Emergence of Lipid Droplets in the Mechanisms of Carcinogenesis and Therapeutic Responses
Abstract
Simple Summary
Abstract
1. Introduction
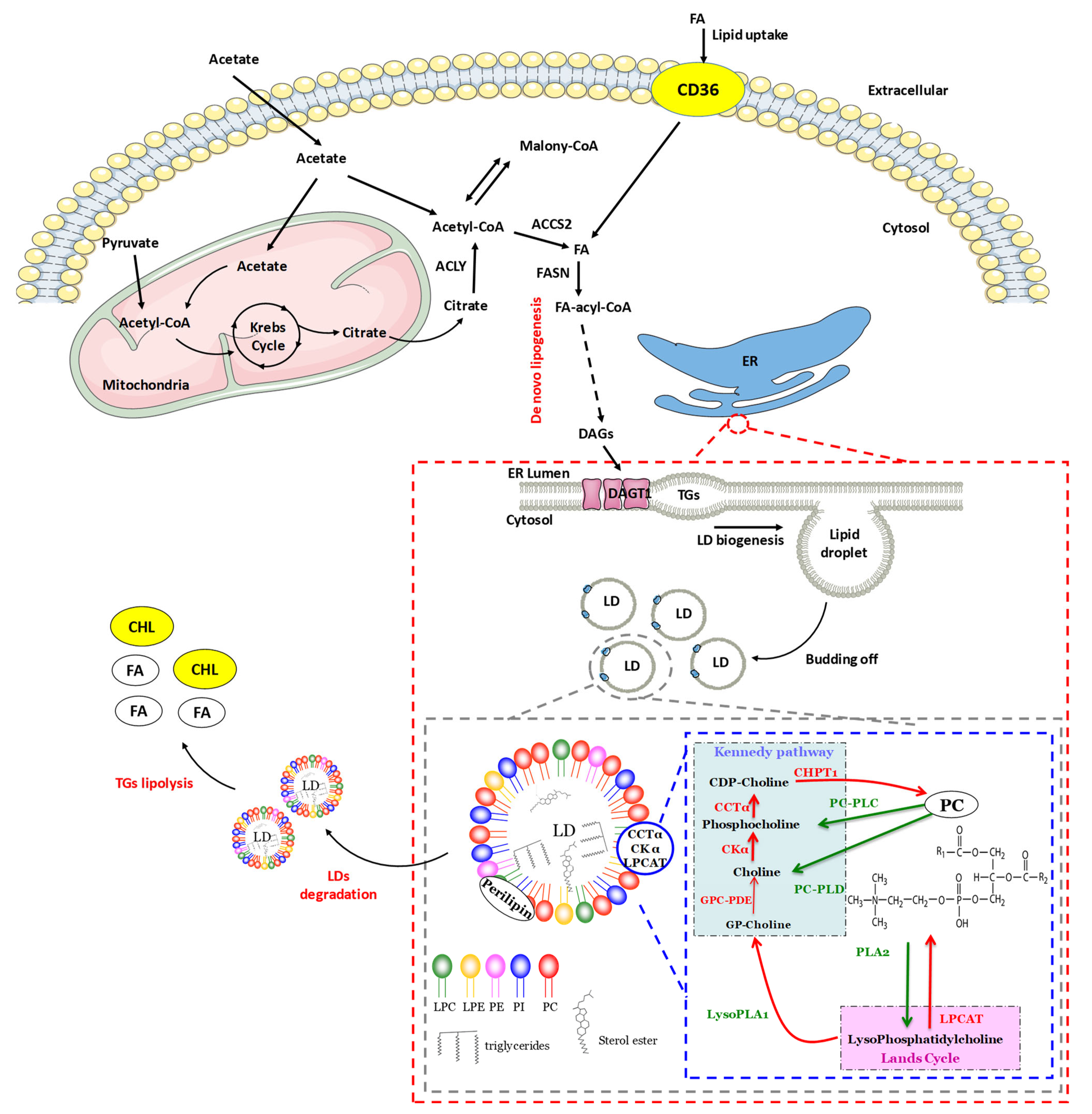
2. Lipid Droplets: Structure and Composition
2.1. Structure and Composition
2.2. Lipogenesis
2.2.1. Initiation of Lipid Droplet Synthesis
2.2.2. Expansion of Lipid Droplets
2.3. Lipolysis
2.4. Lipid Droplet Isolation and Quantification Methods
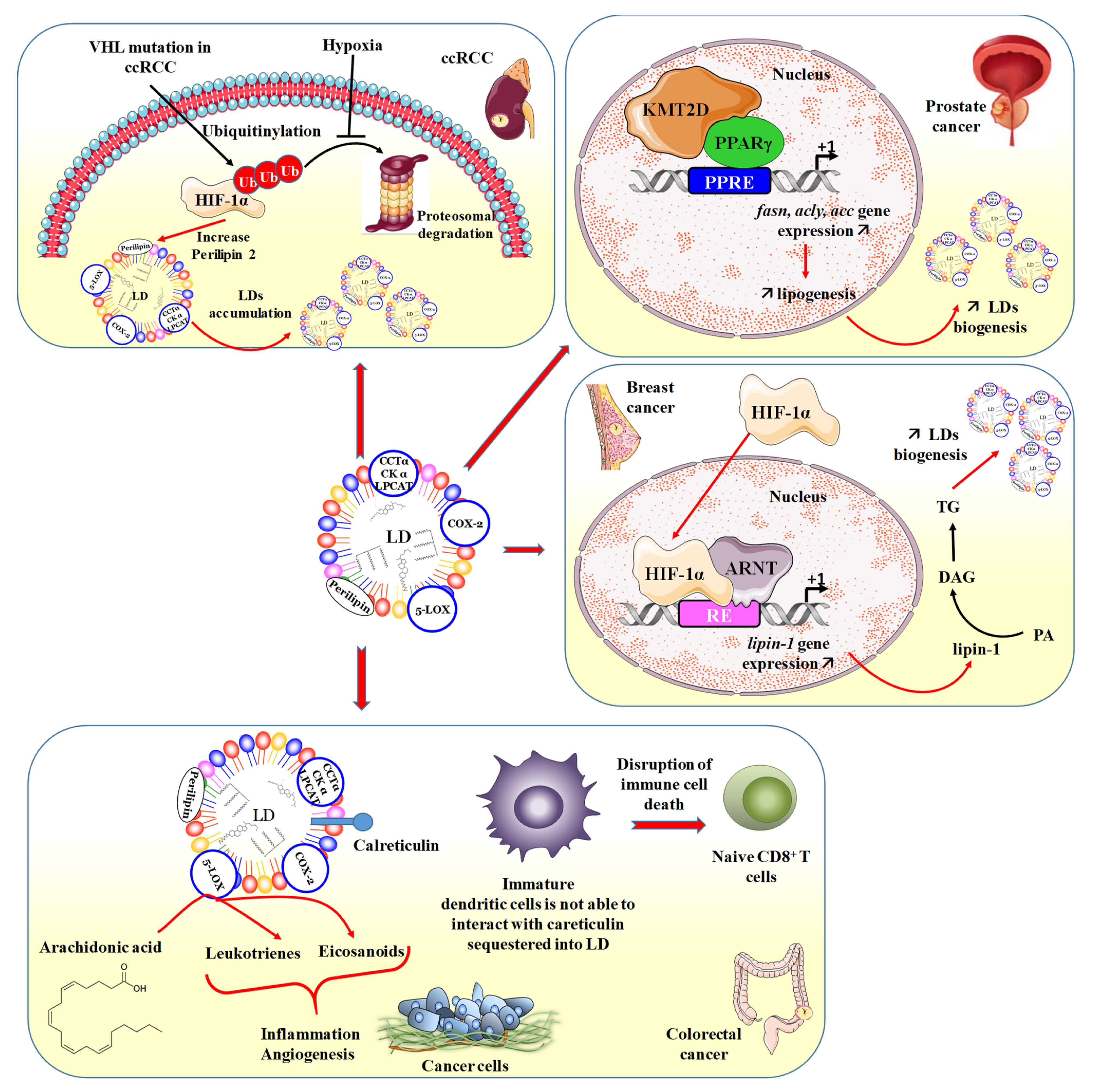
3. Lipid Droplets and Cancers
3.1. Lipotoxicity Reduction/Induction Balance
3.2. LDs and Renal Cancer
3.3. LDs and Prostate Cancer
3.4. LDs and Breast Cancer
3.5. LDs and Hepatocarcinoma
3.6. LDs and Colorectal Cancer
3.7. LDs as Targets to Reverse Chemoresistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gross, D.A.; Silver, D.L. Cytosolic lipid droplets: From mechanisms of fat storage to disease. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 304–326. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, F.G.; McReynolds, M.; Couvillon, A.; Kam, Y.; Holla, V.R.; Dubois, R.N.; Exton, J.H. Requirement of phospholipase D1 activity in H-RasV12-induced transformation. Proc. Natl. Acad. Sci. USA 2005, 102, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Fei, W.; Zhong, L.; Ta, M.T.; Shui, G.; Wenk, M.R.; Yang, H. The size and phospholipid composition of lipid droplets can influence their proteome. Biochem. Biophys. Res. Commun. 2011, 415, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Brasaemle, D.L. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 2007, 48, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, N.A.; Bickel, P.E. Lipid droplets in lipogenesis and lipolysis. Endocrinology 2008, 149, 942–949. [Google Scholar] [CrossRef]
- Robenek, H.; Robenek, M.J.; Troyer, D. PAT family proteins pervade lipid droplet cores. J. Lipid Res. 2005, 46, 1331–1338. [Google Scholar] [CrossRef]
- Szlasa, W.; Zendran, I.; Zalesinska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef]
- Moessinger, C.; Klizaite, K.; Steinhagen, A.; Philippou-Massier, J.; Shevchenko, A.; Hoch, M.; Ejsing, C.S.; Thiele, C. Two different pathways of phosphatidylcholine synthesis, the Kennedy Pathway and the Lands Cycle, differentially regulate cellular triacylglycerol storage. BMC Cell Biol. 2014, 15, 43. [Google Scholar] [CrossRef]
- Cheng, M.; Bhujwalla, Z.M.; Glunde, K. Targeting Phospholipid Metabolism in Cancer. Front. Oncol. 2016, 6, 266. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, D.; Rao, B.; Jian, L.; Chen, Y.; Hu, K.; Xia, Y.; Li, S.; Shen, Y.; Qin, A.; et al. The structural basis for the phospholipid remodeling by lysophosphatidylcholine acyltransferase 3. Nat. Commun. 2021, 12, 6869. [Google Scholar] [CrossRef]
- Wilfling, F.; Wang, H.; Haas, J.T.; Krahmer, N.; Gould, T.J.; Uchida, A.; Cheng, J.X.; Graham, M.; Christiano, R.; Frohlich, F.; et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev. Cell 2013, 24, 384–399. [Google Scholar] [CrossRef] [PubMed]
- Ploegh, H.L. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature 2007, 448, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.C.; Farese, R.V., Jr. The life of lipid droplets. Biochim. Biophys. Acta 2009, 1791, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.F.; Goodman, J.M. The life cycle of lipid droplets. Curr. Opin. Cell Biol. 2015, 33, 119–124. [Google Scholar] [CrossRef]
