Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Endometrial Cancer Staging in FIGO System
1.2. Histology of Endometrial Cancer: An Independent Prognostic Marker
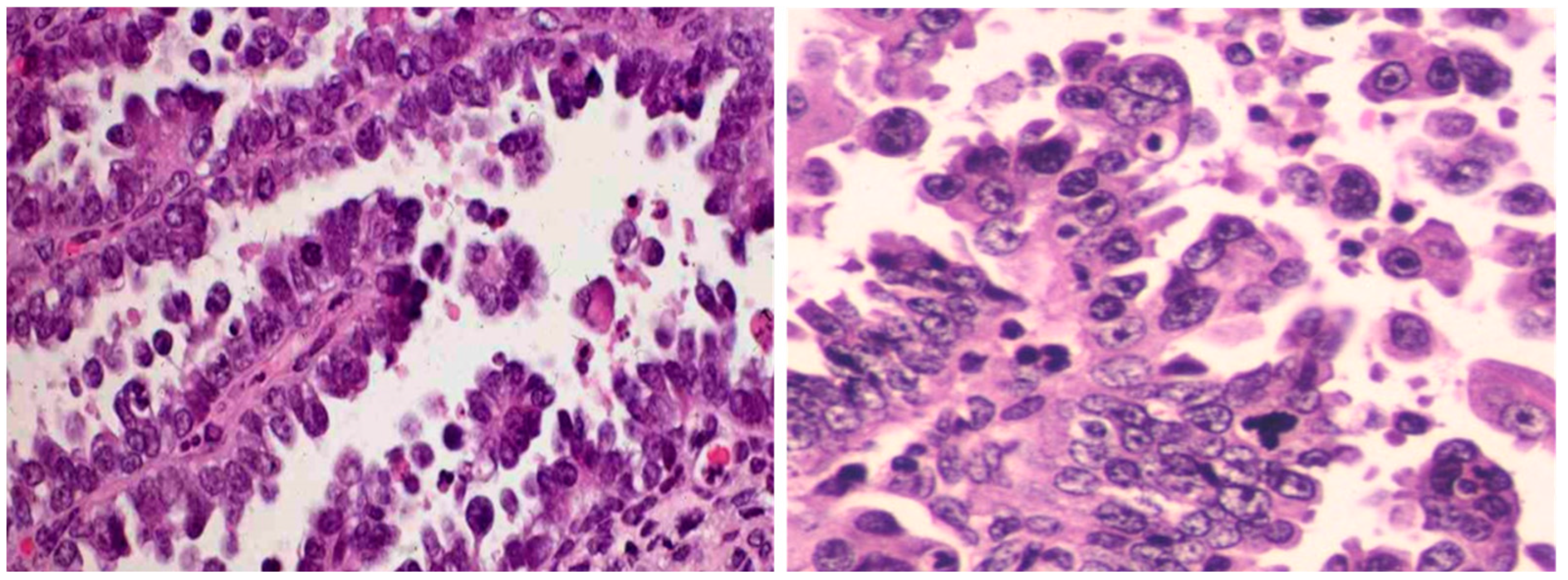
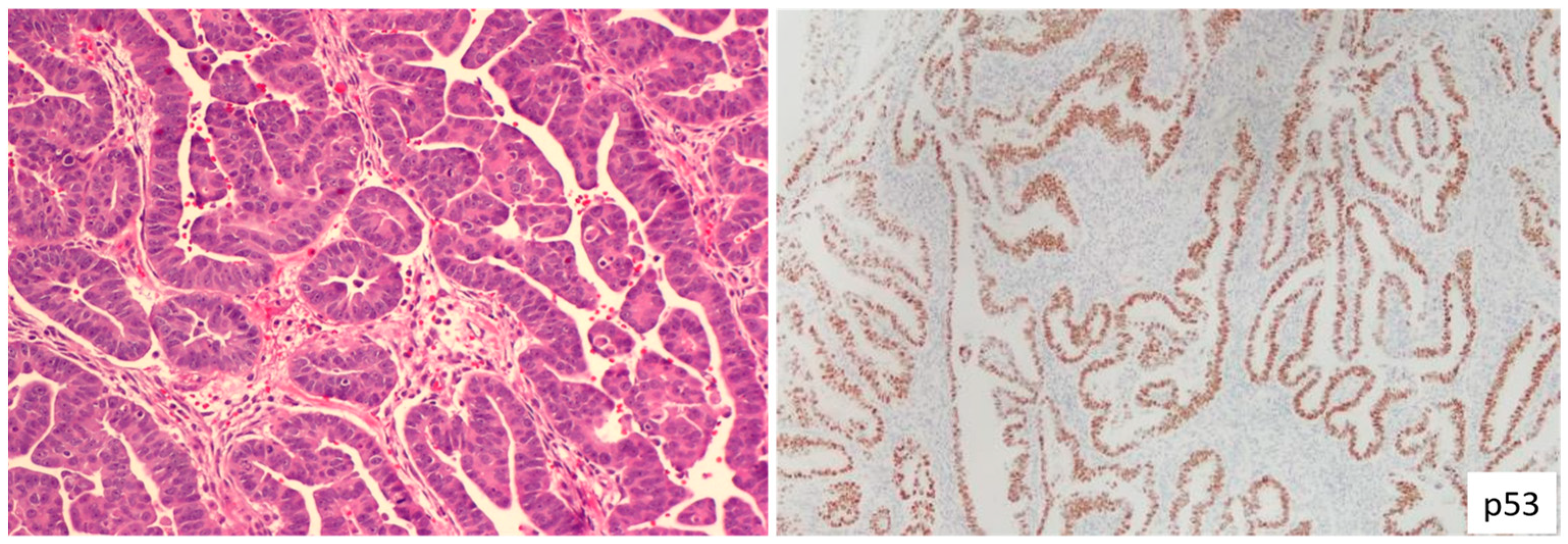
1.3. Endometrial Serous Carcinoma
1.4. Endometrial Clear Cell Carcinoma
1.5. Endometrial Carcinosarcoma
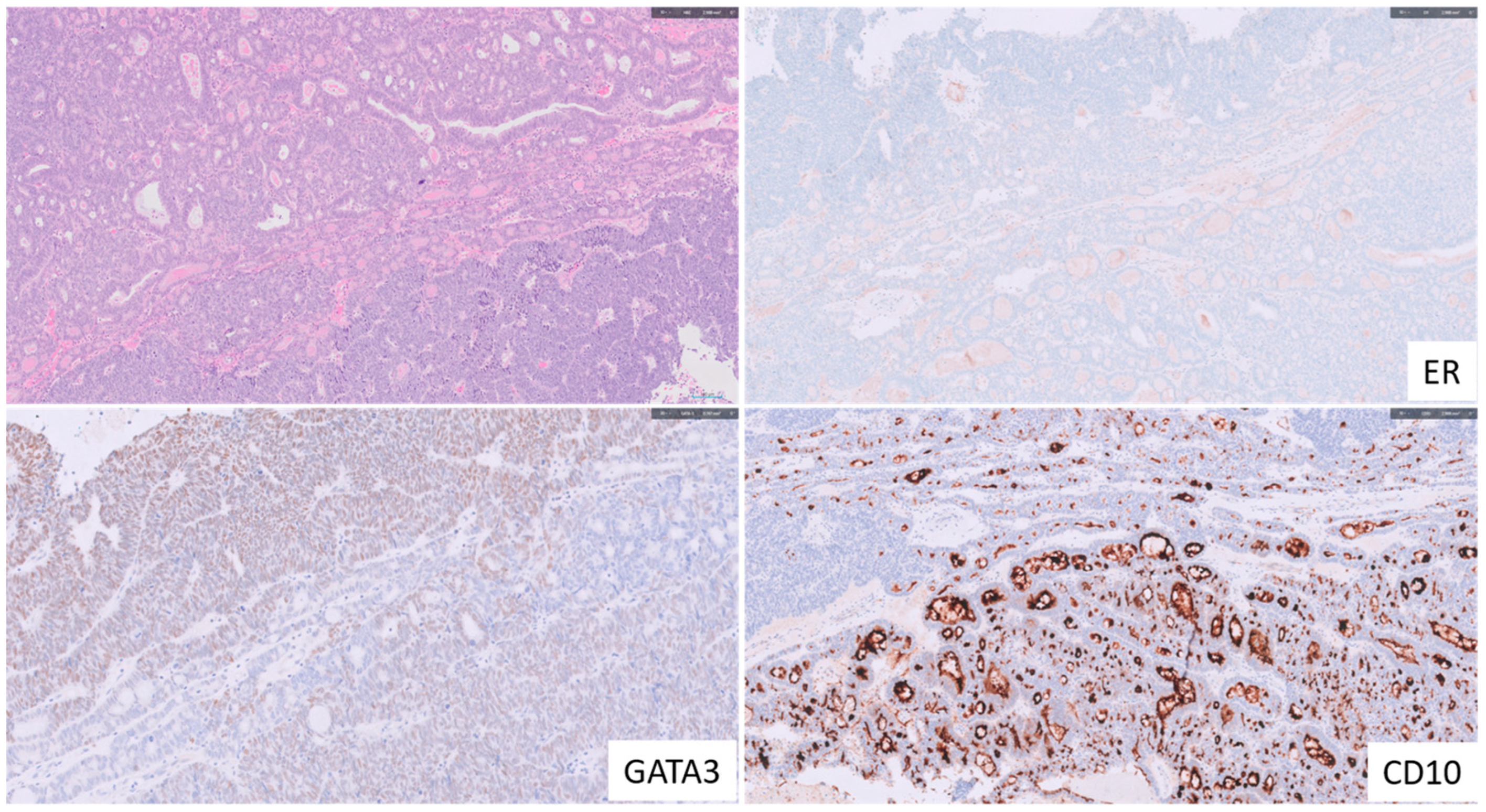
1.6. Endometrial Mesonephric-like Adenocarcinoma
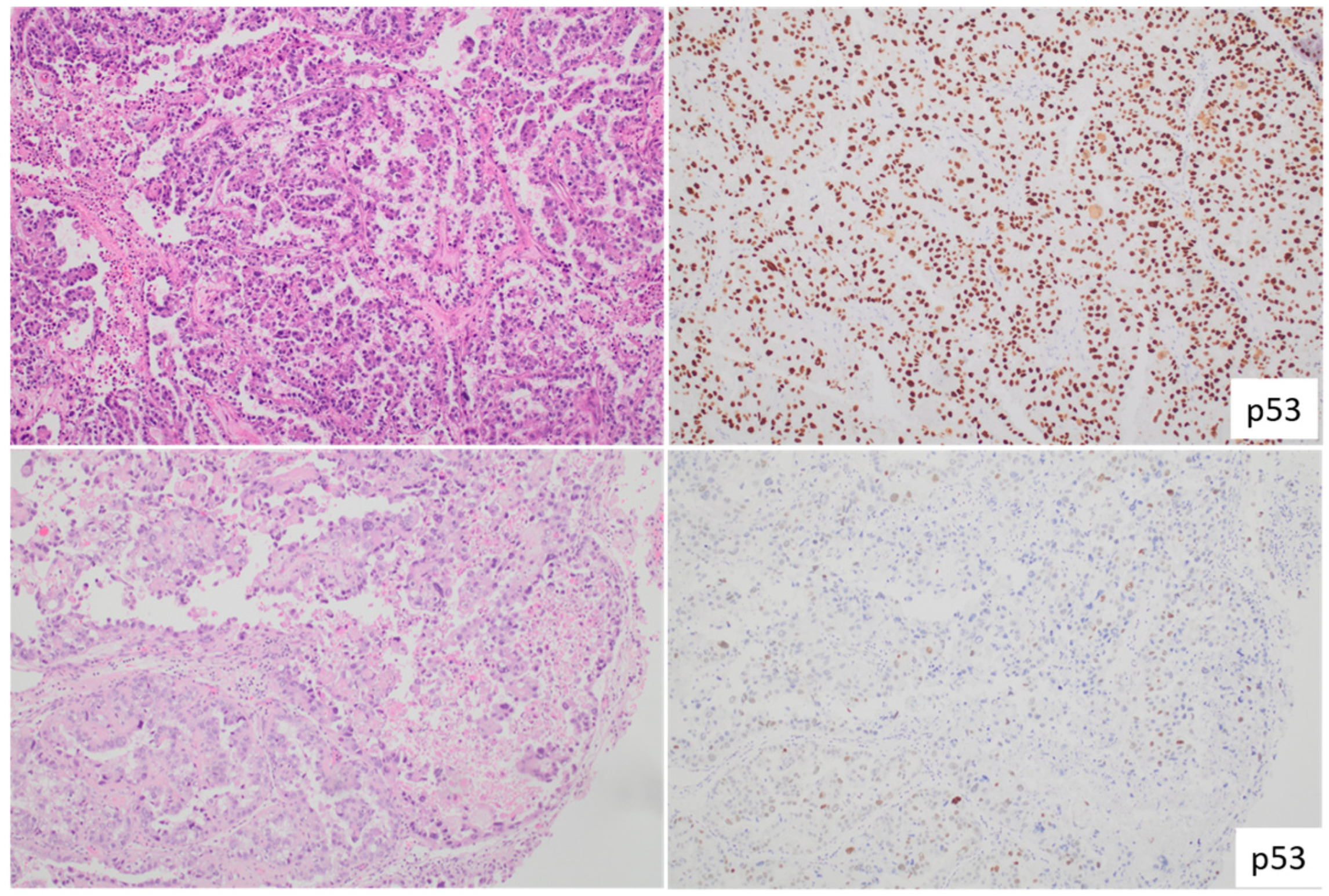
2. Recent Advancements and Insights in Endometrial Cancer with Lymphovascular Space Invasion
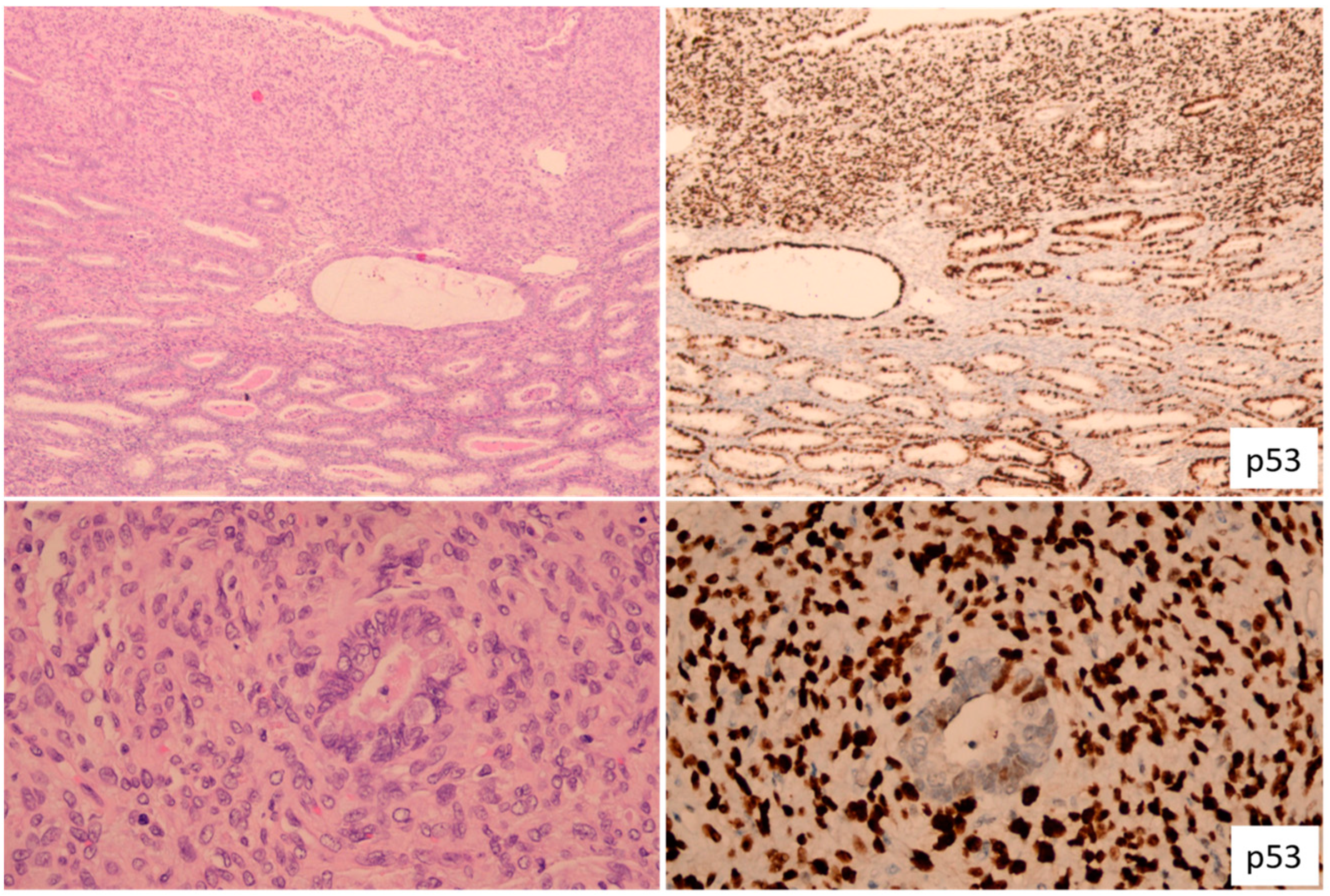
3. The Importance of Lymph Node Metastasis Size in Endometrial Cancer
4. Synchronous Endometrial and Ovarian Endometrioid Carcinomas: Staging Advances and Recent Insights
5. Integrating Molecular Classifications into FIGO 2023 Staging of Endometrial Cancer
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Devesa, S.S.; Hammer, A.; Wentzensen, N. Racial and Ethnic Differences in Hysterectomy-Corrected Uterine Corpus Cancer Mortality by Stage and Histologic Subtype. JAMA Oncol. 2022, 8, 895–903. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [PubMed]
- Leon-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit from Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.; Leung, S.; Li-Chang, H.; Kwon, J.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee; et al. Endometrial Cancer Staging Subcommittee, FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Zhou, Q.; Iasonos, A.; Alektiar, K.M.; Leitao, M.M., Jr.; Chi, D.S.; Sonoda, Y.; Soslow, R.; Hensley, M.; Barakat, R.R. The revised 2009 FIGO staging system for endometrial cancer: Should the 1988 FIGO stages IA and IB be altered? Int. J. Gynecol. Cancer 2011, 21, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zheng, W. High-grade endometrial carcinomas: Morphologic spectrum and molecular classification. Semin. Diagn. Pathol. 2022, 39, 176–186. [Google Scholar] [CrossRef]
- Lucas, E.; Carrick, K.S. Low grade endometrial endometrioid adenocarcinoma: A review and update with emphasis on morphologic variants, mimics, immunohistochemical and molecular features. Semin. Diagn. Pathol. 2022, 39, 159–175. [Google Scholar] [CrossRef]
