Planning CT Identifies Patients at Risk of High Prostate Intrafraction Motion
Abstract
Simple Summary
Abstract
1. Introduction
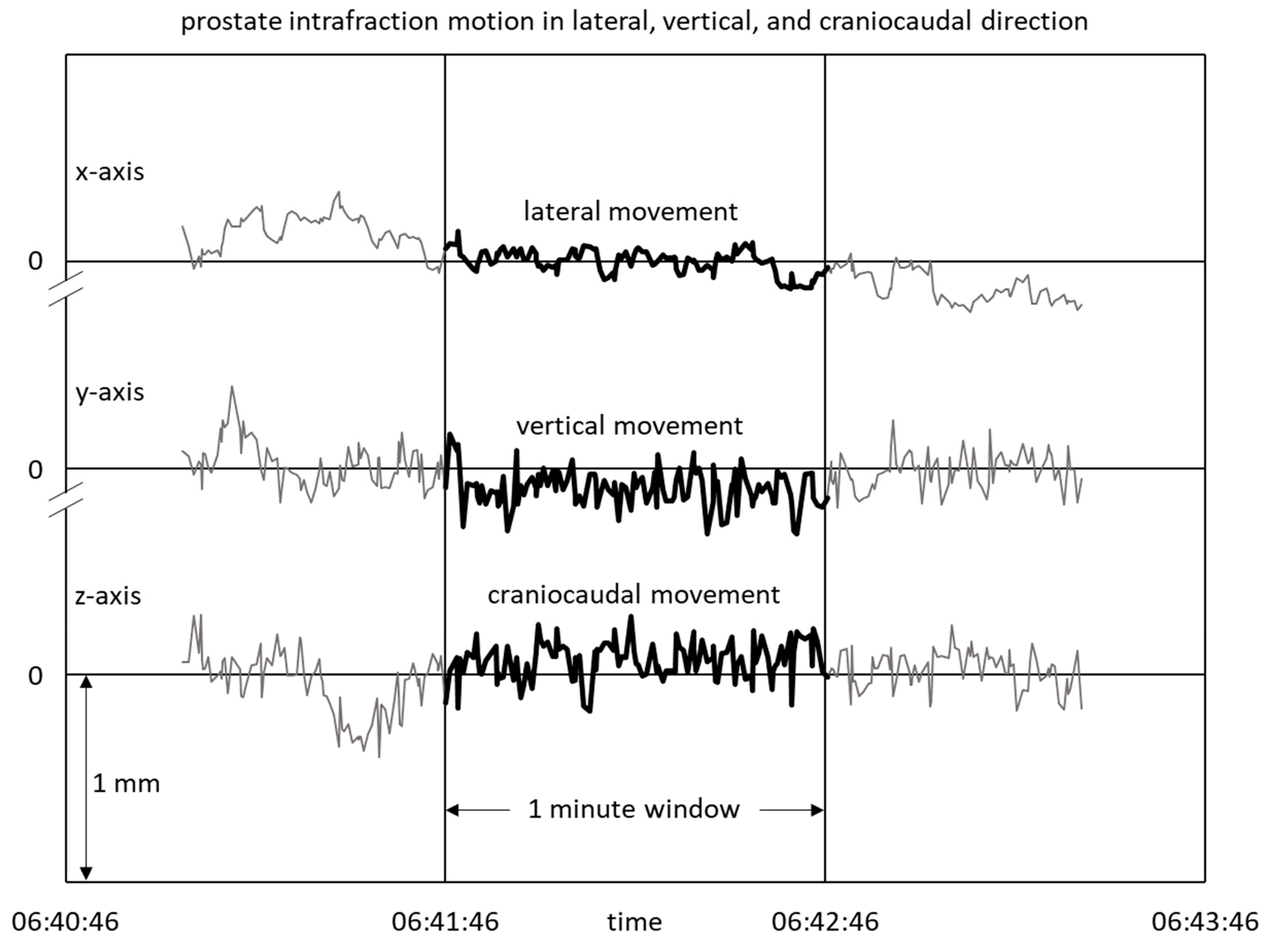
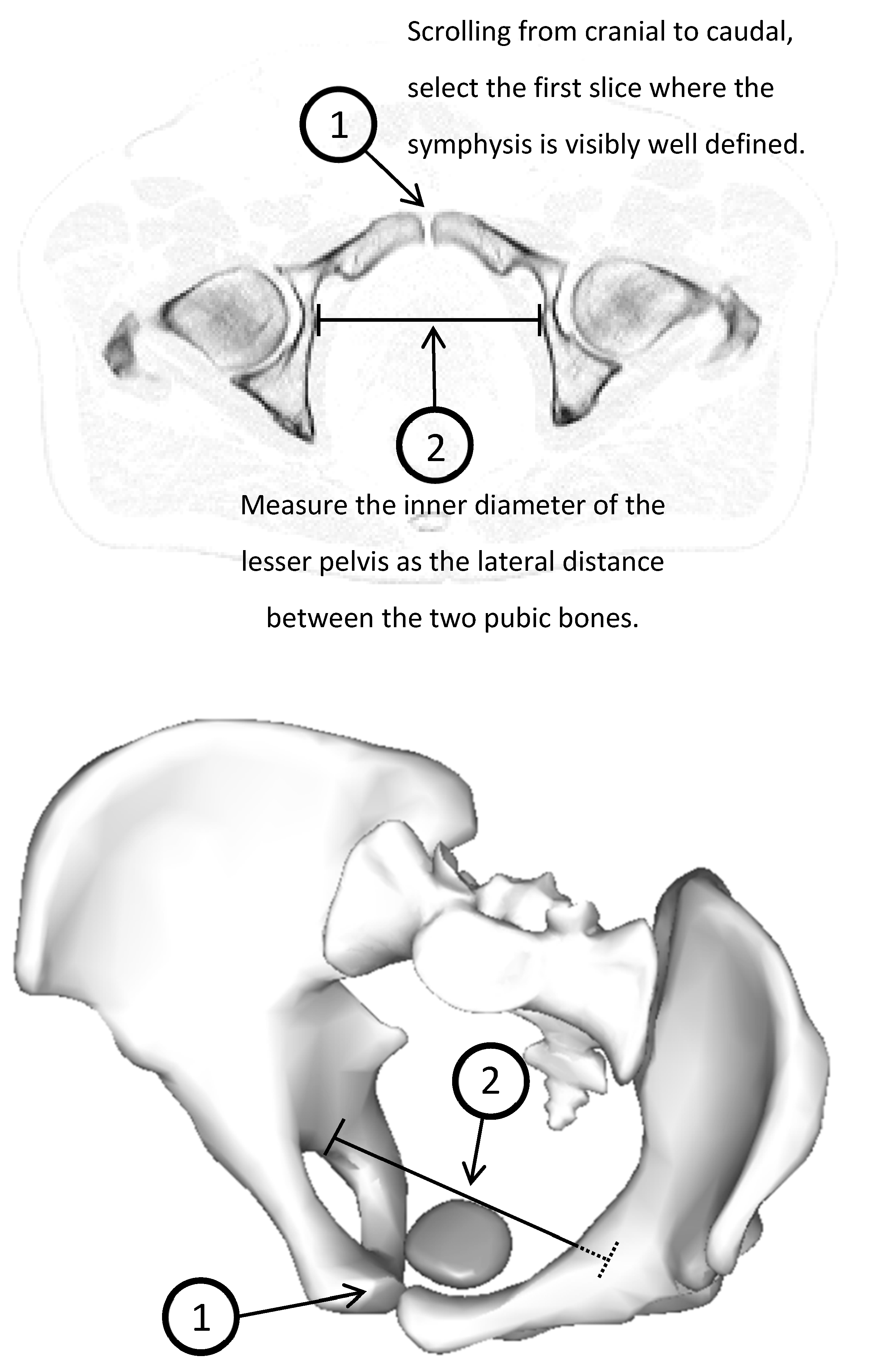
2. Materials and Methods
3. Results
3.1. Available Data
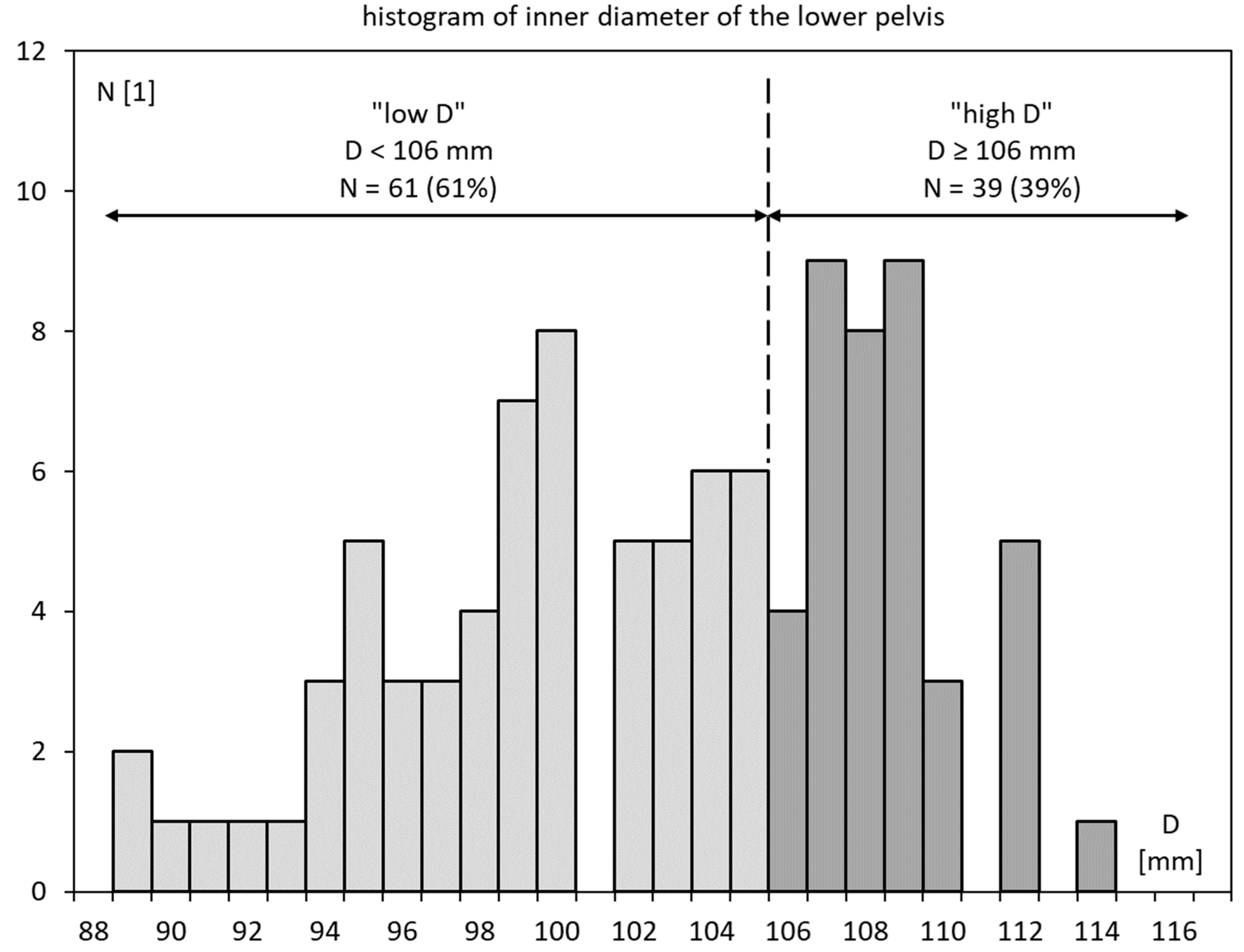
3.2. Inner Diameter of the Lesser Pelvis
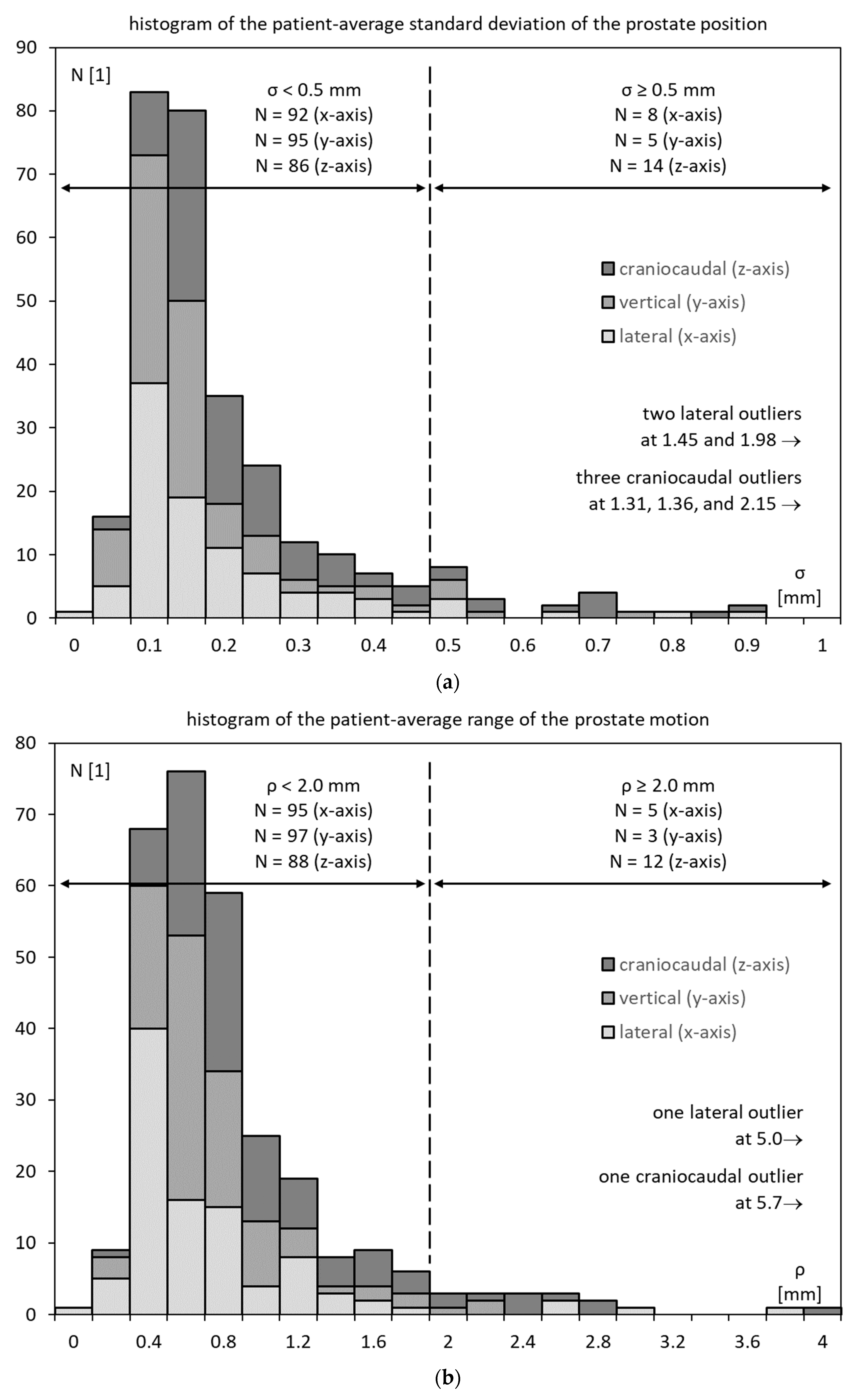
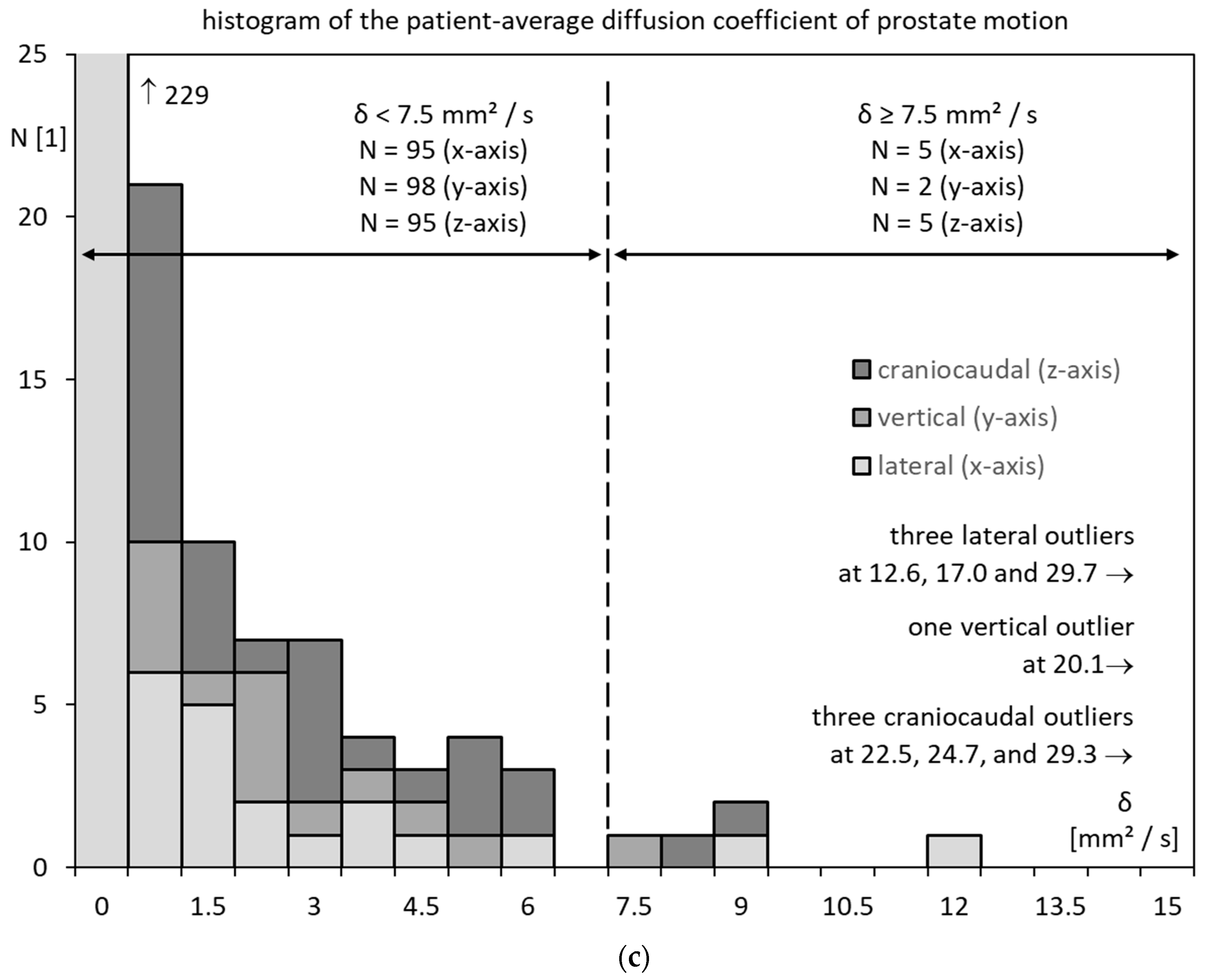
3.3. Prostate Motility
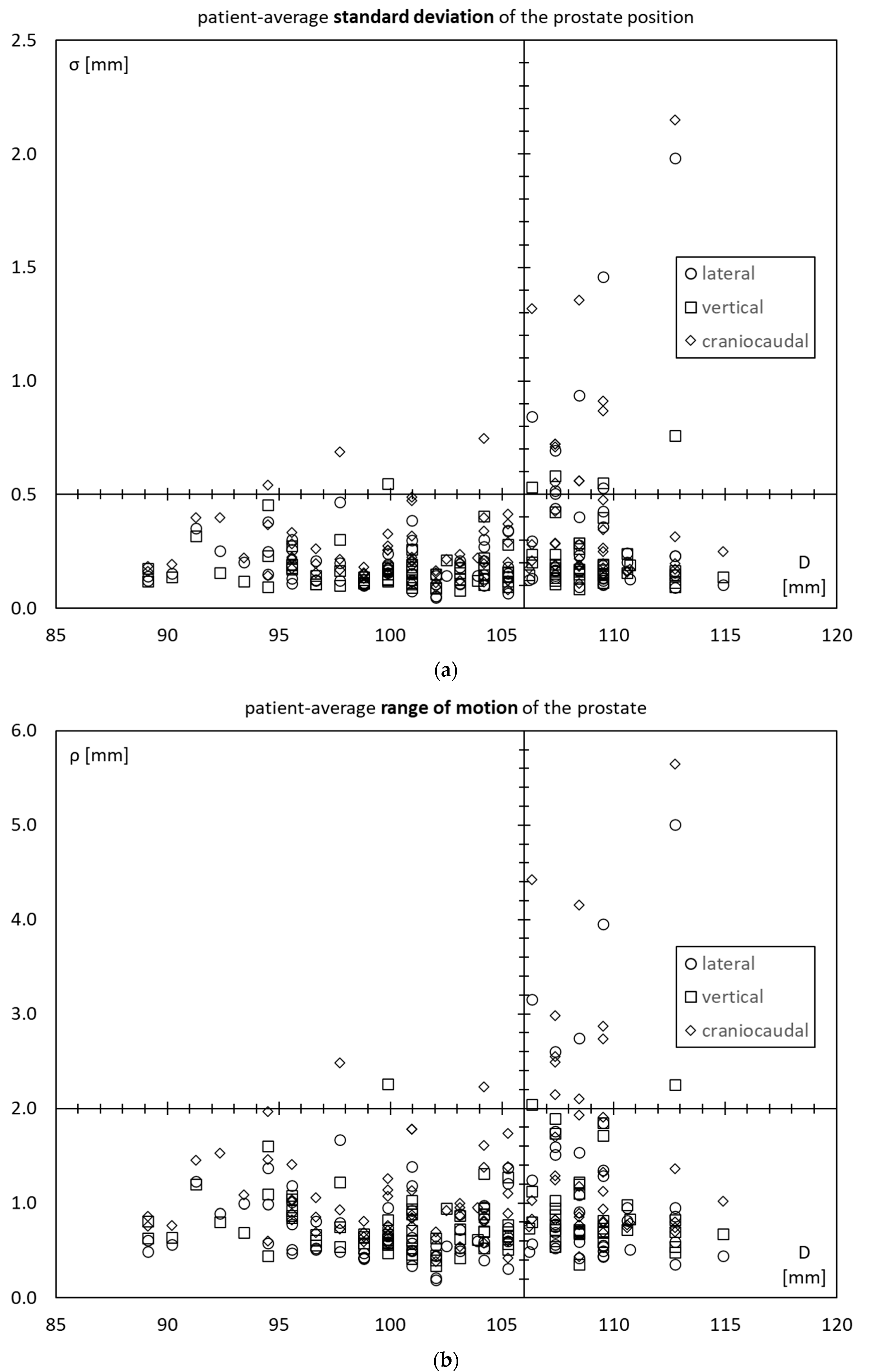
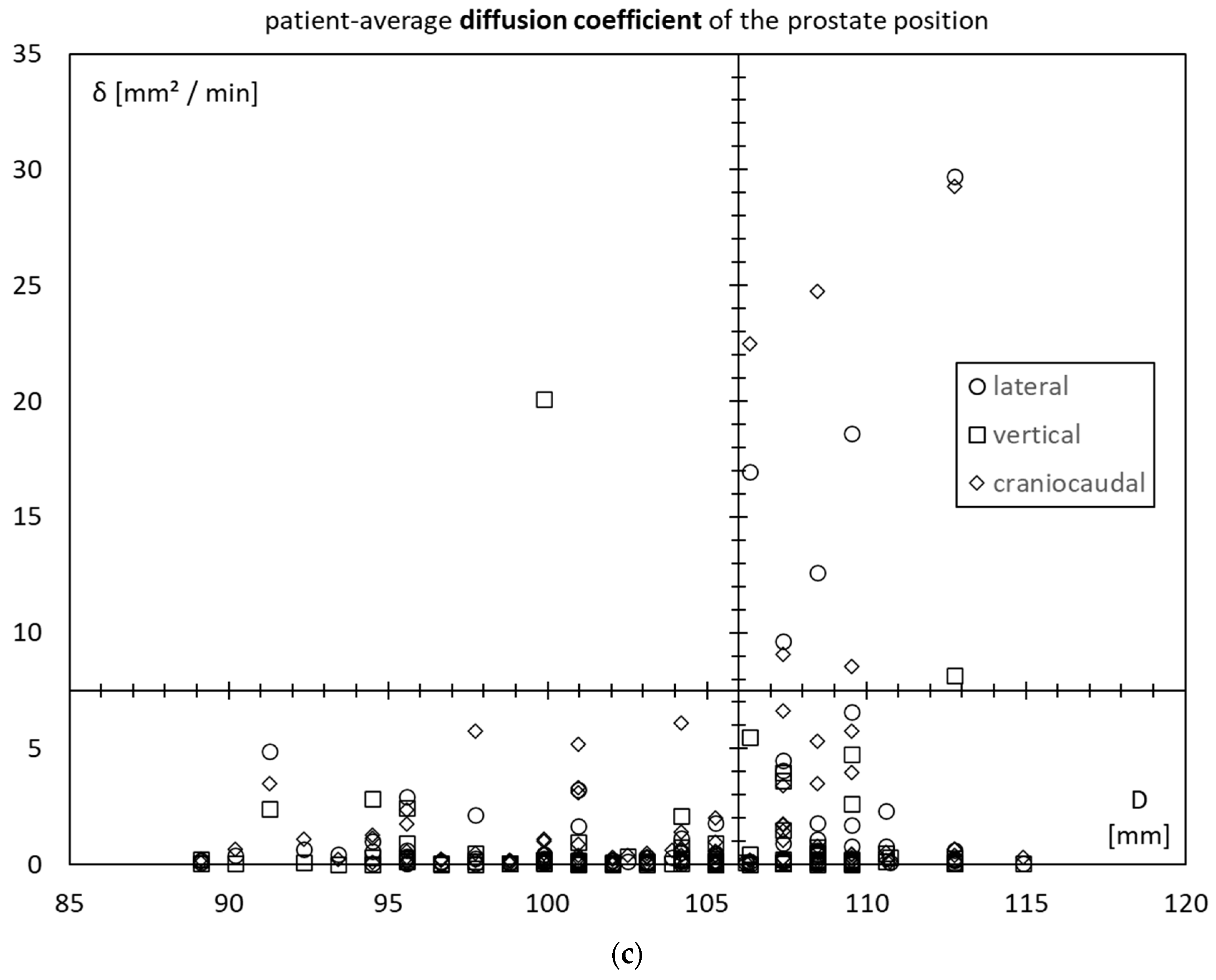
3.4. Prostate Motility vs. Inner Diameter of the Lesser Pelvis
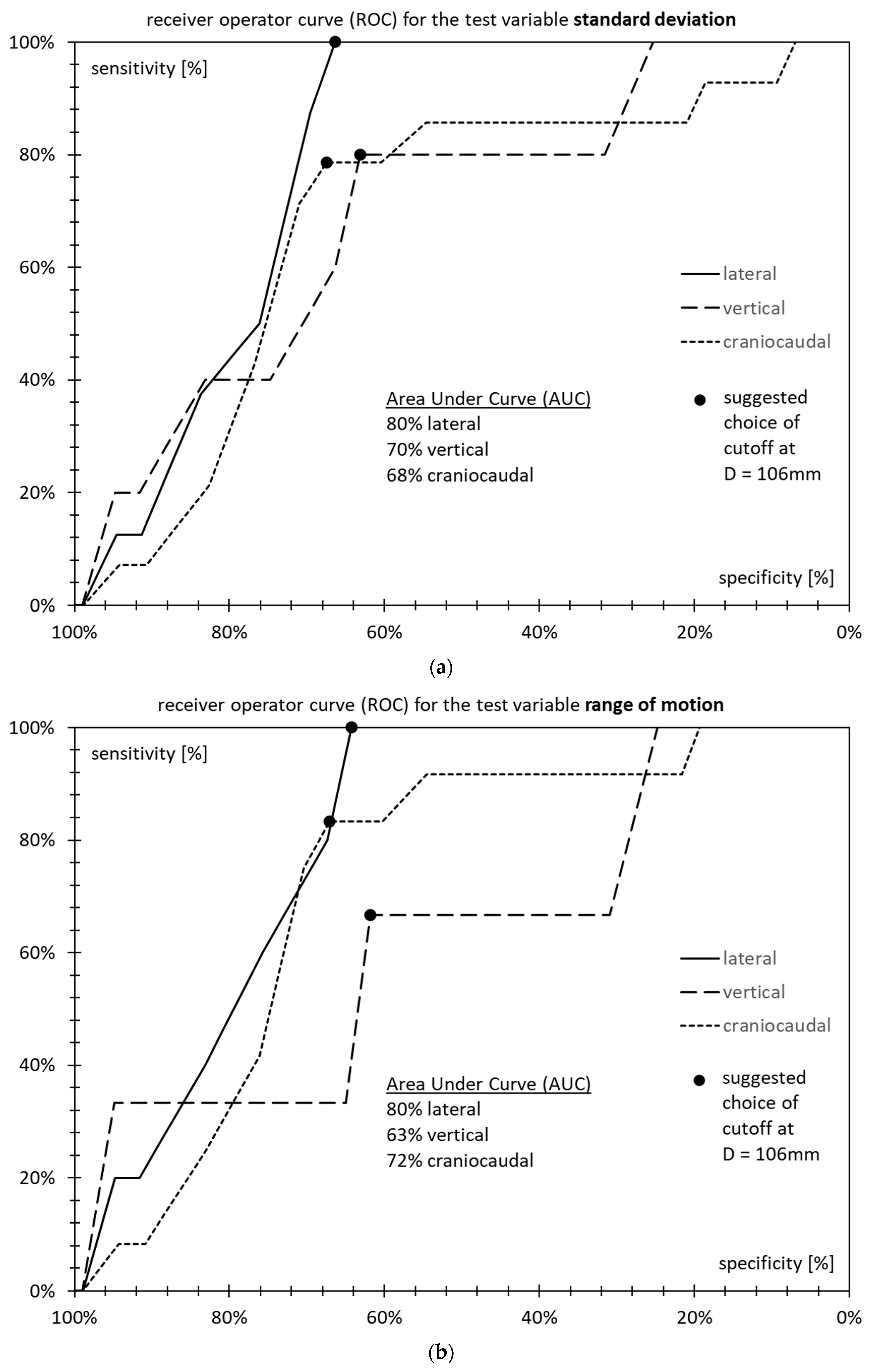
3.5. Receiver Operator Characteristics
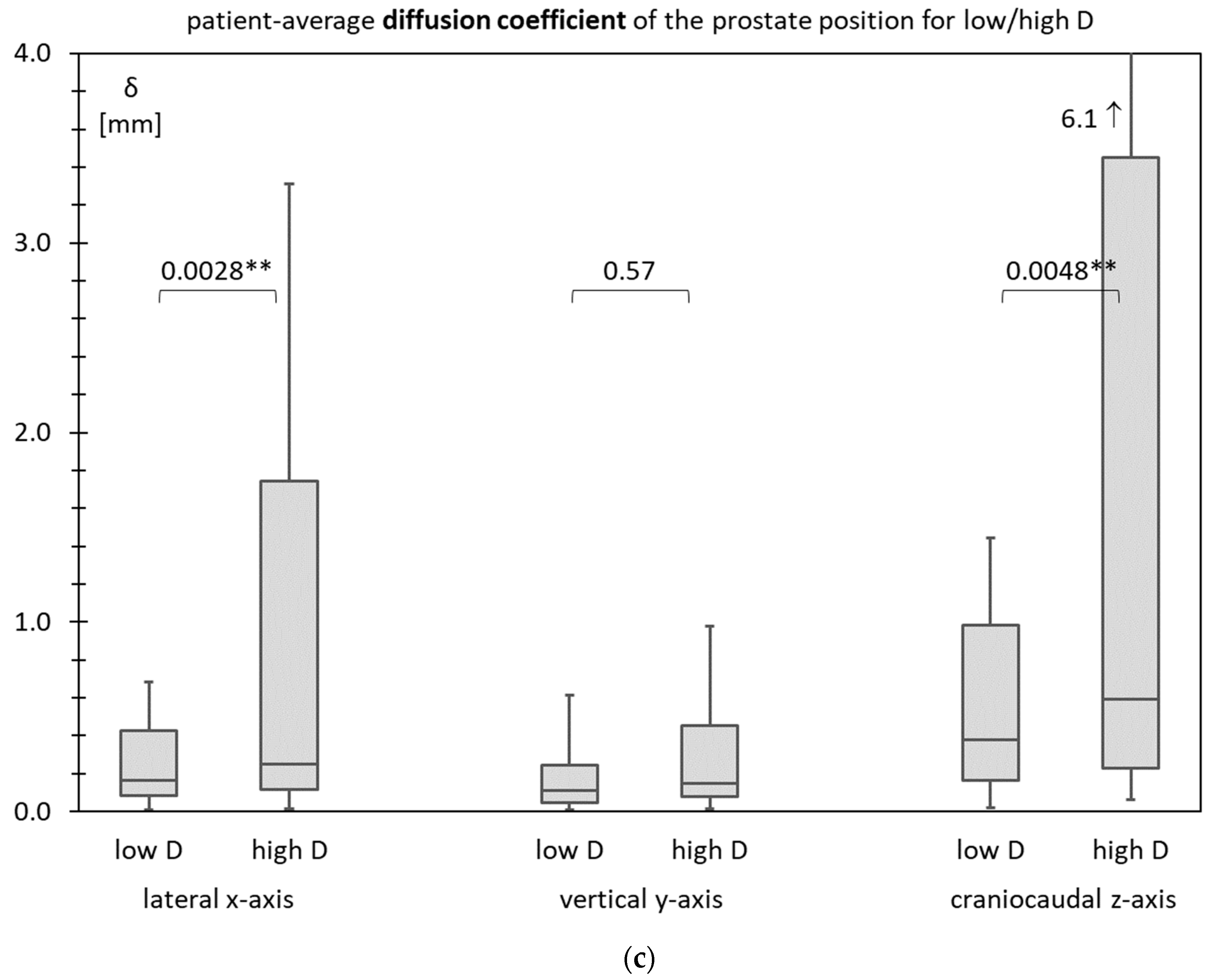
3.6. Prostate Motility for Low D and High D
3.7. Test Statistics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagshaw, M.A.; Ray, G.R.; Pistenma, D.A.; Castellino, R.A.; Meares, E.M. External beam radiation therapy of primary carcinoma of the prostate. Cancer 1975, 36, 723–728. [Google Scholar] [CrossRef]
- Terlizzi, M.; Limkin, E.J.; Moukasse, Y.; Blanchard, P. Adjuvant or Salvage Radiation Therapy for Prostate Cancer after Prostatectomy: Current Status, Controversies and Perspectives. Cancers 2022, 14, 1688. [Google Scholar] [CrossRef] [PubMed]
- Kissel, M.; Créhange, G.; Graff, P. Stereotactic Radiation Therapy versus Brachytherapy: Relative Strengths of Two Highly Efficient Options for the Treatment of Localized Prostate Cancer. Cancers 2022, 14, 2226. [Google Scholar] [CrossRef] [PubMed]
