Locoregional Treatment in Intrahepatic Cholangiocarcinoma: Which Treatment for Which Patient?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Narrative Review about Current Data Regarding LRT of iCC
2.1. Radiofrequency Ablation (RFA) and Microwave Ablation (MWA)
| Authors | Methods | Retrospective or Prospective Study | Patients (n) | Tumor (n) | Median Tumor Size (cm) | Number of Lesions | Extrahepatic Disease Patients (n) or % of Patients | Efficacy: Local Tumor Progression | Grade 3–5 Treatment Related Toxicities |
|---|---|---|---|---|---|---|---|---|---|
| One arm cohort | |||||||||
| Butros [16] | RFA | Retrospective study | 7 | 9 | 2.4 (1.3–3.3) | 1–2 | 1/9 (11%) | No major complication | |
| Fu [17] | RFA | Retrospective study | 17 | 26 | 4.4 (2.1–6.9) | 1–5 | 7% | 3/17 (17.6%) | 1 major compli- cation occurred (3.6%, 1 of 28 sessions) (pleural effusion) |
| Kim [15] | RFA | Retrospective study | 13 | 17 | 0.8–8 | 1–2 | 6/17 (35.3%) | 1 patient died following liver abscess | |
| Kim [18] | RFA | Retrospective study | 20 | 29 | 1.5 (0.7–4.4) | 6/29 (20.7%) | 2 major complications occurred (7%) (liver abscess, bile duct stenosis) | ||
| Carrafiello [19] | RFA | Retrospective study | 6 | 6 | 3.45 (1.0–5.8) | 3/6 (50%) | No major complication | ||
| Chiou [20] | RFA | Prospective cohort | 10 | 10 | 1.9–6.8 | 1 | 2/10 (20%) | No major complication | |
| Haidu [21] | RFA | Retrospective study | 11 | 36 | 3 (0.5–10) | 3% | 3/36 (8%) | Major complication rate: 13% (3/23) (bleeding, pseudoaneurysm, pulmonary embolism) | |
| Brandi [8] | RFA | Retrospective observational cohort study | 29 | 117 | 1.7 (0.5–4.8) | Local tumor progression-free survival: 9.27 months (7.34–11.15) | Major complication rate: 7% (8/117) (liver abscess, pleural effusion, biloma, intrahepatic hematoma) | ||
| Díaz-González [9] | MWA (microwave) and RFA | Retrospective analysis | 27 | 2.1 | 1–2 | 0 | 21/27 (77.8%) | Data not known | |
| Ni [11] | MWA | Retrospective study | 78 | 106 | 3.1 (0.8–5.0) | 1–3 | 0 | 3 patients (3.8%) had major complication (liver abscess, pleural effusion) | |
| Takahashi [22] | 6 MWA and 44 RFA | Retrospective review | 20 | 50 | 1.8 (0.5–4.7) | 11/50 tumor Or 5/20 patients | No major complication | ||
| Zhang [13] | MWA | Retrospective study | 107 | 171 | <5 | 1–3 | 3 patients (2.8%) presented major complication (pleural effusion, liver abscess) | ||
| Xu [14] | MWA and RFA | Retrospective study | 18 | 25 | 2.8 (0.7–6.9) | 1–4 | 0 | 12/18 | 1 patient presented major complication (fever) |
| Ge [23] | MWA | Retrospective study | 92 (compared to 183 TACE) | 3.3–8.1 | Data not known | ||||
| Giorgio [24] | MWA versus RFA | Retrospective study | 71 36 RFA 35 MWA | 98 | 3.6 (2.2–7.2) | 0 | No major complication | ||
| Xu [10] | MWA versus surgery | Retrospective study | 121 56 MWA 65 surgery | 2.7 (0.8–5.0) | 11/56 (19.6%) | MWA: rate of major complication was 5.6% (3/56) (hepatic failure, ascites, liver absces) | |||
| Zhang [12] | Ablation vs. surgery | Retrospective study | 32 surgery 77 ablation | <5 | 0 | Major complication rate: 3.9% (3/77) (hepatic failure, liver abscesses) | |||
2.2. External Beam Radiotherapy (EBRT)
2.3. Intra-Arterial Treatment (IAT)
2.3.1. Yttrium-90 Microsphere Selective Internal Radiation Therapy
| Authors | Retrospective or Prospective Study | Treatment Dose | Patients (n) | Extrahepatic Disease Patients (n) or % of Patients | Median Tumor Size (cm) | Efficacy | Grade 3–5 Treatment-Related Toxicities |
|---|---|---|---|---|---|---|---|
| Non-comparative arm | |||||||
| Shimizu [25] | Retrospective study | Range: 46.6 Gy in 12 fractions to 74.0 Gy in 37 fractions 16 patients received concomitant CT | 37 | 5.7 (1.5–14) | The 1-year local control rate: 97.3% (95% CI: 92.0–100%) | 3 patients experienced grade 3 biliary tract infections | |
| Smart [31] | Retrospective study | Median dose: 58.05 Gy (37.5–67.5) | 66 | 23/66 patients | 5.6 (2.5–16) | Disease recurrence: 42/66 (74%) Local failure: 5/66 | 11% (7/66) patients had grade 3 and 4 toxicities (thrombocytopenia, neutropenia, nausea, anorexia, abdominal pain, dehydration, fever, RILD) |
| Kozak [27] | Retrospective study | Median dose: 40 Gy (26–50) Median fractions: 5 (1–5) | 40 | 4.2 (1.0–12.5) | Local failure: 12/40 | 16 patients (40%) experienced grade 3 toxicity (abdominal pain, infection, biliary complication, liver abscess, cholecystitis, elevated liver enzymes) | |
| Kasuya [37] | Retrospective study | Carbon-ion radiotherapy Most commonly prescribed dose: 76 Gy in 20 fractions | 56 | 3.7 (1.5–11) | The 1-year local control rate: 79.4% (IC 95%: 62.7–89.2) | 1 patient died of liver failure, 3 patients had liver dysfunction, and 1 patient presented a bile duct stenosis | |
| Shen [34] | Retrospective study | Median dose: 45 Gy (36–54) | 28 | The 1-year PFS rate: 50% | 28 patients had at least one grade 3 toxicity (gastrointestinal ulcers elevated liver enzyme, hematological toxicity). | ||
| Cho [26] | Retrospective study | Concomitantly with chemotherapy: For IMRT: 45 Gy to the PTV For 3D-CRT: 45 Gy in 25 fractions to the PTV | 64 patients concomitantly with chemotherapy, and 56 underwent surgery | 0/120 patients | The 3-year locoregional failure-free survival: 50% for patients who underwent surgery after radio–chemotherapy | 7.8% (5/64) patients had grade 3 toxicities (nausea, vomiting, epigastric pain, gastric bleeding) | |
| Weiner [28] | Prospective trial | Median dose: 55 Gy (40–55) | 26 patients but 12 HCC, 12 iCC and 2 mixed | 5.5 (1.6–12.3) | The 1-year local control rate: 91% 1-year PFS: 50% (95% IC: 29–69%) | 11 patients presented ≥ grade 3 toxicities (hematological toxicity, hepatic failure, abdominal pain, elevated liver enzymes, ascites, vomiting and skin fibrosis) | |