- Eastman, S.W.; Yassaee, M.; Bieniasz, P.D. A role for ubiquitin ligases and Spartin/SPG20 in lipid droplet turnover. J. Cell Biol. 2009, 184, 881–894. [Google Scholar] [CrossRef]
- Lind, G.E.; Raiborg, C.; Danielsen, S.A.; Rognum, T.O.; Thiis-Evensen, E.; Hoff, G.; Nesbakken, A.; Stenmark, H.; Lothe, R.A. SPG20, a novel biomarker for early detection of colorectal cancer, encodes a regulator of cytokinesis. Oncogene 2011, 30, 3967–3978. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, W.; Xie, X.; Song, Y.; Dang, C.; Zhang, H. Methylation-induced silencing of SPG20 facilitates gastric cancer cell proliferation by activating the EGFR/MAPK pathway. Biochem. Biophys. Res. Commun. 2018, 500, 411–417. [Google Scholar] [CrossRef]
- Cusenza, V.Y.; Bonora, E.; Amodio, N.; Frazzi, R. Spartin: At the crossroad between ubiquitination and metabolism in cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188813. [Google Scholar] [CrossRef]
- Shockey, J.M.; Gidda, S.K.; Chapital, D.C.; Kuan, J.C.; Dhanoa, P.K.; Bland, J.M.; Rothstein, S.J.; Mullen, R.T.; Dyer, J.M. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 2006, 18, 2294–2313. [Google Scholar] [CrossRef]
- Stone, S.J.; Levin, M.C.; Zhou, P.; Han, J.; Walther, T.C.; Farese, R.V., Jr. The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J. Biol. Chem. 2009, 284, 5352–5361. [Google Scholar] [CrossRef]
- Wolins, N.E.; Brasaemle, D.L.; Bickel, P.E. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006, 580, 5484–5491. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, B.R.; Binns, D.D.; Hilton, C.L.; Han, S.; Gao, Q.; Goodman, J.M. Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology. Mol. Biol. Cell 2015, 26, 726–739. [Google Scholar] [CrossRef]
- Zhang, X.D.; Li, W.; Zhang, N.; Hou, Y.L.; Niu, Z.Q.; Zhong, Y.J.; Zhang, Y.P.; Yang, S.Y. Identification of adipophilin as a potential diagnostic tumor marker for lung adenocarcinoma. Int. J. Clin. Exp. Med. 2014, 7, 1190–1196. [Google Scholar] [PubMed]
- Chang, B.H.; Li, L.; Paul, A.; Taniguchi, S.; Nannegari, V.; Heird, W.C.; Chan, L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol. Cell Biol. 2006, 26, 1063–1076. [Google Scholar] [CrossRef]
- Bostrom, P.; Andersson, L.; Rutberg, M.; Perman, J.; Lidberg, U.; Johansson, B.R.; Fernandez-Rodriguez, J.; Ericson, J.; Nilsson, T.; Boren, J.; et al. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat. Cell Biol. 2007, 9, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Itabe, H.; Sakai, J.; Makita, M.; Noda, J.; Mori, M.; Higashi, Y.; Kojima, S.; Takano, T. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim. Biophys. Acta 2004, 1644, 47–59. [Google Scholar] [CrossRef]
- Fei, W.; Shui, G.; Zhang, Y.; Krahmer, N.; Ferguson, C.; Kapterian, T.S.; Lin, R.C.; Dawes, I.W.; Brown, A.J.; Li, P.; et al. A role for phosphatidic acid in the formation of “supersized” lipid droplets. PLoS Genet. 2011, 7, e1002201. [Google Scholar] [CrossRef]
- Xu, Y.; Mak, H.Y.; Lukmantara, I.; Li, Y.E.; Hoehn, K.L.; Huang, X.; Du, X.; Yang, H. CDP-DAG synthase 1 and 2 regulate lipid droplet growth through distinct mechanisms. J. Biol. Chem. 2019, 294, 16740–16755. [Google Scholar] [CrossRef]
- Guo, Y.; Walther, T.C.; Rao, M.; Stuurman, N.; Goshima, G.; Terayama, K.; Wong, J.S.; Vale, R.D.; Walter, P.; Farese, R.V. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 2008, 453, 657–661. [Google Scholar] [CrossRef]
