Small Molecules against Metastatic Tumors: Concrete Perspectives and Shattered Dreams
Abstract
:Simple Summary
Abstract
1. Introduction
2. Tyrosine Kinase Inhibitors
3. Cyclin-Dependent Kinase Inhibitors and KRAS Inhibitors
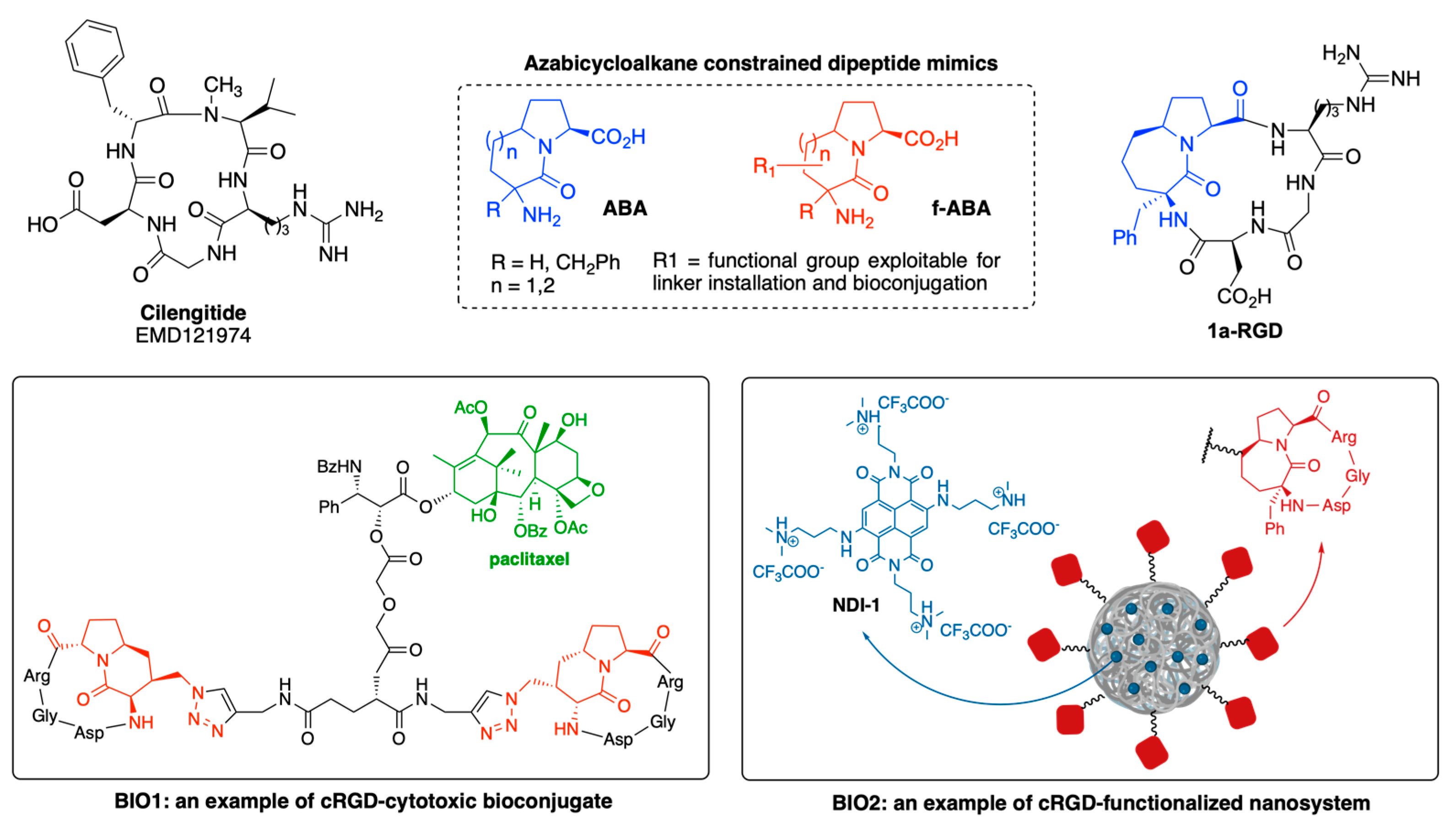
4. Integrin Antagonists
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R. Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, J.; Jiang, L.; Kang, Y.J. Cancer and Stem Cells. Exp. Biol. Med. 2021, 246, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting Cancer Stem Cell Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Sleeman, J.; Steeg, P.S. Cancer Metastasis as a Therapeutic Target. Eur. J. Cancer 2010, 46, 1177–1180. [Google Scholar] [CrossRef]
- Liu, C.; Wang, M.; Xu, C.; Li, B.; Chen, J.; Chen, J.; Wang, Z. Immune Checkpoint Inhibitor Therapy for Bone Metastases: Specific Microenvironment and Current Situation. J. Immunol. Res. 2021, 2021, 8970173. [Google Scholar] [CrossRef]
- Lange, A.; Prenzler, A.; Frank, M.; Kirstein, M.; Vogel, A.; Von Der Schulenburg, J.M. A Systematic Review of Cost-Effectiveness of Monoclonal Antibodies for Metastatic Colorectal Cancer. Eur. J. Cancer 2014, 50, 40–49. [Google Scholar] [CrossRef]
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small Molecules, Big Impact: 20 Years of Targeted Therapy in Oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef]
- Kobelt, D.; Dahlmann, M.; Dumbani, M.; Güllü, N.; Kortüm, B.; Vílchez, M.E.A.; Stein, U.; Walther, W. Small Ones to Fight a Big Problem—Intervention of Cancer Metastasis by Small Molecules. Cancers 2020, 12, 1454. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Jeong, J.-H.; Chen, Z.; Chen, Z.; Luo, J.-L. Targeting Tumor Microenvironment by Small-Molecule Inhibitors. Transl. Oncol. 2020, 13, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Gandalovičová, A.; Rosel, D.; Fernandes, M.; Veselý, P.; Heneberg, P.; Čermák, V.; Petruželka, L.; Kumar, S.; Sanz-Moreno, V.; Brábek, J. Migrastatics—Anti-Metastatic and Anti-Invasion Drugs: Promises and Challenges. Trends Cancer 2017, 3, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-Y.; Wang, N.; Lam, W.; Guo, W.; Feng, Y.; Cheng, Y.-C. Targeting Tumour Microenvironment by Tyrosine Kinase Inhibitor. Mol. Cancer 2018, 17, 43. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, S.; Shi, Y. Tyrosine Kinase Inhibitors for Solid Tumors in the Past 20 Years (2001–2020). J. Hematol. Oncol. 2020, 13, 143. [Google Scholar] [CrossRef]
- Angeli, E.; Bousquet, G. Brain Metastasis Treatment: The Place of Tyrosine Kinase Inhibitors and How to Facilitate Their Diffusion across the Blood–Brain Barrier. Pharmaceutics 2021, 13, 1446. [Google Scholar] [CrossRef]
- Esteban-Villarrubia, J.; Soto-Castillo, J.J.; Pozas, J.; San Román-Gil, M.; Orejana-Martín, I.; Torres-Jiménez, J.; Carrato, A.; Alonso-Gordoa, T.; Molina-Cerrillo, J. Tyrosine Kinase Receptors in Oncology. Int. J. Mol. Sci. 2020, 21, 8529. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, M.; Lonati, V.; Tomasello, G.; Borgonovo, K.; Ghilardi, M.; Cabiddu, M.; Barni, S. The Efficacy of Lapatinib and Capecitabine in HER-2 Positive Breast Cancer with Brain Metastases: A Systematic Review and Pooled Analysis. Eur. J. Cancer 2017, 84, 141–148. [Google Scholar] [CrossRef]
