Effectiveness of Therapeutic Education in Patients with Cancer Pain: Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
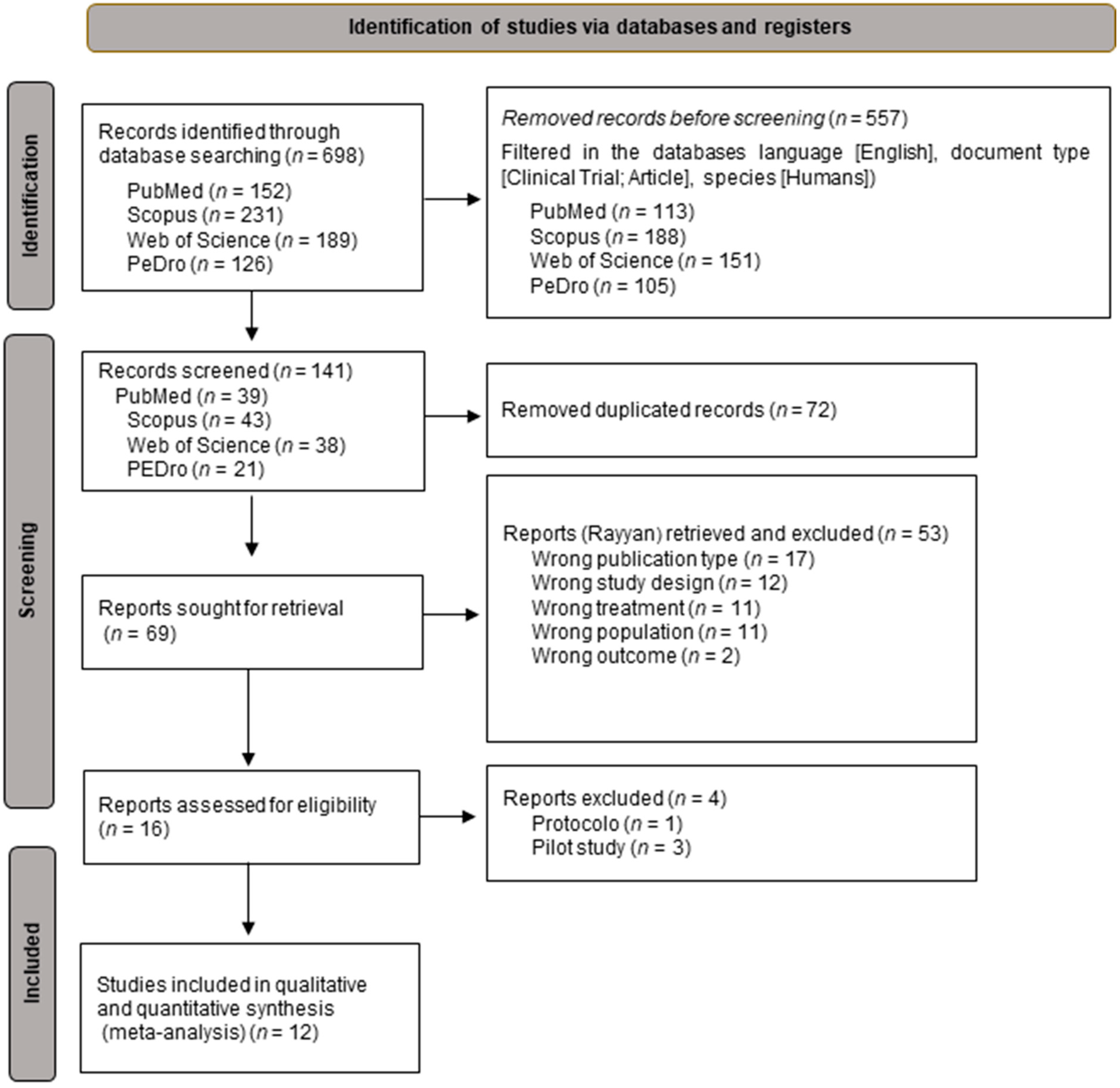
2.4. Study Selection Process
2.5. Data Extraction
2.6. Methodological Quality Assessment
2.7. Analytical Decisions for Meta-Analysis
3. Results
3.1. Selection of the Studies
3.2. Methodological Quality
3.3. Characteristics of the Studies
3.4. Study Results
3.4.1. Medical Information and Type of Cancer