| Hong [30] | Prospective trial | 83 patients: 44 HCC 39 iCC | 0 | 5.7 (1.9–12.0) For iCC: 6 (2.2–10.9) | 4 patients (4.8%) presented grade 3 toxicity (thrombocytopenia, liver failure, ascites, gastric ulcer and elevated bilirubin) | ||
| Tao [38] | Retrospective study | 58.05 Gy (35–100) | 79 | 7.9 (2.2–17) | The 1-year local control rate: 81% | 4 patients had major complication (cholangitis, gastric bleeding) | |
| Ohkawa [29] | Retrospective study | Median total proton dose: 72.6 Gy in 22 fractions for intrahepatic region | 20 12 curative 8 palliative (4 stage IV and 4 stage IIIC for which the irradiation was not sufficient due to a too-wide tumor size | 4/20 patients | 5.0 (1.5–14) | The 1-year local control rate: 88% for the curative group | 5% (1/20) patients had grade 3 toxicities (bone marrow suppression) |
| Jung [32] | Retrospective study | 45 Gy in 3 fractions (range: 15 to 60 Gy in 1–5 fractions) SBRT alone or EBRT and SBRT | 58 | The 1-year local control rate: 85% | 6 patients (10%) experienced a toxicity ≥ grade 3 (cholangitis and bile duct stenosis, gastric perforation). | ||
| Kim [39] | Retrospective study | In association with chemotherapy: 44 Gy (25–60): 5 fractions of 2–3 Gy | 92:25 in the arm chemo-radiation | 7.6 ± 3.9 | Disease control rate: 56% | Grade 3 neutropenia occurred in 3/25 patients (12%) Grade 3 thrombopenia occurred in 5 (20%) 6/25 (24%) patients had > grade 3 toxicities. | |
| Ibarra [40] | Retrospective study | iCC: 30 Gy (22–50) 1–10 fractions | 32:21 HCC 11 iCC | 45.5% | The 1-year disease-free local progression: 50% | 9% (3/32) patients had grade 3 or 4 toxicities | |
| Tse [33] | Prospective trial | 36 Gy (24–54) | 41:31 HCC and 10 iCC | 2/10 iCC | Tumor volume: 172 cm3 (10–465) | The 1-year local control rate: 65% (95% IC 44–79%) | 18 events of grade 3 or 4 toxicities were observed (liver toxicity and nausea) |
| Yi [41] | Retrospective study | Chemoradiation | 176 | 0 | Response rate: 19.8% | Grade 3 thrombocytopenia occurred in 10.4% of patients | |
| Authors | Retrospective or Prospective Study | Patients (n) | Localisation | Patients with Extrahepatic Disease n (%) | Previous Treatment | Mean Activity (GBq) | Median Tumor size (cm) | Efficacy mOS or meanOS from the 1st RE | Grade 3–5 Treatment-Related Toxicities |
|---|---|---|---|---|---|---|---|---|---|
| Helmberger [42] | Prospective observational study | 1050 in the whole cohort 120 iCC | 36/120 (30%) | ICC patients: Chemotherapy: 39.2% received combined regimens based on gemcitabine Locoregional treatments: 34.2% (surgery for 26.7%) | mOS: 14.7 months (95% CI: 10.9–17.9) | Less than 2.5% patients presented grade 3–4 toxicities (gastritis, gastrointestinal ulcerations, radiation cholecystitis and REILD) | |||
| Azar [43] | Retrospective review | 96 in whole cohort and 22 iCC | Bilobar: 63.6% Unilobar: 35.4% | 2/22 (9.1%) | 16/22 (72.7%): Surgery: 8/22 (36.4%) Radiotherapy: 5/22 (22.7%) Chemotherapy: 12/22 (54.5%) Locoregional treatment: 1/22 (4.5%) | 1.5 (0.5–2.8) | Data not known | ||
| Bargellini [44] | Retrospective study SirSphere | 81 in whole cohort: 35 (42.2%) in group A: first-line treatment at first diagnosis or at recurrence after surgery 19 (23.5%) in group B: SIRT as consolidation treatment after radio-logical disease control following first-line chemotherapy 27 (33.3%) in group C: SIRT because of tumor progression after first-line chemotherapy | Bilobar: 49.4% | 8/81 (10%) | Surgery: 32/81 (39.5%) | 1.46 ± 0.49 | 59.8 ± 32.5 | mOS: 14.5 months (11.1–16.9) | No toxicity grade ≥ 3 was recorded |
| Buettner [45] | Retrospective study | 115 92: SIR-Sphere 22: resin microsphere 1: with both | Bilobar: 72% | 27/115 (24%) | Chemotherapy: 91/115 (79%) | Median administered activity measurements were 1.6 GBq (IQR (interquartile range), 1.3–1.9 GBq) for patients who received resin microspheres and 2.6 GBq (IQR, 1.5–3.8 GBq; p = 0.0017) for patients who received glass microspheres. | 7.2 (5.4–10.0) | Median OS after treatment was 11 months (95% CI: 8–13) | 4 patients experienced grade 3 toxicity (4%) (REILD) |
| Filippi [46] | Retrospective study | 20 SIR-Sphere | 8/20 (40%) | Chemotherapy: 11 patients Surgery (liver resection): 8 patients Ablation: 1 RT on metastatic site: 1 patient | 1.6 ± 0.4 GBq | meanOS 12.5 ± 1.5 months | Data not known | ||
| Köhler [47] | Retrospective study | 46 SIR-Sphere | Bilobar: 63% | 14/46 (30.4%) | 30/46 (65.2%) Chemotherapy: 28 patients Immunotherapy: 1 Radiotherapy: 4 Liver resection: 9 TACE: 1 | Median: 1.74 (0.51–3.26) | mOS: 9.5 months (95% CI: 6.1–12.9) | Data not known | |
| Edeline [36] | Phase 2 clinical trial SIRT in association with chemotherapy (GEMCIS) in 1st line | 41 | Unifocal: 34% | 7/41 (17%) (lung metastasis ≤ 1 cm) | Resection: 5 | The median dose delivered to the tumor was 317 Gy (range: 64–1673 Gy) | mOS: 22 months (95% CI: 14–52) | 29 patients (71%) experienced grade 3 or 4 toxicities (gastrointestinal, hematological, hepatobiliary and general toxicities). | |
| White [48] | Prospective single-arm observational | 61 SIR-sphere (74%) and therasphere (26%) | Bilobar: 64% | 22/61 (36%) | Chemotherapy: 56/61 (92%) | mOS: 8.7 months (95% CI: 5.3–12.1) | 7 events of grade 3–4 toxicities (fatigue, fever and perturbation of liver function) | ||
| Bourien [49] | Retrospective study | 64 | Bilobar: 56% | 10/64 (16%) | Resection: 15/64 (23%) Chemotherapy: 27/64 (42%) | 2.5 (0.6–7.7) | 7.7 (1.4–18.2) | 16.4 months (95% CI: 7.8–25.0) | 10 patients (16%) experienced grade 3 fatigue; 6 patients (9%) experienced grade 3 liver pain; 2 patients (3%) had grade 3 nausea; and 2 patients (3%) had hepatic failure. No grade 4 toxicity was reported |