- Tevaarwerk, A.J.; Kolesar, J.M. Lapatinib: A Small-Molecule Inhibitor of Epidermal Growth Factor Receptor and Human Epidermal Growth Factor Receptor—2 Tyrosine Kinases Used in the Treatment of Breast Cancer. Clin. Ther. 2009, 31, 2332–2348. [Google Scholar] [CrossRef]
- Hackshaw, M.D.; Danysh, H.E.; Henderson, M.; Wang, E.; Tu, N.; Islam, Z.; Ladner, A.; Ritchey, M.E.; Salas, M. Prognostic Factors of Brain Metastasis and Survival among HER2-Positive Metastatic Breast Cancer Patients: A Systematic Literature Review. BMC Cancer 2021, 21, 967. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-Targeted Therapy with Trastuzumab and Lapatinib in Treatment-Refractory, KRAS Codon 12/13 Wild-Type, HER2-Positive Metastatic Colorectal Cancer (HERACLES): A Proof-of-Concept, Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, P.; Mimansa, Jaganathan, M.; Munawara, R.; Aggarwal, A.; Shanavas, A. Glycol Chitosan Stabilized Nanomedicine of Lapatinib and Doxorubicin for the Management of Metastatic Breast Tumor. Drug Deliv. Transl. Res. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lavi, O. Redundancy: A Critical Obstacle to Improving Cancer Therapy. Cancer Res. 2015, 75, 808–812. [Google Scholar] [CrossRef]
- Sun, C.; Bernards, R. Feedback and Redundancy in Receptor Tyrosine Kinase Signaling: Relevance to Cancer Therapies. Trends Biochem. Sci. 2014, 39, 465–474. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chu, P.-Y.; Huang, C.-T.; Chen, J.-L.; Yang, H.-P.; Wang, W.-L.; Lau, K.-Y.; Lee, C.-H.; Lan, T.-Y.; Huang, T.-T.; et al. Varlitinib Downregulates HER/ERK Signaling and Induces Apoptosis in Triple Negative Breast Cancer Cells. Cancers 2019, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.X.; Wong, A.L.A.; Ow, S.; Sundar, R.; Tan, D.S.P.; Soo, R.A.; Chee, C.E.; Lim, J.S.J.; Yong, W.P.; Lim, S.E.; et al. Phase Ib Dose-Finding Study of Varlitinib Combined with Weekly Paclitaxel With or Without Carboplatin ± Trastuzumab in Advanced Solid Tumors. Target Oncol. 2022, 17, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.M.; Oh, D.Y.; Ikeda, M.; Yong, W.P.; Hsu, K.; Lindmark, B.; McIntyre, N.; Firth, C. Varlitinib plus capecitabine in second-line advanced biliary tract cancer: A randomized, phase II study (TreeTopp). ESMO Open 2022, 7, 100314. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, H.; Kitadai, Y.; Shinagawa, K.; Yuge, R.; Higashi, Y.; Tanaka, S.; Yasui, W.; Chayama, K. Multikinase Inhibitor Regorafenib Inhibits the Growth and Metastasis of Colon Cancer with Abundant Stroma. Cancer Sci. 2016, 107, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wei, L.; Yu, J.; Zhang, L. Regorafenib Inhibits Colorectal Tumor Growth through PUMA-Mediated Apoptosis. Clin. Cancer Res. 2014, 20, 3472–3484. [Google Scholar] [CrossRef]