- Ridgway, N.D. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 20–38. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Zhao, Y.; Cheng, J.; Zhao, C.; Wang, Z. CCT alpha is a novel biomarker for diagnosis of laryngeal squamous cell cancer. Sci. Rep. 2019, 9, 11823. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Ding, Y.; Hui, N.; Su, B.; Yang, M. LPCAT1 acts as an independent prognostic biomarker correlated with immune infiltration in hepatocellular carcinoma. Eur. J. Med. Res. 2022, 27, 216. [Google Scholar] [CrossRef] [PubMed]
- Lebok, P.; von Hassel, A.; Meiners, J.; Hube-Magg, C.; Simon, R.; Hoflmayer, D.; Hinsch, A.; Dum, D.; Fraune, C.; Gobel, C.; et al. Up-regulation of lysophosphatidylcholine acyltransferase 1 (LPCAT1) is linked to poor prognosis in breast cancer. Aging 2019, 11, 7796–7804. [Google Scholar] [CrossRef] [PubMed]
- Krahmer, N.; Guo, Y.; Wilfling, F.; Hilger, M.; Lingrell, S.; Heger, K.; Newman, H.W.; Schmidt-Supprian, M.; Vance, D.E.; Mann, M.; et al. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 2011, 14, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.L.; Lingrell, S.; Zhao, Y.; Francis, G.A.; Vance, D.E. Hepatic CTP:phosphocholine cytidylyltransferase-alpha is a critical predictor of plasma high density lipoprotein and very low density lipoprotein. J. Biol. Chem. 2008, 283, 2147–2155. [Google Scholar] [CrossRef]
- Barbosa, A.D.; Savage, D.B.; Siniossoglou, S. Lipid droplet-organelle interactions: Emerging roles in lipid metabolism. Curr. Opin. Cell Biol. 2015, 35, 91–97. [Google Scholar] [CrossRef]
- Moessinger, C.; Kuerschner, L.; Spandl, J.; Shevchenko, A.; Thiele, C. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J. Biol. Chem. 2011, 286, 21330–21339. [Google Scholar] [CrossRef]
- Anderson, N.; Borlak, J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol. Rev. 2008, 60, 311–357. [Google Scholar] [CrossRef]
- Guijas, C.; Rodriguez, J.P.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Phospholipase A2 regulation of lipid droplet formation. Biochim. Biophys. Acta 2014, 1841, 1661–1671. [Google Scholar] [CrossRef]
- Zimmermann, R.; Lass, A.; Haemmerle, G.; Zechner, R. Fate of fat: The role of adipose triglyceride lipase in lipolysis. Biochim. Biophys. Acta 2009, 1791, 494–500. [Google Scholar] [CrossRef]
- Londos, C.; Brasaemle, D.L.; Schultz, C.J.; Segrest, J.P.; Kimmel, A.R. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin. Cell Dev. Biol. 1999, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kuma, A.; Sugiura, Y.; Ichimura, Y.; Obata, M.; Kitamura, H.; Okuda, S.; Lee, H.C.; Ikeda, K.; Kanegae, Y.; et al. Autophagy regulates lipid metabolism through selective turnover of NCoR1. Nat. Commun. 2019, 10, 1567. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Brasaemle, D.L.; Wolins, N.E. Isolation of Lipid Droplets from Cells by Density Gradient Centrifugation. Curr. Protoc. Cell Biol. 2016, 72, 3.15.11–13.15.13. [Google Scholar] [CrossRef] [PubMed]
- Rosch, K.; Kwiatkowski, M.; Schluter, H.; Herker, E. Lipid Droplet Isolation for Quantitative Mass Spectrometry Analysis. J. Vis. Exp. 2017, 122, e55585. [Google Scholar] [CrossRef]