- Haisen, S.L.; Chetty, I.J.; Enke, C.A.; Foster, R.D.; Willoughby, T.R.; Kupellian, P.A.; Solberg, T.D. Consequences of Intrafraction Prostate Motion. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 801–812. [Google Scholar]
- Faccenda, V.; Panizza, D.; Daniotti, M.C.; Pellegrini, R.; Trivellato, S.; Caricato, P.; Lucchini, R.; De Ponti, E.; Arcangeli, S. Dosimetric Impact of Intrafraction Prostate Motion and Interfraction Anatomical Changes in Dose-Escalated Linac-Based SBRT. Cancers 2023, 15, 1153. [Google Scholar] [CrossRef] [PubMed]
- Litzenberg, D.W.; Balter, J.M.; Hadley, S.W.; Sandler, H.M.; Willoughby, T.R.; Kupelian, P.A.; Levine, L. Influence of intrafraction motion on margins for prostate radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 548–553. [Google Scholar] [CrossRef]
- Higuchi, D.; Ono, T.; Kakino, R.; Aizawa, R.; Nakayasu, N.; Ito, H.; Sakamoto, T. Evaluation of internal margins for prostate for step and shoot intensity-modulated radiation therapy and volumetric modulated arc therapy using different margin formulas. J. Appl. Clin. Med. Phys. 2022, 23, e13707. [Google Scholar] [CrossRef]
- Huang, E.; Dong, L.; Chandra, A.; Kuban, D.A.; Rosen, I.I.; Evans, A.; Pollack, A. Intrafraction prostate motion during IMRT for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 261–268. [Google Scholar] [CrossRef]
- Kron, T.; Thomas, J.; Fox, C.; Thompson, A.; Owen, R.; Herschtal, A.; Haworth, A.; Tai, K.-H.; Foroudi, F. Intra-fraction prostate displacement in radiotherapy estimated from pre- and post-treatment imaging of patients with implanted fiducial markers. Radiother. Oncol. 2010, 95, 191–197. [Google Scholar] [CrossRef]
