Inhibition of MTOR Complex 1/P70 S6 Kinase Signaling Elevates PD-L1 Levels in Human Cancer Cells through Enhancing Protein Stabilization Accompanied with Enhanced β-TrCP Degradation. Oncogene 2019, 38, 6270–6282. [Google Scholar] [CrossRef] [PubMed]
- Orian, A.; Gonen, H.; Bercovich, B.; Fajerman, I.; Eytan, E.; Israël, A.; Mercurio, F.; Iwai, K.; Schwartz, A.L.; Ciechanover, A. SCFβ-TrCP Ubiquitin Ligase-mediated Processing of NF-κB p 105 Requires Phosphorylation of Its C-terminus by IκB Kinase. EMBO J. 2000, 19, 2580–2591. [Google Scholar] [CrossRef]
- Orian, A.; Schwartz, A.L.; Israël, A.; Whiteside, S.; Kahana, C.; Ciechanover, A. Structural Motifs Involved in Ubiquitin-Mediated Processing of the NF-ΚB Precursor P105: Roles of the Glycine-Rich Region and a Downstream Ubiquitination Domain. Mol. Cell. Biol. 1999, 19, 3664–3673. [Google Scholar] [CrossRef]
- Lin, L.; Ghosh, S. A Glycine-Rich Region in NF-KappaB P105 Functions as a Processing Signal for the Generation of the P50 Subunit. Mol. Cell. Biol. 1996, 16, 2248–2254. [Google Scholar] [CrossRef]
- Ciechanover, A.; Gonen, H.; Bercovich, B.; Cohen, S.; Fajerman, I.; Israël, A.; Mercurio, F.; Kahana, C.; Schwartz, A.L.; Iwai, K.; et al. Mechanisms of Ubiquitin-Mediated, Limited Processingof the NF-ΚB1 Precursor Protein P105. Biochimie 2001, 83, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Iwai, K.; Ciechanover, A. The NEDD8 Pathway Is Essential for SCFβ-TrCP-Mediated Ubiquitination and Processing of the NF-ΚB Precursor P105*. J. Biol. Chem. 2002, 277, 23253–23259. [Google Scholar] [CrossRef]
- Fong, A.; Sun, S.-C. Genetic Evidence for the Essential Role of β-Transducin Repeat-Containing Protein in the Inducible Processing of NF-ΚB2/P100*. J. Biol. Chem. 2002, 277, 22111–22114. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Achbert-Weiner, H.; Ciechanover, A. Dual Effects of IκB Kinase β-Mediated Phosphorylation on P105 Fate: SCFβ-TrCP-Dependent Degradation and SCFβ-TrCP-Independent Processing. Mol. Cell. Biol. 2004, 24, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Fong, A.; Sun, S.-C. Induction of P100 Processing by NF-ΚB-Inducing Kinase Involves Docking IκB Kinase α (IKKα) to P100 and IKKα-Mediated Phosphorylation*. J. Biol. Chem. 2004, 279, 30099–30105. [Google Scholar] [CrossRef]
- Spengler, M.L.; Brattain, M.G. Sumoylation Inhibits Cleavage of Sp1 N-Terminal Negative Regulatory Domain and Inhibits Sp1-Dependent Transcription*. J. Biol. Chem. 2006, 281, 5567–5574. [Google Scholar] [CrossRef]
- Spengler, M.L.; Guo, L.-W.; Brattain, M.G. Phosphorylation Mediates Sp1 Coupled Activities of Proteolytic Processing, Desumoylation and Degradation. Cell Cycle 2008, 7, 623–630. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y. Evidence for the Direct Involvement of ΒTrCP in Gli3 Protein Processing. Proc. Natl. Acad. Sci. USA 2006, 103, 33–38. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, M.; Sun, S.-C. β-TrCP Binding and Processing of NF-ΚB2/P100 Involve Its Phosphorylation at Serines 866 and 870. Cell Signal. 2006, 18, 1309–1317. [Google Scholar] [CrossRef]
- Kimbrel, E.A.; Kung, A.L. The F-Box Protein β-TrCp1/Fbw1a Interacts with P300 to Enhance β-Catenin Transcriptional Activity*. J. Biol. Chem. 2009, 284, 13033–13044. [Google Scholar] [CrossRef]
- Popov, N.; Schülein, C.; Jaenicke, L.A.; Eilers, M. Ubiquitylation of the Amino Terminus of Myc by SCF(β-TrCP) Antagonizes SCF(Fbw7)-Mediated Turnover. Nat. Cell Biol. 2010, 12, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Welcker, M.; Orian, A.; Jin, J.; Grim, J.A.; Harper, J.W.; Eisenman, R.N.; Clurman, B.E. The Fbw7 Tumor Suppressor Regulates Glycogen Synthase Kinase 3 Phosphorylation-Dependent c-Myc Protein Degradation. Proc. Natl. Acad. Sci. USA 2004, 101, 9085–9090. [Google Scholar] [CrossRef] [PubMed]
- Yada, M.; Hatakeyama, S.; Kamura, T.; Nishiyama, M.; Tsunematsu, R.; Imaki, H.; Ishida, N.; Okumura, F.; Nakayama, K.; Nakayama, K.I. Phosphorylation-dependent Degradation of C-Myc Is Mediated by the F-box Protein Fbw7. EMBO J. 2004, 23, 2116–2125. [Google Scholar] [CrossRef]
- Cohen, M.; Amir, S.; Golan, M.; Ben-Neriah, Y.; Mabjeesh, N.J. β-TrCP Upregulates HIF-1 in Prostate Cancer Cells. Prostate 2019, 79, 403–413. [Google Scholar] [CrossRef]
- Bhatia, N.; Herter, J.R.; Slaga, T.J.; Fuchs, S.Y.; Spiegelman, V.S. Mouse Homologue of HOS (MHOS) Is Overexpressed in Skin Tumors and Implicated in Constitutive Activation of NF-ΚB. Oncogene 2002, 21, 1501–1509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saitoh, T.; Katoh, M. Expression Profiles of βTRCP1 and βTRCP2, and Mutation Analysis of ΒTRCP2 in Gastric Cancer. Int. J. Oncol. 2001, 18, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Ougolkov, A.; Zhang, B.; Yamashita, K.; Bilim, V.; Mai, M.; Fuchs, S.Y.; Minamoto, T. Associations Among β-TrCP, an E3 Ubiquitin Ligase Receptor, β-Catenin, and NF-ΚB in Colorectal Cancer. J. Natl. Cancer Inst. 2004, 96, 1161–1170. [Google Scholar] [CrossRef]
- Spiegelman, V.S.; Slaga, T.J.; Pagano, M.; Minamoto, T.; Ronai, Z.; Fuchs, S.Y. Wnt/β-Catenin Signaling Induces the Expression and Activity of ΒTrCP Ubiquitin Ligase Receptor. Mol. Cell 2000, 5, 877–882. [Google Scholar] [CrossRef]
- Belaïdouni, N.; Peuchmaur, M.; Perret, C.; Florentin, A.; Benarous, R.; Besnard-Guérin, C. Overexpression of Human Beta TrCP1 Deleted of Its F Box Induces Tumorigenesis in Transgenic Mice. Oncogene 2005, 24, 2271–2276. [Google Scholar] [CrossRef][Green Version]
- Koch, A.; Waha, A.; Hartmann, W.; Hrychyk, A.; Schüller, U.; Waha, A.; Wharton, K.A.; Fuchs, S.Y.; von Schweinitz, D.; Pietsch, T. Elevated Expression of Wnt Antagonists Is a Common Event in Hepatoblastomas. Clin. Cancer Res. 2005, 11, 4295–4304. [Google Scholar] [CrossRef]
- Kim, C.J.; Song, J.H.; Cho, Y.G.; Kim, Y.S.; Kim, S.Y.; Nam, S.W.; Yoo, N.J.; Lee, J.Y.; Park, W.S. Somatic Mutations of the β-TrCP Gene in Gastric Cancer. Apmis 2007, 115, 127–133. [Google Scholar] [CrossRef]
- Chen, S.; He, Y.; Ding, J.; Jiang, Y.; Jia, S.; Xia, W.; Zhao, J.; Lu, M.; Gu, Z.; Gao, Y. An Insertion/Deletion Polymorphism in the 3′ Untranslated Region of β-Transducin Repeat-Containing Protein (ΒTrCP) Is Associated with Susceptibility for Hepatocellular Carcinoma in Chinese. Biochem. Biophys. Res. Commun. 2010, 391, 552–556. [Google Scholar] [CrossRef]
- Huo, Z.H.; Zhong, H.J.; Zhu, Y.S.; Xing, B.; Tang, H. Roles of Functional NFKB1 and ΒTrCP Insertion/Deletion Polymorphisms in MRNA Expression and Epithelial Ovarian Cancer Susceptibility. Genet. Mol. Res. 2013, 12, 3435–3443. [Google Scholar] [CrossRef]
- Bi, H.; Tian, T.; Zhu, L.; Zhou, H.; Hu, H.; Liu, Y.; Li, X.; Hu, F.; Zhao, Y.; Wang, G. Copy Number Variation of E3 Ubiquitin Ligase Genes in Peripheral Blood Leukocyte and Colorectal Cancer. Sci. Rep. 2016, 6, 29869. [Google Scholar] [CrossRef][Green Version]