3.4.2. Painful Experience
3.4.3. Pain Intensity
3.4.4. Knowledge about Pain
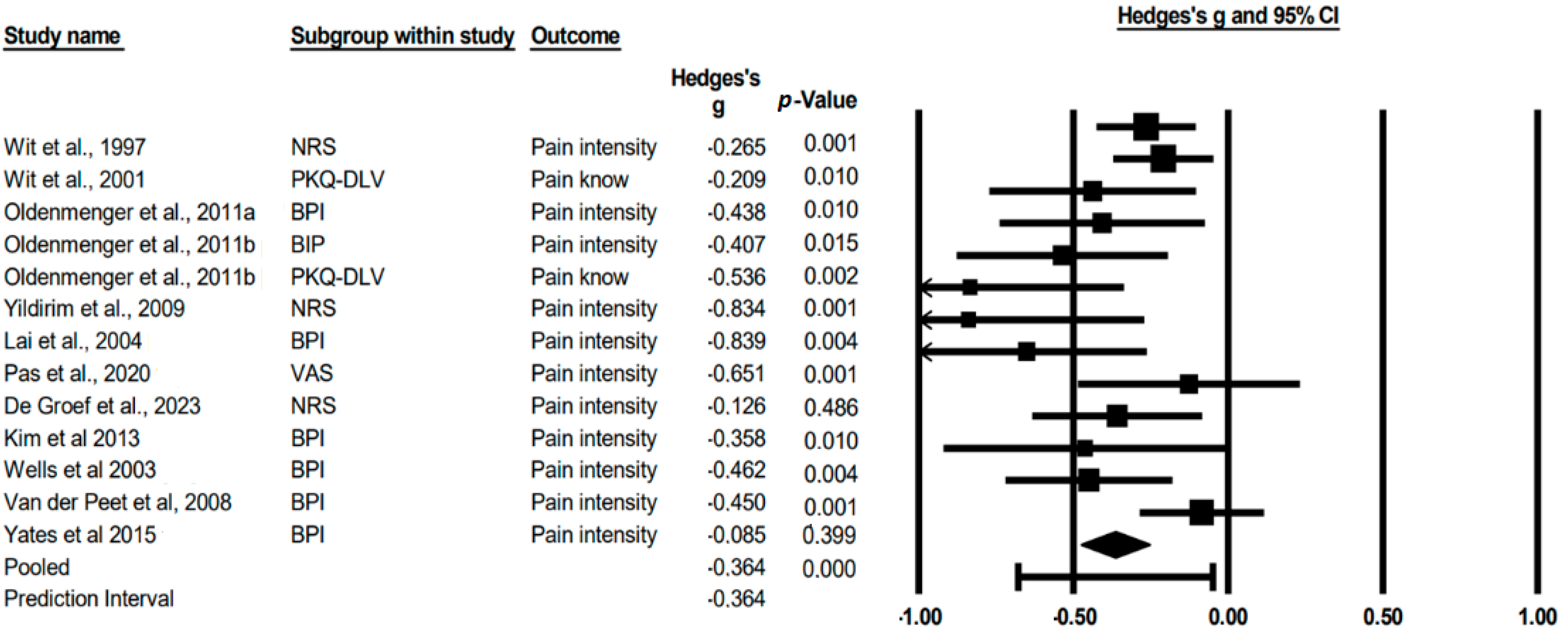
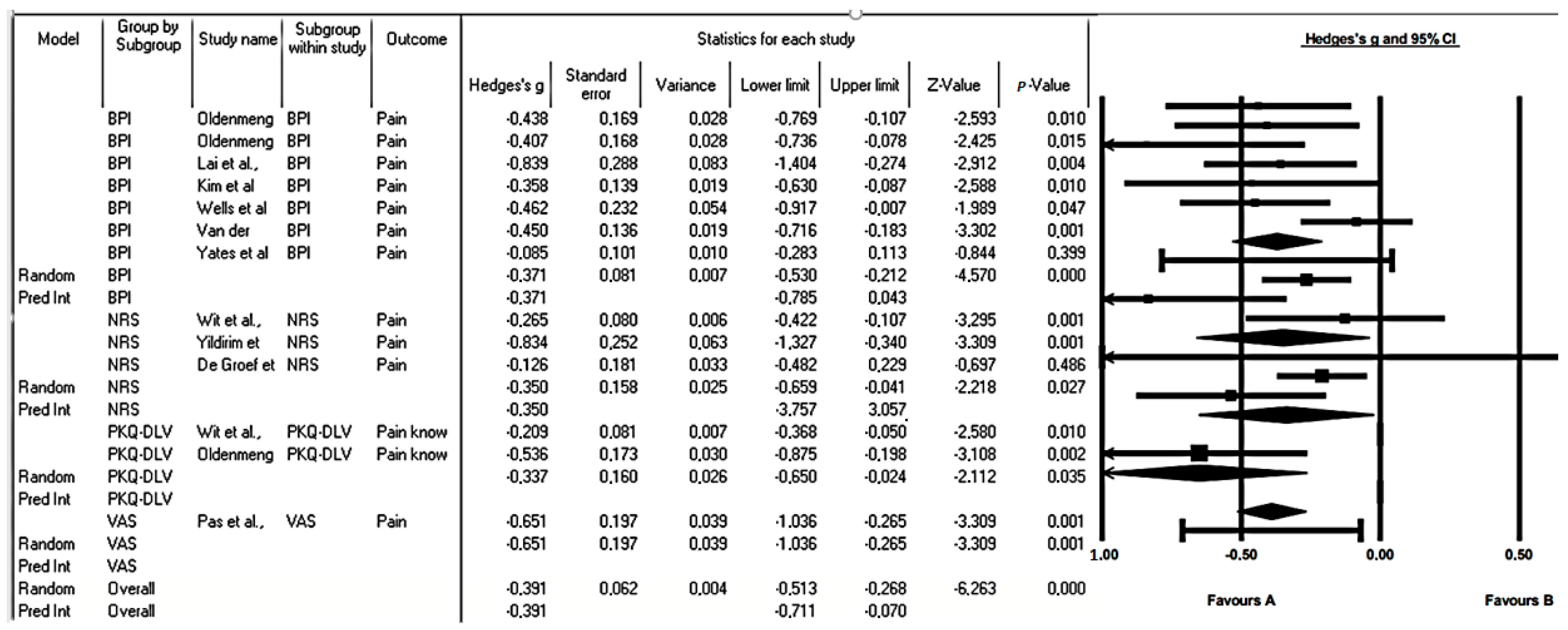
3.5. Meta-Analysis
3.6. Subgroup Analysis
3.7. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- WCRF International. Worldwide Cancer Data|World Cancer Research Fund International. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ (accessed on 2 February 2023).