- Wang, H.; Quiroga, A.D.; Lehner, R. Analysis of lipid droplets in hepatocytes. Methods Cell Biol. 2013, 116, 107–127. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Perumal, S.K.; Rasineni, K.; Donohue, T.M., Jr.; Osna, N.A.; Kharbanda, K.K. Lipidomic Analysis of Liver Lipid Droplets after Chronic Alcohol Consumption with and without Betaine Supplementation. Biology 2023, 12, 462. [Google Scholar] [CrossRef]
- Kieu, T.L.; Pierre, L.; Derangere, V.; Perrey, S.; Truntzer, C.; Jalil, A.; Causse, S.; Groetz, E.; Dumont, A.; Guyard, L.; et al. Downregulation of Elovl5 promotes breast cancer metastasis through a lipid-droplet accumulation-mediated induction of TGF-beta receptors. Cell Death Dis. 2022, 13, 758. [Google Scholar] [CrossRef]
- Hara, M.; Wu, W.; Malechka, V.V.; Takahashi, Y.; Ma, J.X.; Moiseyev, G. PNPLA2 mobilizes retinyl esters from retinosomes and promotes the generation of 11-cis-retinal in the visual cycle. Cell Rep. 2023, 42, 112091. [Google Scholar] [CrossRef]
- Gupta, A.; Dorlhiac, G.F.; Streets, A.M. Quantitative imaging of lipid droplets in single cells. Analyst 2019, 144, 753–765. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, K.; Yao, Y.; Reilly, P.T.; Kannan, K.; Kiarash, R.; Mason, J.; Huang, P.; Sawyer, S.K.; Fuerth, B.; Faubert, B.; et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes. Dev. 2011, 25, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sreenivasan, U.; Hu, H.; Saladino, A.; Polster, B.M.; Lund, L.M.; Gong, D.W.; Stanley, W.C.; Sztalryd, C. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J. Lipid Res. 2011, 52, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Surmacki, J.; Kopec, M.; Olejnik, A.K.; Lubecka-Pietruszewska, K.; Fabianowska-Majewska, K. The role of lipid droplets and adipocytes in cancer. Raman imaging of cell cultures: MCF10A, MCF7, and MDA-MB-231 compared to adipocytes in cancerous human breast tissue. Analyst 2015, 140, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Andersson, E.; Anand, S.K.; Cansby, E.; Caputo, M.; Kumari, S.; Porosk, R.; Kilk, K.; Nair, S.; Marschall, H.U.; et al. Silencing of STE20-type kinase TAOK1 confers protection against hepatocellular lipotoxicity through metabolic rewiring. Hepatol. Commun. 2023, 7, e0037. [Google Scholar] [CrossRef]
- Wu, C.; Dai, C.; Li, X.; Sun, M.; Chu, H.; Xuan, Q.; Yin, Y.; Fang, C.; Yang, F.; Jiang, Z.; et al. AKR1C3-dependent lipid droplet formation confers hepatocellular carcinoma cell adaptability to targeted therapy. Theranostics 2022, 12, 7681–7698. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Fan, J.; Li, Q.; Guo, R.; Pan, J.; Liu, Y.; Peng, J.; Zhu, Q.; Feng, Y.; et al. Inhibition of integrated stress response protects against lipid-induced senescence in hypothalamic neural stem cells in adamantinomatous craniopharyngioma. Neuro Oncol. 2023, 25, 720–732. [Google Scholar] [CrossRef]
- Eyme, K.M.; Sammarco, A.; Jha, R.; Mnatsakanyan, H.; Pechdimaljian, C.; Carvalho, L.; Neustadt, R.; Moses, C.; Alnasser, A.; Tardiff, D.F.; et al. Targeting de novo lipid synthesis induces lipotoxicity and impairs DNA damage repair in glioblastoma mouse models. Sci. Transl. Med. 2023, 15, eabq6288. [Google Scholar] [CrossRef]
- Alannan, M.; Trezeguet, V.; Amoedo, N.D.; Rossignol, R.; Mahfouf, W.; Rezvani, H.R.; Dittrich-Domergue, F.; Moreau, P.; Lacomme, S.; Gontier, E.; et al. Rewiring Lipid Metabolism by Targeting PCSK9 and HMGCR to Treat Liver Cancer. Cancers 2022, 15, 3. [Google Scholar] [CrossRef]