| Gangi [50] | Retrospective study | 85 | Bilobar: 36.5% Solitary tumor: 61.2% | 36/85 (42.4%) | Chemotherapy: 61/85 (71.8%) Liver resection: 14/85 (16.5%) Radiotherapy: 4/85 (4.7%) | Median delivered dose was 136.0 Gy | 12.0 months (95% CI: 8.0–15.2) | 1 patient developed a grade 3 toxicity (liver abscess) | |
| Shaker [51] | Retrospective study | 17 9 SIR-sphere 8 therasphere | 5/17 (29.4%) | Chemotherapy: 5/17 patients | The thera-Sphere and SIR-Sphere groups were 158.2 ± 128.1 Gy and 34.5 ± 16.3 Gy, respectively | 7.4 cm ± 3.3 | mOS from the diagnosis 33.6 months (95% CI: 4–64.8) | Data not known | |
| Reimer [52] | Retrospective study | 21 | Bilobar: 19% Solitary: 57% | 3/21 (14%) | 0 | Median survival was 15 months | One of the patients had an ulceration of gastric mucosa. | ||
| Akinwande [53] | Retrospective study | 25 SIRT 15 TACE | 11/25 (44%) | Chemotherapy: 16/25 (64%) Surgery/ablation: 5/25 (20%) | 1.56 GBq (0.41–5.31) | TACE: 3/33 (9%) grade 3 or more treatment-related toxicities (grade 3 or more treatment related toxicities) SIRT: 4/39 (10%) grade 3 or more treatment-related toxicities (abdominal pain) | |||
| Swinburne [54] | Retrospective study | 34, but 5 patients were excluded without histological confirmation of ICC | 11/29 (37.9%) | Surgery: 7 patients Chemotherapy: 15 patients TACE: 1 patient EBRT: 2 patients. | 6.8–4.1 | Median survival: 9.1 months (95% CI: 1.7–16.4) | No major toxicity was observed | ||
| Jia [55] | Retrospective study | 24 | 1.6 ± 0.4 GBq. | 9.0 months (5.6–12.4) | Grade 3 toxicities were observed in 20.8% patients (5/24) (abdominal pain and vomiting) | ||||
| Soydal [56] | Retrospective study | 16 | 3/16 (19%) | Chemotherapy: 9/16 patients TACE: 1 patient Surgery: 2 patients | Mean 1.7 ± 0.1 GBq | The median overall survival time was calculated as 293 ± 70 days (154–431, 95% CI) | Data not known | ||
| Saxena [57] | Retrospective study | 25 | Bilobar: 80% | 12/25 (48%) | Liver resection: 10/25 (40%) Chemotherapy: 18/25 (72%) Ablation: 2/25 (8%) TACE/SIRT: 2/25 (8%) | 1.76 GBq (SD = 0.33; range, 1.0–2.21 GBq | Median survival: 9.3 months | 3/25 patients (12%) developed grade III albumin, alkaline phosphatase and bilirubin toxicity. 1 patient (4%) developed a duodenal ulcer. | |
| Manceau [58] | Retrospective study Concomitantly with chemotherapy | 35 | Bilobar: 71.4% | 2.6 ± 1.4GBq | Mean: 7.85 ± 3.47 | Median OS was 28.6 months (95% CI: 21.8 to ∞) | 6/35 (17%) patients presented hepatic dysfunction 1 patient presented with grade 3 cholecystitis. |
2.3.2. Transarterial Chemoembolization (TACE)
| Authors | Retrospective or Prospective Study | Chemotherapy | Patients (n) | Median Number of Tumor | Median Tumor Size (cm) | Localisation | Mean Number of Sessions/Patients | Extrahepatic Disease (%) of Patients | Efficacy | Grade 3–5 Toxicities |
|---|---|---|---|---|---|---|---|---|---|---|
| One arm | ||||||||||
| Zhou [66] | Retrospective study | DEB-TACE Epirubicin | 88 | Bilateral 33% | 50% | ORR = 65.9% mOS 9 months | No grade 3–4 toxicity was observed | |||
| Luo [67] | Prospective trial | DEB TACE | 37 | 5.7 (3–8.3) | Bilobar 27% Multifocal 67.6% | ORR = 66.7% after DEB-TACE treatment, mean OS of iCC patients was 376 days (95% CI: 341–412 days) | Data not known | |||
| Goerg [68] | Retrospective study | 100 mg cisplatin (CDDP), 50 mg doxorubicin and 10 mg mitomycin C | 18 | Bilobar 52% | 3.4 | mOS: 13.3 months (0.95-CI 8.9–17.7 months ORR = 61% | 1 severe toxicity 2 patient deaths due to liver abscess and sudden cardiac arrest. | |||
| Aliberti [69] | Prospective cohort | Doxorubicin DEBDOX and LIFDOX | 127 | 0 | Median OS of the LIFDOX group was 14.53 (95% confidence interval = 9.17–15.23) months. | DEBDOX: grade 3 toxicities were nausea/vomiting (24%) and fever (7%) LIFDOX: grade 3 toxicities were pain (7%). No grade 4 toxicity | ||||
| Hyder [65] | Retrospective study | Gemcitabine + cisplatin/ Cisplatin + doxorubicin + mitomycin Gemcitabine alone Cisplatin alone | 198 TACE 128 DEB TACE 11 Embolization 13 RE 46 | mOS 13.2 months (95% CI 10.8–15.8) | 16.8% patients developed a major complication (acute renal and hepatic failure, pulmonary embolism and liver abscess). | |||||
| Vogl [64] | Retrospective study | Mitomycin C Gemcitabine Mitomycin C and Gemcitabine Mitomycin C, Gemcitabine and Cisplatin | 115 | Bilobar 77.4% 59.6% multiple (>5) | Mean of 7.1 (range, 3–30) | 0 | mOS: 13 months | No major complication was reported. | ||
| Kiefer [70] | Retrospective study | Mitomycin-C, doxorubicin and cisplatinum | 62 | Mean, 2.0; range, 1–4 | 19% | Median survival from time of first chemoembolization was 15 months | Major complications occurred following 5 of the 165 procedures (3%) (pulmonary edema and elevated cardiac enzymes post procedure, a pulmonary infarct, postembolization syndrome, acute renal failure and dehydration post procedure.) | |||
| Schiffman [60] | Retrospective study | Irinotecan doxorubicin | 24 | 11.5 (4–33.3) | Median number of liver lesions was 3 but ranged from 1 to 25 lesions | 10% | ORR: 79% mOS: 17.5 months | 4 events of grade 3–5 toxicities were observed (hepatorenal syndrome led to death, sepsis from a port infection, hepatic insufficiency) | ||
| Shitara [71] | Prospective cohort | Mitomycin | 7.8 (range 3.0–16.0) | Number of tumors 3 (1 × 1010) | ORR: 50.0% mOS: 4.1 months | 5 patients (25%) presented grade 3–4 toxicities (gastroduodenal ulcer, epigastralgia) | ||||