- Cancer Research UK. Cancer Statistics for the UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk (accessed on 2 February 2023).
- Ventafridda, V.; Tamburini, M.; Caraceni, A.; De Conno, F.; Naldi, F. A validation study of the WHO method for cancer pain relief. Cancer 1987, 59, 850–856. [Google Scholar] [CrossRef] [PubMed]
- WCRF International. Cancer Survival Statistics. World Cancer Research Fund International. Available online: https://www.wcrf.org/cancer-trends/cancer-survival-statistics/ (accessed on 4 February 2023).
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in cancer mortality, incidence, and survival: A global overview. Eur. J. Cancer Prev. 2020, 29, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Breast Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-bycancer-type/breast-cancer (accessed on 5 February 2023).
- Luo, Y.H.; Luo, L.; Wampfler, J.A.; Wang, Y.; Liu, D.; Chen, Y.M.; Adjei, A.A.; Midthun, D.E.; Yang, P. 5-year overall survival in patients with lung cancer eligible or ineligible for screening according to US Preventive Services Task Force criteria: A prospective, observational cohort study. Lancet Oncol. 2019, 20, 1098–1108. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Amador, M.; Matias-Guiu, X.; Sancho-Pardo, G.; Contreras Martinez, J.; de la Torre-Montero, J.C.; Peñuelas Saiz, A.; Garrido, P.; García-Sanz, R.; Rodríguez-Lescure, Á.; Paz-Ares, L. Impact of the COVID-19 pandemic on the care of cancer patients in Spain. ESMO Open 2021, 6, 100157. [Google Scholar] [CrossRef] [PubMed]
- The Effects of Cancer Delays during the COVID-19 Pandemic—Collateral Global. (7 October 2021). Collateral Global. Available online: https://collateralglobal.org/article/effects-of-cancer-delays-during-the-covid-19-pandemic (accessed on 5 February 2023).
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Clohisy, D.R.; Mantyh, P.W. Bone cancer pain. Clin. Orthop. Relat. Res. 2003, 415, 279–288. [Google Scholar] [CrossRef]
- Van den Beuken-van Everdingen, M.H.J.; de Rijke, J.M.; Kessels, A.G.; Schouten, H.C.; van Kleef, M.; Patijn, J. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain 2007, 132, 312–320. [Google Scholar] [CrossRef]
- van den Beuken-van Everdingen, M.H.J.; de Rijke, J.M.; Kessels, A.G.; Schouten, H.C.; van Kleef, M.; Patijn, J. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann. Oncol. 2007, 18, 1437–1449. [Google Scholar] [CrossRef]
- Taber, J.M.; Leyva, B.; Persoskie, A. Why do people avoid medical care? A qualitative study using national data. J. Gen. Intern. Med. 2015, 30, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, N.J.; Elnahal, S.M.; Alvarez, R.H. Cancer pain: A review of epidemiology, clinical quality and value impact. Future Oncol. 2017, 13, 833–841. [Google Scholar] [CrossRef]
- Smith, T.J.; Staats, P.S.; Deer, T.; Stearns, L.J.; Rauck, R.L.; Boortz-Marx, R.L.; Buchser, E.; Català, E.; Bryce, D.A.; Coyne, P.J.; et al. Implantable Drug Delivery Systems Study Group. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: Impact on pain, drug-related toxicity, and survival. J. Clin. Oncol. 2002, 20, 4040–4049. [Google Scholar] [CrossRef]
- Kurita, G.P.; Sjøgren, P. Pain management in cancer survivorship. Acta Oncol. 2015, 54, 629–634. [Google Scholar] [CrossRef]
- Pidgeon, T.; Johnson, C.E.; Currow, D.; Yates, P.; Banfield, M.; Lester, L.; Allingham, S.F.; Bird, S.; Eagar, K. A survey of patients’ experience of pain and other symptoms while receiving care from palliative care services. BMJ Support. Palliat. Care 2016, 6, 315–322. [Google Scholar] [CrossRef]
- Concepción Pérez Hernández, D.; Babarro, A.A.; Aguerri, A.R.; Villegas Estévez, F.; Virizuela Echaburu, J.A. Guía Para el Abordaje Interdisciplinar del Dolor Oncológico. Available online: https://seom.org/seomcms/images/stories/recursos/Guia_GADO_dolor_oncologico.pdf (accessed on 18 February 2023).
- World Health Organization. Cancer Pain Relief. 1986. Available online: https://apps.who.int/iris/handle/10665/43944 (accessed on 7 February 2023).