- Wong, A.L.A.; Lim, J.S.J.; Sinha, A.; Gopinathan, A.; Lim, R.; Tan, C.-S.; Soh, T.; Venkatesh, S.; Titin, C.; Sapari, N.S.; et al. Tumour Pharmacodynamics and Circulating Cell Free DNA in Patients with Refractory Colorectal Carcinoma Treated with Regorafenib. J. Transl. Med. 2015, 13, 57. [Google Scholar] [CrossRef]
- Crona, D.J.; Keisler, M.D.; Walko, C.M. Regorafenib: A Novel Multitargeted Tyrosine Kinase Inhibitor for Colorectal Cancer and Gastrointestinal Stromal Tumors. Ann. Pharmacother. 2013, 47, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Marinelli, S.; Forgione, A.; Renzulli, M.; Benevento, F.; Piscaglia, F.; Tovoli, F. Regorafenib Combined with Other Systemic Therapies: Exploring Promising Therapeutic Combinations in HCC. J. Hepatocell. Carcinoma 2021, 8, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Kiyota, N.; Hoff, A.O.; Badiu, C.; Owonikoko, T.K.; Dutcus, C.E.; Suzuki, T.; Ren, M.; Wirth, L.J. Impact of Lung Metastases on Overall Survival in the Phase 3 SELECT Study of Lenvatinib in Patients with Radioiodine-Refractory Differentiated Thyroid Cancer. Eur. J. Cancer 2021, 147, 51–57. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, E. Use of Multikinase Inhibitors/Lenvatinib Concomitant with Antiresorptive Therapy for Bone Metastases from Radioiodine-resistant Differentiated Thyroid Cancer. Cancer Med. 2022, 11, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, V.; Powles, T.; Choueiri, T.K.; Hutson, T.E.; Porta, C.; Eto, M.; Sternberg, C.N.; Rha, S.Y.; He, C.S.; Dutcus, C.E.; et al. Lenvatinib plus Everolimus or Pembrolizumab versus Sunitinib in Advanced Renal Cell Carcinoma: Study Design and Rationale. Future Oncol. 2019, 15, 929–941. [Google Scholar] [CrossRef]
- Rehman, O.; Jaferi, U.; Padda, I.; Khehra, N.; Atwal, H.; Mossabeh, D.; Bhangu, R. Overview of Lenvatinib as a Targeted Therapy for Advanced Hepatocellular Carcinoma. Clin. Exp. Hepatol. 2021, 7, 249–257. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, W.; Wang, J.; Zhai, Y.; Xiong, F.; Cai, Y.; Gong, X.; Zhu, B.; Zhu, H.H.; Wang, H.; et al. Lenvatinib- and Vadimezan-Loaded Synthetic High-Density Lipoprotein for Combinational Immunochemotherapy of Metastatic Triple-Negative Breast Cancer. Acta Pharm. Sin. B 2022, 12, 3726–3738. [Google Scholar] [CrossRef]
- Johnston, S.R.; Toi, M.; O’Shaughnessy, J.; Rastogi, P.; Campone, M.; Neven, P.; Huang, C.-S.; Huober, J.; Jaliffe, J.J.; Cicin, I.; et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023, 24, 77–90. [Google Scholar] [CrossRef]
- Jacobs, F.; Cani, M.; Malapelle, U.; Novello, S.; Napoli, V.M.; Bironzo, P. Targeting KRAS in NSCLC: Old Failures and New Options for “Non-G12c” Patients. Cancers 2021, 13, 6332. [Google Scholar] [CrossRef]