- Huang, K.; Palma, D.A.; Scott, D.; McGregor, D.; Gaede, S.; Yartsev, S.; Bauman, G.; Louie, A.V.; Rodrigues, G. Inter- and intrafraction uncertainty in prostate bed image-guided radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 402–407. [Google Scholar] [CrossRef]
- Dang, A.; Kupelian, P.A.; Cao, M.; Agazaryan, N.; Kishan, A.U. Image-guided radiotherapy for prostate cancer. Transl. Androl. Urol. 2018, 7, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Lohr, F.; Boda-Heggemann, J.; Wenz, F.; Wiegel, T. Image-guided radiotherapy for prostate cancer. Aktuelle Urol. 2007, 38, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Pang, E.P.P.; Knight, K.; Hussain, A.; Fan, Q.; Baird, M.; Tan, S.X.F.; Mui, W.H.; Leung, R.W.; Seah, I.K.L.; Master, Z.; et al. Reduction of intra-fraction prostate motion—Determining optimal bladder volume and filling for prostate radiotherapy using daily 4D TPUS and CBCT. Tech. Innov. Patient Support Radiat. Oncol. 2018, 5, 9–15. [Google Scholar] [CrossRef]
- Both, S.; Wang, K.K.; Plastaras, J.P.; Deville, C.; Bar Ad, V.; Tochner, Z.; Vapiwala, N. Real-time study of prostate intrafraction motion during external beam radiotherapy with daily endorectal balloon. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1302–1309. [Google Scholar] [CrossRef]
- Smeenk, R.J.; Louwe, R.J.; Langen, K.M.; Shah, A.P.; Kupelian, P.A.; van Lin, E.N.; Kaanders, J.H. An endorectal balloon reduces intrafraction prostate motion during radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 661–669. [Google Scholar] [CrossRef]