- Meuser, T.; Pietruck, C.; Radbruch, L.; Stute, P.; Lehmann, K.A.; Grond, S. Symptoms during cancer pain treatment following WHO-guidelines: A longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 2001, 93, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Jara, C.; Del Barco, S.; Grávalos, C.; Hoyos, S.; Hernández, B.; Muñoz, M.; Quintanar, T.; Meana, J.A.; Rodriguez, C.; de Las Peñas, R. SEOM clinical guideline for treatment of cancer pain (2017). Clin. Transl. Oncol. 2018, 20, 97–107. [Google Scholar] [CrossRef] [PubMed]
- DiPiro, J.T.; Talbert, R.L.; Yee, G.C.; Wells, B.G.; Posey, L.M. Pharmacotherapy: A Pathophysiologic Approach 9/E; McGraw Hill: New York, NY, USA, 2014. [Google Scholar]
- Crawford, C.; Boyd, C.; Paat, C.F.; Price, A.; Xenakis, L.; Yang, E.; Zhang, W.; Evidence for Massage Therapy (EMT) Working Group. The Impact of Massage Therapy on Function in Pain Populations—A Systematic Review and Meta-Analysis of Randomized Controlled Trials: Part I, Patients Experiencing Pain in the General Population. Pain Med. 2016, 17, 1353–1375. [Google Scholar] [CrossRef]
- Nijs, J.; Wijma, A.J.; Leysen, L.; Pas, R.; Willaert, W.; Hoelen, W.; Ickmans, K.; Van Wilgen, C.P. Explaining pain following cancer: A practical guide for clinicians. Braz. J. Phys. Ther. 2019, 23, 367–377. [Google Scholar] [CrossRef]
- Petticrew, M.; Anderson, L.; Elder, R.; Grimshaw, J.; Hopkins, D.; Hahn, R.; Krause, L.; Kristjansson, E.; Mercer, S.; Sipe, T.; et al. Complex interventions and their implications for systematic reviews: A pragmatic approach. J. Clin. Epidemiol. 2013, 66, 1209–1214. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hyun, M.K.; Jung, Y.J.; Kang, M.J.; Keam, B.; Go, S.J. Effectiveness of education interventions for the management of cancer pain: A systematic review. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 4787–4793. [Google Scholar] [CrossRef] [PubMed]
- Marie, N.; Luckett, T.; Davidson, P.M.; Lovell, M.; Lal, S. Optimal patient education for cancer pain: A systematic review and theory-based meta-analysis. Support. Care Cancer 2013, 21, 3529–3537. [Google Scholar] [CrossRef] [PubMed]
- Champarnaud, M.; Villars, H.; Girard, P.; Brechemier, D.; Balardy, L.; Nourhashemi, F. Effectiveness of Therapeutic Patient Education Interventions for Older Adults with Cancer: A Systematic Review. J. Nutr. Health Aging 2020, 24, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- De Wit, R.; Van Dam, F.S.; Zandbelt, L.C.; Van Buuren, A.; Van Der Heijden, K.; Leenhouts, G.; Loonstra, S. A Pain Education Program for chronic cancer pain patients: Follow-up results from a randomized controlled trial. Pain 1997, 73, 55–69. [Google Scholar] [CrossRef]
- De Wit, R.; Van Dam, F.S.; Loonstra, S.; Zandbelt, L.C.; Van Buuren, A.; Van Der Heijden, K.; Leenhouts, G.; Duivenvoorden, H.J.; Abu-Saad, H.H. Improving the quality of pain treatment by a tailored pain education programme for cancer patients in chronic pain. Eur. J. Pain 2001, 5, 241–256. [Google Scholar] [CrossRef]
- Oldenmenger, W.H.; Van Der Rijt, C.C. Feasibility of assessing patients’ acceptable pain in a randomized controlled trial on a patient pain education program. Palliat. Med. 2016, 31, 553–558. [Google Scholar] [CrossRef]
- Oldenmenger, W.H.; Smitt, P.A.E.S.; Van Montfort, C.; De Raaf, P.J.; Van Der Rijt, C.C. A combined pain consultation and pain education program decreases average and current pain and decreases interference in daily life by pain in oncology outpatients: A randomized controlled trial. Pain 2011, 152, 2632–2639. [Google Scholar] [CrossRef]
- Yildirim, Y.; Cicek, F.; Uyar, M. Effects of Pain Education Program on Pain Intensity, Pain Treatment Satisfaction, and Barriers in Turkish Cancer Patients. Pain Manag. Nurs. 2009, 10, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Guo, S.; Keefe, F.J.; Tsai, S.; Chien, C.; Sung, Y.; Chen, M. Effects of brief pain education on hospitalized cancer patients with moderate to severe pain. Support. Care Cancer 2004, 12, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Pas, R.; Leysen, L.; De Goeij, W.; Vossebeld, L.; Van Wilgen, P.; De Groef, A.; De Kooning, M. Pain Neuroscience Education in cancer survivors with persistent pain: A pilot study. J. Bodyw. Mov. Ther. 2020, 24, 239–244. [Google Scholar] [CrossRef]
- De Groef, A.; Evenepoel, M.; Van Dijck, S.; Dams, L.; Haenen, V.; Wiles, L.; Catley, M.; Vogelzang, A.A.; Olver, I.; Hibbert, P.; et al. Feasibility and pilot testing of a personalized eHealth intervention for pain science education and self-management for breast cancer survivors with persistent pain: A mixed-method study. Support. Care Cancer 2023, 31, 119. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Shin, S.J.; Kim, S.H.; An, S.; Rha, S.Y.; Ahn, J.B.; Cho, B.C.; Choi, H.J.; Sohn, J.; Rha, S.; et al. Randomized controlled trial of standardized education and telemonitoring for pain in outpatients with advanced solid tumors. Support. Care Cancer 2013, 21, 1751–1759. [Google Scholar] [CrossRef]