- Yuan, Y.; Shah, N.; Almohaisin, M.I.; Saha, S.; Lu, F. Assessing fatty acid-induced lipotoxicity and its therapeutic potential in glioblastoma using stimulated Raman microscopy. Sci. Rep. 2021, 11, 7422. [Google Scholar] [CrossRef]
- Cheng, X.; Geng, F.; Pan, M.; Wu, X.; Zhong, Y.; Wang, C.; Tian, Z.; Cheng, C.; Zhang, R.; Puduvalli, V.; et al. Targeting DGAT1 Ameliorates Glioblastoma by Increasing Fat Catabolism and Oxidative Stress. Cell Metab. 2020, 32, 229–242.e228. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Fujiwara, Y.; Komohara, Y. Cholesterol metabolism and lipid droplet vacuoles; a potential target for the therapy of aggressive lymphoma. J. Clin. Exp. Hematop. 2022, 62, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.M.; Nguyen, T.H.; Kim, H.S.; Dao, T.T.P.; Moon, Y.; Seo, M.; Kang, S.; Mai, V.H.; An, Y.J.; Jung, C.R.; et al. GPX8 regulates clear cell renal cell carcinoma tumorigenesis through promoting lipogenesis by NNMT. J. Exp. Clin. Cancer Res. 2023, 42, 42. [Google Scholar] [CrossRef]
- Quan, Y.; Dai, J.; Zhou, S.; Zhao, L.; Jin, L.; Long, Y.; Liu, S.; Hu, Y.; Liu, Y.; Zhao, J.; et al. HIF2alpha-induced upregulation of RNASET2 promotes triglyceride synthesis and enhances cell migration in clear cell renal cell carcinoma. FEBS Open Bio 2023, 13, 638–654. [Google Scholar] [CrossRef]
- Zhou, J.; Simon, J.M.; Liao, C.; Zhang, C.; Hu, L.; Zurlo, G.; Liu, X.; Fan, C.; Hepperla, A.; Jia, L.; et al. An oncogenic JMJD6-DGAT1 axis tunes the epigenetic regulation of lipid droplet formation in clear cell renal cell carcinoma. Mol. Cell 2022, 82, 3030–3044.e3038. [Google Scholar] [CrossRef]
- Klasson, T.D.; LaGory, E.L.; Zhao, H.; Huynh, S.K.; Papandreou, I.; Moon, E.J.; Giaccia, A.J. ACSL3 regulates lipid droplet biogenesis and ferroptosis sensitivity in clear cell renal cell carcinoma. Cancer Metab. 2022, 10, 14. [Google Scholar] [CrossRef]
- Nitta, S.; Kandori, S.; Tanaka, K.; Sakka, S.; Siga, M.; Nagumo, Y.; Negoro, H.; Kojima, T.; Mathis, B.J.; Shimazui, T.; et al. ELOVL5-mediated fatty acid elongation promotes cellular proliferation and invasion in renal cell carcinoma. Cancer Sci. 2022, 113, 2738–2752. [Google Scholar] [CrossRef]
- Sundelin, J.P.; Stahlman, M.; Lundqvist, A.; Levin, M.; Parini, P.; Johansson, M.E.; Boren, J. Increased expression of the very low-density lipoprotein receptor mediates lipid accumulation in clear-cell renal cell carcinoma. PLoS ONE 2012, 7, e48694. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhang, L.; Brett-Morris, A.; Aguila, B.; Kerner, J.; Hoppel, C.L.; Puchowicz, M.; Serra, D.; Herrero, L.; Rini, B.I.; et al. HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat. Commun. 2017, 8, 1769. [Google Scholar] [CrossRef]
- Liao, M.; Li, Y.; Xiao, A.; Lu, Q.; Zeng, H.; Qin, H.; Zheng, E.; Luo, X.; Chen, L.; Ruan, X.Z.; et al. HIF-2alpha-induced upregulation of CD36 promotes the development of ccRCC. Exp. Cell Res. 2022, 421, 113389. [Google Scholar] [CrossRef]
- Qiu, B.; Ackerman, D.; Sanchez, D.J.; Li, B.; Ochocki, J.D.; Grazioli, A.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Keith, B.; Simon, M.C. HIF2alpha-Dependent Lipid Storage Promotes Endoplasmic Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2015, 5, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, J.J.; Cross, J.R.; Fan, J.; de Stanchina, E.; Mathew, R.; White, E.P.; Thompson, C.B.; Rabinowitz, J.D. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl. Acad. Sci. USA 2013, 110, 8882–8887. [Google Scholar] [CrossRef] [PubMed]