- Hedrick, S.G.; Fagundes, M.; Robison, B.; Blakey, M.; Renegar, J.; Artz, M.; Schreuder, N. A comparison between hydrogel spacer and endorectal balloon: An analysis of intrafraction prostate motion during proton therapy. J. Appl. Clin. Med. Phys. 2017, 18, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Ballhausen, H.; Li, M.; Ganswindt, U.; Belka, C. Shorter treatment times reduce the impact of intra-fractional motion: A real-time 4DUS study comparing VMAT vs. step-and-shoot IMRT for prostate cancer. Strahlenther. Onkol. 2018, 194, 664–674. [Google Scholar] [CrossRef]
- Kupelian, P.; Willoughby, T.; Mahadevan, A.; Djemil, T.; Weinstein, G.; Jani, S.; Enke, C.; Solberg, T.; Flores, N.; Liu, D.; et al. Multi-institutional clinical experience with the Calypso system in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1088–1098. [Google Scholar] [CrossRef]
- Richardson, A.K.; Jacobs, P. Intrafraction monitoring of prostate motion during radiotherapy using the Clarity® Autoscan Transperineal Ultrasound (TPUS) system. Radiography 2017, 23, 310–313. [Google Scholar] [CrossRef]
- Sihono, D.S.K.; Ehmann, M.; Heitmann, S.; von Swietochowski, S.; Grimm, M.; Boda-Heggemann, J.; Lohr, F.; Wenz, F.; Wertz, H. Determination of Intrafraction Prostate Motion During External Beam Radiation Therapy with a Transperineal 4-Dimensional Ultrasound Real-Time Tracking System. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 136–143. [Google Scholar] [CrossRef]
- Richter, A.; Exner, F.; Weick, S.; Lawrenz, I.; Polat, B.; Flentje, M.; Mantel, F. Evaluation of intrafraction prostate motion tracking using the Clarity Autoscan system for safety margin validation. Z. Med. Phys. 2020, 30, 135–141. [Google Scholar] [CrossRef]