- Gilks, C.B.; Oliva, E.; Soslow, R.A. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am. J. Surg. Pathol. 2013, 37, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; Nout, R.A.; McAlpine, J.N.; McConechy, M.K.; Britton, H.; Hussein, Y.R.; Gonzalez, C.; Ganesan, R.; Steele, J.C.; Harrison, B.T.; et al. Molecular Classification of Grade 3 Endometrioid Endometrial Cancers Identifies Distinct Prognostic Subgroups. Am. J. Surg. Pathol. 2018, 42, 561–568. [Google Scholar] [CrossRef] [PubMed]
- DeLair, D.F.; Burke, K.A.; Selenica, P.; Lim, R.S.; Scott, S.N.; Middha, S.; Mohanty, A.S.; Cheng, D.T.; Berger, M.F.; Soslow, R.A.; et al. The genetic landscape of endometrial clear cell carcinomas. J. Pathol. 2017, 243, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Baniak, N.; Fadare, O.; Kobel, M.; DeCoteau, J.; Parkash, V.; Hecht, J.L.; Hanley, K.Z.; Gwin, K.; Zheng, W.; Quick, C.M.; et al. Targeted Molecular and Immunohistochemical Analyses of Endometrial Clear Cell Carcinoma Show that POLE Mutations and DNA Mismatch Repair Protein Deficiencies Are Uncommon. Am. J. Surg. Pathol. 2019, 43, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Cloutier, B.T.; Leung, S.; Cochrane, D.; Britton, H.; Pina, A.; Storness-Bliss, C.; Farnell, D.; Huang, L.; Shum, K.; et al. Molecular subtypes of clear cell carcinoma of the endometrium: Opportunities for prognostic and predictive stratification. Gynecol. Oncol. 2020, 158, 3–11. [Google Scholar] [CrossRef]
- Espinosa, I.; Lee, C.H.; D’Angelo, E.; Palacios, J.; Prat, J. Undifferentiated and Dedifferentiated Endometrial Carcinomas with POLE Exonuclease Domain Mutations Have a Favorable Prognosis. Am. J. Surg. Pathol. 2017, 41, 1121–1128. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Raimondo, D.; Arciuolo, D.; Angelico, G.; Valente, M.; Scaglione, G.; D’Alessandris, N.; Casadio, P.; Inzani, F.; et al. Prognostic value of the TCGA molecular classification in uterine carcinosarcoma. Int. J. Gynecol. Obstet. 2022, 158, 13–20. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; Horeweg, N.; Peters, E.E.M.; Rutten, T.; Haar, N.T.; Smit, V.; Kroon, C.D.; Boennelycke, M.; Hogdall, E.; Hogdall, C.; et al. Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol. Oncol. 2022, 164, 577–586. [Google Scholar] [CrossRef]
- Hoang, L.N.; McConechy, M.K.; Kobel, M.; Han, G.; Rouzbahman, M.; Davidson, B.; Irving, J.; Ali, R.H.; Leung, S.; McAlpine, J.N.; et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am. J. Surg. Pathol. 2013, 37, 1421–1432. [Google Scholar] [CrossRef]