| Gusani [72] | Retrospective study | GEMZAR CDDP OXALIPLATIN | 42 | 9.8 cm (range 1.3–17.0) | Median of 3.5 TACE treatments per patient (range 1–16) | 19% | Median overall survival from the date of first TACE treatment was 9.1 months | 2 patients presented grade 4 toxicities (acute myocardial infarction and hepatic abscess) | ||
| Comparative arm | ||||||||||
| Martin [62] | Prospective trial | IRINOTECAN and GEMCIS (GEMZAR and CISPLATIN) concomitant IV prospective, multicenter, open-label, randomized phase II study | 48 patients: 24 treated with GEMCIS and DEBIRI and 22 with GEMCIS alone | Median OS: 33.7 months (95% CI 13.5–54.5) | Data not known | |||||
| Ge [23] | Retrospective study | Epirubicine + 5FU Comparison with MWA | mOS 26.9 months (6.6–44.2) | Data not known | ||||||
| Wright [61] | Retrospective study | Surgery vs. IAT GEMCIS (63%) GEMZAR (19.5%) IRINOTECAN (4.9%) CDDP-DOXORUBICIN-MITOMYCIN C 2.1% | 59 patients underwent intra-arterial treatment (IAT) (41 = TACE, 16 = HAIC and 2 = SIRT ) vs. 57 patients who benefited from surgery | IAT: 5 (2–50) HAIC: 7 (2–50) TACE: 4 (2–27) | 10.6 (3.3–25.3) HAIC 9.4 (4.1–19.2) TACE 11.0 (3.3–25.3) | Bilobar: 88% HAIC 81.3% TACE 90.2% | Median of 3 for the whole cohort of IAT (1–15) | mOS for IAT: 16 months (95% CI 13.3–18.7, p = 0.627) For TACE: mOS: 15 months (95% CI 11.4–18.6) HAIC pump = 39 months (95% CI 32.7–51.3) | Data not known | |
| Akinwande [53] | Retrospective study | SIRT: 25 TACE: 15 DOXORUBICIN | ORR 6% | TACE: 3/33 (9%) grade 3 or more treatment-related toxicities (fever and abdominal pain) SIRT: 4/39 (10%) grade 3 or more treatment-related toxicities (abdominal pain) | ||||||
| Scheuermann [73] | Retrospective study | Surgery vs. TACE | 273 130 surgery 32 TACE 111 palliative | 8.7 (2.0–18.0) | Unilobar 13/32 | Median: 3 (range: 1–18 sessions) | Median survival of TACE patients: 11 months | 1 liver dysfunction (ascites) and 2 vascular complications (dissection or occlusion of the hepatic artery) | ||
| Park [63] | Retrospective study | TACE vs. palliative treatment CDDP | 155 72 TACE 83 palliative | Mean 8.1 ± 3.4 | Bilobar 37/72 Multiple or diffuse 41/72 | 2.5 per patient (range: 1–17 sessions) | 12.2 months (95% CI 9.8–14.6) | 11 grade 3 hematological toxicities occurred in 9 patients (13%, 9/72), and 25 grade 3 non-hematological toxicities occurred in 17 patients (24%, 17/72) (elevation of liver enzymes, pain and nausea) | ||
2.3.3. Hepatic Arterial Infusion Chemotherapy (HAIC)
| Authors | Retrospective or Prospective Study | Chemotherapy | Patients (n) | Tumor Size Median (cm) | Extrahepatic Disease (%) of Patients | Number of Sessions per Patient | Efficacy | Grade 3–5 Treatment Related Toxicities |
|---|---|---|---|---|---|---|---|---|
| Cercek [75] | Phase 2 clinical trial | HAIC floxuridine and systemic gemcitabine and oxaliplatin | 42 included and 38 treated | 8.3 (1.7–24.8) Bilobar: 66% | 18% | The median OS was 25.0 months (95% CI, 20.6-not reached) | The most common grade 3 and 4 adverse events were related to elevated liver enzymes (5% grade 4 elevated bilirubin level, 5% grade 4 elevated AST (aspartate aminotransferase), and 5% grade 4 elevated ALT (alanine aminotransferase)). No grade 4 non-biological toxicities were observed. | |
| Marquardt [78] | Retrospective study | Melphalan | 15 | Range: 1–5 | Median OS was 26.9 months from initial diagnosis and 7.6 months from first PHP | 13 patients (50%) presented grade 3–5 toxicities (hematological, pneumonia, acute renal failure, ascites, bleeding, oedema, multi-organ failure, otitis, pseudoaneurysm and stroke) | ||
| Higaki [76] | Retrospective study | CDDP + oral S1 | 12 | Multiple (35.7%) | Median survival time = 10.1 months (range, 3.6–23.2) | 1 patient (4.5%) experienced a grade 3 toxicity (anemia) | ||
| Konstantinidis [79] | Retrospective study | 5FU pump concomitantly with chemotherapy IV | 167 | 8.5 cm (range: 1.5–16.4 cm) multifocal (63.5%) | mOS: 30.8 months | Data not known | ||
| Massani [80] | Retrospective study | 11 | 0 | mOS: 17.6 months (6–40) | 4 patients experienced a major complication (hepatic decompensation and hand–foot syndrome) | |||
| Kasai [77] | Retrospective study | Fluorouracil and oxaliplatin after placement of an + HAIC pump + PEG-IFNa-2b SC | 20 | 5% | Mean: 2 cycles (range: 1–8 cycles) | ORR 50% Median survival time: 14.6 months (95 % CI 5.5–16.8) | 6 patients experienced grade 3 hematological toxicity | |
| Ghiringhelli [81] | Retrospective study | HAIC GEMOX | 12 | Multifocal 5/62 | ORR 66.6% (95% CI 29–100%) Median OS: 20.3 months (95% CI 13.2–49.7) | 7 grade 3–4 hematological adverse events and 6 grade 3–4 non-hematological adverse events were reported (oxaliplatin-related peripheral neuropathy, infection and oxaliplatin-allergy) | ||
| Inaba [74] | Phase I/II clinical trial | HAIC GEMZAR | 11 | 9% | The incidence of adverse events of grade 3–4 was 20% neutropenia, 22% elevated liver enzymes, 4% nausea and 4% fatigue. | |||
| Mambrini [82] | Retrospective study | EPIRUBICIN AND CDDP + CAPECITABINE oral | 20 | OS 18 months | One grade 5 toxicity (diarrhea) and one grade 3 toxicity (vomiting). | |||
| Vogl [83] | Retrospective study | GEMZAR | 24 | OS 20.2 months | 1 severe adverse event occurred (allergic or toxic lung edema) | |||
| Cantore [84] | Retrospective study | EPIRUBICIN + CDPP 5FU IV | 30 | Median 4 (2–8) | ORR 40% mOS 13.2 months | Grade 3 toxicity observed in 11 of 30 patients (37%) (hematological toxicity, stomatitis, nausea, diarrhea, alopecia) | ||
| Tanaka [85] | Retrospective study | Epirubicin and cisplatin 5FU | 11 | Mean tumor size: 7.0 ± 2.6 cm (range: 3.8–13.5) | 4% | One severe cholangitis was observed |