- Watanabe, N.; Arai, H.; Nishihara, Y.; Taniguchi, M.; Watanabe, N.; Hunter, T.; Osada, H. M-Phase Kinases Induce Phospho-Dependent Ubiquitination of Somatic Wee1 by SCFbeta-TrCP. Proc. Natl. Acad. Sci. USA 2004, 101, 4419–4424. [Google Scholar] [CrossRef]
- Guardavaccaro, D.; Kudo, Y.; Boulaire, J.; Barchi, M.; Busino, L.; Donzelli, M.; Margottin-Goguet, F.; Jackson, P.K.; Yamasaki, L.; Pagano, M. Control of Meiotic and Mitotic Progression by the F Box Protein β-Trcp1 In Vivo. Dev. Cell 2003, 4, 799–812. [Google Scholar] [CrossRef]
- Margottin-Goguet, F.; Hsu, J.Y.; Loktev, A.; Hsieh, H.-M.; Reimann, J.D.R.; Jackson, P.K. Prophase Destruction of Emi1 by the SCFβTrCP/Slimb Ubiquitin Ligase Activates the Anaphase Promoting Complex to Allow Progression beyond Prometaphase. Dev. Cell 2003, 4, 813–826. [Google Scholar] [CrossRef]
- Müerköster, S.; Arlt, A.; Sipos, B.; Witt, M.; Großmann, M.; Klöppel, G.; Kalthoff, H.; Fölsch, U.R.; Schäfer, H. Increased Expression of the E3-Ubiquitin Ligase Receptor Subunit ΒTRCP1 Relates to Constitutive Nuclear Factor-ΚB Activation and Chemoresistance in Pancreatic Carcinoma Cells. Cancer Res. 2005, 65, 1316–1324. [Google Scholar] [CrossRef]
- Cai, N.; Chen, Z.; Huang, Y.; Shao, S.; Yu, H.; Wang, Y.; He, S. β-TrCP1 Promotes Cell Proliferation via TNF-Dependent NF-ΚB Activation in Diffuse Large B Cell Lymphoma. Cancer Biol. Ther. 2019, 21, 241–247. [Google Scholar] [CrossRef]
- Kudo, Y.; Guardavaccaro, D.; Santamaria, P.G.; Koyama-Nasu, R.; Latres, E.; Bronson, R.; Yamasaki, L.; Pagano, M. Role of F-Box Protein BetaTrcp1 in Mammary Gland Development and Tumorigenesis. Mol. Cell. Biol. 2004, 24, 8184–8194. [Google Scholar] [CrossRef]
- LIANG, J.; WANG, W.-F.; XIE, S.; ZHANG, X.-L.; QI, W.-F.; ZHOU, X.-P.; HU, J.-X.; SHI, Q.; YU, R.-T. Expression of β-Transducin Repeat-Containing E3 Ubiquitin Protein Ligase in Human Glioma and Its Correlation with Prognosis. Oncol. Lett. 2015, 9, 2651–2656. [Google Scholar] [CrossRef]
- Liang, J.; Wang, W.-F.; Xie, S.; Zhang, X.-L.; Qi, W.-F.; Zhou, X.-P.; Hu, J.-X.; Shi, Q.; Yu, R.-T. β-Transducin Repeat-Containing E3 Ubiquitin Protein Ligase Inhibits Migration, Invasion and Proliferation of Glioma Cells. Oncol. Lett. 2017, 14, 3131–3135. [Google Scholar] [CrossRef]
- He, N.; Li, C.; Zhang, X.; Sheng, T.; Chi, S.; Chen, K.; Wang, Q.; Vertrees, R.; Logrono, R.; Xie, J. Regulation of Lung Cancer Cell Growth and Invasiveness by β-TRCP. Mol. Carcinog. 2005, 42, 18–28. [Google Scholar] [CrossRef]
- Spiegelman, V.S.; Tang, W.; Chan, A.M.; Igarashi, M.; Aaronson, S.A.; Sassoon, D.A.; Katoh, M.; Slaga, T.J.; Fuchs, S.Y. Induction of Homologue of Slimb Ubiquitin Ligase Receptor by Mitogen Signaling*. J. Biol. Chem. 2002, 277, 36624–36630. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, H.; Zhang, X.; Liu, Z.; Ma, X. Association of FBXW11 Levels with Tumor Development and Prognosis in Chondrosarcoma. Cancer Biomark. 2022, 35, 429–437. [Google Scholar] [CrossRef]
- Wang, L.; Feng, W.; Yang, X.; Yang, F.; Wang, R.; Ren, Q.; Zhu, X.; Zheng, G. Fbxw11 Promotes the Proliferation of Lymphocytic Leukemia Cells through the Concomitant Activation of NF-ΚB and β-Catenin/TCF Signaling Pathways. Cell Death Dis. 2018, 9, 427. [Google Scholar] [CrossRef]
- Polakis, P. The Oncogenic Activation of β-Catenin. Curr. Opin. Genet. Dev. 1999, 9, 15–21. [Google Scholar] [CrossRef]
- Tseng, R.-C.; Lin, R.-K.; Wen, C.-K.; Tseng, C.; Hsu, H.-S.; Hsu, W.-H.; Wang, Y.-C. Epigenetic Silencing of AXIN2/BetaTrCP and Deregulation of P53-Mediated Control Lead to Wild-Type β-Catenin Nuclear Accumulation in Lung Tumorigenesis. Oncogene 2008, 27, 4488–4496. [Google Scholar] [CrossRef]
- Li, Y.; Clevenger, C.V.; Minkovsky, N.; Kumar, K.G.S.; Raghunath, P.N.; Tomaszewski, J.E.; Spiegelman, V.S.; Fuchs, S.Y. Stabilization of Prolactin Receptor in Breast Cancer Cells. Oncogene 2006, 25, 1896–1902. [Google Scholar] [CrossRef]
- Goffin, V.; Bernichtein, S.; Touraine, P.; Kelly, P.A. Development and Potential Clinical Uses of Human Prolactin Receptor Antagonists. Endocr. Rev. 2005, 26, 400–422. [Google Scholar] [CrossRef]
- Kang, T.; Wei, Y.; Honaker, Y.; Yamaguchi, H.; Appella, E.; Hung, M.-C.; Piwnica-Worms, H. GSK-3β Targets Cdc25A for Ubiquitin-Mediated Proteolysis, and GSK-3β Inactivation Correlates with Cdc25A Overproduction in Human Cancers. Cancer Cell 2008, 13, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, A.; Li, Y.; Tran, T.H.; Tang, W.; Palazzo, J.P.; Rui, H.; Fuchs, S.Y. Oncogene-Mediated Inhibition of Glycogen Synthase Kinase 3β Impairs Degradation of Prolactin Receptor. Cancer Res. 2008, 68, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, V.S.; Tang, W.; Katoh, M.; Slaga, T.J.; Fuchs, S.Y. Inhibition of HOS Expression and Activities by Wnt Pathway. Oncogene 2002, 21, 856–860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spiegelman, V.S.; Stavropoulos, P.; Latres, E.; Pagano, M.; Ronai, Z.; Slaga, T.J.; Fuchs, S.Y. Induction of β-Transducin Repeat-Containing Protein by JNK Signaling and Its Role in the Activation of NF-ΚB*. J. Biol. Chem. 2001, 276, 27152–27158. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Dutta, P.; Sahay, O.; Santra, M.K. β-TrCP1 Facilitates Cell Cycle Checkpoint Activation, DNA Repair, and Cell Survival through Ablation of β-TrCP2 in Response to Genotoxic Stress. J. Biol. Chem. 2021, 296, 100511. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Dai, X.; Shu, J.; Ma, Y.; Wei, D.; Xiong, X.; Zhao, Y. The Cross Talk of Two Family Members of β-TrCP in the Regulation of Cell Autophagy and Growth. Cell Death Differ. 2020, 27, 1119–1133. [Google Scholar] [CrossRef]
- Ji, J.; Xu, R.; Zhang, X.; Han, M.; Xu, Y.; Wei, Y.; Ding, K.; Wang, S.; Huang, B.; Chen, A.; et al. Actin Like-6A Promotes Glioma Progression through Stabilization of Transcriptional Regulators YAP/TAZ. Cell Death Dis. 2018, 9, 517. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Q.; Yang, S.; Zheng, T.; Shao, J.; Guan, W.; Chen, F.; Zhang, S. Energy Deprivation-Induced AMPK Activation Inhibits Milk Synthesis by Targeting PrlR and PGC-1α. Cell Commun. Signal. 2022, 20, 25. [Google Scholar] [CrossRef]
- Liu, J.; Kumar, K.G.S.; Yu, D.; Molton, S.A.; McMahon, M.; Herlyn, M.; Thomas-Tikhonenko, A.; Fuchs, S.Y. Oncogenic BRAF Regulates β-Trcp Expression and NF-ΚB Activity in Human Melanoma Cells. Oncogene 2007, 26, 1954–1958. [Google Scholar] [CrossRef]
- Bu, X.; Qu, X.; Guo, K.; Meng, X.; Yang, X.; Huang, Q.; Dou, W.; Feng, L.; Wei, X.; Gao, J.; et al. CD147 Confers Temozolomide Resistance of Glioma Cells via the Regulation of β-TrCP/Nrf2 Pathway. Int. J. Biol. Sci. 2021, 17, 3013–3023. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.; Xu, Y.; Qiao, Y.; Zhang, X.; Wang, Y.; Guan, Y.; Sun, F.; Wang, J. CD166 Positively Regulates MCAM via Inhibition to Ubiquitin E3 Ligases Smurf1 and ΒTrCP through PI3K/AKT and c-Raf/MEK/ERK Signaling in Bel-7402 Hepatocellular Carcinoma Cells. Cell Signal. 2015, 27, 1694–1702. [Google Scholar] [CrossRef]