- Nakajima, E.C.; Drezner, N.; Li, X.; Mishra-Kalyani, P.S.; Liu, Y.; Zhao, H.; Bi, Y.; Liu, J.; Rahman, A.; Wearne, E.; et al. FDA Approval Summary: Sotorasib for KRAS G12C-Mutated Metastatic NSCLC. Clin Cancer Res. 2022, 28, 1482–1486. [Google Scholar] [CrossRef]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Weiss, J.; Pelster, M.S.; Spira, A.I.; Barve, M.; Ou, S.I.; Leal, T.A.; Bekaii-Saab, T.S.; Paweletz, C.P.; Heavey, G.A.; et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 388, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, S.A. Acquired Resistance to Drugs Targeting Tyrosine Kinases. Adv. Cancer Res. 2018, 138, 71–98. [Google Scholar]
- Lovly, C.M.; Shaw, A.T. Molecular Pathways: Resistance to Kinase Inhibitors and Implications for Therapeutic Strategies. Clin. Cancer Res. 2014, 20, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y. Advances in Studies of Tyrosine Kinase Inhibitors and Their Acquired Resistance. Mol. Cancer 2018, 17, 36. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate Prediction of Protein Structures and Interactions Using a Three-Track Neural Network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Wang, Y.; Zhao, Y.; Li, Q. Protein Tyrosine Kinase Inhibitor Resistance in Malignant Tumors: Molecular Mechanisms and Future Perspective. Signal Transduct. Target. Ther. 2022, 7, 329. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Hatley, R.J.D.; Macdonald, S.J.F.; Slack, R.J.; Le, J.; Ludbrook, S.B.; Lukey, P.T. An Av-RGD Integrin Inhibitor Toolbox: Drug Discovery Insight, Challenges and Opportunities. Angew. Chem. Int. Ed. 2018, 57, 3298–3321. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging Therapeutic Opportunities for Integrin Inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef]
- Gu, Y.; Dong, B.; He, X.; Qiu, Z.; Zhang, J.; Zhang, M.; Liu, H.; Pang, X.; Cui, Y. The Challenges and Opportunities of Avβ3-Based Therapeutics in Cancer: From Bench to Clinical Trials. Pharmacol. Res. 2023, 189, 106694. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Rechenmacher, F.; Kessler, H. Cilengitide: The First Anti-Angiogenic Small Molecule Drug Candidate. Design, Synthesis and Clinical Evaluation. Anti-Cancer Agents Med. Chem. 2010, 10, 753–768. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.-K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide Combined with Standard Treatment for Patients with Newly Diagnosed Glioblastoma with Methylated MGMT Promoter (CENTRIC EORTC 26071-22072 Study): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.R.; Hart, I.R.; Watson, A.R.; Welti, J.C.; Silva, R.G.; Robinson, S.D.; Da Violante, G.; Gourlaouen, M.; Salih, M.; Jones, M.C.; et al. Stimulation of Tumor Growth and Angiogenesis by Low Concentrations of RGD-Mimetic Integrin Inhibitors. Nat. Med. 2009, 15, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fukase, Y.; Shang, Y.; Zou, W.; Muñoz-Félix, J.M.; Buitrago, L.; Van Agthoven, J.; Zhang, Y.; Hara, R.; Coller, B.S.; et al. Novel Pure AVβ3 Integrin Antagonists That Do Not Induce Receptor Extension, Prime the Receptor, or Enhance Angiogenesis at Low Concentrations. ACS Pharmacol. Transl. Sci. 2019, 2, 387–401. [Google Scholar] [CrossRef]