- Ballhausen, H.; Li, M.; Belka, C. The ProMotion LMU dataset, prostate intra-fraction motion recorded by transperineal ultrasound. Sci. Data 2019, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Ballhausen, H.; Kortmann, E.; Li, M.; Belka, C. The ProMotion LMU dataset (2022 edition), prostate intra-fraction motion recorded by transperineal ultrasound. Sci. Data 2022, 9, 455. [Google Scholar] [CrossRef]
- Ballhausen, H.; Reiner, M.; Kantz, S.; Belka, C.; Söhn, M. The random walk model of intrafraction movement. Phys. Med. Biol. 2013, 58, 2413–2427. [Google Scholar] [CrossRef]
- Ballhausen, H.; Li, M.; Hegemann, N.-S.; Ganswindt, U.; Belka, C. Intra-fraction motion of the prostate is a random walk. Phys. Med. Biol. 2015, 60, 549–563. [Google Scholar] [CrossRef]
- Roch, M.; Zapatero, A.; Castro, P.; Büchser, D.; Pérez, L.; Hernández, D.; Ansón, C.; Chevalier, M.; García-Vicente, F. Impact of rectum and bladder anatomy in intrafractional prostate motion during hypofractionated radiation therapy. Clin. Transl. Oncol. 2019, 21, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Oates, R.; Brown, A.; Tan, A.; Foroudi, F.; Lim Joon, M.; Schneider, M.; Herschtal, A.; Kron, T. Real-time image-guided adaptive-predictive prostate radiotherapy using rectal diameter as a predictor of motion. Clin. Oncol. 