- Cheon, Y.; Jeon, S.; Lee, S. Centromere Protein W Interacts with Beta-transducin Repeat-containing Protein 1 and Modulates Its Subcellular Localization. FEBS Lett. 2016, 590, 4441–4452. [Google Scholar] [CrossRef]
- Fang, L.; Lu, W.; Choi, H.H.; Yeung, S.-C.J.; Tung, J.-Y.; Hsiao, C.-D.; Fuentes-Mattei, E.; Menter, D.; Chen, C.; Wang, L.; et al. ERK2-Dependent Phosphorylation of CSN6 Is Critical in Colorectal Cancer Development. Cancer Cell 2015, 28, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Lee, G.-E.; An, H.-J.; Cho, E.S.; Chen, W.; Lee, J.Y.; Kang, H.C.; Lee, H.S.; Cho, Y.-Y. F-Box Protein ΒTrCP1 Is a Substrate of Extracellular Signal-Regulated Kinase 2. J. Cancer Prev. 2021, 26, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, F.; Li, Y.; Drabsch, Y.; Zhang, J.; van Dam, H.; ten Dijke, P. Fas-Associated Factor 1 Is a Scaffold Protein That Promotes β-Transducin Repeat-Containing Protein (β-TrCP)-Mediated β-Catenin Ubiquitination and Degradation*. J. Biol. Chem. 2012, 287, 30701–30710. [Google Scholar] [CrossRef]
- Islam, S.; Dutta, P.; Chopra, K.; Sahay, O.; Rapole, S.; Chauhan, R.; Santra, M.K. Co-Operative Binding of SKP1, Cullin1 and Cullin7 to FBXW8 Results in Cullin1-SKP1-FBXW8-Cullin7 Functional Complex Formation That Monitors Cellular Function of β-TrCP1. Int. J. Biol. Macromol. 2021, 190, 233–243. [Google Scholar] [CrossRef]
- Islam, S.; Dutta, P.; Chopra, K.; Rapole, S.; Chauhan, R.; Santra, M.K. FBXW8 Regulates G1 and S Phases of Cell Cycle Progression by Restricting β-TrCP1 Function. FEBS J. 2021, 288, 5474–5497. [Google Scholar] [CrossRef]
- Zhao, L.; Lei, J.; Gu, S.; Zhang, Y.; Jing, X.; Wang, L.; Zhang, L.; Ning, Q.; Luo, M.; Qi, Y.; et al. A Yes-Associated Protein 1- Notch1 Receptor Positive Feedback Loop Promotes Breast Cancer Lung Metastasis by Attenuating the Bone Morphogenetic Protein 4-SMAD Family Member 1/5 Signaling. Carcinogenesis 2022, 43, 1162–1175. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, K.; Kim, S.A.; Park, S.; Park, B.-O.; Kim, J.-H.; Kim, S.-Y.; Kwon, M.J.; Han, M.H.; Lee, S.B.; et al. Deubiquitinase OTUD5 Is a Positive Regulator of MTORC1 and MTORC2 Signaling Pathways. Cell Death Differ. 2021, 28, 900–914. [Google Scholar] [CrossRef] [PubMed]
- Whitehurst, A.W.; Ram, R.; Shivakumar, L.; Gao, B.; Minna, J.D.; White, M.A. The RASSF1A Tumor Suppressor Restrains Anaphase-Promoting Complex/Cyclosome Activity during the G 1 /S Phase Transition To Promote Cell Cycle Progression in Human Epithelial Cells. Mol. Cell. Biol. 2008, 28, 3190–3197. [Google Scholar] [CrossRef]
- Estrabaud, E.; Lassot, I.; Blot, G.; Rouzic, E.L.; Tanchou, V.; Quemeneur, E.; Daviet, L.; Margottin-Goguet, F.; Benarous, R. RASSF1C, an Isoform of the Tumor Suppressor RASSF1A, Promotes the Accumulation of β-Catenin by Interacting with ΒTrCP. Cancer Res. 2007, 67, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Kuang, P.; Tan, M.; Zhou, W.; Zhang, Q.; Sun, Y. SAG/RBX2 E3 Ligase Complexes with UBCH10 and UBE2S E2s to Ubiquitylate β-TrCP1 via K11-Linkage for Degradation. Sci. Rep. 2016, 6, 37441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, F.; Shi, W.; Zhai, C.; Wang, J.; Yan, X.; Wang, Q.; Zhang, Q.; Yang, L.; Gao, L.; et al. COP9 Signalosome Subunit 6 Mediates PDGF -Induced Pulmonary Arterial Smooth Muscle Cells Proliferation. Exp. Cell Res. 2018, 371, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Xiaoyun, S.; Yuyuan, Z.; Jie, X.; Yingjie, N.; Qing, X.; Yuezhen, D.; Haiguang, X. PHF19 Activates Hedgehog Signaling and Promotes Tumorigenesis in Hepatocellular Carcinoma. Exp. Cell Res. 2021, 406, 112690. [Google Scholar] [CrossRef]
- Woo, S.R.; Byun, J.G.; Kim, Y.H.; Park, E.-R.; Joo, H.-Y.; Yun, M.; Shin, H.-J.; Kim, S.-H.; Shen, Y.N.; Park, J.-E.; et al. SIRT1 Suppresses Cellular Accumulation of β-TrCP E3 Ligase via Protein Degradation. Biochem. Biophys. Res. Commun. 2013, 441, 831–837. [Google Scholar] [CrossRef]
- Wei, S.; Chu, P.-C.; Chuang, H.-C.; Hung, W.-C.; Kulp, S.K.; Chen, C.-S. Targeting the Oncogenic E3 Ligase Skp2 in Prostate and Breast Cancer Cells with a Novel Energy Restriction-Mimetic Agent. PLoS ONE 2012, 7, e47298. [Google Scholar] [CrossRef]
- Deng, W.; Vanderbilt, D.B.; Lin, C.-C.; Martin, K.H.; Brundage, K.M.; Ruppert, J.M. SOX9 Inhibits β-TrCP-Mediated Protein Degradation to Promote Nuclear GLI1 Expression and Cancer Stem Cell Properties. J. Cell Sci. 2015, 128, 1123–1138. [Google Scholar] [CrossRef]
- Shanzer, M.; Adler, J.; Ricardo-Lax, I.; Reuven, N.; Shaul, Y. The Nonreceptor Tyrosine Kinase C-Src Attenuates SCF(β-TrCP) E3-Ligase Activity Abrogating Taz Proteasomal Degradation. Proc. Natl. Acad. Sci. USA 2017, 114, 1678–1683. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Cai, S.; Chen, Y.; Wang, Q.; Pan, Q.; Sun, F.; Wang, J. Reciprocal Regulation between ΒTrCP and Smurf1 Suppresses Proliferative Capacity of Liver Cancer Cells. J. Cell. Physiol. 2017, 232, 3347–3359. [Google Scholar] [CrossRef]
- Shukla, S.; Allam, U.S.; Ahsan, A.; Chen, G.; Krishnamurthy, P.M.; Marsh, K.; Rumschlag, M.; Shankar, S.; Whitehead, C.; Schipper, M.; et al. KRAS Protein Stability Is Regulated through SMURF2: UBCH5 Complex-Mediated β-TrCP1 Degradation. Neoplasia 2014, 16, 115–128. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Z.; Li, L.; Qin, Y.-R.; Liu, H.; Jiang, C.; Zeng, T.-T.; Li, M.-Q.; Xie, D.; Li, Y.; et al. TSPAN15 Interacts with BTRC to Promote Oesophageal Squamous Cell Carcinoma Metastasis via Activating NF-ΚB Signaling. Nat. Commun. 2018, 9, 1423. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wu, Y.-S.; Hung, C.-Y.; Wang, S.-A.; Young, M.-J.; Hsu, T.-I.; Hung, J.-J. USP24 Induces IL-6 in Tumor-Associated Microenvironment by Stabilizing P300 and β-TrCP and Promotes Cancer Malignancy. Nat. Commun. 2018, 9, 3996. [Google Scholar] [CrossRef]
- Peschiaroli, A.; Skaar, J.R.; Pagano, M.; Melino, G. The Ubiquitin-Specific Protease USP47 Is a Novel β-TRCP Interactor Regulating Cell Survival. Oncogene 2010, 29, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.X.; Lin, H.; Chu, T.; Lim, Y.P. WBP2 Promotes BTRC MRNA Stability to Drive Migration and Invasion in Triple-negative Breast Cancer via NF-κB Activation. Mol. Oncol. 2022, 16, 422–446. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Lu, L.; Wu, C.; Pan, X.; Liu, B.; Zhang, Y.; Wang, Y.; Wu, W.; Yan, B.; Zhang, Y.; et al. CircHIPK3 Prevents Cardiac Senescence by Acting as a Scaffold to Recruit Ubiquitin Ligase to Degrade HuR. Theranostics 2022, 12, 7550–7566. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Wang, Y.; Wang, Y.; Deng, X.; Yan, Q.; Fan, C.; Zhang, S.; Zhang, S.; Gong, Z.; Shi, L.; et al. Circular RNA CircPVT1 Promotes Nasopharyngeal Carcinoma Metastasis via the β-TrCP/c-Myc/SRSF1 Positive Feedback Loop. Mol. Cancer 2022, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yang, Z.; Yang, B.; Xiong, H.; Ye, W. LINC00460 Promotes Cutaneous Squamous Cell Carcinoma Progression Through Stabilizing ELAVL1 Protein. Mol. Biotechnol. 2022, 65, 1296–1305. [Google Scholar] [CrossRef]