- Gimm, T.; Wiese, M.; Teschemacher, B.; Deggerich, A.; Schodel, J.; Knaup, K.X.; Hackenbeck, T.; Hellerbrand, C.; Amann, K.; Wiesener, M.S.; et al. Hypoxia-inducible protein 2 is a novel lipid droplet protein and a specific target gene of hypoxia-inducible factor-1. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 4443–4458. [Google Scholar] [CrossRef]
- Bensaad, K.; Favaro, E.; Lewis, C.A.; Peck, B.; Lord, S.; Collins, J.M.; Pinnick, K.E.; Wigfield, S.; Buffa, F.M.; Li, J.L.; et al. Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014, 9, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Cheng, J.; Fujita, A.; Tokumoto, T.; Fujimoto, T. Cytoplasmic lipid droplets are sites of convergence of proteasomal and autophagic degradation of apolipoprotein B. Mol. Biol. Cell 2006, 17, 2674–2683. [Google Scholar] [CrossRef] [PubMed]
- Herms, A.; Bosch, M.; Ariotti, N.; Reddy, B.J.; Fajardo, A.; Fernandez-Vidal, A.; Alvarez-Guaita, A.; Fernandez-Rojo, M.A.; Rentero, C.; Tebar, F.; et al. Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Curr. Biol. 2013, 23, 1489–1496. [Google Scholar] [CrossRef]
- Kaini, R.R.; Sillerud, L.O.; Zhaorigetu, S.; Hu, C.A. Autophagy regulates lipolysis and cell survival through lipid droplet degradation in androgen-sensitive prostate cancer cells. Prostate 2012, 72, 1412–1422. [Google Scholar] [CrossRef]
- Basavaraj, P.; Ruangsai, P.; Hsieh, P.F.; Jiang, W.P.; Bau, D.T.; Huang, G.J.; Huang, W.C. Alpinumisoflavone Exhibits the Therapeutic Effect on Prostate Cancer Cells by Repressing AR and Co-Targeting FASN- and HMGCR-Mediated Lipid and Cholesterol Biosynthesis. Life 2022, 12, 1769. [Google Scholar] [CrossRef]
- Cardoso, H.J.; Figueira, M.I.; Carvalho, T.M.A.; Serra, C.D.M.; Vaz, C.V.; Madureira, P.A.; Socorro, S. Androgens and low density lipoprotein-cholesterol interplay in modulating prostate cancer cell fate and metabolism. Pathol. Res. Pract. 2022, 240, 154181. [Google Scholar] [CrossRef]
- Gu, Y.; Xue, M.; Wang, Q.; Hong, X.; Wang, X.; Zhou, F.; Sun, J.; Wang, G.; Peng, Y. Novel Strategy of Proxalutamide for the Treatment of Prostate Cancer through Coordinated Blockade of Lipogenesis and Androgen Receptor Axis. Int. J. Mol. Sci. 2021, 22, 13222. [Google Scholar] [CrossRef]
- Zhai, Q.; Luo, M.; Zhang, Y.; Zhang, W.; Wu, C.; Lv, S.; Wei, Q. Histone methyltransferase KMT2D mediated lipid metabolism via peroxisome proliferator-activated receptor gamma in prostate cancer. Transl. Cancer Res. 2022, 11, 2607–2621. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Ono, M.; Iesato, A.; Hanamura, T.; Watanabe, T.; Ito, T.; Kanai, T.; Maeno, K.; Ito, K.; Tateishi, A.; et al. Lipid-rich carcinoma of the breast that is strongly positive for estrogen receptor: A case report and literature review. OncoTargets Ther. 2016, 9, 1641–1646. [Google Scholar] [CrossRef][Green Version]
- Pucer, A.; Brglez, V.; Payre, C.; Pungercar, J.; Lambeau, G.; Petan, T. Group X secreted phospholipase A(2) induces lipid droplet formation and prolongs breast cancer cell survival. Mol. Cancer 2013, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Burgermeister, E.; Tencer, L.; Liscovitch, M. Peroxisome proliferator-activated receptor-gamma upregulates caveolin-1 and caveolin-2 expression in human carcinoma cells. Oncogene 2003, 22, 3888–3900. [Google Scholar] [CrossRef] [PubMed]