2017, 29, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Rios, R.; De Crevoisier, R.; Ospina, J.D.; Commandeur, F.; Lafond, C.; Simon, A.; Haigron, P.; Espinosa, R.; Acosta, O. Population model of bladder motion and deformation based on dominant eigenmodes and mixed-effects models in prostate cancer radiotherapy. Med. Image Anal. 2017, 38, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Futaguchi, M.; Haga, A.; Sakumi, A.; Sasaki, K.; Yamamoto, K.; Igaki, H.; Ohtomo, K.; Yoda, K.; Nakagawa, K. Validation of planning target volume margins by analyzing intrafractional localization errors for 14 prostate cancer patients based on three-dimensional cross-correlation between the prostate images of planning CT and intrafraction cone-beam CT during volumetric modulated arc therapy. BioMed Res. Int. 2014, 2014, 960928. [Google Scholar]
- Rose, C.; Ebert, M.A.; Mukwada, G.; Skorska, M.; Gill, S. Intrafraction motion during CyberKnife® prostate SBRT: Impact of imaging frequency and patient factors. Phys. Eng. Sci. Med. 2023, 46, 669–685. [Google Scholar] [CrossRef]


| Patient | D [mm] | [mm] | [mm] | [mm] | [mm] | [mm] | [mm] | [mm2/s] | [mm2/s] | [mm2/s] |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 102.0 | 0.143 | 0.086 | 0.155 | 0.549 | 0.455 | 0.633 | 0.181 | 0.034 | 0.223 |
| 2 | 99.9 | 0.169 | 0.125 | 0.148 | 0.615 | 0.579 | 0.661 | 0.084 | 0.181 | 0.099 |
| 3 | 97.7 | 0.467 | 0.300 | 0.688 | 1.668 | 1.219 | 2.484 | 2.140 | 0.493 | 5.739 |
| 4 | 109.5 | 0.427 | 0.154 | 0.866 | 1.344 | 0.694 | 2.869 | 1.688 | 0.067 | 5.759 |
| 5 | 109.5 | 0.103 | 0.110 | 0.178 | 0.444 | 0.596 | 0.810 | 0.057 | 0.013 | 0.132 |
| … | … | … | … | … | … | … | … | … | … | … |
| 100 | 101.0 | 0.300 | 0.181 | 0.487 | 1.183 | 0.887 | 1.782 | 1.678 | 0.098 | 3.107 |
