Dynamic Optical Coherence Tomography of Blood Vessels in Cutaneous Melanoma—Correlation with Histology, Immunohistochemistry and Dermoscopy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
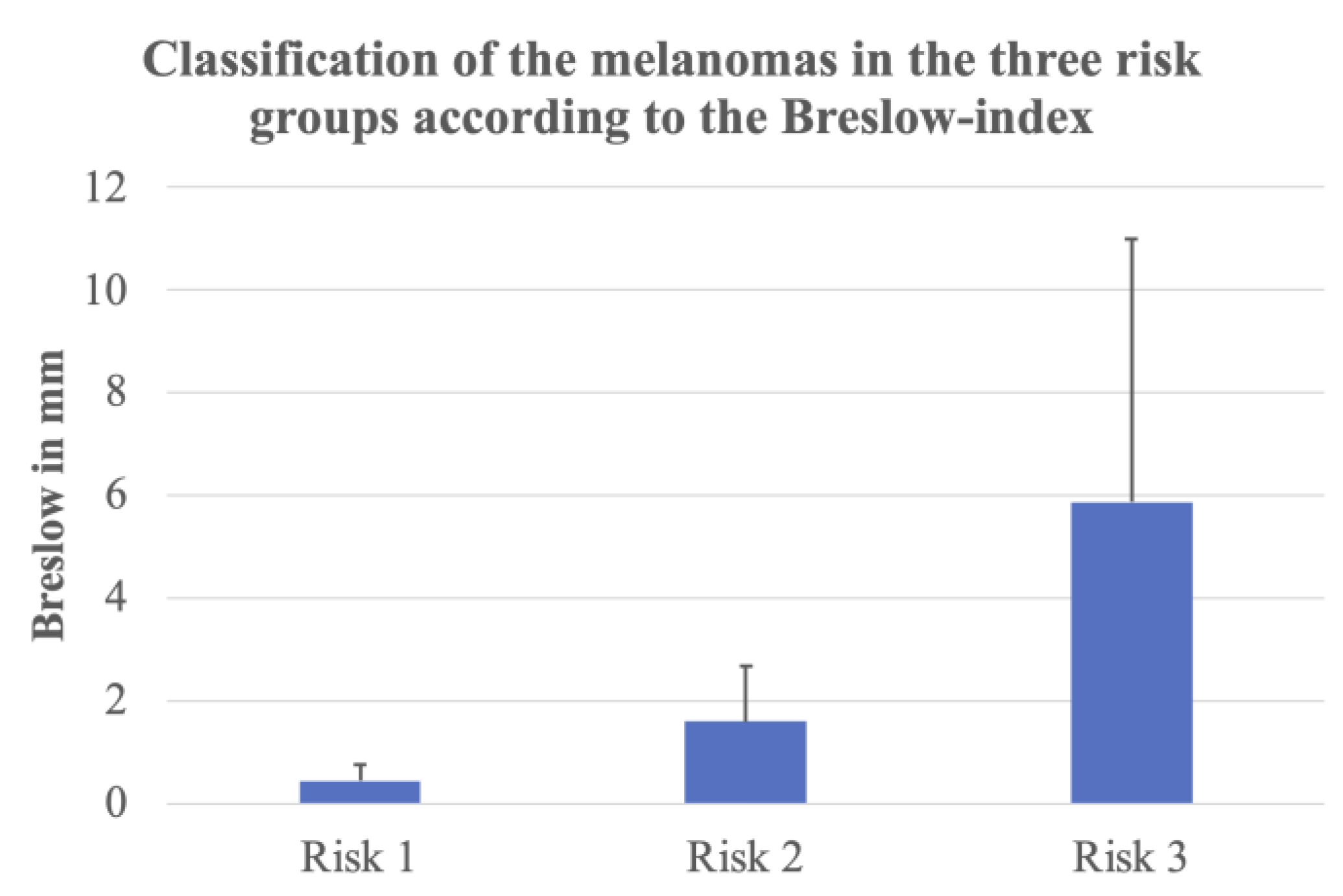
3.1. Cutaneous Melanomas
3.2. Blood Vessels in D-OCT and Comparison with Histology
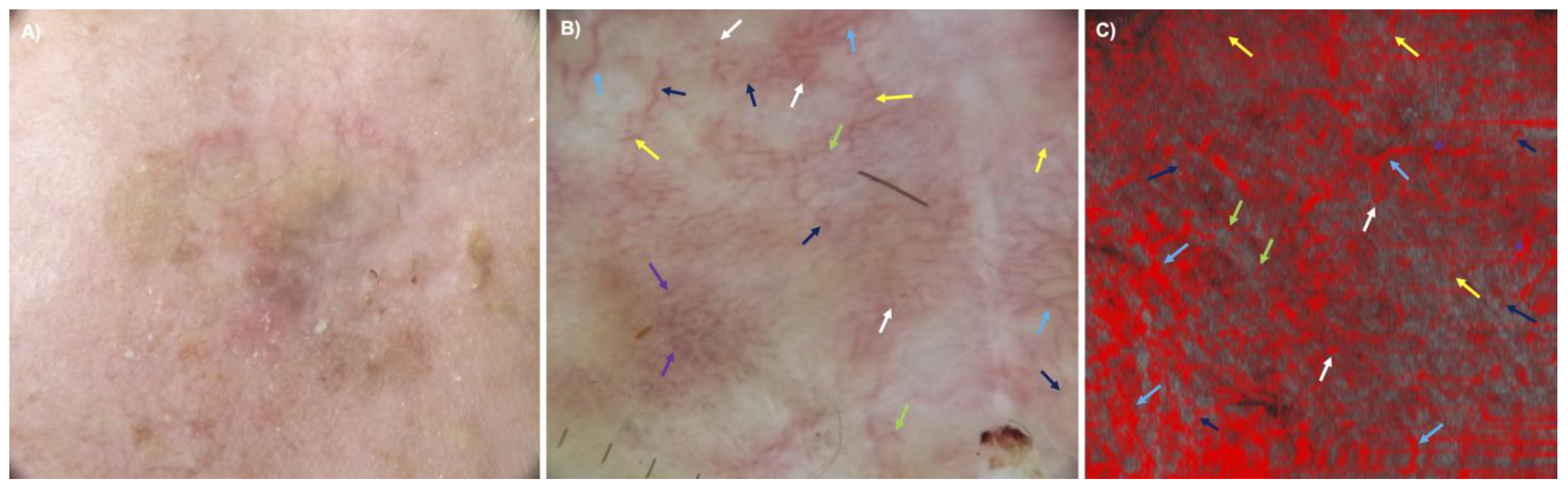
3.3. Comparison between Dermoscopy and D-OCT
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, M.; Geller, A.C.; Tucker, M.A.; Guy, G.P., Jr.; Weinstock, M.A. Melanoma burden and recent trends among non-Hispanic whites aged 15–49 years, United States. Prev. Med. 2016, 91, 294–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ulrich, M.; Braunmuehl, T.; Kurzen, H.; Dirschka, T.; Kellner, C.; Sattler, E.; Berking, C.; Welzel, J.; Reinhold, U. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: An observational study. Br. J. Dermatol. 2015, 173, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Nguyen, B.T. The utility of optical coherence tomography for diagnosis of basal cell carcinoma: A quantitative review. Br. J. Dermatol. 2019, 18, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Themstrup, L.; De Carvalho, N.; Nielsen, S.M.; Olsen, J.; Ciardo, S.; Schuh, S.; Nørnberg, B.M.-H.; Welzel, J.; Ulrich, M.; Pellacani, G.; et al. In vivo differentiation of common basal cell carcinoma subtypes by microvascular and structural imaging using dynamic optical coherence tomography. Exp. Dermatol. 2018, 27, 156–165. [Google Scholar] [CrossRef]
- Markowitz, O.; Schwartz, M.; Minhas, S.; Siegel, D.M. Speckle-variance optical coherence tomography: A novel approach to skin cancer characterization using vascular patterns. Dermatol. Online J. 2016, 22. [Google Scholar] [CrossRef]
- De Carvalho, N.; Ciardo, S.; Cesinaro, A.M.; Jemec, G.B.E.; Ulrich, M.; Welzel, J.; Holmes, J.; Pellacani, G. In vivo micro-angiography by means of speckle-variance optical coherence tomography (SV-OCT) is able to detect microscopic vascular changes in naevus to melanoma transition. J. Eur. Acad. Dermatol. Venereol. 2016, 30, e67–e68. [Google Scholar] [CrossRef]
- Welzel, J.; Schuh, S.; De Carvalho, N.; Themstrup, L.; Ulrich, M.; Jemec, G.; Holmes, J.; Pellacani, G. Dynamic optical coherence tomography shows characteristic alterations of blood vessels in malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1087–1093. [Google Scholar] [CrossRef]