- Paolillo, M.; Serra, M.; Schinelli, S. Integrins in Glioblastoma: Still an Attractive Target? Pharmacol. Res. 2016, 113, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Arosio, D.; Casagrande, C. Advancement in Integrin Facilitated Drug Delivery. Adv. Drug Deliv. Rev. 2016, 97, 111–143. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Baneshi, M.; Hosseinkhani, S.; Mahmoudi, R.; Jabari Arabzadeh, A.; Akrami, M.; Mehrzad, J.; Bardania, H. Recent Progress in Biomedical Applications of RGD-based Ligand: From Precise Cancer Theranostics to Biomaterial Engineering: A Systematic Review. J. Biomed. Mater. Res. A 2020, 108, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Battistini, L.; Bugatti, K.; Sartori, A.; Curti, C.; Zanardi, F. RGD Peptide-Drug Conjugates as Effective Dual Targeting Platforms: Recent Advances. Eur. J. Org. Chem. 2021, 2021, 2506–2528. [Google Scholar] [CrossRef]
- Paolillo, M.; Russo, M.; Serra, M.; Colombo, L.; Schinelli, S. Small Molecule Integrin Antagonists in Cancer Therapy. Mini-Rev. Med. Chem. 2009, 9, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Khashper, A.; Lubell, W.D. Design, Synthesis, Conformational Analysis and Application of Indolizidin-2-One Dipeptide Mimics. Org Biomol Chem 2014, 12, 5052–5070. [Google Scholar] [CrossRef] [PubMed]
- Arosio, D.; Belvisi, L.; Colombo, L.; Colombo, M.; Invernizzi, D.; Manzoni, L.; Potenza, D.; Serra, M.; Castorina, M.; Pisano, C.; et al. A Potent Integrin Antagonist from a Small Library of Cyclic RGD Pentapeptide Mimics Including Benzyl-Substituted Azabicycloalkane Amino Acids. ChemMedChem 2008, 3, 1589–1603. [Google Scholar] [CrossRef]
- Serra, M.; Peviani, E.G.; Bernardi, E.; Colombo, L. Synthesis of Variously Functionalized Azabicycloalkane Scaffolds by Domino Metathesis Reactions. J. Org. Chem. 2017, 82, 11091–11101. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Bernardi, E.; Lorenzi, E.D.; Colombo, L. Synthesis of Functionalized 6,5- and 7,5-Azabicycloalkane Amino Acids by Metathesis Reactions. J. Org. Chem. 2019, 84, 15726–15734. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Tambini, S.M.; Di Giacomo, M.; Peviani, E.G.; Belvisi, L.; Colombo, L. Synthesis of Easy-to-Functionalize Aza bicycloalkane Scaffolds as Dipeptide Turn Mimics En Route to CRGD-Based Bioconjugates: Synthesis of Easily Functionalizable Azabicycloalkane Scaffolds. Eur. J. Org. Chem. 2015, 2015, 7557–7570. [Google Scholar] [CrossRef]
- Serra, M.; Terreni, M.; Bernardi, E.; Colombo, L. Synthesis of Functionalized Proline-Derived Azabicycloalkane Amino Acids and Their Applications in Drug Discovery: Recent Advances. Eur. J. Org. Chem. 2023, 26, e202201394. [Google Scholar] [CrossRef]
- Pilkington-Miksa, M.; Arosio, D.; Battistini, L.; Belvisi, L.; De Matteo, M.; Vasile, F.; Burreddu, P.; Carta, P.; Rassu, G.; Perego, P.; et al. Design, Synthesis, and Biological Evaluation of Novel CRGD–Paclitaxel Conjugates for Integrin-Assisted Drug Delivery. Bioconjug. Chem. 2012, 23, 1610–1622. [Google Scholar] [CrossRef]