- Wells, N.; Hepworth, J.T.; Murphy, B.A.; Wujcik, D.; Johnson, R. Improving Cancer Pain Management Through Patient and Family Education. J. Pain Symptom Manag. 2003, 25, 344–356. [Google Scholar] [CrossRef]
- Van Der Peet, E.H.; Van Den Beuken-Van Everdingen, M.H.J.; Patijn, J.; Schouten, H.C.; Van Kleef, M.; Courtens, A.M. Randomized clinical trial of an intensive nursing-based pain education program for cancer outpatients suffering from pain. Support. Care Cancer 2009, 17, 1089–1099. [Google Scholar] [CrossRef][Green Version]
- Yates, P.; Edwards, H.; Nash, R.; Aranda, S.; Purdie, D.M.; Najman, J.M.; Skerman, H.; Walsh, A.M. A randomized controlled trial of a nurse-administered educational intervention for improving cancer pain management in ambulatory settings. Patient Educ. Couns. 2004, 53, 227–237. [Google Scholar] [CrossRef]
- Lovell, M.R.; Luckett, T.; Boyle, F.M.; Phillips, J.; Agar, M.; Davidson, P.M. Patient education, coaching, and self-management for cancer pain. J. Clin. Oncol. 2014, 32, 1712–1720. [Google Scholar] [CrossRef]
- Oldenmenger, W.H.; Geerling, J.I.; Mostovaya, I.; Vissers, K.C.P.; de Graeff, A.; Reyners, A.K.L.; Van der Linden, Y.M. A systematic review of the effectiveness of patient-based educational interventions to improve cancer-related pain. Cancer Treat. Rev. 2018, 63, 96–103. [Google Scholar] [CrossRef]
- Prevost, V.; Delorme, C.; Grach, M.; Chvetzoff, G.; Hureau, M. Therapeutic Education in Improving Cancer Pain Management. Am. J. Hosp. Palliat. Med. 2016, 33, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.I.; Bagnall, A.M.; Closs, J.S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and metaanalysis. Pain 2009, 143, 192–199. [Google Scholar] [CrossRef]
- Lepri, B.; Romani, D.; Storari, L.; Barbari, V. Effectiveness of Pain Neuroscience Education in Patients with Chronic Musculoskeletal Pain and Central Sensitization: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 4098. [Google Scholar] [CrossRef]
- Van Ittersum, M.; Van Wilgen, C.P.; Van Der Schans, C.P.; Lambrecht, L.; Groothoff, J.W.; Nijs, J. Written Pain Neuroscience Education in Fibromyalgia: A Multicenter Randomized Controlled Trial. Pain Pract. 2014, 14, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Louw, A.; Diener, I.; Butler, D.; Puentedura, E.J. The Effect of Neuroscience Education on Pain, Disability, Anxiety, and Stress in Chronic Musculoskeletal Pain. Phys. Med. Rehabil. 2011, 92, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.Y.; Pisu, M.; Kvale, E.A.; Johns, S.A. Developing effective cancer pain education programs. Curr. Pain Headache Rep. 2012, 16, 332–342. [Google Scholar] [CrossRef]
- Mosadeghrad, A.M. Factors influencing healthcare service quality. Int. J. Health Policy Manag. 2014, 3, 77–89. [Google Scholar] [CrossRef]
- Nijs, J.; Van Wilgen, C.P.; Van Oosterwijck, J.; Van Ittersum, M.; Meeus, M. How to explain central sensitization to patients with ‘unexplained’ chronic musculoskeletal pain: Practice guidelines. Man. Ther. 2011, 16, 413–418. [Google Scholar] [CrossRef]
- Caraceni, A.; Hanks, G.; Kaasa, S.; Bennett, M.I.; Brunelli, C.; Cherny, N.; Zeppetella, G. Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC. Lancet Oncol. 2012, 13, e58–e68. [Google Scholar] [CrossRef]
- Fernández-Lao, C.; Cantarero-Villanueva, I.; Fernández-De-Las-Peñas, C.; Del-Moral-Ávila, R.; Menjón-Beltrán, S.; Arroyo-Morales, M. Widespread Mechanical Pain 46 Hypersensitivity as a Sign of Central Sensitization after Breast Cancer Surgery: Comparison between Mastectomy and Lumpectomy. Pain Med. 2011, 12, 72–78. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.W.; Yue, D.; Liu, G. Protease-activated receptor 2 in dorsal root ganglion contributes to peripheral sensitization of bone cancer pain. Eur. J. Pain 2014, 18, 326–337. [Google Scholar] [CrossRef] [PubMed]
| Article | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total Score on PEDro Scale |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wit et al., 1997 [35] | Y | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Wit et al., 2001 [36] | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Oldenmenger et al., 2011a [37] | Y | Y | N | N | N | N | N | N | N | Y | Y | 3 |
| Oldenmenger et al., 2011b [38] | Y | Y | N | Y | N | N | N | Y | N | Y | N | 4 |