- Ulrich, M.; Themstrup, L.; de Carvalho, N.; Ciardo, S.; Holmes, J.; Whitehead, R.; Welzel, J.; Jemec, G.; Pellacani, G. Dynamic optical coherence tomography of skin blood vessels—Proposed terminology and practical guidelines. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 152–155. [Google Scholar] [CrossRef]
- Mariampillai, A.; Standish, B.A.; Moriyama, E.H.; Khurana, M.; Munce, N.R.; Leung, M.K.K.; Jiang, J.; Cable, A.; Wilson, B.C.; Vitkin, I.A.; et al. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt. Lett. 2008, 33, 1530–1532. [Google Scholar] [CrossRef]
- Manfredi, M.G.C.; Pellacani, G. Skin Surface Reconstruction and 3D Vessels Segmentation in Speckle Variance Optical Coherence Tomography. In Proceedings of the 11th Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications, Rome, Italy, 27–29 February 2016. [Google Scholar]
- Ulrich, M.; Themstrup, L.; de Carvalho, N.; Manfredi, M.; Grana, C.; Ciardo, S.; Kästle, R.; Holmes, J.; Whitehead, R.; Jemec, G.B.; et al. Dynamic Optical Coherence Tomography in Dermatology. Dermatology 2016, 232, 298–311. [Google Scholar] [CrossRef]
- Zalaudek, I.; Kreusch, J.; Giacomel, J.; Ferrara, G.; Catricalà, C.; Argenziano, G. How to diagnose nonpigmented skin tumors: Review of vascular structures seen with dermoscopy: Part I. Melanocytic skin tumors. J. Am. Acad. Dermatol. 2010, 63, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Bichakjian, C.K.; Halpern, A.C.; Johnson, T.M.; Hood, A.F.; Grichnik, J.M.; Swetter, S.M.; Tsao, H.; Barbosa, V.H.; Chuang, T.-Y.; Duvic, M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2011, 65, 1032–1047. [Google Scholar] [CrossRef] [PubMed]
- Breslow, A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann. Surg. 1970, 172, 902–908. [Google Scholar] [CrossRef]
- German Guideline Program in Oncology (German Cancer Society GCA, AWMF). Evidence-Based Guideline on Prevention of Skin Cancer Long Version 2.1. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/hautkrebs-praevention/ (accessed on 26 March 2023).
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Lindsø Andersen, P.; Olsen, J.; Friis, K.B.E.; Themstrup, L.; Grandahl, K.; Mortensen, O.S.; Jemec, G.B.E. Vascular morphology in normal skin studied with dynamic optical coherence tomography. Exp. Dermatol. 2018, 27, 966–972. [Google Scholar] [CrossRef]
- De Carvalho, N.; Welzel, J.; Schuh, S.; Themstrup, L.; Ulrich, M.; Jemec, G.B.E.; Holmes, J.; Kaleci, S.; Chester, J.; Bigi, L.; et al. The vascular morphology of melanoma is related to Breslow index: An in vivo study with dynamic optical coherence tomography. Exp. Dermatol. 2018, 27, 1280–1286. [Google Scholar] [CrossRef]