- Lanzardo, S.; Conti, L.; Brioschi, C.; Bartolomeo, M.P.; Arosio, D.; Belvisi, L.; Manzoni, L.; Maiocchi, A.; Maisano, F.; Forni, G. A New Optical Imaging Probe Targeting AVβ3 Integrin in Glioblastoma Xenografts: Optical Imaging Of Avβ3 Integrin. Contrast Media Mol. Imaging 2011, 6, 449–458. [Google Scholar] [CrossRef]
- Manzoni, L.; Belvisi, L.; Arosio, D.; Bartolomeo, M.P.; Bianchi, A.; Brioschi, C.; Buonsanti, F.; Cabella, C.; Casagrande, C.; Civera, M.; et al. Synthesis of Gd and 68Ga Complexes in Conjugation with a Conformationally Optimized RGD Sequence as Potential MRI and PET Tumor-Imaging Probes. ChemMedChem 2012, 7, 1084–1093. [Google Scholar] [CrossRef]
- Battistini, L.; Burreddu, P.; Sartori, A.; Arosio, D.; Manzoni, L.; Paduano, L.; D’Errico, G.; Sala, R.; Reia, L.; Bonomini, S.; et al. Enhancement of the Uptake and Cytotoxic Activity of Doxorubicin in Cancer Cells by Novel CRGD-Semipeptide-Anchoring Liposomes. Mol. Pharm. 2014, 11, 2280–2293. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Serra, M.; Paolillo, M.; Bernardi, E.; Tengattini, S.; Piccinini, F.; Lanni, C.; Sorlini, M.; Bisbano, G.; Calleri, E.; et al. Silk Fibroin Nanoparticle Functionalization with Arg-Gly-Asp Cyclopentapeptide Promotes Active Targeting for Tumor Site-Specific Delivery. Cancers 2021, 13, 1185. [Google Scholar] [CrossRef] [PubMed]
- Spinello, A.; Barone, G.; Grunenberg, J. Molecular Recognition of Naphthalene Diimide Ligands by Telomeric Quadruplex-DNA: The Importance of the Protonation State and Mediated Hydrogen Bonds. Phys. Chem. Chem. Phys. 2016, 18, 2871–2877. [Google Scholar] [CrossRef]
- Doria, F.; Salvati, E.; Pompili, L.; Pirota, V.; D’Angelo, C.; Manoli, F.; Nadai, M.; Richter, S.N.; Biroccio, A.; Manet, I.; et al. Dyads of G-Quadruplex Ligands Triggering DNA Damage Response and Tumour Cell Growth Inhibition at Subnanomolar Concentration. Chem. Eur. J. 2019, 25, 11085–11097. [Google Scholar] [CrossRef] [PubMed]
- Micco, M.; Collie, G.W.; Dale, A.G.; Ohnmacht, S.A.; Pazitna, I.; Gunaratnam, M.; Reszka, A.P.; Neidle, S. Structure-Based Design and Evaluation of Naphthalene Diimide G-Quadruplex Ligands As Telomere Targeting Agents in Pancreatic Cancer Cells. J. Med. Chem. 2013, 56, 2959–2974. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Angell, R.; Oxenford, S.; Worthington, J.; Williams, N.; Barton, N.; Fowler, T.G.; O’Flynn, D.E.; Sunose, M.; McConville, M.; et al. Asymmetrically Substituted Quadruplex-Binding Naphthalene Diimide Showing Potent Activity in Pancreatic Cancer Models. ACS Med. Chem. Lett. 2020, 11, 1634–1644. [Google Scholar] [CrossRef]
- Pirota, V.; Nadai, M.; Doria, F.; Richter, S. Naphthalene Diimides as Multimodal G-Quadruplex-Selective Ligands. Molecules 2019, 24, 426. [Google Scholar] [CrossRef] [PubMed]
- Pirota, V.; Bisbano, G.; Serra, M.; Torre, M.L.; Doria, F.; Bari, E.; Paolillo, M. CRGD-Functionalized Silk Fibroin Nanoparticles: A Strategy for Cancer Treatment with a Potent Unselective Naphthalene Diimide Derivative. Cancers 2023, 15, 1725. [Google Scholar] [CrossRef]
- Information about Past and Ongoing Clinical Trials. Available online: https://www.clinicaltrials.gov/ct2/home (accessed on 20 June 2023).