- Soslow, R.A. Endometrial carcinomas with ambiguous features. Semin. Diagn. Pathol. 2010, 27, 261–273. [Google Scholar] [CrossRef]
- Matsuo, K.; Ross, M.S.; Machida, H.; Blake, E.A.; Roman, L.D. Trends of uterine carcinosarcoma in the United States. J. Gynecol. Oncol. 2018, 29, e22. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Klar, M.; Matsuzaki, S.; Roman, L.D.; Sood, A.K.; Matsuo, K. Uterine carcinosarcoma: Contemporary clinical summary, molecular updates, and future research opportunity. Gynecol. Oncol. 2021, 160, 586–601. [Google Scholar] [CrossRef]
- Horn, L.C.; Hohn, A.K.; Krucken, I.; Stiller, M.; Obeck, U.; Brambs, C.E. Mesonephric-like adenocarcinomas of the uterine corpus: Report of a case series and review of the literature indicating poor prognosis for this subtype of endometrial adenocarcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Kolin, D.L.; Costigan, D.C.; Dong, F.; Nucci, M.R.; Howitt, B.E. A Combined Morphologic and Molecular Approach to Retrospectively Identify KRAS-Mutated Mesonephric-like Adenocarcinomas of the Endometrium. Am. J. Surg. Pathol. 2019, 43, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Euscher, E.D.; Bassett, R.; Duose, D.Y.; Lan, C.; Wistuba, I.; Ramondetta, L.; Ramalingam, P.; Malpica, A. Mesonephric-like Carcinoma of the Endometrium: A Subset of Endometrial Carcinoma with an Aggressive Behavior. Am. J. Surg. Pathol. 2020, 44, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Pors, J.; Cheng, A.; Leo, J.M.; Kinloch, M.A.; Gilks, B.; Hoang, L. A Comparison of GATA3, TTF1, CD10, and Calretinin in Identifying Mesonephric and Mesonephric-like Carcinomas of the Gynecologic Tract. Am. J. Surg. Pathol. 2018, 42, 1596–1606. [Google Scholar] [CrossRef]
- Deolet, E.; Van Dorpe, J.; Van de Vijver, K. Mesonephric-like Adenocarcinoma of the Endometrium: Diagnostic Advances to Spot This Wolf in Sheep’s Clothing. A Review of the Literature. J. Clin. Med. 2021, 10, 698. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; Gonzalez-Martin, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Bosse, T.; Peters, E.E.; Creutzberg, C.L.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Smit, V.T.; Nout, R.A. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer—A pooled analysis of PORTEC 1 and 2 trials. Eur. J. Cancer 2015, 51, 1742–1750. [Google Scholar] [CrossRef]