- Wang, S.Q.; Dusza, S.W.; Scope, A.; Braun, R.P.; Kopf, A.W.; Marghoob, A.A. Differences in dermoscopic images from nonpolarized dermoscope and polarized dermoscope influence the diagnostic accuracy and confidence level: A pilot study. Dermatol. Surg. 2008, 34, 1389–1395. [Google Scholar] [CrossRef][Green Version]
- Benvenuto-Andrade, C.; Dusza, S.W.; Agero, A.L.C.; Scope, A.; Rajadhyaksha, M.; Halpern, A.C.; Marghoob, A.A. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch. Dermatol. 2007, 143, 329–338. [Google Scholar] [CrossRef]
- Pan, Y.; Gareau, D.S.; Scope, A.; Rajadhyaksha, M.; Mullani, N.A.; Marghoob, A.A. Polarized and nonpolarized dermoscopy: The explanation for the observed differences. Arch. Dermatol. 2008, 144, 828–829. [Google Scholar] [CrossRef]
- Themstrup, L.; Pellacani, G.; Welzel, J.; Holmes, J.; Jemec, G.B.E.; Ulrich, M. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Regeniter, P.; Bechara, F.G.; Orlikov, A.; Vasa, R.; Moussa, G.; Stücker, M.; Altmeyer, P.; Hoffmann, K. Characterization of benign and malignant melanocytic skin lesions using optical coherence tomography in vivo. J. Am. Acad. Dermatol. 2007, 57, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Jaedicke, V.; Terras, S. Optical coherence tomography in dermatology: Technical and clinical aspects. Arch. Dermatol. Res. 2011, 303, 457–473. [Google Scholar] [CrossRef]
- Gambichler, T.; Schmid-Wendtner, M.; Plura, I.; Kampilafkos, P.; Stücker, M.; Berking, C.; Maier, T. A multicentre pilot study investigating high-definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Plura, I.; Schmid-Wendtner, M.; Valavanis, K.; Kulichova, D.; Stücker, M.; Pljakic, A.; Berking, C.; Maier, T. High-definition optical coherence tomography of melanocytic skin lesions. J. Biophotonics 2015, 8, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Welzel, J. Optical coherence tomography in dermatology: A review. Ski. Res. Technol. 2001, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tejera-Vaquerizo, A.; Arias-Santiago, S.; Nagore, E.; Martín-Cuevas, P.; Orgaz-Molina, J.; Traves, V.; Herrera-Acosta, E.; Naranjo-Sintes, R.; Guillén, C.; Herrera-Ceballos, E. Defining the dermoscopic characteristics of fast-growing cutaneous melanomas. Melanoma Res. 2015, 25, 269–272. [Google Scholar] [CrossRef]