- Furuta, E.; Pai, S.K.; Zhan, R.; Bandyopadhyay, S.; Watabe, M.; Mo, Y.Y.; Hirota, S.; Hosobe, S.; Tsukada, T.; Miura, K.; et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008, 68, 1003–1011. [Google Scholar] [CrossRef]
- Yoshii, Y.; Furukawa, T.; Yoshii, H.; Mori, T.; Kiyono, Y.; Waki, A.; Kobayashi, M.; Tsujikawa, T.; Kudo, T.; Okazawa, H.; et al. Cytosolic acetyl-CoA synthetase affected tumor cell survival under hypoxia: The possible function in tumor acetyl-CoA/acetate metabolism. Cancer Sci. 2009, 100, 821–827. [Google Scholar] [CrossRef]
- Berardi, D.E.; Bock-Hughes, A.; Terry, A.R.; Drake, L.E.; Bozek, G.; Macleod, K.F. Lipid droplet turnover at the lysosome inhibits growth of hepatocellular carcinoma in a BNIP3-dependent manner. Sci. Adv. 2022, 8, eabo2510. [Google Scholar] [CrossRef]
- Uzbekov, R.; Roingeard, P. Nuclear lipid droplets identified by electron microscopy of serial sections. BMC Res. Notes 2013, 6, 386. [Google Scholar] [CrossRef]
- Ueno, M.; Shen, W.J.; Patel, S.; Greenberg, A.S.; Azhar, S.; Kraemer, F.B. Fat-specific protein 27 modulates nuclear factor of activated T cells 5 and the cellular response to stress. J. Lipid Res. 2013, 54, 734–743. [Google Scholar] [CrossRef]
- Cermelli, S.; Guo, Y.; Gross, S.P.; Welte, M.A. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr. Biol. 2006, 16, 1783–1795. [Google Scholar] [CrossRef]
- Salloum, S.; Wang, H.; Ferguson, C.; Parton, R.G.; Tai, A.W. Rab18 binds to hepatitis C virus NS5A and promotes interaction between sites of viral replication and lipid droplets. PLoS Pathog. 2013, 9, e1003513. [Google Scholar] [CrossRef] [PubMed]
- Tirinato, L.; Liberale, C.; Di Franco, S.; Candeloro, P.; Benfante, A.; La Rocca, R.; Potze, L.; Marotta, R.; Ruffilli, R.; Rajamanickam, V.P.; et al. Lipid droplets: A new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells 2015, 33, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Penrose, H.; Heller, S.; Cable, C.; Makboul, R.; Chadalawada, G.; Chen, Y.; Crawford, S.E.; Savkovic, S.D. Epidermal growth factor receptor mediated proliferation depends on increased lipid droplet density regulated via a negative regulatory loop with FOXO3/Sirtuin6. Biochem. Biophys. Res. Commun. 2016, 469, 370–376. [Google Scholar] [CrossRef]
- Accioly, M.T.; Pacheco, P.; Maya-Monteiro, C.M.; Carrossini, N.; Robbs, B.K.; Oliveira, S.S.; Kaufmann, C.; Morgado-Diaz, J.A.; Bozza, P.T.; Viola, J.P. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 2008, 68, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Boren, J.; Brindle, K.M. Apoptosis-induced mitochondrial dysfunction causes cytoplasmic lipid droplet formation. Cell Death Differ. 2012, 19, 1561–1570. [Google Scholar] [CrossRef]
- Cotte, A.K.; Aires, V.; Fredon, M.; Limagne, E.; Derangere, V.; Thibaudin, M.; Humblin, E.; Scagliarini, A.; de Barros, J.P.; Hillon, P.; et al. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat. Commun. 2018, 9, 322. [Google Scholar] [CrossRef]
- Cotte, A.K.; Aires, V.; Ghiringhelli, F.; Delmas, D. LPCAT2 controls chemoresistance in colorectal cancer. Mol. Cell. Oncol. 2018, 5, e1448245. [Google Scholar] [CrossRef]
- Yosef, H.K.; Mavarani, L.; Maghnouj, A.; Hahn, S.; El-Mashtoly, S.F.; Gerwert, K. In vitro prediction of the efficacy of molecularly targeted cancer therapy by Raman spectral imaging. Anal. Bioanal. Chem. 2015, 407, 8321–8331. [Google Scholar] [CrossRef]