2.3.4. Comparisons of the Different Intra-Arterial Therapies
2.4. Existing Guidelines
2.4.1. NCCN (National Comprehensive Cancer Network)
2.4.2. ESMO (European Society for Medical Oncology)
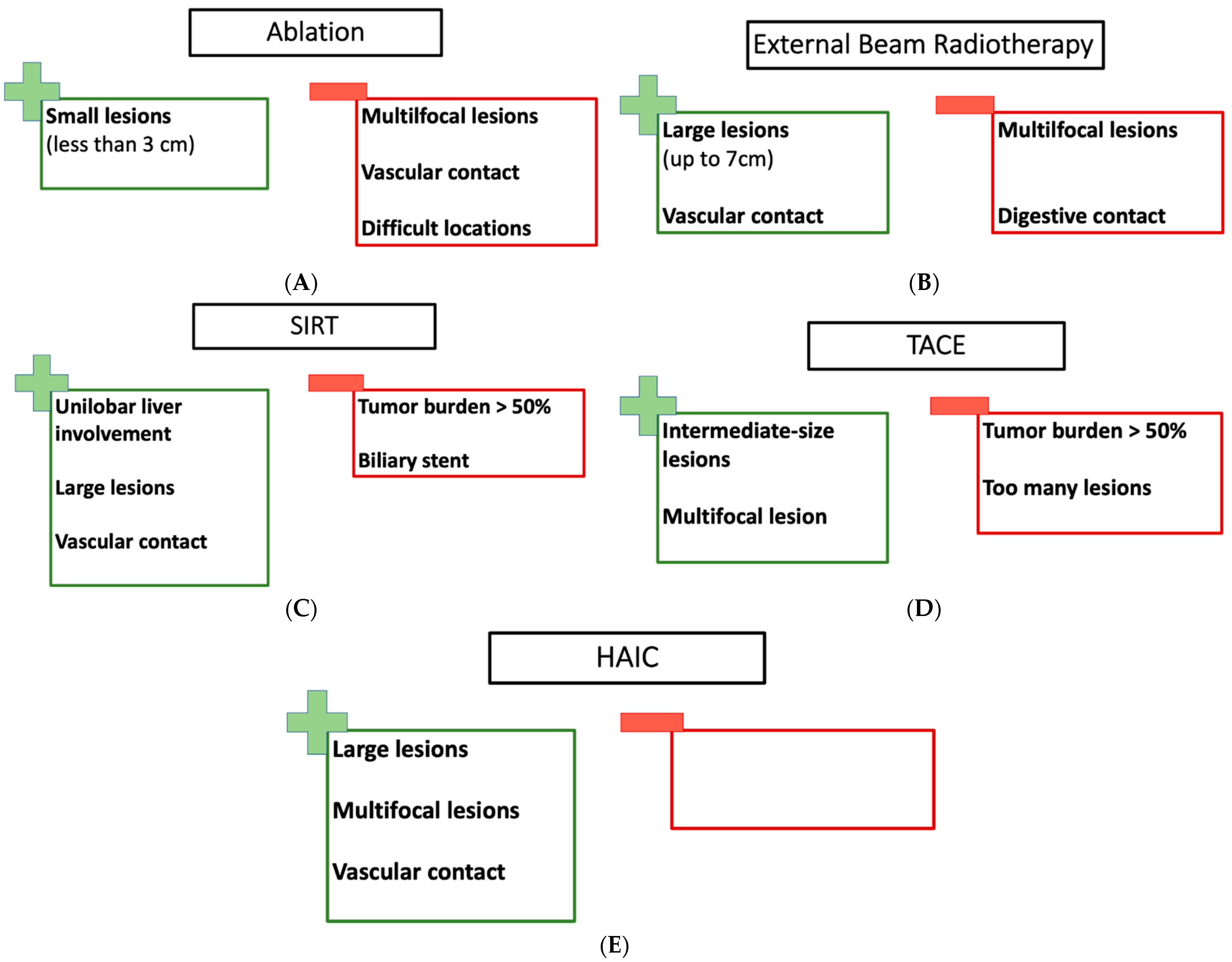
3. Which Treatment for Which Patient?
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Nakachi, K.; Fukutomi, A.; Mizuno, N.; Ohkawa, S.; Funakoshi, A.; Nagino, M.; Kondo, S.; Nagaoka, S.; Funai, J.; et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br. J. Cancer 2010, 103, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Ruth, H.A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah, L.M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Fong, Y. Outcomes in biliary malignancy. J. Surg. Oncol. 2014, 110, 585–591. [Google Scholar] [CrossRef]
- Cillo, U.; Fondevila, C.; Donadon, M.; Gringeri, E.; Mocchegiani, F.; Schlitt, H.J.; Ijzermans, J.N.M.; Vivarelli, M.; Zieniewicz, K.; Olde Damink, S.W.M.; et al. Surgery for cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. S1), 143–155. [Google Scholar] [CrossRef]
- Bridgewater, J.; Fletcher, P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Long-Term Outcomes and Exploratory Analyses of the Randomized Phase III BILCAP Study. J. Clin. Oncol. 2022, 40, 2048–2057. [Google Scholar] [CrossRef]
- Edeline, J.; Lamarca, A.; McNamara, M.G.; Jacobs, T.; Hubner, R.A.; Palmer, D.; Groot Koerkamp, B.; Johnson, P.; Guiu, B.; Valle, J.W. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: A systematic review and pooled analysis. Cancer Treat. Rev. 2021, 99, 102258. [Google Scholar] [CrossRef]
- Brandi, G.; Rizzo, A.; Dall’Olio, F.G.; Felicani, C.; Ercolani, G.; Cescon, M.; Frega, G.; Tavolari, S.; Palloni, A.; De Lorenzo, S.; et al. Percutaneous radiofrequency ablation in intrahepatic cholangiocarcinoma: A retrospective single-center experience. Int. J. Hyperth. 2020, 37, 479–485. [Google Scholar] [CrossRef]
- Díaz-González, Á.; Vilana, R.; Bianchi, L.; García-Criado, Á.; Rimola, J.; Rodríguez de Lope, C.; Ferrer, J.; Ayuso, C.; Da Fonseca, L.G.; Reig, M.; et al. Thermal Ablation for Intrahepatic Cholangiocarcinoma in Cirrhosis: Safety and Efficacy in Non-Surgical Patients. J. Vasc. Interv. Radiol. 2020, 31, 710–719. [Google Scholar] [CrossRef]
- Xu, C.; Li, L.; Xu, W.; Du, C.; Yang, L.; Tong, J.; Yi, Y. Ultrasound-guided percutaneous microwave ablation versus surgical resection for recurrent intrahepatic cholangiocarcinoma: Intermediate-term results. Int. J. Hyperth. 2019, 36, 351–358. [Google Scholar] [CrossRef]
- Ni, J.-Y.; An, C.; Zhang, T.-Q.; Huang, Z.-M.; Jiang, X.-Y.; Huang, J.-H. Predictive value of the albumin-bilirubin grade on long-term outcomes of CT-guided percutaneous microwave ablation in intrahepatic cholangiocarcinoma. Int. J. Hyperth. 2019, 36, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-J.; Hu, P.; Wang, N.; Shen, Q.; Sun, A.-X.; Kuang, M.; Qian, G.-J. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2013, 20, 3596–3602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yu, J.; Yu, X.; Han, Z.; Cheng, Z.; Liu, F.; Liang, P. Clinical and survival outcomes of percutaneous microwave ablation for intrahepatic cholangiocarcinoma. Int. J. Hyperth. 2018, 34, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-X.; Wang, Y.; Lu, M.-D.; Liu, L.-N. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br. J. Radiol. 2012, 85, 1078–1084. [Google Scholar] [CrossRef]
- Kim, J.H.; Won, H.J.; Shin, Y.M.; Kim, K.-A.; Kim, P.N. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am. J. Roentgenol. 2011, 196, W205–W209. [Google Scholar] [CrossRef]
- Butros, S.R.; Shenoy-Bhangle, A.; Mueller, P.R.; Arellano, R.S. Radiofrequency ablation of intrahepatic cholangiocarcinoma: Feasability, local tumor control, and long-term outcome. Clin. Imaging 2014, 38, 490–494. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, W.; Wu, W.; Yan, K.; Xing, B.C.; Chen, M.H. Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. J. Vasc. Interv. Radiol. 2012, 23, 642–649. [Google Scholar] [CrossRef]
- Kim, J.H.; Won, H.J.; Shin, Y.M.; Kim, P.N.; Lee, S.-G.; Hwang, S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur. J. Radiol. 2011, 80, e221–e225. [Google Scholar] [CrossRef]
- Carrafiello, G.; Laganà, D.; Cotta, E.; Mangini, M.; Fontana, F.; Bandiera, F.; Fugazzola, C. Radiofrequency ablation of intrahepatic cholangiocarcinoma: Preliminary experience. Cardiovasc. Interv. Radiol. 2010, 33, 835–839. [Google Scholar] [CrossRef]
- Chiou, Y.-Y.; Hwang, J.-I.; Chou, Y.-H.; Wang, H.-K.; Chiang, J.-H.; Chang, C.-Y. Percutaneous ultrasound-guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung J. Med. Sci. 2005, 21, 304–309. [Google Scholar] [CrossRef]
- Haidu, M.; Dobrozemsky, G.; Schullian, P.; Widmann, G.; Klaus, A.; Weiss, H.; Margreiter, R.; Bale, R. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: A retrospective study. Cardiovasc. Interv. Radiol. 2012, 35, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.A.; Kinsman, K.A.; Schmit, G.D.; Atwell, T.D.; Schmitz, J.J.; Welch, B.T.; Callstrom, M.R.; Geske, J.R.; Kurup, A.N. Thermal ablation of intrahepatic cholangiocarcinoma: Safety, efficacy, and factors affecting local tumor progression. Abdom Radiol (NY) 2018, 43, 3487–3492. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Jeong, S.; Luo, G.-J.; Ren, Y.-B.; Zhang, B.-H.; Zhang, Y.-J.; Shen, F.; Cheng, Q.-B.; Sui, C.-J.; Wang, H.-Y.; et al. Transarterial chemoembolization versus percutaneous microwave coagulation therapy for recurrent unresectable intrahepatic cholangiocarcinoma: Development of a prognostic nomogram. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; Gatti, P.; Montesarchio, L.; Santoro, B.; Dell’Olio, A.; Crucinio, N.; Coppola, C.; Scarano, F.; Biase, F.D.; Ciracì, E.; et al. Intrahepatic Cholangiocarcinoma and Thermal Ablation: Long-term Results of An Italian Retrospective Multicenter Study. J. Clin. Transl. Hepatol. 2019, 7, 287–292. [Google Scholar] [CrossRef]
- Shimizu, S.; Okumura, T.; Oshiro, Y.; Fukumitsu, N.; Fukuda, K.; Ishige, K.; Hasegawa, N.; Numajiri, H.; Murofushi, K.; Ohnishi, K.; et al. Clinical outcomes of previously untreated patients with unresectable intrahepatic cholangiocarcinoma following proton beam therapy. Radiat. Oncol. 2019, 14, 241. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, T.H.; Seong, J. Improved oncologic outcome with chemoradiotherapy followed by surgery in unresectable intrahepatic cholangiocarcinoma. Strahlenther. Onkol. 2017, 193, 620–629. [Google Scholar] [CrossRef]