- Wu, N.; Jiang, M.; Liu, H.; Chu, Y.; Wang, D.; Cao, J.; Wang, Z.; Xie, X.; Han, Y.; Xu, B. LINC00941 Promotes CRC Metastasis through Preventing SMAD4 Protein Degradation and Activating the TGF-β/SMAD2/3 Signaling Pathway. Cell Death Differ. 2021, 28, 219–232. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, B.; Hu, X.; Ying, S.; Zhou, Q.; Xu, W.; Feng, L.; Hou, T.; Wang, X.; Zhu, L.; et al. LncRNA LINC00942 Promotes Chemoresistance in Gastric Cancer by Suppressing MSI2 Degradation to Enhance C-Myc MRNA Stability. Clin. Transl. Med. 2022, 12, e703. [Google Scholar] [CrossRef]
- Fang, Y.; Shi, C.; Manduchi, E.; Civelek, M.; Davies, P.F. MicroRNA-10a Regulation of Proinflammatory Phenotype in Athero-Susceptible Endothelium in Vivo and in Vitro. Proc. Natl. Acad. Sci. USA 2010, 107, 13450–13455. [Google Scholar] [CrossRef]
- Savita, U.; Karunagaran, D. MicroRNA-106b-25 Cluster Targets β-TRCP2, Increases the Expression of Snail and Enhances Cell Migration and Invasion in H1299 (Non Small Cell Lung Cancer) Cells. Biochem. Biophys. Res. Commun. 2013, 434, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chang, Y.-L.; Chang, Y.-C.; Lin, J.-C.; Chen, C.-C.; Pan, S.-H.; Wu, C.-T.; Chen, H.-Y.; Yang, S.-C.; Hong, T.-M.; et al. MicroRNA-135b Promotes Lung Cancer Metastasis by Regulating Multiple Targets in the Hippo Pathway and LZTS1. Nat. Commun. 2013, 4, 1877. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ji, J.; Song, J.; Li, X.; Han, S.; Lian, W.; Cao, C.; Zhang, X.; Li, M. MicroRNA-182 Promotes Pancreatic Cancer Cell Proliferation and Migration by Targeting β-TrCP2. Acta Biochim. Biophys. Sin. 2016, 48, 1085–1093. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, H.; Li, W.; Jia, C.; Zhang, H.; Sun, Y.; Chen, X.; Song, X. Overexpression of TP53 Mutation-Associated MicroRNA-182 Promotes Tumor Cell Proliferation and Migration in Head and Neck Squamous Cell Carcinoma. Arch. Oral Biol. 2017, 73, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Liu, W.-H.; Chang, L.-S. Hydroquinone-Induced FOXP3-ADAM17-Lyn-Akt-P21 Signaling Axis Promotes Malignant Progression of Human Leukemia U937 Cells. Arch. Toxicol. 2017, 91, 983–997. [Google Scholar] [CrossRef]
- Huang, C.-H.; Lee, Y.-C.; Chen, Y.-J.; Wang, L.-J.; Shi, Y.-J.; Chang, L.-S. Quinacrine Induces the Apoptosis of Human Leukemia U937 Cells through FOXP3/MiR-183/β-TrCP/SP1 Axis-Mediated BAX Upregulation. Toxicol. Appl. Pharmacol. 2017, 334, 35–46. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Li, H.-L.; Liu, W.; Zhang, L.-P.; Zheng, Q.; Bai, J.; Hu, Y.-Q.; Yin, C.-G.; Lv, S.-J.; Zhang, B.-G. MiR-193a-3p Promotes the Invasion, Migration, and Mesenchymal Transition in Glioma through Regulating BTRC. BioMed Res. Int. 2021, 2021, 8928509. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, X.; Zhang, Y. MicroRNA-221 Promotes Cell Proliferation and Inhibits Apoptosis in Osteosarcoma Cells by Directly Targeting FBXW11 and Regulating Wnt Signaling. Arch. Med. Res. 2021, 52, 191–199. [Google Scholar] [CrossRef]
- Zheng, Q.; Yu, J.J.; Li, C.; Li, J.; Wang, J.; Wang, S. MiR-224 Targets BTRC and Promotes Cell Migration and Invasion in Colorectal Cancer. 3 Biotech 2020, 10, 485. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, D.; Tang, B.; Xu, A.; Huang, H.; Su, Y.; Xu, J.; Deng, J.; Tang, L.; Sun, C.; et al. MicroRNA-324-5p Suppresses the Migration and Invasion of MM Cells by Inhibiting the SCFβ−TrCP E3 Ligase. Oncol. Lett. 2018, 16, 5331–5338. [Google Scholar] [CrossRef]
- Yang, Q.; Li, K.; Huang, X.; Zhao, C.; Mei, Y.; Li, X.; Jiao, L.; Yang, H. LncRNA SLC7A11-AS1 Promotes Chemoresistance by Blocking SCFβ-TRCP-Mediated Degradation of NRF2 in Pancreatic Cancer. Mol. Ther. Nucleic Acids 2020, 19, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Svensson, C.; Ceder, J.; Iglesias-Gato, D.; Chuan, Y.-C.; Pang, S.T.; Bjartell, A.; Martinez, R.M.; Bott, L.; Helczynski, L.; Ulmert, D.; et al. REST Mediates Androgen Receptor Actions on Gene Repression and Predicts Early Recurrence of Prostate Cancer. Nucleic Acids Res. 2014, 42, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, G.; Kuan, S.-F.; Zhang, D.H.; Schlaepfer, D.D.; Hu, J. FAK/PYK2 Promotes the Wnt/β-Catenin Pathway and Intestinal Tumorigenesis by Phosphorylating GSK3β. eLife 2015, 4, e10072. [Google Scholar] [CrossRef] [PubMed]
- Amiri, K.I.; Richmond, A. Role of Nuclear Factor-κ B in Melanoma. Cancer Metastasis Rev. 2005, 24, 301–313. [Google Scholar] [CrossRef]
- Bi, Y.; Chen, X.; Wei, B.; Wang, L.; Gong, L.; Li, H.; Xiong, X.; Zhao, Y. DEPTOR Stabilizes ErbB2 to Promote the Proliferation and Survival of ErbB2-Positive Breast Cancer Cells. Theranostics 2021, 11, 6355–6369. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.; Xu, J.; Xiao, K.; Xu, Y.; Guo, T.; Zhang, L.; Wang, J.; Zheng, H. β-TrCP Restricts Lipopolysaccharide (LPS)-Induced Activation of TRAF6-IKK Pathway Upstream of IκBα Signaling. Front. Immunol. 2018, 9, 2930. [Google Scholar] [CrossRef]
- Shivakumar, L.; Minna, J.; Sakamaki, T.; Pestell, R.; White, M.A. The RASSF1A Tumor Suppressor Blocks Cell Cycle Progression and Inhibits Cyclin D1 Accumulation. Mol. Cell. Biol. 2002, 22, 4309–4318. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, W.; Chuang, K.; Shen, Y.; Hu, W.S.; Ho, C.; Chen, Y.; Hsu, M.; Hsu, H.; Lieu, C. Blockade of JAK2 Activity Suppressed Accumulation of β-catenin in Leukemic Cells. J. Cell. Biochem. 2010, 111, 402–411. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Zha, Z.-Y.; Zhou, X.; Zhang, H.; Huang, W.; Zhao, D.; Li, T.; Chan, S.W.; Lim, C.J.; Hong, W.; et al. The Hippo Tumor Pathway Promotes TAZ Degradation by Phosphorylating a Phosphodegron and Recruiting the SCFβ-TrCP E3 Ligase*. J. Biol. Chem. 2010, 285, 37159–37169. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Yang, S. Trp53 Controls Chondrogenesis and Endochondral Ossification by Negative Regulation of TAZ Activity and Stability via β-TrCP-Mediated Ubiquitination. Cell Death Dis. 2022, 8, 317. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; White, G.; Fadlullah, M.Z.H.; Dreger, M.; Pickering, K.; Maltas, J.; Ashton, G.; MacLeod, R.; Baillie, G.S.; Kouskoff, V.; et al. TIAM1 Antagonizes TAZ/YAP Both in the Destruction Complex in the Cytoplasm and in the Nucleus to Inhibit Invasion of Intestinal Epithelial Cells. Cancer Cell 2017, 31, 621–634.e6. [Google Scholar] [CrossRef] [PubMed]