- Veglia, F.; Tyurin, V.A.; Mohammadyani, D.; Blasi, M.; Duperret, E.K.; Donthireddy, L.; Hashimoto, A.; Kapralov, A.; Amoscato, A.; Angelini, R.; et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat. Commun. 2017, 8, 2122. [Google Scholar] [CrossRef]
- Bandeira-Melo, C.; Phoofolo, M.; Weller, P.F. Extranuclear lipid bodies, elicited by CCR3-mediated signaling pathways, are the sites of chemokine-enhanced leukotriene C4 production in eosinophils and basophils. J. Biol. Chem. 2001, 276, 22779–22787. [Google Scholar] [CrossRef]
- Bozza, P.T.; Viola, J.P. Lipid droplets in inflammation and cancer. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 243–250. [Google Scholar] [CrossRef]
- Nistico, C.; Pagliari, F.; Chiarella, E.; Fernandes Guerreiro, J.; Marafioti, M.G.; Aversa, I.; Genard, G.; Hanley, R.; Garcia-Calderon, D.; Bond, H.M.; et al. Lipid Droplet Biosynthesis Impairment through DGAT2 Inhibition Sensitizes MCF7 Breast Cancer Cells to Radiation. Int. J. Mol. Sci. 2021, 22, 102. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Cruz, A.; Barbosa, J.; Bonifacio, V.D.B.; Pinto, S.N. Lipid Droplets in Cancer: From Composition and Role to Imaging and Therapeutics. Molecules 2022, 27, 991. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, C. Recent Advances in Activatable Organic Photosensitizers for Specific Photodynamic Therapy. Chempluschem 2020, 85, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, R.; Hu, Y.; Liu, W.; Liu, T.; Sun, W.; Fan, J.; Peng, X. A Novel Photosensitizer for Lipid Droplet-Location Photodynamic Therapy. Front. Chem. 2021, 9, 701771. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Thery, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Kreger, B.T.; Dougherty, A.L.; Greene, K.S.; Cerione, R.A.; Antonyak, M.A. Microvesicle Cargo and Function Changes upon Induction of Cellular Transformation. J. Biol. Chem. 2016, 291, 19774–19785. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Bordanaba-Florit, G.; Royo, F.; Kruglik, S.G.; Falcon-Perez, J.M. Using single-vesicle technologies to unravel the heterogeneity of extracellular vesicles. Nat. Protoc. 2021, 16, 3163–3185. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Johansson, H.J.; Mager, I.; Lee, Y.; Blomberg, K.E.; Sadik, M.; Alaarg, A.; Smith, C.I.; Lehtio, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delmas, D.; Cotte, A.K.; Connat, J.-L.; Hermetet, F.; Bouyer, F.; Aires, V. Emergence of Lipid Droplets in the Mechanisms of Carcinogenesis and Therapeutic Responses. Cancers 2023, 15, 4100. https://doi.org/10.3390/cancers15164100
Delmas D, Cotte AK, Connat J-L, Hermetet F, Bouyer F, Aires V. Emergence of Lipid Droplets in the Mechanisms of Carcinogenesis and Therapeutic Responses. Cancers. 2023; 15(16):4100. https://doi.org/10.3390/cancers15164100
Chicago/Turabian StyleDelmas, Dominique, Alexia K. Cotte, Jean-Louis Connat, François Hermetet, Florence Bouyer, and Virginie Aires. 2023. "Emergence of Lipid Droplets in the Mechanisms of Carcinogenesis and Therapeutic Responses" Cancers 15, no. 16: 4100. https://doi.org/10.3390/cancers15164100
APA StyleDelmas, D., Cotte, A. K., Connat, J.-L., Hermetet, F., Bouyer, F., & Aires, V. (2023). Emergence of Lipid Droplets in the Mechanisms of Carcinogenesis and Therapeutic Responses. Cancers, 15(16), 4100. https://doi.org/10.3390/cancers15164100