| Yildirim et al., 2009 [39] | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Lai et al., 2004 [40] | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Pas et al., 2020 [41] | Y | Y | N | Y | Y | Y | N | Y | N | Y | Y | 7 |
| De Groef et al., 2023 [42] | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
| Kim et al., 2013 [43] | Y | Y | N | Y | Y | Y | N | Y | Y | Y | Y | 8 |
| Wells et al., 2003 [44] | Y | Y | N | Y | N | N | N | N | Y | Y | Y | 5 |
| Van der Peet et al., 2008 [45] | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Yates et al., 2015 [46] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 |
| Control Group | Experimental Group | Variables | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author and Year | Country | Sample Size | Age Mean (SD) | N | Relevant Characteristics | Intervention | Sessions/Method | N | Relevant Character-istics | Intervention | Sessions/Method | Time | Outcome | Measuring Instrument | Assess-Ments | Results |
| Wit et al., 1997 [35] | Netherlands | 313 | 15.5 | 154 | With nursing district: 51 No nursing district: 103 65.6% women Mean age 55.9 Breast and genitourinary cancer as the most frequent tumors. | Standard cancer care | - | 159 | With nursing district: 53 No nursing district: 106 59.7% women Mean age 57 Breast and genito-urinary cancer as the most frequent tumors. | Standard cancer care + PEP | Initial instruction 30–60 min. Telephone sessions. 5–15 min, theoretical brochure, and lectures recorded in audio as reinforce-ment. | 1 week | Painful experience | MPQ-DLV | At baseline [T0] and 2 weeks [T1]. | Although all patients’ pain intensity scores decreased after discharge, scores in the intervention group decreased significantly more than for patients in the control group at follow-up (p < 0.01). Mean pain intensity T0 = 58 vs. T3 = 16.9 p = 0.01 |
| Medium and present pain intensity | NRS 0–10 | At baseline [T0], 2 weeks [T1], 4 weeks [T2], and eighth [T3]. | ||||||||||||||
| Quality of life | EORTC QLC-C30 (+3) | At baseline [T0] and at 4 weeks [T2]. | ||||||||||||||
| Pain knowledge | PKQ-DLV | At baseline [T0] and 2 weeks [T1]. | ||||||||||||||
| Wit et al., 2001 [36] | Netherlands | 313 | 15.5 | 154 | With nursing district: 51 No nursing district: 103 65.6% women Mean age 55.9 Breast and genitourinary cancer was the most frequent tumors. | Standard cancer care | - | 159 | With nursing district: 53 No nursing district: 106 59.7% women Mean age 57 Breast and genito-urinary cancer was the most frequent tumors. | Standard cancer care + PEP | 1 week | Pain intensity present/mean pain intensity | AMPI | At baseline [T0], 2 weeks [T1], 4 weeks [T2], and eighth [T3]. | AMPI R2 60% Short term BETA −0.47 < 0.001 Long term R2 56% beta 0.72 < 0.001 Pain knowledge beta 0.12 < 0.01 Pain impact beta 0.11 < 0.05 | |
| Worst pain intensity | (MPQ-DLV) | |||||||||||||||
| Tolerable intensity of pain | (PKQ-DLV) | |||||||||||||||
| Experience of pain | List of cancer patients | |||||||||||||||
| Pain Knowledge | (PCL-C) | |||||||||||||||
| Olden-menger et al., 2011 [37] | Netherlands | 72 | 59 (11) | 37 | 62% women Mean age 61 Breast and gastroin-testinal cancer was the most frequent tumors. All have nociceptive pain. | Standard cancer care | - | 35 | 69% women Average age 56 Breast cancer was the most common tumor. All have nociceptive pain. Tumor status: Locally advanced 14 (19%) Metastatic 58 (81%) Pain duration (months; median, IQR) 5 (3–14) | Standard cancer care + pain consultation + PEP | Previous PEP + Compre-hensive face-to-face assessment at the beginning of treatment. | 1 week | Pain intensity | BPI | At the start of the study [T0], after two weeks [T1], after 4 weeks [T2] and after 8 weeks [T3]. | Mean score for intensidad media aceptable del dolor. CG T0 = 4.6 (standard deviation (SD) = 1.7), T3 = 5.0 (SD = 1.9) IG acceptable pain decreased significantly T0 = 4.6 (SD = 1.7) T = 3 3.8 (SD = 1.7; p < 0.01). Difference between the groups was significant at 8 weeks (p < 0.05). |
| Use of analgesics | OMS analgesic ladder + MEDD (table of equiv-alences | |||||||||||||||
| Need for analgesic prescription | ATC + PRN | |||||||||||||||
| Adequacy of analgesic prescription | PMI | |||||||||||||||
| Analgesic adherence | MEMS | Measured in each of the 8 weeks. | ||||||||||||||
| Knowledge about pain | PKQ-DLV | At baseline [T0] and at 2 weeks [T1]. | ||||||||||||||