- Cirkel, G.A.; Kerklaan, B.M.; Vanhoutte, F.; Der Aa, A.V.; Lorenzon, G.; Namour, F.; Pujuguet, P.; Darquenne, S.; De Vos, F.Y.F.; Snijders, T.J.; et al. A Dose Escalating Phase I Study of GLPG0187, a Broad Spectrum Integrin Receptor Antagonist, in Adult Patients with Progressive High-Grade Glioma and Other Advanced Solid Malignancies. Investig. New Drugs 2016, 34, 184–192. [Google Scholar] [CrossRef]
- Rosenthal, M.A.; Davidson, P.; Rolland, F.; Campone, M.; Xue, L.; Han, T.H.; Mehta, A.; Berd, Y.; He, W.; Lombardi, A. Evaluation of the Safety, Pharmacokinetics and Treatment Effects of an αν β3 Integrin Inhibitor on Bone Turnover and Disease Activity in Men with Hormone-Refractory Prostate Cancer and Bone Metastases. Asia Pac. J. Clin. Oncol. 2010, 6, 42–48. [Google Scholar]
- Zhou, X.; Zhang, J.; Haimbach, R.; Zhu, W.; Mayer-Ezell, R.; Garcia-Calvo, M.; Smith, E.; Price, O.; Kan, Y.; Zycband, E.; et al. An Integrin Antagonist (MK-0429) Decreases Proteinuria and Renal Fibrosis in the ZSF1 Rat Diabetic Nephropathy Model. Pharmacol. Res. Perspect. 2017, 5, e00354. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.M.; Ma, L.; Zhou, X.; Haimbach, R.E.; Coleman, P.J.; Zhou, H.; Kelley, D.E.; Stoch, S.A.; Duong, L.T.; Hoek, M. Composition and Methods for Treating Chronic kidney Disease. US 20190307735, 10 October 2019. [Google Scholar]
- Stoeltzing, O.; Liu, W.; Reinmuth, N.; Fan, F.; Parry, G.C.; Parikh, A.A.; McCarty, M.F.; Bucana, C.D.; Mazar, A.P.; Ellis, L.M. Inhibition of Integrin ?5?1 Function with a Small Peptide (ATN-161) plus Continuous 5-FU Infusion Reduces Colorectal Liver Metastases and Improves Survival in Mice. Int. J. Cancer 2003, 104, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Khalili, P.; Arakelian, A.; Chen, G.; Plunkett, M.L.; Beck, I.; Parry, G.C.; Doñate, F.; Shaw, D.E.; Mazar, A.P.; Rabbani, S.A. A Non–RGD-Based Integrin Binding Peptide (ATN-161) Blocks Breast Cancer Growth and Metastasis In Vivo. Mol. Cancer Ther. 2006, 5, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Doñate, F.; Parry, G.C.; Shaked, Y.; Hensley, H.; Guan, X.; Beck, I.; Tel-Tsur, Z.; Plunkett, M.L.; Manuia, M.; Shaw, D.E.; et al. Pharmacology of the Novel Antiangiogenic Peptide ATN-161 (Ac-PHSCN-NH2): Observation of a U-Shaped Dose-Response Curve in Several Preclinical Models of Angiogenesis and Tumor Growth. Clin. Cancer Res. 2008, 14, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Beddingfield, B.J.; Iwanaga, N.; Chapagain, P.P.; Zheng, W.; Roy, C.J.; Hu, T.Y.; Kolls, J.K.; Bix, G.J. The Integrin Binding Peptide, ATN-161, as a Novel Therapy for SARS-CoV-2 Infection. JACC Basic Transl. Sci. 2021, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Makowski, L.; Olson-Sidford, W.; Weisel, J.W. Biological and Clinical Consequences of Integrin Binding via a Rogue RGD Motif in the SARS CoV-2 Spike Protein. Viruses 2021, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, C.J.; Bridge, A.; Le Mercier, P. A Potential Role for Integrins in Host Cell Entry by SARS-CoV-2. Antivir. Res. 2020, 177, 104759. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Vanderslice, P.; Market, R.V.; Biediger, R.J.; Woodside, D.G.; Marathi, U.K.; Overwijk, W.W. Abstract 5010: Potentiating Immune Checkpoint Blockade Therapeutic Efficacy Using a Small Molecule Activator of Integrin Cell Adhesion Receptors. Cancer Res. 2019, 79 (Suppl. S13), 5010. [Google Scholar] [CrossRef]
- 7 Hills Pharma’s Clinical-stage Novel Immunostimulant 7HP349 Granted FDA Fast Track Designation for Anti-PD-1-resistant Metastatic Melanoma. Press Releases on BioSpace, 8 March 2022.