| N = 100 | D [mm] | [mm] | [mm] | [mm] | [mm] | [mm] | [mm] | [mm2/s] | [mm2/s] | [mm2/s] |
|---|---|---|---|---|---|---|---|---|---|---|
| average | 103.3 | 0.25 | 0.19 | 0.32 | 0.90 | 0.84 | 1.22 | 1.46 | 0.75 | 1.91 |
| std. dev. | 5.9 | 0.26 | 0.12 | 0.29 | 0.72 | 0.39 | 0.85 | 4.16 | 2.32 | 4.59 |
| minimum | 89.1 | 0.05 | 0.08 | 0.08 | 0.19 | 0.34 | 0.38 | 0.01 | 0.01 | 0.02 |
| 1st quartile | 99.9 | 0.12 | 0.12 | 0.17 | 0.51 | 0.61 | 0.76 | 0.09 | 0.05 | 0.18 |
| median | 104.2 | 0.16 | 0.16 | 0.22 | 0.65 | 0.71 | 0.89 | 0.20 | 0.12 | 0.42 |
| 3rd quartile | 108.5 | 0.26 | 0.19 | 0.34 | 0.98 | 0.94 | 1.38 | 0.61 | 0.28 | 1.16 |
| maximum | 114.9 | 1.98 | 0.76 | 2.15 | 5.01 | 2.26 | 5.65 | 29.70 | 20.08 | 29.30 |
| Patient-Average Standard Deviation σ [mm] | Inner Diameter of the Lesser Pelvis D [mm] | Significance p-Value | Sensitivity Specificity | NPV PPV | ||
|---|---|---|---|---|---|---|
| “Low D” < 106 mm | “High D” ≥ 106 mm | |||||
| (a) | ||||||
| lateral (x-axis) | <0.5 | 0 | 8 | 0.0003 | 100% | 100% |
| ≥0.5 | 61 | 31 | 66% | 21% | ||
| vertical (y-axis) | <0.5 | 1 | 4 | 0.0743 | 80% | 98% |
| ≥0.5 | 60 | 35 | 63% | 10% | ||
| craniocaudal (z-axis) | <0.5 | 3 | 11 | 0.0020 | 79% | 95% |
| ≥0.5 | 58 | 28 | 67% | 28% | ||
| Patient-Average Range of Motion ρ [mm] | Inner Diameter of the Lesser Pelvis D [mm] | Significance p-Value | Sensitivity Specificity | NPV PPV | ||
| “Low D” < 106 mm | “High D” ≥106 mm | |||||
| (b) | ||||||
| lateral (x-axis) | <2.0 | 0 | 5 | 0.0076 | 100% | 100% |
| ≥2.0 | 61 | 34 | 64% | 13% | ||