- Kozak, M.M.; Toesca, D.A.S.; von Eyben, R.; Pollom, E.L.; Chang, D.T. Stereotactic Body Radiation Therapy for Cholangiocarcinoma: Optimizing Locoregional Control With Elective Nodal Irradiation. Adv. Radiat. Oncol. 2020, 5, 77–84. [Google Scholar] [CrossRef]
- Weiner, A.A.; Olsen, J.; Ma, D.; Dyk, P.; DeWees, T.; Myerson, R.J.; Parikh, P. Stereotactic body radiotherapy for primary hepatic malignancies—Report of a phase I/II institutional study. Radiother. Oncol. 2016, 121, 79–85. [Google Scholar] [CrossRef]
- Ohkawa, A.; Mizumoto, M.; Ishikawa, H.; Abei, M.; Fukuda, K.; Hashimoto, T.; Sakae, T.; Tsuboi, K.; Okumura, T.; Sakurai, H. Proton beam therapy for unresectable intrahepatic cholangiocarcinoma. J. Gastroenterol. Hepatol. 2015, 30, 957–963. [Google Scholar] [CrossRef]
- Hong, T.S.; Wo, J.Y.; Yeap, B.Y.; Ben-Josef, E.; McDonnell, E.I.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Goyal, L.; et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Clin. Oncol. 2016, 34, 460–468. [Google Scholar] [CrossRef]
- Smart, A.C.; Goyal, L.; Horick, N.; Petkovska, N.; Zhu, A.X.; Ferrone, C.R.; Tanabe, K.K.; Allen, J.N.; Drapek, L.C.; Qadan, M.; et al. Hypofractionated Radiation Therapy for Unresectable/Locally Recurrent Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Kim, M.-S.; Cho, C.K.; Yoo, H.J.; Jang, W.I.; Seo, Y.S.; Paik, E.K.; Kim, K.B.; Han, C.J.; Kim, S.B. Outcomes of stereotactic body radiotherapy for unresectable primary or recurrent cholangiocarcinoma. Radiat. Oncol. J. 2014, 32, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Tse, R.V.; Hawkins, M.; Lockwood, G.; Kim, J.J.; Cummings, B.; Knox, J.; Sherman, M.; Dawson, L.A. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Clin. Oncol. 2008, 26, 657–664. [Google Scholar] [CrossRef]
- Shen, Z.-T.; Zhou, H.; Li, A.-M.; Li, B.; Shen, J.-S.; Zhu, X.-X. Clinical outcomes and prognostic factors of stereotactic body radiation therapy for intrahepatic cholangiocarcinoma. Oncotarget 2017, 8, 93541–93550. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.M.; Mulcahy, M.F.; Lewandowski, R.J.; Sato, K.T.; Ryu, R.K.; Masterson, E.J.; Newman, S.B.; Benson, A.; Omary, R.A.; Salem, R. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: Results from a pilot study. Cancer 2008, 113, 2119–2128. [Google Scholar] [CrossRef]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 51–59. [Google Scholar] [CrossRef]
- Kasuya, G.; Terashima, K.; Shibuya, K.; Toyama, S.; Ebner, D.K.; Tsuji, H.; Okimoto, T.; Ohno, T.; Shioyama, Y.; Nakano, T.; et al. Carbon-ion radiotherapy for cholangiocarcinoma: A multi-institutional study by and the Japan carbon-ion radiation oncology study group (J-CROS). Oncotarget 2019, 10, 4369–4379. [Google Scholar] [CrossRef]
- Tao, R.; Krishnan, S.; Bhosale, P.R.; Javle, M.M.; Aloia, T.A.; Shroff, R.T.; Kaseb, A.O.; Bishop, A.J.; Swanick, C.W.; Koay, E.J.; et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J. Clin. Oncol. 2016, 34, 219–226. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Park, J.-W.; Kim, B.H.; Woo, S.M.; Kim, T.H.; Koh, Y.H.; Lee, W.J.; Kim, C.-M. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for advanced-stage unresectable intrahepatic cholangiocarcinoma. Radiat. Oncol. 2013, 8, 292. [Google Scholar] [CrossRef]
- Ibarra, R.A.; Rojas, D.; Snyder, L.; Yao, M.; Fabien, J.; Milano, M.; Katz, A.; Goodman, K.; Stephans, K.; El-Gazzaz, G.; et al. Multicenter results of stereotactic body radiotherapy (SBRT) for non-resectable primary liver tumors. Acta Oncol. 2012, 51, 575–583. [Google Scholar] [CrossRef]
- Yi, S.W.; Kang, D.R.; Kim, K.S.; Park, M.S.; Seong, J.; Park, J.Y.; Bang, S.M.; Song, S.Y.; Chung, J.B.; Park, S.W. Efficacy of concurrent chemoradiotherapy with 5-fluorouracil or gemcitabine in locally advanced biliary tract cancer. Cancer Chemother. Pharmacol. 2014, 73, 191–198. [Google Scholar] [CrossRef]
- Helmberger, T.; Golfieri, R.; Pech, M.; Pfammatter, T.; Arnold, D.; Cianni, R.; Maleux, G.; Munneke, G.; Pellerin, O.; Peynircioglu, B.; et al. Clinical Application of Trans-Arterial Radioembolization in Hepatic Malignancies in Europe: First Results from the Prospective Multicentre Observational Study CIRSE Registry for SIR-Spheres Therapy (CIRT). Cardiovasc. Interv. Radiol. 2021, 44, 21–35. [Google Scholar] [CrossRef]
- Azar, A.; Devcic, Z.; Paz-Fumagalli, R.; Vidal, L.L.C.; McKinney, J.M.; Frey, G.; Lewis, A.R.; Ritchie, C.; Starr, J.S.; Mody, K.; et al. Albumin-bilirubin grade as a prognostic indicator for patients with non-hepatocellular primary and metastatic liver malignancy undergoing Yttrium-90 radioembolization using resin microspheres. J. Gastrointest. Oncol. 2020, 11, 715–723. [Google Scholar] [CrossRef]
- Bargellini, I.; Mosconi, C.; Pizzi, G.; Lorenzoni, G.; Vivaldi, C.; Cappelli, A.; Vallati, G.E.; Boni, G.; Cappelli, F.; Paladini, A.; et al. Yttrium-90 Radioembolization in Unresectable Intrahepatic Cholangiocarcinoma: Results of a Multicenter Retrospective Study. Cardiovasc. Interv. Radiol. 2020, 43, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Buettner, S.; Braat, A.J.A.T.; Margonis, G.A.; Brown, D.B.; Taylor, K.B.; Borgmann, A.J.; Kappadath, S.C.; Mahvash, A.; IJzermans, J.N.M.; Weiss, M.J.; et al. Yttrium-90 Radioembolization in Intrahepatic Cholangiocarcinoma: A Multicenter Retrospective Analysis. J. Vasc. Interv. Radiol. 2020, 31, 1035–1043.e2. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Di Costanzo, G.G.; Tortora, R.; Pelle, G.; Saltarelli, A.; Marino Marsilia, G.; Cianni, R.; Schillaci, O.; Bagni, O. Prognostic value of neutrophil-to-lymphocyte ratio and its correlation with fluorine-18-fluorodeoxyglucose metabolic parameters in intrahepatic cholangiocarcinoma submitted to 90Y-radioembolization. Nucl. Med. Commun. 2020, 41, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.; Harders, F.; Lohöfer, F.; Paprottka, P.M.; Schaarschmidt, B.M.; Theysohn, J.; Herrmann, K.; Heindel, W.; Schmidt, H.H.; Pascher, A.; et al. Prognostic Factors for Overall Survival in Advanced Intrahepatic Cholangiocarcinoma Treated with Yttrium-90 Radioembolization. J. Clin. Med. 2019, 9, 56. [Google Scholar] [CrossRef]
- White, J.; Carolan-Rees, G.; Dale, M.; Patrick, H.E.; See, T.C.; Bell, J.K.; Manas, D.M.; Crellin, A.; Slevin, N.J.; Sharma, R.A. Yttrium-90 Transarterial Radioembolization for Chemotherapy-Refractory Intrahepatic Cholangiocarcinoma: A Prospective, Observational Study. J. Vasc. Interv. Radiol. 2019, 30, 1185–1192. [Google Scholar] [CrossRef]