- Ombrato, L.; Nolan, E.; Kurelac, I.; Mavousian, A.; Bridgeman, V.L.; Heinze, I.; Chakravarty, P.; Horswell, S.; Gonzalez-Gualda, E.; Matacchione, G.; et al. Metastatic-Niche Labelling Reveals Parenchymal Cells with Stem Features. Nature 2019, 572, 603–608. [Google Scholar] [CrossRef]
- Passaro, D.; Garcia-Albornoz, M.; Diana, G.; Chakravarty, P.; Ariza-McNaughton, L.; Batsivari, A.; Borràs-Eroles, C.; Abarrategi, A.; Waclawiczek, A.; Ombrato, L.; et al. Integrated OMICs Unveil the Bone-Marrow Microenvironment in Human Leukemia. Cell Rep. 2021, 35, 109119. [Google Scholar] [CrossRef]
- Han, Y.; Wang, D.; Peng, L.; Huang, T.; He, X.; Wang, J.; Ou, C. Single-Cell Sequencing: A Promising Approach for Uncovering the Mechanisms of Tumor Metastasis. J Hematol Oncol 2022, 15, 59. [Google Scholar] [CrossRef] [PubMed]



| Molecule | Target/Mechanism | Cancer Type | Clinical Trials/FDA Approval |
|---|---|---|---|
| Lapatinib | Inhibition of phosphorylation of EGFR1, HER2, HER4. | Ovarian cancer, Head and neck cancer, Prostate cancer, High-grade gliomas, Metastatic thyroid gland cancer | 337 trials, 29 in progress. |
| Metastatic Breast cancer | FDA approved 2010 | ||
| Advance Breast cancer | FDA approved 2006 | ||
| Regorafenib | Inhibition of multiple membrane-bound and intracellular kinases. | Metastatic solid malignancies, Colon cancer, Gastro-oesophageal cancer | 299 trials, 143 in progress. |
| Hepatocellular carcinoma | FDA approved 2017 | ||
| Gastrointestinal stromal tumor | FDA approved 2013 | ||
| Advanced colorectal cancer | FDA approved 2012 | ||
| Lenvatinib | Inhibition of the kinase activities of VEGF receptors VEGFR1, 2, 3; inhibition of FGF receptors FGFR1, 2, 3, 4. | Metastatic thyroid cancer, Hepatocellular carcinoma, Children and adolescents with refractory or relapsed solid malignancies, including differentiated thyroid carcinoma and osteosarcoma… | 429 trials, 298 in progress. |
| Advanced renal carcinoma | FDA approved 2021 | ||
| Advanced endometrial carcinoma | FDA approved 2021 | ||
| Unresectable hepatocellular carcinoma | FDA approved 2018 | ||
| Varlitinib | Inhibition of ErbB-2 (Her-2/neu) and EGFR. | Metastatic breast cancer | 12 trials (first study started in 2015), 2 in progress. |
| Abemaciclib | Dual inhibition of cyclin-dependent kinases 4 (CDK4) and 6 (CDK6) | Recurrent primary brain tumors, breast cancer... | 199 trials, 135 in progress. |
| (HR)-positive, HER2-negative advanced or metastatic breast cancer | FDA approved 2021 | ||
| Advanced or metastatic breast cancer | FDA approved 2017 | ||
| Sotorasib | KRAS G12C-mutated inhibition | Non-small cell lung cancer | 46 trials, 28 in progress |
| KRAS G12C mutated locally advanced or metastatic non-small cell lung cancer | FDA approved 2021 | ||
| Adagrasib | KRAS G12C-mutated inhibition | Gastrointestinal cancers | 24 trials (first study started in 2019), 22 in progress. |
| Locally advanced or metastatic KRAS G12C-mutated non-small cell lung cancer | FDA approved 2022 |
| Molecule | Target/Mechanism | Cancer Type | Clinical Trials |
|---|---|---|---|
| Cilengitide | Inhibition of phosphorylation of EGFR1, HER2, HER4. | Gliomas, lung cancer, childhood high-grade cerebellar astrocytoma, recurrent prostate cancer, glioblastoma multiforme | 30 trials |
| GLPG0187 | Non-selective αv antagonist | Solid tumors Cystic fribrosis | (phase I) (phase II) |
| MK-0429 | Non-selective αv antagonist | Prostatic neoplasms with metastatic bone disease | (phase I) |
| ATN-161 | α5β1 and αvβ3 integrins antagonist | Intracranial malignant glioma Renal cell cancer | (phaseI/II) (phase II) |
| 7HP349 | allosteric activator of αLβ2 and α4β1 integrins | Solid tumor | (phase I) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, M.; Rubes, D.; Schinelli, S.; Paolillo, M. Small Molecules against Metastatic Tumors: Concrete Perspectives and Shattered Dreams. Cancers 2023, 15, 4173. https://doi.org/10.3390/cancers15164173
Serra M, Rubes D, Schinelli S, Paolillo M. Small Molecules against Metastatic Tumors: Concrete Perspectives and Shattered Dreams. Cancers. 2023; 15(16):4173. https://doi.org/10.3390/cancers15164173
Chicago/Turabian StyleSerra, Massimo, Davide Rubes, Sergio Schinelli, and Mayra Paolillo. 2023. "Small Molecules against Metastatic Tumors: Concrete Perspectives and Shattered Dreams" Cancers 15, no. 16: 4173. https://doi.org/10.3390/cancers15164173
APA StyleSerra, M., Rubes, D., Schinelli, S., & Paolillo, M. (2023). Small Molecules against Metastatic Tumors: Concrete Perspectives and Shattered Dreams. Cancers, 15(16), 4173. https://doi.org/10.3390/cancers15164173