- Bufalieri, F.; Infante, P.; Bernardi, F.; Caimano, M.; Romania, P.; Moretti, M.; Severini, L.L.; Talbot, J.; Melaiu, O.; Tanori, M.; et al. ERAP1 Promotes Hedgehog-Dependent Tumorigenesis by Controlling USP47-Mediated Degradation of ΒTrCP. Nat. Commun. 2019, 10, 3304. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Xu, X.; Zhang, W.; Yu, T.; Jia, J.; Liu, C. Deubiquitinase USP47/UBP64E Regulates β-Catenin Ubiquitination and Degradation and Plays a Positive Role in Wnt Signaling. Mol. Cell. Biol. 2015, 35, 3301–3311. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, H. Functional Characterization of SAG/RBX2/ROC2/RNF7, an Antioxidant Protein and an E3 Ubiquitin Ligase. Protein Cell 2013, 4, 103–116. [Google Scholar] [CrossRef]
- Banerjee, S.; Zmijewski, J.W.; Lorne, E.; Liu, G.; Sha, Y.; Abraham, E. Modulation of SCFβ-TrCP-Dependent IκBα Ubiquitination by Hydrogen Peroxide*. J. Biol. Chem. 2010, 285, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Shi, Y.; Xue, C.; Li, M.; Wei, J.; Li, G.; Xiong, W.; Zhou, M. Understanding the Dual Roles of CircHIPK3 in Tumorigenesis and Tumor Progression. J. Cancer 2022, 13, 3674–3686. [Google Scholar] [CrossRef]
- Shao, Q.; Huang, Y.; Zhang, C.; Gao, X.; Gao, S. Emerging Landscape of CircHIPK3 and Its Role in Cancer and Other Diseases. Mol. Med. Rep. 2021, 23, 409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Liao, Q. CircHIPK3: A Promising Cancer-Related Circular RNA. Am. J. Transl. Res. 2020, 12, 6694–6704. [Google Scholar]
- Chen, C.-H.; Chuang, S.-M.; Yang, M.-F.; Liao, J.-W.; Yu, S.-L.; Chen, J.J.W. A Novel Function of YWHAZ/β-Catenin Axis in Promoting Epithelial–Mesenchymal Transition and Lung Cancer Metastasis. Mol. Cancer Res. 2012, 10, 1319–1331. [Google Scholar] [CrossRef]
- Wang, F.; Huang, W.; Hu, X.; Chen, C.; Li, X.; Qiu, J.; Liang, Z.; Zhang, J.; Li, L.; Wang, X.; et al. Transcription Factor AP-2β Suppresses Cervical Cancer Cell Proliferation by Promoting the Degradation of Its Interaction Partner β-catenin. Mol. Carcinog. 2017, 56, 1909–1923. [Google Scholar] [CrossRef]
- Chen, Y.; Velmurugan, B.K.; Wang, H.; Tu, C.; Che, R.; Chen, M.; Jen, L.; Vishwanadha, V.P.; Hsu, H.; Huang, C. Estrogen and ERα Enhanced β-catenin Degradation and Suppressed Its Downstream Target Genes to Block the Metastatic Function of HA22T Hepatocellular Carcinoma Cells via Modulating GSK-3β and β-TrCP Expression. Environ. Toxicol. 2017, 32, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.L.; Donninger, H.; Clark, G.J. Ras Regulates SCFβ-TrCP Protein Activity and Specificity via Its Effector Protein NORE1A*. J. Biol. Chem. 2014, 289, 31102–31110. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Park, J.-S.; Wei, Y.; Rajurkar, M.; Cotton, J.L.; Fan, Q.; Lewis, B.C.; Ji, H.; Mao, J. TRIB2 Acts Downstream of Wnt/TCF in Liver Cancer Cells to Regulate YAP and C/EBPα Function. Mol. Cell 2013, 51, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, S.; Lin, C.; Li, Y.; Ye, L.; Wu, X.; Jian, Y.; Dai, Y.; Ouyang, Y.; Zhao, L.; et al. TRIB3 Confers Radiotherapy Resistance in Esophageal Squamous Cell Carcinoma by Stabilizing TAZ. Oncogene 2020, 39, 3710–3725. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Cho, H.; Inn, K.-S.; Yang, A.; Zhao, Z.; Liang, Q.; Versteeg, G.A.; Amini-Bavil-Olyaee, S.; Wong, L.-Y.; Zlokovic, B.V.; et al. Negative Regulation of NF-ΚB Activity by Brain-Specific TRIpartite Motif Protein 9. Nat. Commun. 2014, 5, 4820. [Google Scholar] [CrossRef]
- Fan, W.; Liu, X.; Zhang, J.; Qin, L.; Du, J.; Li, X.; Qian, S.; Chen, H.; Qian, P. TRIM67 Suppresses TNFalpha-Triggered NF-KB Activation by Competitively Binding Beta-TrCP to IkBa. Front. Immunol. 2022, 13, 793147. [Google Scholar] [CrossRef]
- Yang, N.; Chen, T.; Wang, L.; Liu, R.; Niu, Y.; Sun, L.; Yao, B.; Wang, Y.; Yang, W.; Liu, Q.; et al. CXCR4 Mediates Matrix Stiffness-Induced Downregulation of UBTD1 Driving Hepatocellular Carcinoma Progression via YAP Signaling Pathway. Theranostics 2020, 10, 5790–5801. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Schmidt, L.; Clark, G. RASSF5 (Ras Association (RalGDS/AF-6) Domain Family Member 5). Atlas Genet. Cytogenet. Oncol. Haematol. 2012. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Y.; Wang, J. Ubiquitin E3 Ligase SCFβ-TRCP Regulates TRIB2 Stability in Liver Cancer Cells. Biochem. Biophys. Res. Commun. 2013, 441, 555–559. [Google Scholar] [CrossRef]
- Xu, S.; Tong, M.; Huang, J.; Zhang, Y.; Qiao, Y.; Weng, W.; Liu, W.; Wang, J.; Sun, F. TRIB2 Inhibits Wnt/β-Catenin/TCF4 Signaling through Its Associated Ubiquitin E3 Ligases, β-TrCP, COP1 and Smurf1, in Liver Cancer Cells. FEBS Lett. 2014, 588, 4334–4341. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, J.; Rychahou, P.G.; Qiu, S.; Evers, B.M.; Zhou, B.P. Stabilization of Snail by NF-ΚB Is Required for Inflammation-Induced Cell Migration and Invasion. Cancer Cell 2009, 15, 416–428. [Google Scholar] [CrossRef]
- Liu, S.; An, H.; Li, N.; Yu, Y.; Lin, N.; Wan, T.; Zhang, M.; Wang, W.; Cao, X. Cloning and Identification of a Novel Human Ubiquitin-like Protein, DC-UbP, from Dendritic Cells. Biochem. Biophys. Res. Commun. 2003, 300, 800–805. [Google Scholar] [CrossRef]
- Uhler, J.P.; Spåhr, H.; Farge, G.; Clavel, S.; Larsson, N.-G.; Falkenberg, M.; Samuelsson, T.; Gustafsson, C.M. The UbL Protein UBTD1 Stably Interacts with the UBE2D Family of E2 Ubiquitin Conjugating Enzymes. Biochem. Biophys. Res. Commun. 2014, 443, 7–12. [Google Scholar] [CrossRef]
- Ballout, F.; Lu, H.; Chen, L.; Sriramajayam, K.; Que, J.; Meng, Z.; Wang, T.C.; Giordano, S.; Zaika, A.; McDonald, O.; et al. APE1 Redox Function Is Required for Activation of Yes-Associated Protein 1 under Reflux Conditions in Barrett’s-Associated Esophageal Adenocarcinomas. J. Exp. Clin. Cancer Res. 2022, 41, 264. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.P.Z.P.; Rodrigues, A.M.; Ribeiro-Filho, H.V.; Manucci, A.C.; Ribeiro, P.d.F.; Botelho, M.C.S.; Vogel, C.; Lopes-de-Oliveira, P.S.; Pagano, M.; Bruni-Cardoso, A. Extracellular Matrix Stiffness Regulates Degradation of MST2 via SCF ΒTrCP. Biochim. Biophys. Acta (BBA) Gen. Subj. 2022, 1866, 130238. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Zhang, Y.; Zhang, Y.; Ma, L.; Weng, W.; Qiao, Y.; Xiao, W.; Wang, H.; Yu, W.; et al. MEK1 Promotes YAP and Their Interaction Is Critical for Tumorigenesis in Liver Cancer. FEBS Lett. 2013, 587, 3921–3927. [Google Scholar] [CrossRef] [PubMed]
- Mansur, D.S.; de Motes, C.M.; Unterholzner, L.; Sumner, R.P.; Ferguson, B.J.; Ren, H.; Strnadova, P.; Bowie, A.G.; Smith, G.L. Poxvirus Targeting of E3 Ligase β-TrCP by Molecular Mimicry: A Mechanism to Inhibit NF-ΚB Activation and Promote Immune Evasion and Virulence. PLoS Pathog. 2013, 9, e1003183. [Google Scholar] [CrossRef]