- Winer, I.; Ahmed, Q.F.; Mert, I.; Bandyopadhyay, S.; Cote, M.; Munkarah, A.R.; Hussein, Y.; Al-Wahab, Z.; Elshaikh, M.A.; Alosh, B.; et al. Significance of lymphovascular space invasion in uterine serous carcinoma: What matters more; extent or presence? Int. J. Gynecol. Pathol. 2015, 34, 47–56. [Google Scholar] [PubMed]
- Logani, S.; Herdman, A.V.; Little, J.V.; Moller, K.A. Vascular “pseudo invasion” in laparoscopic hysterectomy specimens: A diagnostic pitfall. Am. J. Surg. Pathol. 2008, 32, 560–565. [Google Scholar]
- Peters, E.E.M.; Leon-Castillo, A.; Smit, V.; Boennelycke, M.; Hogdall, E.; Hogdall, C.; Creutzberg, C.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Mens, J.W.M.; et al. Defining Substantial Lymphovascular Space Invasion in Endometrial Cancer. Int. J. Gynecol. Pathol. 2022, 41, 220–226. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Classification of Tumours Editorial Board. Female Genital Tumours; World Health Organization Classification of Tumours: Lyon, France, 2020. [Google Scholar]
- ASTEC Study Group; Kitchener, H.; Swart, A.M.C.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Panici, P.B.; Basile, S.; Maneschi, F.; Lissoni, A.A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. Gynecol. Oncol. 2008, 100, 1707–1716. [Google Scholar]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Darai, E.; Dubernard, G.; Bats, A.S.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Golfier, F.; Leblanc, E.; Rouzier, R.; et al. Sentinel node biopsy for the management of early stage endometrial cancer: Long-term results of the SENTI-ENDO study. Gynecol. Oncol. 2015, 136, 54–59. [Google Scholar] [CrossRef]
- Frumovitz, M.; Plante, M.; Lee, P.S.; Sandadi, S.; Lilja, J.F.; Escobar, P.F.; Gien, L.T.; Urbauer, D.L.; Abu-Rustum, N.R. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): A randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018, 19, 1394–1403. [Google Scholar]
- Holloway, R.W.; Gupta, S.; Stavitzski, N.M.; Zhu, X.; Takimoto, E.L.; Gubbi, A.; Bigsby, G.E.; Brudie, L.A.; Kendrick, J.E.; Ahmad, S. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol. Oncol. 2016, 141, 206–210. [Google Scholar]
- Kim, C.H.; Soslow, R.A.; Park, K.J.; Barber, E.L.; Khoury-Collado, F.; Barlin, J.N.; Sonoda, Y.; Hensley, M.L.; Barakat, R.R.; Abu-Rustum, N.R. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int. J. Gynecol. Cancer 2013, 23, 964–970. [Google Scholar] [CrossRef]