| Olden-menger et al., 2011 [38] | Netherlands | 72 | 59 (11) | 37 | 62% women Mean age 61 Breast and gastro-intestinal cancer was the most frequent tumors. All have nociceptive pain. | Standard cancer care | - | 35 | 69% women Average age 56 Breast cancer was the most common tumor. All have nociceptive pain. Tumor status: Locally advanced 14 (19%) Metastatic 58 (81%) Pain duration (months; median, IQR) 5 (3–14) | Standard cancer care + Pain consultation + PEP | Previous PEP + Compre-hensive face-to-face assessment at the beginning of treatment. | 1 week | Acceptable intensity of pain Knowledge of pain | BPI + Question (NRS) | Measured at 2 weeks [T1], 4 weeks [T2], and 8 weeks [T3]. | For mean pain intensity, the mean reduction was 1.13 for CG and 1.95 for GI (20% vs. 31%; p = 0.03). For current pain intensity, the mean reduction was 0.67 for CS and 1.50 for PC-PEP (16% vs. 30%; p = 0.016). At week 2, the level of pain awareness (0 to 100) was significantly better after random GI assignment (71 ± 13). |
| Yildirim et al., 2009 [39] | Turkey | 40 | 20 | 20 | 75% < 60 years 55% men Tumors of digestive organs and peritoneum was the most common. | Standard cancer care | - | 20 | 95% < 60 years old 55% men Tumors of digestive organs and peritoneum was the most common. | Standard cancer care + PEP (modified) | Initial instruction 30–40 min. Sessions of 5–15 min and theoretical brochure as reinforce-ment. | 1 week | Pain intensity (present, worst, and last) | NRS | At the beginning of the study and weeks 2, 4, and 8 of the application. | The effect of the PEP on patients’ pain intensity (present, worst, and least pain intensities) and satisfaction with pain treatment was assessed at baseline 3.1 (±1.55) and after 2 weeks (mean 1.10 ± 0.85), 4 weeks (1.20 ± 1.02), and 8 weeks (1.20 ± 1.06). |
| Painful experience | MPQ | At the beginning of the study and 2 weeks after application. | ||||||||||||||
| Patient barriers in relation to oncological pain management | BQ-r | |||||||||||||||
| Patient fitness | KPS | Measured at baseline. | ||||||||||||||
| Lai et al., 2004 [40] | Taiwan | 30 | 53.5 | 15 | 60% women Mean age 56 Half are undergoing chemotherapy and all have metastases. | Standard cancer care | The control group was visited by the researchers once a day for 10–15 min. However, researchers did not provide the group with any information regarding concepts of pain. | 15 | 53% women Mean age 51.7 Half are undergoing chemo-therapy and all have metastases. | Standard cancer care + PEP | 5 days with individual talk of 10–15 min. Explain a theoretical brochure and reinforce-ment of concepts. | 5 days | Mean, current and last pain intensity | BPI-T brief pain inventory | Measured at the beginning of the study and the end of the intervention (5 days). | BPIT CG = pre = 4.33 ± 2.88 pos = 3.73 ± 1.83 T = 0.98 IG pre = 5.00 ± 1.07 pos = 2.80 ± 1.61 t = 2.40 Between groups = t = 0.94 F = 4.01 |
| Interference with daily life | ||||||||||||||||
| Pain tolerance | POAB-CA | |||||||||||||||
| Catastro-phism | CSQ-Cat | |||||||||||||||
| Beliefs about sense of pain control | ||||||||||||||||
| Pas et al., 2020 [41] | Belgium | Not Compared To Control Group | 30 | Cancer survivors. 24 women and 6 men. Breast cancer was the most common tumor. | Pain neuroscience education program | Individual talk of at least 30–45 min. Theoretical pamphlet as reinforce-ment. | 2 weeks | Pain intensity | VAS | Measured at baseline and 2 weeks after application. | Levels of the VAS were significantly higher pre = mean 47 (RIQ 43–65) compared to post = 40 (RIQ 34–55) mean difference = (p = 0.001, r = −0.44). | |||||
| Catastrophist pain | PCS | |||||||||||||||
| Quality of life | SF-36 | |||||||||||||||
| De Groef et al., 2023 [42] | Germany | Not Compared To Control Group | 29 | Mean age 50.8. All participants were breast cancer survivors. 90% with unilateral breast involvement. | eHealth intervention: personalized pain neuroscience education program for each breast cancer survivor. | 22 individual sessions with graphic support material. | 6 weeks | Pain-related functionality | PDI | Measure-ments at the beginning of the study (T0), post-intervention (T1), and at 3 months (T2). | 1. Pain-related functioning (PDI) Pre = 27.6 (13.0) Post 1 = 22.6 (±12.3) T2 = 21.2 (±14.0) p = 0.0038 | |||||
| Aggravating pain | NRS 0–10 | |||||||||||||||
| Self-reported symptoms of hyperalgesia and allodynia | CSI | |||||||||||||||
| Physical function | PROMIS-PF-SF | |||||||||||||||
| Pain concern | PCS | |||||||||||||||
| Depression, anxiety, and stress. | DASS-21 | |||||||||||||||
| Symptoms | BTMS | |||||||||||||||
| Quality of life | 0–10 | |||||||||||||||
| Health related to quality of life | MqoL | |||||||||||||||
| Self-efficacy | PSEQ | |||||||||||||||