- Mouli, S.; Memon, K.; Baker, T.; Benson, A.B.; Mulcahy, M.F.; Gupta, R.; Ryu, R.K.; Salem, R.; Lewandowski, R.J. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: Safety, response, and survival analysis. J. Vasc. Interv. Radiol. 2013, 24, 1227–1234. [Google Scholar] [CrossRef]
- Gangi, A.; Shah, J.; Hatfield, N.; Smith, J.; Sweeney, J.; Choi, J.; El-Haddad, G.; Biebel, B.; Parikh, N.; Arslan, B.; et al. Intrahepatic Cholangiocarcinoma Treated with Transarterial Yttrium-90 Glass Microsphere Radioembolization: Results of a Single Institution Retrospective Study. J. Vasc. Interv. Radiol. 2018, 29, 1101–1108. [Google Scholar] [CrossRef]
- Shaker, T.M.; Chung, C.; Varma, M.K.; Doherty, M.G.; Wolf, A.M.; Chung, M.H.; Assifi, M.M. Is there a role for Ytrrium-90 in the treatment of unresectable and metastatic intrahepatic cholangiocarcinoma? Am. J. Surg. 2018, 215, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Manceau, V.; Palard, X.; Rolland, Y.; Pracht, M.; Le Sourd, S.; Laffont, S.; Boudjema, K.; Lievre, A.; Mesbah, H.; Haumont, L.-A.; et al. A MAA-based dosimetric study in patients with intrahepatic cholangiocarcinoma treated with a combination of chemotherapy and 90Y-loaded glass microsphere selective internal radiation therapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Akinwande, O.; Shah, V.; Mills, A.; Noda, C.; Weiner, E.; Foltz, G.; Saad, N. Chemoembolization versus radioembolization for the treatment of unresectable intrahepatic cholangiocarcinoma in a single institution image-based efficacy and comparative toxicity. Hepat. Oncol. 2017, 4, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Swinburne, N.C.; Biederman, D.M.; Besa, C.; Tabori, N.E.; Fischman, A.M.; Patel, R.S.; Nowakowski, F.S.; Gunasekaran, G.; Schwartz, M.E.; Lookstein, R.A.; et al. Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma: Review of Safety, Response Evaluation Criteria in Solid Tumors 1.1 Imaging Response and Survival. Cancer Biother. Radiopharm. 2017, 32, 161–168. [Google Scholar] [CrossRef]
- Jia, Z.; Paz-Fumagalli, R.; Frey, G.; Sella, D.M.; McKinney, J.M.; Wang, W. Resin-based Yttrium-90 microspheres for unresectable and failed first-line chemotherapy intrahepatic cholangiocarcinoma: Preliminary results. J. Cancer Res. Clin. Oncol. 2017, 143, 481–489. [Google Scholar] [CrossRef]
- Soydal, C.; Kucuk, O.N.; Bilgic, S.; Ibis, E. Radioembolization with (90)Y resin microspheres for intrahepatic cholangiocellular carcinoma: Prognostic factors. Ann. Nucl. Med. 2016, 30, 29–34. [Google Scholar] [CrossRef]
- Saxena, A.; Bester, L.; Chua, T.C.; Chu, F.C.; Morris, D.L. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: A preliminary assessment of this novel treatment option. Ann. Surg. Oncol. 2010, 17, 484–491. [Google Scholar] [CrossRef]
- Bourien, H.; Palard, X.; Rolland, Y.; Le Du, F.; Beuzit, L.; Uguen, T.; Le Sourd, S.; Pracht, M.; Manceau, V.; Lièvre, A.; et al. Yttrium-90 glass microspheres radioembolization (RE) for biliary tract cancer: A large single-center experience. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 669–676. [Google Scholar] [CrossRef]
- Reimer, P.; Virarkar, M.K.; Binnenhei, M.; Justinger, M.; Schön, M.R.; Tatsch, K. Prognostic Factors in Overall Survival of Patients with Unresectable Intrahepatic Cholangiocarcinoma Treated by Means of Yttrium-90 Radioembolization: Results in Therapy-Naïve Patients. Cardiovasc. Interv. Radiol. 2018, 41, 744–752. [Google Scholar] [CrossRef]
- Schiffman, S.C.; Metzger, T.; Dubel, G.; Andrasina, T.; Kralj, I.; Tatum, C.; McMasters, K.M.; Scoggins, C.R.; Martin, R.C.G. Precision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: Optimal tolerance and prolonged overall survival. Ann. Surg. Oncol. 2011, 18, 431–438. [Google Scholar] [CrossRef]
- Wright, G.P.; Perkins, S.; Jones, H.; Zureikat, A.H.; Marsh, J.W.; Holtzman, M.P.; Zeh, H.J.; Bartlett, D.L.; Pingpank, J.F. Surgical Resection Does Not Improve Survival in Multifocal Intrahepatic Cholangiocarcinoma: A Comparison of Surgical Resection with Intra-Arterial Therapies. Ann. Surg. Oncol. 2018, 25, 83–90. [Google Scholar] [CrossRef]
- Martin, R.C.G.; Simo, K.A.; Hansen, P.; Rocha, F.; Philips, P.; McMasters, K.M.; Tatum, C.M.; Kelly, L.R.; Driscoll, M.; Sharma, V.R.; et al. Drug-Eluting Bead, Irinotecan Therapy of Unresectable Intrahepatic Cholangiocarcinoma (DELTIC) with Concomitant Systemic Gemcitabine and Cisplatin. Ann. Surg. Oncol. 2022, 29, 5462–5473. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, J.H.; Yoon, H.-J.; Lee, I.-S.; Yoon, H.-K.; Kim, K.-P. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin. Radiol. 2011, 66, 322–328. [Google Scholar] [CrossRef]
- Vogl, T.J.; Naguib, N.N.N.; Nour-Eldin, N.-E.A.; Bechstein, W.O.; Zeuzem, S.; Trojan, J.; Gruber-Rouh, T. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: Results and prognostic factors governing treatment success. Int. J. Cancer 2012, 131, 733–740. [Google Scholar] [CrossRef]
- Hyder, O.; Marsh, J.W.; Salem, R.; Petre, E.N.; Kalva, S.; Liapi, E.; Cosgrove, D.; Neal, D.; Kamel, I.; Zhu, A.X.; et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: A multi-institutional analysis. Ann. Surg. Oncol. 2013, 20, 3779–3786. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.-Y.; Zhou, G.-H.; Zhang, Y.-L.; Nie, C.-H.; Zhu, T.-Y.; Wang, H.-L.; Chen, S.-Q.; Wang, B.-Q.; Yu, Z.-N.; Wu, L.-M.; et al. Drug-eluting beads transarterial chemoembolization with CalliSpheres microspheres for treatment of unresectable intrahepatic cholangiocarcinoma. J. Cancer 2020, 11, 4534–4541. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zheng, J.; Shi, C.; Fang, J.; Peng, Z.; Huang, J.; Sun, J.; Zhou, G.; Li, T.; Zhu, D.; et al. Drug-eluting beads transarterial chemoembolization by CalliSpheres is effective and well tolerated in treating intrahepatic cholangiocarcinoma patients: A preliminary result from CTILC study. Medicine 2020, 99, e19276. [Google Scholar] [CrossRef]
- Goerg, F.; Zimmermann, M.; Bruners, P.; Neumann, U.; Luedde, T.; Kuhl, C. Chemoembolization with Degradable Starch Microspheres for Treatment of Patients with Primary or Recurrent Unresectable, Locally Advanced Intrahepatic Cholangiocarcinoma: A Pilot Study. Cardiovasc. Interv. Radiol. 2019, 42, 1709–1717. [Google Scholar] [CrossRef]