- Pineda, V.G.; Gutierrez, A.H.; Segovia, M.G.; Ridruejo, J.S.; Tejeda, M.D.D.; Zapardiel, I. Low-Volume Nodal Metastasis in Endometrial Cancer: Risk Factors and Prognostic Significance. J. Clin. Med. 2020, 9, 1999. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Mariani, A.; Paolini, B.; Ditto, A.; Raspagliesi, F. Low-volume disease in endometrial cancer: The role of micrometastasis and isolated tumor cells. Gynecol. Oncol. 2019, 153, 670–675. [Google Scholar] [CrossRef]
- Gomez-Hidalgo, N.R.; Ramirez, P.T.; Ngo, B.; Perez-Hoyos, S.; Coreas, N.; Sanchez-Iglesias, J.L.; Cabrera, S.; Franco, S.; Benavente, A.P.; Gil-Moreno, A. Oncologic impact of micrometastases or isolated tumor cells in sentinel lymph nodes of patients with endometrial cancer: A meta-analysis. Clin. Transl. Oncol. 2020, 22, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Malpica, A.; Euscher, E.D.; Hecht, J.L.; Ali-Fehmi, R.; Quick, C.M.; Singh, N.; Horn, L.C.; Alvarado-Cabrero, I.; Matias-Guiu, X.; Hirschowitz, L.; et al. Endometrial Carcinoma, Grossing and Processing Issues: Recommendations of the International Society of Gynecologic Pathologists. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. 1), S9–S24. [Google Scholar] [CrossRef] [PubMed]
- Clair, C.M.S.; Eriksson, A.G.; Ducie, J.A.; Jewell, E.L.; Alektiar, K.M.; Hensley, M.L.; Soslow, R.A.; Abu-Rustum, N.R.; Leitao, M.M., Jr. Low-Volume Lymph Node Metastasis Discovered during Sentinel Lymph Node Mapping for Endometrial Carcinoma. Ann. Surg. Oncol. 2016, 23, 1653–1659. [Google Scholar] [CrossRef]
- Soumerai, T.E.; Donoghue, M.T.A.; Bandlamudi, C.; Srinivasan, P.; Chang, M.T.; Zamarin, D.; Cadoo, K.A.; Grisham, R.N.; O’Cearbhaill, R.E.; Tew, W.P.; et al. Clinical Utility of Prospective Molecular Characterization in Advanced Endometrial Cancer. Clin. Cancer Res. 2018, 24, 5939–5947. [Google Scholar] [CrossRef]
- Edmondson, R.J.; Crosbie, E.J.; Nickkho-Amiry, M.; Kaufmann, A.; Stelloo, E.; Nijman, H.W.; Leary, A.; Auguste, A.; Mileshkin, L.; Pollock, P.; et al. Markers of the p53 pathway further refine molecular profiling in high-risk endometrial cancer: A TransPORTEC initiative. Gynecol. Oncol. 2017, 146, 327–333. [Google Scholar] [CrossRef]