| vertical (y-axis) | <2.0 | 1 | 2 | 0.5586 | 67% | 98% |
| ≥2.0 | 60 | 37 | 62% | 5% | ||
| craniocaudal (z-axis) | <2.0 | 2 | 10 | 0.0012 | 83% | 97% |
| ≥2.0 | 59 | 29 | 67% | 26% | ||
| Patient-Average Diffusion Coefficient δ [mm2/s] | Inner Diameter of the Lesser Pelvis D [mm] | Significance p-Value | Sensitivity Specificity | NPV PPV | ||
| “Low D” < 106 mm | “High D” ≥ 106 mm | |||||
| (c) | ||||||
| lateral (x-axis) | <7.5 | 0 | 5 | 0.0076 | 100% | 100% |
| ≥7.5 | 61 | 34 | 64% | 13% | ||
| vertical (y-axis) | <7.5 | 1 | 1 | 1.0000 | 50% | 98% |
| ≥7.5 | 60 | 38 | 61% | 3% | ||
| craniocaudal (z-axis) | <7.5 | 0 | 5 | 0.0076 | 100% | 100% |
| ≥7.5 | 61 | 34 | 64% | 13% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballhausen, H.; Li, M.; Lombardo, E.; Landry, G.; Belka, C. Planning CT Identifies Patients at Risk of High Prostate Intrafraction Motion. Cancers 2023, 15, 4103. https://doi.org/10.3390/cancers15164103
Ballhausen H, Li M, Lombardo E, Landry G, Belka C. Planning CT Identifies Patients at Risk of High Prostate Intrafraction Motion. Cancers. 2023; 15(16):4103. https://doi.org/10.3390/cancers15164103
Chicago/Turabian StyleBallhausen, Hendrik, Minglun Li, Elia Lombardo, Guillaume Landry, and Claus Belka. 2023. "Planning CT Identifies Patients at Risk of High Prostate Intrafraction Motion" Cancers 15, no. 16: 4103. https://doi.org/10.3390/cancers15164103
APA StyleBallhausen, H., Li, M., Lombardo, E., Landry, G., & Belka, C. (2023). Planning CT Identifies Patients at Risk of High Prostate Intrafraction Motion. Cancers, 15(16), 4103. https://doi.org/10.3390/cancers15164103