- Aliberti, C.; Carandina, R.; Sarti, D.; Pizzirani, E.; Ramondo, G.; Mulazzani, L.; Mattioli, G.M.; Fiorentini, G. Chemoembolization with Drug-eluting Microspheres Loaded with Doxorubicin for the Treatment of Cholangiocarcinoma. Anticancer. Res. 2017, 37, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, M.V.; Albert, M.; McNally, M.; Robertson, M.; Sun, W.; Fraker, D.; Olthoff, K.; Christians, K.; Pappas, S.; Rilling, W.; et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: A 2-center study. Cancer 2011, 117, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Ikami, I.; Munakata, M.; Muto, O.; Sakata, Y. Hepatic arterial infusion of mitomycin C with degradable starch microspheres for unresectable intrahepatic cholangiocarcinoma. Clin. Oncol. (R. Coll. Radiol.) 2008, 20, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Gusani, N.J.; Balaa, F.K.; Steel, J.L.; Geller, D.A.; Marsh, J.W.; Zajko, A.B.; Carr, B.I.; Gamblin, T.C. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): A single-institution experience. J. Gastrointest. Surg. 2008, 12, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, U.; Kaths, J.M.; Heise, M.; Pitton, M.B.; Weinmann, A.; Hoppe-Lotichius, M.; Otto, G. Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma—A single-center experience. Eur. J. Surg. Oncol. 2013, 39, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Arai, Y.; Yamaura, H.; Sato, Y.; Najima, M.; Aramaki, T.; Sone, M.; Kumada, T.; Tanigawa, N.; Anai, H.; et al. Phase I/II study of hepatic arterial infusion chemotherapy with gemcitabine in patients with unresectable intrahepatic cholangiocarcinoma (JIVROSG-0301). Am. J. Clin. Oncol. 2011, 34, 58–62. [Google Scholar] [CrossRef]
- Cercek, A.; Boerner, T.; Tan, B.R.; Chou, J.F.; Gönen, M.; Boucher, T.M.; Hauser, H.F.; Do, R.K.G.; Lowery, M.A.; Harding, J.J.; et al. Assessment of Hepatic Arterial Infusion of Floxuridine in Combination With Systemic Gemcitabine and Oxaliplatin in Patients With Unresectable Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 60–67. [Google Scholar] [CrossRef]
- Higaki, T.; Aramaki, O.; Moriguchi, M.; Nakayama, H.; Midorikawa, Y.; Takayama, T. Arterial infusion of cisplatin plus S-1 against unresectable intrahepatic cholangiocarcinoma. Biosci. Trends 2018, 12, 73–78. [Google Scholar] [CrossRef]
- Kasai, K.; Kooka, Y.; Suzuki, Y.; Suzuki, A.; Oikawa, T.; Ushio, A.; Kasai, Y.; Sawara, K.; Miyamoto, Y.; Oikawa, K.; et al. Efficacy of hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic pegylated interferon α-2b for advanced intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2014, 21, 3638–3645. [Google Scholar] [CrossRef]
- Marquardt, S.; Kirstein, M.M.; Brüning, R.; Zeile, M.; Ferrucci, P.F.; Prevoo, W.; Radeleff, B.; Trillaud, H.; Tselikas, L.; Vicente, E.; et al. Percutaneous hepatic perfusion (chemosaturation) with melphalan in patients with intrahepatic cholangiocarcinoma: European multicentre study on safety, short-term effects and survival. Eur. Radiol. 2019, 29, 1882–1892. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Groot Koerkamp, B.; Do, R.K.G.; Gönen, M.; Fong, Y.; Allen, P.J.; D’Angelica, M.I.; Kingham, T.P.; DeMatteo, R.P.; Klimstra, D.S.; et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 2016, 122, 758–765. [Google Scholar] [CrossRef]
- Massani, M.; Nistri, C.; Ruffolo, C.; Bonariol, R.; Pauletti, B.; Bonariol, L.; Caratozzolo, E.; Morana, G.; Bassi, N. Intrahepatic chemotherapy for unresectable cholangiocarcinoma: Review of literature and personal experience. Updates Surg. 2015, 67, 389–400. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Lorgis, V.; Vincent, J.; Ladoire, S.; Guiu, B. Hepatic arterial infusion of gemcitabine plus oxaliplatin as second-line treatment for locally advanced intrahepatic cholangiocarcinoma: Preliminary experience. Chemotherapy 2013, 59, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Mambrini, A.; Guglielmi, A.; Pacetti, P.; Iacono, C.; Torri, T.; Auci, A.; Nicoli, N.; Orlandi, M.; Guadagni, S.; Fiorentini, G.; et al. Capecitabine plus hepatic intra-arterial epirubicin and cisplatin in unresectable biliary cancer: A phase II study. Anticancer Res. 2007, 27, 3009–3013. [Google Scholar] [PubMed]
- Vogl, T.J.; Schwarz, W.; Eichler, K.; Hochmuth, K.; Hammerstingl, R.; Jacob, U.; Scheller, A.; Zangos, S.; Heller, M. Hepatic intraarterial chemotherapy with gemcitabine in patients with unresectable cholangiocarcinomas and liver metastases of pancreatic cancer: A clinical study on maximum tolerable dose and treatment efficacy. J. Cancer Res. Clin. Oncol. 2006, 132, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Cantore, M.; Mambrini, A.; Fiorentini, G.; Rabbi, C.; Zamagni, D.; Caudana, R.; Pennucci, C.; Sanguinetti, F.; Lombardi, M.; Nicoli, N. Phase II study of hepatic intraarterial epirubicin and cisplatin, with systemic 5-fluorouracil in patients with unresectable biliary tract tumors. Cancer 2005, 103, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Yamakado, K.; Nakatsuka, A.; Fujii, A.; Matsumura, K.; Takeda, K. Arterial chemoinfusion therapy through an implanted port system for patients with unresectable intrahepatic cholangiocarcinoma—Initial experience. Eur. J. Radiol. 2002, 41, 42–48. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network® (NCCN®). NCCN Guidelines for Patients Gallbladder and Bile Duct Cancers; National Comprehensive Cancer Network® (NCCN®): Stanford, CA, USA, 2021; p. 102. [Google Scholar]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klümpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 34, 127–140. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. JCO 2022, 40, 378. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourien, H.; Pircher, C.C.; Guiu, B.; Lamarca, A.; Valle, J.W.; Niger, M.; Edeline, J. Locoregional Treatment in Intrahepatic Cholangiocarcinoma: Which Treatment for Which Patient? Cancers 2023, 15, 4217. https://doi.org/10.3390/cancers15174217
Bourien H, Pircher CC, Guiu B, Lamarca A, Valle JW, Niger M, Edeline J. Locoregional Treatment in Intrahepatic Cholangiocarcinoma: Which Treatment for Which Patient? Cancers. 2023; 15(17):4217. https://doi.org/10.3390/cancers15174217
Chicago/Turabian StyleBourien, Héloïse, Chiara Carlotta Pircher, Boris Guiu, Angela Lamarca, Juan W Valle, Monica Niger, and Julien Edeline. 2023. "Locoregional Treatment in Intrahepatic Cholangiocarcinoma: Which Treatment for Which Patient?" Cancers 15, no. 17: 4217. https://doi.org/10.3390/cancers15174217
APA StyleBourien, H., Pircher, C. C., Guiu, B., Lamarca, A., Valle, J. W., Niger, M., & Edeline, J. (2023). Locoregional Treatment in Intrahepatic Cholangiocarcinoma: Which Treatment for Which Patient? Cancers, 15(17), 4217. https://doi.org/10.3390/cancers15174217