- Yasunaga, M.; Yamasaki, F.; Tokunaga, O.; Iwasaka, T. Endometrial carcinomas with lymph node involvement: Novel histopathologic factors for predicting prognosis. Int. J. Gynecol. Pathol. 2003, 22, 341–346. [Google Scholar] [CrossRef]
- Schultheis, A.M.; Ng, C.K.; De Filippo, M.R.; Piscuoglio, S.; Macedo, G.S.; Gatius, S.; Mies, B.P.; Soslow, R.A.; Lim, R.S.; Viale, A.; et al. Massively Parallel Sequencing-Based Clonality Analysis of Synchronous Endometrioid Endometrial and Ovarian Carcinomas. J. Natl. Cancer Inst. 2016, 108, djv427. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.S.; Wang, Y.K.; Maassen, M.; Horlings, H.M.; Bashashati, A.; Senz, J.; Mackenzie, R.; Grewal, D.S.; Li-Chang, H.; Karnezis, A.N.; et al. Synchronous Endometrial and Ovarian Carcinomas: Evidence of Clonality. J. Natl. Cancer Inst. 2016, 108, djv428. [Google Scholar] [CrossRef]
- Casey, L.; Singh, N. Metastases to the ovary arising from endometrial, cervical and fallopian tube cancer: Recent advances. Histopathology 2020, 76, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Church, D.N.; Stelloo, E.; Nout, R.A.; Valtcheva, N.; Depreeuw, J.; Haar, N.T.; Noske, A.; Amant, F.; Tomlinson, I.P.; Wild, P.J. Prognostic significance of POLE proofreading mutations in endometrial cancer. Gynecol. Oncol. 2015, 107, dju402. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; Britton, H.; McConechy, M.K.; McAlpine, J.N.; Nout, R.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; Rau, T.T.; et al. Interpretation of somatic POLE mutations in endometrial carcinoma. J. Pathol. 2020, 250, 323–335. [Google Scholar] [CrossRef]
- Talhouk, A.; Hoang, L.N.; McConechy, M.K.; Nakonechny, Q.; Leo, J.; Cheng, A.; Leung, S.; Yang, W.; Lum, A.; Kobel, M.; et al. Molecular classification of endometrial carcinoma on diagnostic specimens is highly concordant with final hysterectomy: Earlier prognostic information to guide treatment. Gynecol. Oncol. 2016, 143, 46–53. [Google Scholar] [CrossRef]
- Van Gool, I.C.; Ubachs, J.E.H.; Stelloo, E.; de Kroon, C.D.; Goeman, J.J.; Smit, V.; Creutzberg, C.L.; Bosse, T. Blinded histopathological characterisation of POLE exonuclease domain-mutant endometrial cancers: Sheep in wolf’s clothing. Histopathology 2018, 72, 248–258. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Vermij, L.; Smit, V.; Nout, R.; Bosse, T. Incorporation of molecular characteristics into endometrial cancer management. Histopathology 2020, 76, 52–63. [Google Scholar] [CrossRef]