- Guan, H.; Ricciardi, R.P. Transformation by E1A Oncoprotein Involves Ubiquitin-Mediated Proteolysis of the Neuronal and Tumor Repressor REST in the Nucleus. J. Virol. 2012, 86, 5594–5602. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive Molecular Portraits of Human Breast Tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Fiore, I.J.M.D.; Pane, J.A.; Holloway, G.; Coulson, B.S. NSP1 of Human Rotaviruses Commonly Inhibits NF-ΚB Signalling by Inducing β-TrCP Degradation. J. Gen. Virol. 2015, 96, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.; Dennis, A.F.; Patton, J.T. Putative E3 Ubiquitin Ligase of Human Rotavirus Inhibits NF-ΚB Activation by Using Molecular Mimicry To Target β-TrCP. mBio 2015, 6, e02490-14. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.A.; Morelli, M.; Patton, J.T. Rotavirus NSP1 Requires Casein Kinase II-Mediated Phosphorylation for Hijacking of Cullin-RING Ligases. mBio 2017, 8, e01213-17. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, T.; Malouli, D.; Uebelhoer, L.S.; DeFilippis, V.R.; Früh, K.; Verweij, M.C. The ORF61 Protein Encoded by Simian Varicella Virus and Varicella-Zoster Virus Inhibits NF-ΚB Signaling by Interfering with IκBα Degradation. J. Virol. 2015, 89, 8687–8700. [Google Scholar] [CrossRef] [PubMed]
- Surjit, M.; Varshney, B.; Lal, S.K. The ORF2 Glycoprotein of Hepatitis E Virus Inhibits Cellular NF-ΚB Activity by Blocking Ubiquitination Mediated Proteasomal Degradation of IκBα in Human Hepatoma Cells. BMC Biochem. 2012, 13, 7. [Google Scholar] [CrossRef]
- Spardy, N.; Covella, K.; Cha, E.; Hoskins, E.E.; Wells, S.I.; Duensing, A.; Duensing, S. Human Papillomavirus 16 E7 Oncoprotein Attenuates DNA Damage Checkpoint Control by Increasing the Proteolytic Turnover of Claspin. Cancer Res. 2009, 69, 7022–7029. [Google Scholar] [CrossRef]
- Yan, Q.; Zeng, Z.; Gong, Z.; Zhang, W.; Li, X.; He, B.; Song, Y.; Li, Q.; Zeng, Y.; Liao, Q.; et al. EBV-MiR-BART10-3p Facilitates Epithelial-Mesenchymal Transition and Promotes Metastasis of Nasopharyngeal Carcinoma by Targeting BTRC. Oncotarget 2015, 6, 41766–41782. [Google Scholar] [CrossRef]
- Sun, H.; Wang, K.; Yao, W.; Liu, J.; Lv, L.; Shi, X.; Chen, H. Inter-Fighting between Influenza A Virus NS1 and β-TrCP: A Novel Mechanism of Anti-Influenza Virus. Viruses 2022, 14, 2426. [Google Scholar] [CrossRef]
- Warfel, N.A.; Niederst, M.; Stevens, M.W.; Brennan, P.M.; Frame, M.C.; Newton, A.C. Mislocalization of the E3 Ligase, β-Transducin Repeat-Containing Protein 1 (β-TrCP1), in Glioblastoma Uncouples Negative Feedback between the Pleckstrin Homology Domain Leucine-Rich Repeat Protein Phosphatase 1 (PHLPP1) and Akt*. J. Biol. Chem. 2011, 286, 19777–19788. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, S.; Xia, H.; Yu, H.; Tang, Q.; Bi, F. MEK Nuclear Localization Promotes YAP Stability via Sequestering β-TrCP in KRAS Mutant Cancer Cells. Cell Death Differ. 2019, 26, 2400–2415. [Google Scholar] [CrossRef]
- Tang, W.; Li, Y.; Yu, D.; Thomas-Tikhonenko, A.; Spiegelman, V.S.; Fuchs, S.Y. Targeting β-Transducin Repeat–Containing Protein E3 Ubiquitin Ligase Augments the Effects of Antitumor Drugs on Breast Cancer Cells. Cancer Res. 2005, 65, 1904–1908. [Google Scholar] [CrossRef]
- Sharma, R.; Williams, P.J.; Gupta, A.; McCluskey, B.; Bhaskaran, S.; Muñoz, S.; Oyajobi, B.O. A Dominant-Negative F-Box Deleted Mutant of E3 Ubiquitin Ligase, β-TrCP1/FWD1, Markedly Reduces Myeloma Cell Growth and Survival in Mice. Oncotarget 2015, 6, 21589–21602. [Google Scholar] [CrossRef]
- Hayakawa, M.; Miyashita, H.; Sakamoto, I.; Kitagawa, M.; Tanaka, H.; Yasuda, H.; Karin, M.; Kikugawa, K. Evidence That Reactive Oxygen Species Do Not Mediate NF-κB Activation. EMBO J. 2003, 22, 3356–3366. [Google Scholar] [CrossRef]
- Bhatia, N.; Demmer, T.A.; Sharma, A.K.; Elcheva, I.; Spiegelman, V.S. Role of β-TrCP Ubiquitin Ligase Receptor in UVB Mediated Responses in Skin. Arch. Biochem. Biophys. 2011, 508, 178–184. [Google Scholar] [CrossRef]
- Gluschnaider, U.; Hidas, G.; Cojocaru, G.; Yutkin, V.; Ben-Neriah, Y.; Pikarsky, E. β-TrCP Inhibition Reduces Prostate Cancer Cell Growth via Upregulation of the Aryl Hydrocarbon Receptor. PLoS ONE 2010, 5, e9060. [Google Scholar] [CrossRef] [PubMed]
- Hay-Koren, A.; Bialik, S.; Levin-Salomon, V.; Kimchi, A. Changes in CIAP2, Survivin and BimEL Expression Characterize the Switch from Autophagy to Apoptosis in Prolonged Starvation. J. Intern. Med. 2017, 281, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Dehan, E.; Bassermann, F.; Guardavaccaro, D.; Vasiliver-Shamis, G.; Cohen, M.; Lowes, K.N.; Dustin, M.; Huang, D.C.S.; Taunton, J.; Pagano, M. ΒTrCP- and Rsk1/2-Mediated Degradation of BimEL Inhibits Apoptosis. Mol. Cell 2009, 33, 109–116. [Google Scholar] [CrossRef]
- Gorelik, M.; Orlicky, S.; Sartori, M.A.; Tang, X.; Marcon, E.; Kurinov, I.; Greenblatt, J.F.; Tyers, M.; Moffat, J.; Sicheri, F.; et al. Inhibition of SCF Ubiquitin Ligases by Engineered Ubiquitin Variants That Target the Cul1 Binding Site on the Skp1–F-Box Interface. Proc. Natl. Acad. Sci. USA 2016, 113, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Blees, J.S.; Bokesch, H.R.; Rübsamen, D.; Schulz, K.; Milke, L.; Bajer, M.M.; Gustafson, K.R.; Henrich, C.J.; McMahon, J.B.; Colburn, N.H.; et al. Erioflorin Stabilizes the Tumor Suppressor Pdcd4 by Inhibiting Its Interaction with the E3-Ligase β-TrCP1. PLoS ONE 2012, 7, e46567. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.-T.; Lee, Y.-C.; Wang, L.-J.; Chang, L.-S. BCL2 Inhibitor ABT-199 and BCL2L1 Inhibitor WEHI-539 Coordinately Promote NOXA-Mediated Degradation of MCL1 in Human Leukemia Cells. Chem. Biol. Interact. 2022, 361, 109978. [Google Scholar] [CrossRef]
- Shafique, S.; Rashid, S. Antiviral Drug Acyclovir Exhibits Antitumor Activity via Targeting ΒTrCP1: Molecular Docking and Dynamics Simulation Study. J. Mol. Graph. Model. 2017, 72, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fan, Z.; Tang, Q.; Xia, H.; Zhang, T.; Bi, F. Aspirin Attenuates YAP and β-Catenin Expression by Promoting β-TrCP to Overcome Docetaxel and Vinorelbine Resistance in Triple-Negative Breast Cancer. Cell Death Dis. 2020, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Liu, R.; Lv, C.; Miao, Y.; Yue, M.; Tao, Y.; Wei, Z.; Xia, Y.; Dai, Y. Bergenin Impedes the Generation of Extracellular Matrix in Glomerular Mesangial Cells and Ameliorates Diabetic Nephropathy in Mice by Inhibiting Oxidative Stress via the MTOR/β-TrcP/Nrf2 Pathway. Free Radic. Biol. Med. 2019, 145, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Wonganan, O.; Zhang, Z.; Yu, J.; Xi, R.; Cao, Y.; Suksamrarn, A.; Zhang, G.; Wang, F. A 2-Benzylmalonate Derivative as STAT3 Inhibitor Suppresses Tumor Growth in Hepatocellular Carcinoma by Upregulating β-TrCP E3 Ubiquitin Ligase. Int. J. Mol. Sci. 2021, 22, 3354. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Y.; Xu, Y.; Guan, Y.; Zhang, X.; Chen, Y.; Wu, Q.; Zhu, G.; Chen, Y.; Sun, F.; et al. Blocking Inhibition to YAP by ActinomycinD Enhances Anti-Tumor Efficacy of Corosolic Acid in Treating Liver Cancer. Cell Signal. 2017, 29, 209–217. [Google Scholar] [CrossRef]