| Kim et al., 2013 [43] | Korea | 108 | 59.5 | 54 | 38 (70.4) men 16 (29.6) women | General education | Pain management nurse trained each patient and caregiver together for approxi-mately 30 min. | 54 | 35 (64.8) men 19 (35.2) women | Pain education plus telemonitoring. | Tele-monitoring regarding pain was performed by an NP every day for 1 week. The NP phoned patients and asked for their mean VAS pain score and worst VAS pain score in the last 24 h. | 1 week from the first visit | Pain intensity | BPI | T0 = Baseline T1 = 1 week T2 = 2 months | Pain intensity was significantly improved at 1 week, including worst pain (7.3 to 5.7, p < 0.01) and average pain (4.6 to 3.8, p < 0.01). |
| Wells et al., 2003 [44] | USA | 64 | 53.11 | 56 | 54% men and 70% metastases. | Standard cancer care | - | 47.95 | 76 men in hotlines. 68 men in weekly call. | Weekly call. | All patients and their caregivers participated in the pain education program. Included structured and tailored components. The structured component was a 15-min videotape (‘Taking Charge of Your Pain’), side effect management, and discussion of the patient’s present pain regimen. This education program took 20 to 30 min. | 6 months | Pain intensity | BPI | T0 = baseline T2 to T6 monthly for 6 months | Average pain (0–10) t0 = 4.18 (2.10) T1 = CG 4.50 (2.17) IG1 4.00 (2.05) IG2 3.97 (2.12) F = 0.44 p = 0.05 Continuous access to pain-related information using a patient- or provider-initiated format did not affect long-term pain outcomes. |
| Van der Peet et al., 2008 [45] | Netherlands | 120 | 61 | 62 | Mean age 60 Men = 38.3% | Standard cancer care | - | 58 | Mean age 62 Men = 52.1% | Skilled nursing care at home, at the same time as the care received from their doctor, including PEP and follow-up of symptoms other than pain. The PYP consists of three components: improving pain knowledge and management through a handout, instruction on recording pain in a diary, and behavioral stimulation. | Home visits each lasting 1–1.5 h. Second home visit took place in week 3. The third and final home visit took place at week 6. | 8 weeks | Pain intensity | BPI | l baseline (T0), week 4 (T1) and week 8 (T2) | There was no difference in pain scores at T1 (week 4) and T2 (week 8) between the intervention and control groups in patients with a baseline pain score of 1 to 3. Significant differences in pain were found between the intervention and control groups at T1 (p = 0.00) and T2 (p = 0.00) in patients with a baseline score of 7–10. |
| Yates et al., 2015 [46] | Australia | 189 | 56 | 92 | Two-thirds (66.1%) of the sample were female with a mean age of 56 years. The median time since diagnosis was 3 months (range: 0–331 months). | Standard cancer care | - | 97 | Mean age 57. | PMI: The intervention used instructional and cognitive-behavioral strategies and included the delivery of general information about pain and its management, training to help patients learn more adaptive ways of communicating pain, and the development of a pain management plan that included strategies to address patient barriers to effective pain management. | The intervention was administered in two sessions. The first session, approx-imately 30 min long, was held in the outpatient department, and the second session, approxi-mately 15 min long, was conducted by telephone a week later. | 8 weeks | Pain intensity | BPI | Follow-up assessment was conducted at 1 week (T1) and 8 weeks (T2) following the second intervention session. | t0 = 4.1 (1.8) T1 = 3.9 (1.8) T2 = 3.5 (1.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Martín, A.M.; Aguilera-García, I.; Castellote-Caballero, Y.; Rivas-Campo, Y.; Bernal-Suárez, A.; Aibar-Almazán, A. Effectiveness of Therapeutic Education in Patients with Cancer Pain: Systematic Review and Meta-Analysis. Cancers 2023, 15, 4123. https://doi.org/10.3390/cancers15164123
González-Martín AM, Aguilera-García I, Castellote-Caballero Y, Rivas-Campo Y, Bernal-Suárez A, Aibar-Almazán A. Effectiveness of Therapeutic Education in Patients with Cancer Pain: Systematic Review and Meta-Analysis. Cancers. 2023; 15(16):4123. https://doi.org/10.3390/cancers15164123
Chicago/Turabian StyleGonzález-Martín, Ana María, Iván Aguilera-García, Yolanda Castellote-Caballero, Yulieth Rivas-Campo, Antonio Bernal-Suárez, and Agustín Aibar-Almazán. 2023. "Effectiveness of Therapeutic Education in Patients with Cancer Pain: Systematic Review and Meta-Analysis" Cancers 15, no. 16: 4123. https://doi.org/10.3390/cancers15164123
APA StyleGonzález-Martín, A. M., Aguilera-García, I., Castellote-Caballero, Y., Rivas-Campo, Y., Bernal-Suárez, A., & Aibar-Almazán, A. (2023). Effectiveness of Therapeutic Education in Patients with Cancer Pain: Systematic Review and Meta-Analysis. Cancers, 15(16), 4123. https://doi.org/10.3390/cancers15164123