- Reijnen, C.; Kusters-Vandevelde, H.V.N.; Prinsen, C.F.; Massuger, L.; Snijders, M.; Kommoss, S.; Brucker, S.Y.; Kwon, J.S.; McAlpine, J.N.; Pijnenborg, J.M.A. Mismatch repair deficiency as a predictive marker for response to adjuvant radiotherapy in endometrial cancer. Gynecol. Oncol. 2019, 154, 124–130. [Google Scholar] [CrossRef]
- Concin, N.; Creutzberg, C.L.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.A.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with endometrial carcinoma. Virchows Arch. 2021, 478, 153–190. [Google Scholar] [CrossRef]
- Talhouk, A.; Jamieson, A.; Crosbie, E.J.; Taylor, A.; Chiu, D.; Leung, S.; Grube, M.; Kommoss, S.; Gilks, C.B.; McAlpine, J.N.; et al. Targeted Molecular Testing in Endometrial Carcinoma: Validation of a Clinically Driven Selective ProMisE Testing Protocol. Int. J. Gynecol. Pathol. 2023, 42, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Huvila, J.; Chiu, D.; Thompson, E.F.; Scott, S.; Salvador, S.; Vicus, D.; Helpman, L.; Gotlieb, W.; Kean, S.; et al. Grade and Estrogen Receptor Expression Identify a Subset of No Specific Molecular Profile Endometrial Carcinomas at a Very Low Risk of Disease-Specific Death. Mod. Pathol. 2023, 36, 100085. [Google Scholar] [CrossRef] [PubMed]
- Leon-Castillo, A.; Gilvazquez, E.; Nout, R.; Smit, V.T.; McAlpine, J.N.; McConechy, M.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J. Pathol. 2020, 250, 312–322. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, J.N.; Chiu, D.S.; Nout, R.A.; Church, D.N.; Schmidt, P.; Lam, S.; Leung, S.; Bellone, S.; Wong, A.; Brucker, S.Y. Evaluation of treatment effects in patients with endometrial cancer and POLE mutations: An individual patient data meta-analysis. Cancer 2021, 127, 2409–2422. [Google Scholar] [CrossRef] [PubMed]
- Wortman, B.G.; Bosse, T.; Nout, R.A.; Lutgens, L.; van der Steen-Banasik, E.M.; Westerveld, H.; van den Berg, H.; Slot, A.; De Winter, K.A.J.; Verhoeven-Adema, K.W.; et al. Molecular-integrated risk profile to determine adjuvant radiotherapy in endometrial cancer: Evaluation of the pilot phase of the PORTEC-4a trial. Gynecol. Oncol. 2018, 151, 69–75. [Google Scholar] [CrossRef]
- Dood, R.; Trabert, B.; Werner, T.L.; Gaffney, D.; Jarboe, E. The time is now to start molecular subtyping high-intermediate and high-risk endometrial cancers. Int. J. Gynecol. Cancer 2022, 32, 1087–1088. [Google Scholar] [CrossRef]