- Pizzichetta, M.; Kittler, H.; Stanganelli, I.; Ghigliotti, G.; Corradin, M.; Rubegni, P.; Cavicchini, S.; De Giorgi, V.; Bono, R.; Alaibac, M.; et al. Dermoscopic diagnosis of amelanotic/hypomelanotic melanoma. Br. J. Dermatol. 2017, 177, 538–540. [Google Scholar] [CrossRef]
- Blum, A.; Kreusch, J.; Stolz, W.; Argenziano, G.; Forsea, A.-M.; Marmol, V.D.; Zalaudek, I.; Soyer, H.P.; Haenssle, H.A. The status of dermoscopy in Germany—Results of the cross-sectional Pan-Euro-Dermoscopy Study. J. Dtsch. Dermatol. Ges. 2018, 16, 174–181. [Google Scholar] [CrossRef]
- Babino, G.; Lallas, A.; Longo, C.; Moscarella, E.; Alfano, R.; Argenziano, G. Dermoscopy of melanoma and non-melanoma skin cancer. G. Ital. Dermatol. Venereol. 2015, 150, 507–519. [Google Scholar]
- Russo, T.; Piccolo, V.; Ferrara, G.; Agozzino, M.; Alfano, R.; Longo, C.; Argenziano, G. Dermoscopy pathology correlation in melanoma. J. Dermatol. 2017, 44, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Sattler, E.; Kästle, R.; Welzel, J. Optical coherence tomography in dermatology. J. Biomed. Opt. 2013, 18, 061224. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuh, S.; Sattler, E.C.; Rubeck, A.; Schiele, S.; De Carvalho, N.; Themstrup, L.; Ulrich, M.; Jemec, G.B.E.; Holmes, J.; Pellacani, G.; et al. Dynamic Optical Coherence Tomography of Blood Vessels in Cutaneous Melanoma—Correlation with Histology, Immunohistochemistry and Dermoscopy. Cancers 2023, 15, 4222. https://doi.org/10.3390/cancers15174222
Schuh S, Sattler EC, Rubeck A, Schiele S, De Carvalho N, Themstrup L, Ulrich M, Jemec GBE, Holmes J, Pellacani G, et al. Dynamic Optical Coherence Tomography of Blood Vessels in Cutaneous Melanoma—Correlation with Histology, Immunohistochemistry and Dermoscopy. Cancers. 2023; 15(17):4222. https://doi.org/10.3390/cancers15174222
Chicago/Turabian StyleSchuh, Sandra, Elke Christina Sattler, Anna Rubeck, Stefan Schiele, Nathalie De Carvalho, Lotte Themstrup, Martina Ulrich, Gregor B. E. Jemec, Jon Holmes, Giovanni Pellacani, and et al. 2023. "Dynamic Optical Coherence Tomography of Blood Vessels in Cutaneous Melanoma—Correlation with Histology, Immunohistochemistry and Dermoscopy" Cancers 15, no. 17: 4222. https://doi.org/10.3390/cancers15174222
APA StyleSchuh, S., Sattler, E. C., Rubeck, A., Schiele, S., De Carvalho, N., Themstrup, L., Ulrich, M., Jemec, G. B. E., Holmes, J., Pellacani, G., & Welzel, J. (2023). Dynamic Optical Coherence Tomography of Blood Vessels in Cutaneous Melanoma—Correlation with Histology, Immunohistochemistry and Dermoscopy. Cancers, 15(17), 4222. https://doi.org/10.3390/cancers15174222