- Dang, Y.-Y.; Luo, H.; Li, Y.-M.; Zhou, Y.; Luo, X.; Lin, S.-M.; Liu, S.-P.; Lee, S.M.-Y.; Li, C.-W.; Dai, X.-Y. Curcumin Prevents As3+-Induced Carcinogenesis through Regulation of GSK3β/Nrf2. Chin. Med. 2021, 16, 116. [Google Scholar] [CrossRef]
- Li, X.; Dong, L.; Liu, J.; Wang, C.; Zhang, Y.; Mei, Q.; Han, W.; Xie, P.; Nie, J. Low-Dose Decitabine Augments the Activation and Anti-Tumor Immune Response of IFN-Γ+ CD4+ T Cells Through Enhancing IκBα Degradation and NF-ΚB Activation. Front. Cell Dev. Biol. 2021, 9, 647713. [Google Scholar] [CrossRef]
- Shi, H.; Sun, Y.; Ruan, H.; Ji, C.; Zhang, J.; Wu, P.; Li, L.; Huang, C.; Jia, Y.; Zhang, X.; et al. 3,3′-Diindolylmethane Promotes Gastric Cancer Progression via β-TrCP-Mediated NF-ΚB Activation in Gastric Cancer-Derived MSCs. Front. Oncol. 2021, 11, 603533. [Google Scholar] [CrossRef]
- Shi, S.; Li, C.; Zhang, Y.; Deng, C.; Liu, W.; Du, J.; Li, Q.; Ji, Y.; Guo, L.; Liu, L.; et al. Dihydrocapsaicin Inhibits Cell Proliferation and Metastasis in Melanoma via Down-Regulating β-Catenin Pathway. Front. Oncol. 2021, 11, 648052. [Google Scholar] [CrossRef]
- Liu, W.-H.; Chang, L.-S. Fas/FasL-Dependent and -Independent Activation of Caspase-8 in Doxorubicin-Treated Human Breast Cancer MCF-7 Cells: ADAM10 down-Regulation Activates Fas/FasL Signaling Pathway. Int. J. Biochem. Cell Biol. 2011, 43, 1708–1719. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Wang, Y.; Bailey, C.; Zheng, P.; Liu, Y. Dual Targeting Oncoproteins MYC and HIF1α Regresses Tumor Growth of Lung Cancer and Lymphoma. Cancers 2021, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Mohan, C.D.; Chinnathambi, A.; Alharbi, S.A.; Sethi, G.; Rangappa, K.S.; Ahn, K.S. Euphorbiasteroid Abrogates EGFR and Wnt/β-Catenin Signaling in Non-Small-Cell Lung Cancer Cells to Impart Anticancer Activity. Molecules 2022, 27, 3824. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Afaq, F.; Maddodi, N.; Johnson, J.J.; Sarfaraz, S.; Ahmad, A.; Setaluri, V.; Mukhtar, H. Inhibition of Human Melanoma Cell Growth by the Dietary Flavonoid Fisetin Is Associated with Disruption of Wnt/β-Catenin Signaling and Decreased Mitf Levels. J. Investig. Dermatol. 2011, 131, 1291–1299. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, L. Gallic Acid Downregulates Matrix Metalloproteinase-2 (MMP-2) and MMP-9 in Human Leukemia Cells with Expressed Bcr/Abl. Mol. Nutr. Food Res. 2012, 56, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Fujiwara, H.; Furuichi, Y.; Tanaka, K.; Shimbara, N. A Novel Small-Molecule Inhibitor of NF-ΚB Signaling. Biochem. Biophys. Res. Commun. 2008, 368, 1007–1013. [Google Scholar] [CrossRef]
- Chen, R.; Li, Y.; Buttyan, R.; Dong, X. Implications of PI3K/AKT Inhibition on REST Protein Stability and Neuroendocrine Phenotype Acquisition in Prostate Cancer Cells. Oncotarget 2017, 8, 84863–84876. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, H.; Li, Y.; Tan, M.; Fan, S.; Cao, C.; Meng, F.; Zhu, L.; Zhao, L.; Guan, M.-X.; et al. Inhibiting Neddylation Modification Alters Mitochondrial Morphology and Reprograms Energy Metabolism in Cancer Cells. JCI Insight 2019, 4, e121582. [Google Scholar] [CrossRef]
- Xiong, H.; Zheng, D.; Liu, Y.; Ma, L.; Meng, L.; Yang, Z.; Yang, Z. Activation of the β-TrCP/IκBα/Inflammation Axis Limits the Sensitivity of Liver Cancer Cells to Neddylation Inhibition. Oncol. Rep. 2022, 48, 201. [Google Scholar] [CrossRef]
- Sikka, A.; Kaur, M.; Agarwal, C.; Deep, G.; Agarwal, R. Metformin Suppresses Growth of Human Head and Neck Squamous Cell Carcinoma via Global Inhibition of Protein Translation. Cell Cycle 2012, 11, 1374–1382. [Google Scholar] [CrossRef]
- Arafa, E.A.; Abdelazeem, A.H.; Arab, H.H.; Omar, H.A. OSU-CG5, a Novel Energy Restriction Mimetic Agent, Targets Human Colorectal Cancer Cells in Vitro. Acta Pharmacol. Sin. 2014, 35, 394–400. [Google Scholar] [CrossRef]
- Fernández-Ginés, R.; Encinar, J.A.; Hayes, J.D.; Oliva, B.; Rodríguez-Franco, M.I.; Rojo, A.I.; Cuadrado, A. An Inhibitor of Interaction between the Transcription Factor NRF2 and the E3 Ubiquitin Ligase Adapter β-TrCP Delivers Anti-Inflammatory Responses in Mouse Liver. Redox Biol. 2022, 55, 102396. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-H.; Chang, L.-S. Suppression of Akt/Foxp3-Mediated MiR-183 Expression Blocks Sp1-Mediated ADAM17 Expression and TNFα-Mediated NFκB Activation in Piceatannol-Treated Human Leukemia U937 Cells. Biochem. Pharmacol. 2012, 84, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, A.-Y.; Cho, J.-H.; Yoon, M.-H.; Woo, T.-G.; Kang, S.; Lee, H.-Y.; Jung, Y.; Park, B.-J. Therapeutic Effect of Quinacrine, an Anti-Protozoan Drug, by Selective Suppression of p-CHK1/2 in P53-Negative Malignant Cancers. Mol. Cancer Res. 2018, 16, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rokes, C.; Gireud, M.; Fletcher, S.; Baumgartner, J.; Fuller, G.; Stewart, J.; Zage, P.; Gopalakrishnan, V. Retinoic Acid Induces REST Degradation and Neuronal Differentiation by Modulating the Expression of SCF(β-TRCP) in Neuroblastoma Cells. Cancer 2011, 117, 5189–5202. [Google Scholar] [CrossRef]
- Wei, S.; Yang, H.-C.; Chuang, H.-C.; Yang, J.; Kulp, S.K.; Lu, P.-J.; Lai, M.-D.; Chen, C.-S. A Novel Mechanism by Which Thiazolidinediones Facilitate the Proteasomal Degradation of Cyclin D1 in Cancer Cells*. J. Biol. Chem. 2008, 283, 26759–26770. [Google Scholar] [CrossRef]
- Wei, S.; Lin, L.-F.; Yang, C.-C.; Wang, Y.-C.; Chang, G.-D.; Chen, H.; Chen, C.-S. Thiazolidinediones Modulate the Expression of β-Catenin and Other Cell-Cycle Regulatory Proteins by Targeting the F-Box Proteins of Skp1-Cul1-F-Box Protein E3 Ubiquitin Ligase Independently of Peroxisome Proliferator-Activated Receptor γ. Mol. Pharmacol. 2007, 72, 725–733. [Google Scholar] [CrossRef]
- Simonetta, K.R.; Taygerly, J.; Boyle, K.; Basham, S.E.; Padovani, C.; Lou, Y.; Cummins, T.J.; Yung, S.L.; von Soly, S.K.; Kayser, F.; et al. Prospective Discovery of Small Molecule Enhancers of an E3 Ligase-Substrate Interaction. Nat. Commun. 2019, 10, 1402. [Google Scholar] [CrossRef]
- Omar, H.A.; Tolba, M.F.; Saber-Ayad, M.M. Potential Targets of Energy Restriction Mimetic Agents in Cancer Cells. Future Oncol. 2014, 10, 2547–2550. [Google Scholar] [CrossRef]
- Kuntz, S.; Mazerbourg, S.; Boisbrun, M.; Cerella, C.; Diederich, M.; Grillier-Vuissoz, I.; Flament, S. Energy Restriction Mimetic Agents to Target Cancer Cells: Comparison between 2-Deoxyglucose and Thiazolidinediones. Biochem. Pharmacol. 2014, 92, 102–111. [Google Scholar] [CrossRef]