- Van den Heerik, A.; Haar, N.T.T.; Vermij, L.; Jobsen, J.J.; Brinkhuis, M.; Roothaan, S.M.; Leon-Castillo, A.; Ortoft, G.; Hogdall, E.; Hogdall, C.; et al. QPOLE: A Quick, Simple, and Cheap Alternative for POLE Sequencing in Endometrial Cancer by Multiplex Genotyping Quantitative Polymerase Chain Reaction. JCO Glob. Oncol. 2023, 9, e2200384. [Google Scholar] [CrossRef]
| Protein Change | No. of Cases | Nucleotide Substitution | Exon | MSI-H Cases (%) | Mutation Recurrence in EC | Mutation Recurrence Pan-Cancer | No. of “Benign” Results by In Silico Tools | POLE Score | EDM | Signature 10 Contribution |
|---|---|---|---|---|---|---|---|---|---|---|
| P286R | 21 | c.857C>G | 9 | 1 (4.8) | Recurrent | Recurrent | 0 | 5–6 | Y | 0.225–0.978 |
| V411L | 13 | c.1231G>T/C | 13 | 1 (7.7) | Recurrent | Recurrent | 1 | 4–6 | Y | 0.000–0.751 |
| S297F | 3 | c.890C>T | 9 | 2 (66.7) | Recurrent | Recurrent | 0 | 5–6 | Y | 0.123–0.611 |
| S459F | 2 | c.1376C>T | 14 | 0 (0) | Recurrent | Recurrent | 1 | 5–6 | Y | 0.940–0.955 |
| A456P | 2 | c.1366G>C | 14 | 0 (0) | Recurrent | Recurrent | 0 | 5–6 | Y | 0.277–0.837 |
| F367S | 2 | c.1100T>C | 11 | 2 (100) | Recurrent | Recurrent | 0 | 6 | Y | 0.095–0.100 |
| L424I | 2 | c.1270C>A | 13 | 2 (100) | Recurrent | Recurrent | 1 | 5 or 3 | Y | 0.000–0.000 |
| M295R | 1 | c.884T>G | 9 | 1 (100) | Recurrent | Recurrent | 0 | 6 | Y | 0.785 |
| P436R | 1 | c.1307C>G | 13 | 0 (0) | Recurrent | Recurrent | 0 | 6 | Y | 0.230 |
| M444K | 1 | c.1331T>A | 13 | 0 (0) | Recurrent | Recurrent | 0 | 5 | Y | 1.000 |
| D368Y | 1 | c.1102G>T | 11 | 1 (100) | Novel | Recurrent | 0 | 4 | Y | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W. Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists. Cancers 2023, 15, 4101. https://doi.org/10.3390/cancers15164101
Zheng W. Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists. Cancers. 2023; 15(16):4101. https://doi.org/10.3390/cancers15164101
Chicago/Turabian StyleZheng, Wenxin. 2023. "Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists" Cancers 15, no. 16: 4101. https://doi.org/10.3390/cancers15164101
APA StyleZheng, W. (2023). Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists. Cancers, 15(16), 4101. https://doi.org/10.3390/cancers15164101