- Hersi, F.; Omar, H.A.; Al-Qawasmeh, R.A.; Ahmad, Z.; Jaber, A.M.; Zaher, D.M.; Al-Tel, T.H. Design and Synthesis of New Energy Restriction Mimetic Agents: Potent Anti-Tumor Activities of Hybrid Motifs of Aminothiazoles and Coumarins. Sci. Rep. 2020, 10, 2893. [Google Scholar] [CrossRef]
- Wei, S.; Kulp, S.K.; Chen, C.-S. Energy Restriction as an Antitumor Target of Thiazolidinediones*. J. Biol. Chem. 2010, 285, 9780–9791. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Chuang, H.-C.; Tsai, W.-C.; Yang, H.-C.; Ho, S.-R.; Paterson, A.J.; Kulp, S.K.; Chen, C.-S. Thiazolidinediones Mimic Glucose Starvation in Facilitating Sp1 Degradation through the Up-Regulation of β-Transducin Repeat-Containing Protein. Mol. Pharmacol. 2009, 76, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, W.; Sun, Y.; Jia, L. Protein Neddylation and Its Alterations in Human Cancers for Targeted Therapy. Cell Signal. 2018, 44, 92–102. [Google Scholar] [CrossRef] [PubMed]
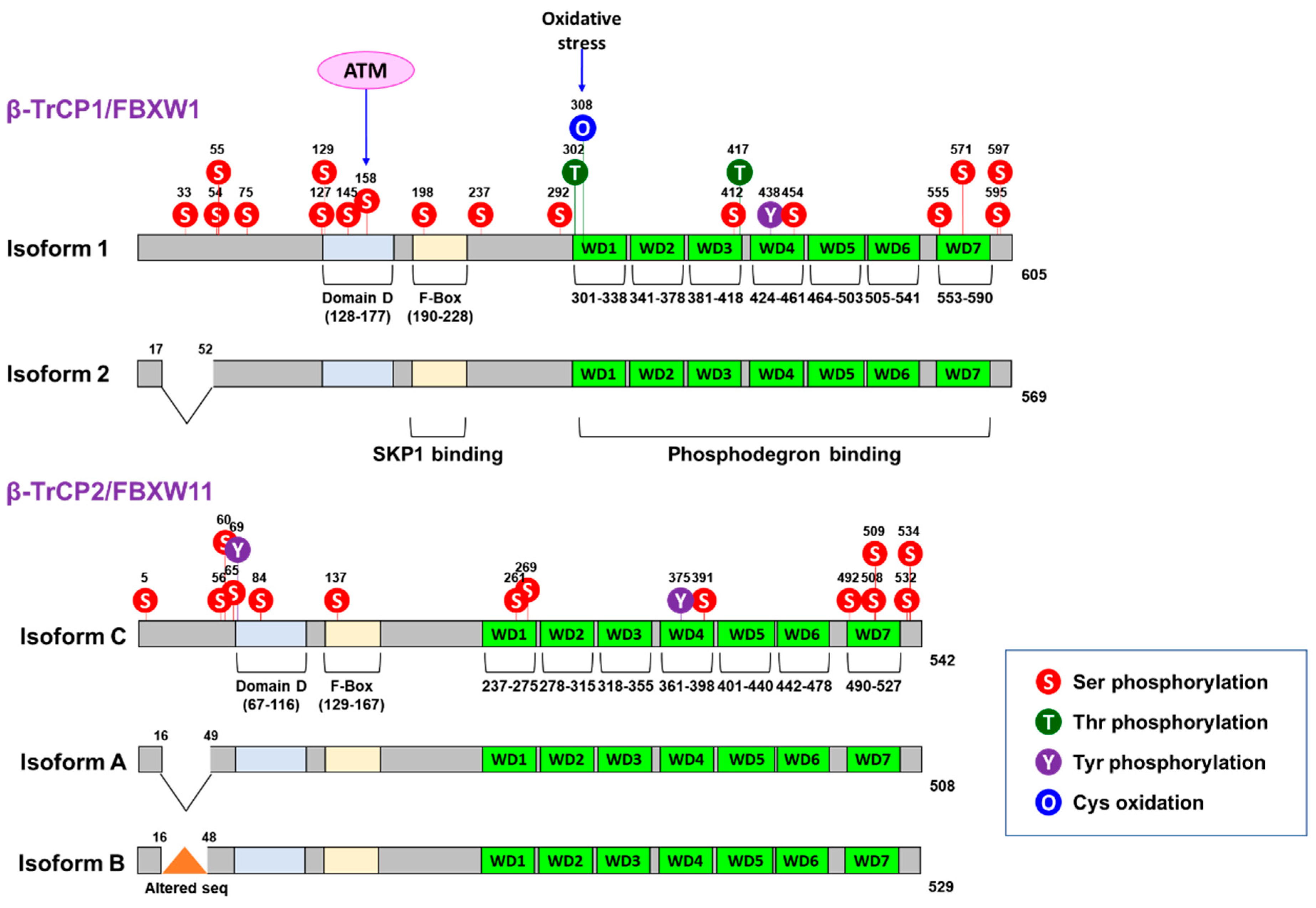
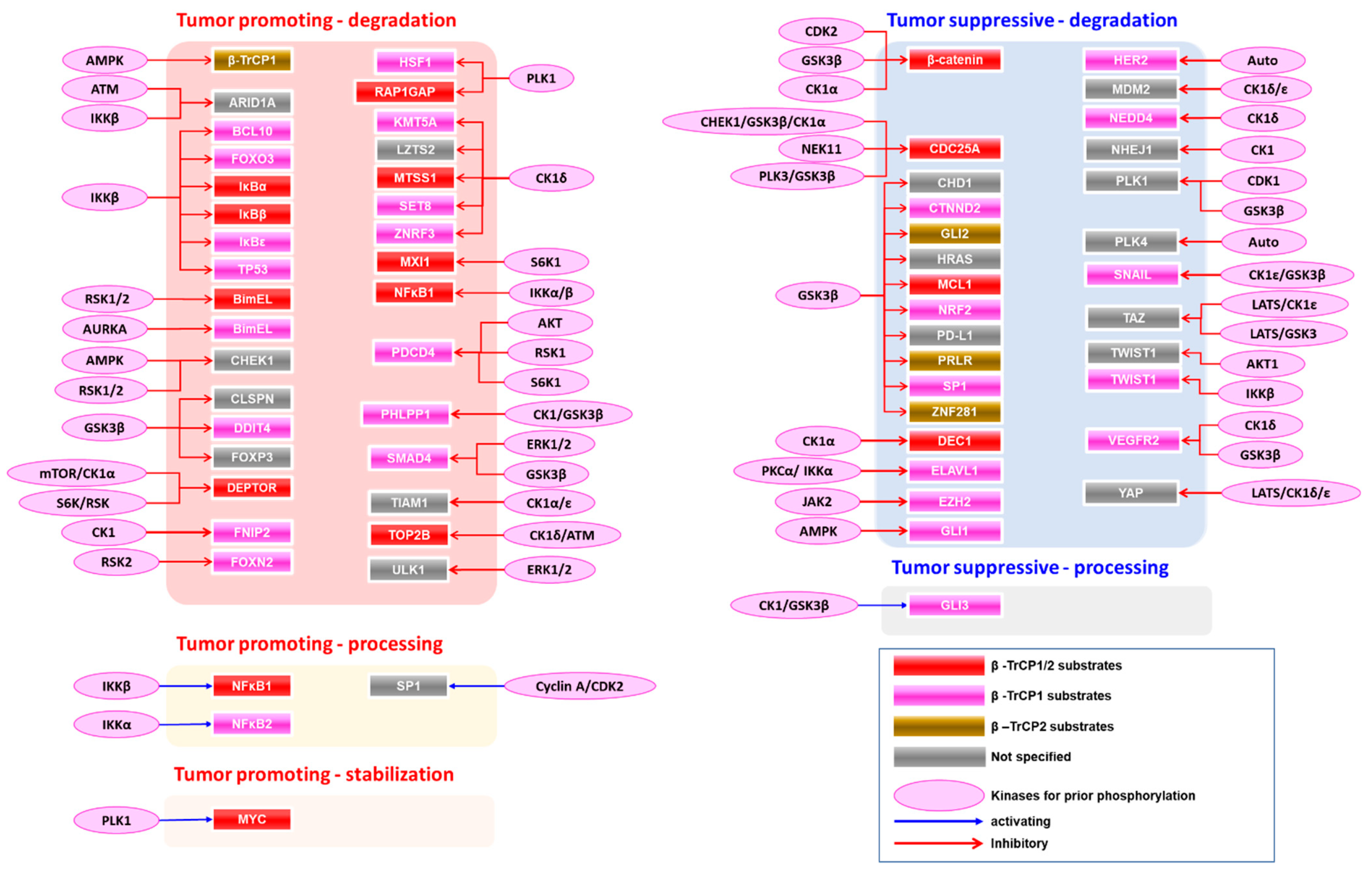
| Effector | β-TrCP Isoform | Biological Consequences | Role in Cancer | Ref. |
|---|---|---|---|---|
| Protein (Also Known as) | ||||
| β-TrCP1 | β-TrCP2 |
| suppressive | [116] |
| β-TrCP2 | β-TrCP1 |
| promoting | [116] |
| ACTL6A (BAF53A) | not specified |
| promoting | [117] |
| AKT | β-TrCP1 [73] |
| promoting | [73,88] |
| AMPK | not specified |
| - | [118] |
| ATM | β-TrCP1 |
| - | [115] |
| BRAFV600E | β-TrCP2 |
| promoting | [119] |
| CD147 | not specified |
| promoting | [120] |
| CD166 | not specified |
| suppressive | [121] |
| CENP-W | β-TrCP1 |
| promoting | [122] |
| CSN6 | not specified |
| suppressive | [123] |
| ERK2 | β-TrCP1 |
| - | [124] |
| FAF1 | not specified |
| suppressive | [125] |
| FBXW8 | β-TrCP1 |
| promoting | [126,127] |
| IGF2BP1 (CRD-BP) | β-TrCP1 |
| promoting | [66] |
| JNK | β-TrCP1 |
| - | [114] |
| JNKK2 | β-TrCP1 |
| - | [114] |
| MKK6 | β-TrCP1 |
| - | [114] |
| mTORC2 | not specified |
| promoting | [27] |
| NOTCH1 | not specified |
| promoting | [128] |
| OTUD5 | β-TrCP1 |
| promoting | [129] |
| P38 | β-TrCP1 |
| - | [114] |
| RASSF1A | β-TrCP1/2 |
| suppressive | [130] |
| RASSF1C | β-TrCP1 |
| promoting | [131] |
| RBX2 (SAG) | β-TrCP1 |
| promoting | [132] |
| PDGF | not specified |
| - | [133] |
| PHF19 | not specified |
| promoting | [134] |
| RPS27L | β-TrCP1 |
| promoting | [134] |
| SIRT1 | β-TrCP1 |
| - | [135] |
| SKP2 | β-TrCP1 |
| - | [136] |
| SOX9 | β-TrCP1 |
| promoting | [137] |
| SRC | not specified |
| promoting | [138] |
| SMURF1 | not specified |
| suppressive | [139] |
| SMURF2 (UBCH5) | β-TrCP1 |
| promoting | [140] |
| TSPAN15 | β-TrCP1 |
| promoting | [141] |
| USP24 | not specified |
| promoting | [142] |
| USP47 | β-TrCP1/2 |
| promoting | [143] |
| WBP2 | β-TrCP1 |
| promoting | [144] |
| WNT/β-catenin | β-TrCP2 |
| - | [113] |
| WNT1/2 | β-TrCP1 |
| promoting | [68] |
| Nucleic acids | ||||
| circHIPK3 | β-TrCP1 |
| - | [145] |
| circPVT1 | not specified |
| promoting | [146] |
| LINC00460 | β-TrCP1 |
| promoting | [147] |
| LINC00941 | β-TrCP1 |
| promoting | [148] |
| LINC00942 | β-TrCP1 |
| promoting | [149] |
| miR-10a | β-TrCP1 |
| - | [150] |
| miR-106b-25 | β-TrCP2 |
| promoting | [151] |
| miR-135b | β-TrCP1 |
| promoting | [152] |
| miR-182 | β-TrCP2 | promoting | [153,154] | |
| miR-183 | β-TrCP1 | promoting [155] suppressive [156] | [155,156] | |
| miR-193a-3p | β-TrCP1 |
| promoting | [157] |
| miR-221 | β-TrCP2 |
| promoting | [158] |
| miR-224 | β-TrCP1 |
| promoting | [159] |
| miR-324-5p | β-TrCP1 |
| suppressive | [160] |
| SLC7A11-AS1 (lncRNA) | β-TrCP1 |
| promoting | [161] |
| Endogenous small molecules | ||||
| Androgen (dihydrotestosterone (DHT)) | not specified |
| promoting | [162] |
| Protein | β-TrCP Isoform | MoA | Biological Consequences | Roles in Cancer |
|---|---|---|---|---|
| 14-3-3ζ (YWHAZ) | not specified | competitive binding to β-catenin |
| promoting |
| AP2-β | not specified | binding to β-catenin and β-TrCP |
| suppressive |
| ERα | not specified | binding to β-catenin |
| suppressive |
| RASS5 (NORE1A) | β-TrCP1 | direct binding to β-TrCP1 |
| suppressive |
| TRIB2 | not specified | direct binding to β-TrCP |
| suppressive |
| TRIB3 | not specified | binding to TAZ |
| promoting |
| TRIM9 | β-TrCP1/2 | direct binding to β-TrCP |
| - |
| TRIM67 | not specified | direct binding to β-TrCP |
| - |
| UBTD1 | not specified | direct binding to β-TrCP |
| - |
| Small Molecule | Known Targets | Functions | Ref. |
|---|---|---|---|
| ABT-199/WEHI-539 combination | BCL2/BCL2L1 (respectively) |
| [220] |
| Acyclovir | β-TrCP1 |
| [221] |
| Aspirin | COX1/2 |
| [222] |
| AZD8055 | mTOR |
| [67] |
| Bergenin | unknown |
| [223] |
| CHIR-99021 | GSK3α/β |
| [27] |
| CIB-6 | STAT3 |
| [224] |
| Corosolic acids | unknown |
| [225] |
| Curcumin | P300/HDAC |
| [226] |
| Decitabine | DNA methyltransferase |
| [227] |
| Diindolylmethane, 3,3′- | AR |
| [228] |
| Dihydrocapsaicin | TRPV1 |
| [229] |
| Doxorubicin | DNA topo II |
| [230] |
| Echinomycin | HIF1 |
| [231] |
| Erioflorin | β-TrCP1 |
| [219] |
| Euphorbiasteroid | AMPK |
| [232] |
| Fisetin | SIRTs |
| [233] |
| Gallic acid | - |
| [234] |
| GS143 | β-TrCP1–p-IκBα interaction |
| [235] |
| Hydroquinone | Melanin synthesis |
| [155] |
| INK128 | mTOR |
| [67] |
| LY294002 | PI3Kα/δ/β |
| [236] |
| MLN4924 (pevonedistat) | NEDD8 |
| [237] |
| [238] | ||
| Metformin | AMPK |
| [239] |
| MK2206 | AKT |
| [27] |
| OSU-CG5 | Energy metabolism |
| [240] |
| PF4708671 | P70S6K |
| [67] |
| PHAR | β-TrCP1/NRF2 interaction |
| [241] |
| PI3Kα inhibitors | PI3Kα |
| [27] |
| PI3K/mTOR dual-inhibitors | PI3K/mTOR |
| [27] |
| Piceatannol | SYK |
| [242] |
| Quinacrine | PLA2 |
| [156] |
| [243] | ||
| Rapamycin | mTORC1 |
| [27] |
| Retinoic acid, all-trans | RAR/RXR |
| [244] |
| STG28 | PPARγ |
| [245,246] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.J.; Yi, Y.W.; Seong, Y.-S. Beta-Transducin Repeats-Containing Proteins as an Anticancer Target. Cancers 2023, 15, 4248. https://doi.org/10.3390/cancers15174248
Kim DJ, Yi YW, Seong Y-S. Beta-Transducin Repeats-Containing Proteins as an Anticancer Target. Cancers. 2023; 15(17):4248. https://doi.org/10.3390/cancers15174248
Chicago/Turabian StyleKim, Dong Joon, Yong Weon Yi, and Yeon-Sun Seong. 2023. "Beta-Transducin Repeats-Containing Proteins as an Anticancer Target" Cancers 15, no. 17: 4248. https://doi.org/10.3390/cancers15174248
APA StyleKim, D. J., Yi, Y. W., & Seong, Y.-S. (2023). Beta-Transducin Repeats-Containing Proteins as an Anticancer Target. Cancers, 15(17), 4248. https://doi.org/10.3390/cancers15174248






