Efficacy and Safety of PD-1/PD-L1 Inhibitor as Single-Agent Immunotherapy in Endometrial Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Eligibility Criteria
2.2. Information Sources and Search Criteria
2.3. Study Selection
2.4. Data Collection Process and Data Items
2.5. Assessment of the Risk of Bias of Individual Studies
2.6. Statistical Methods
3. Results
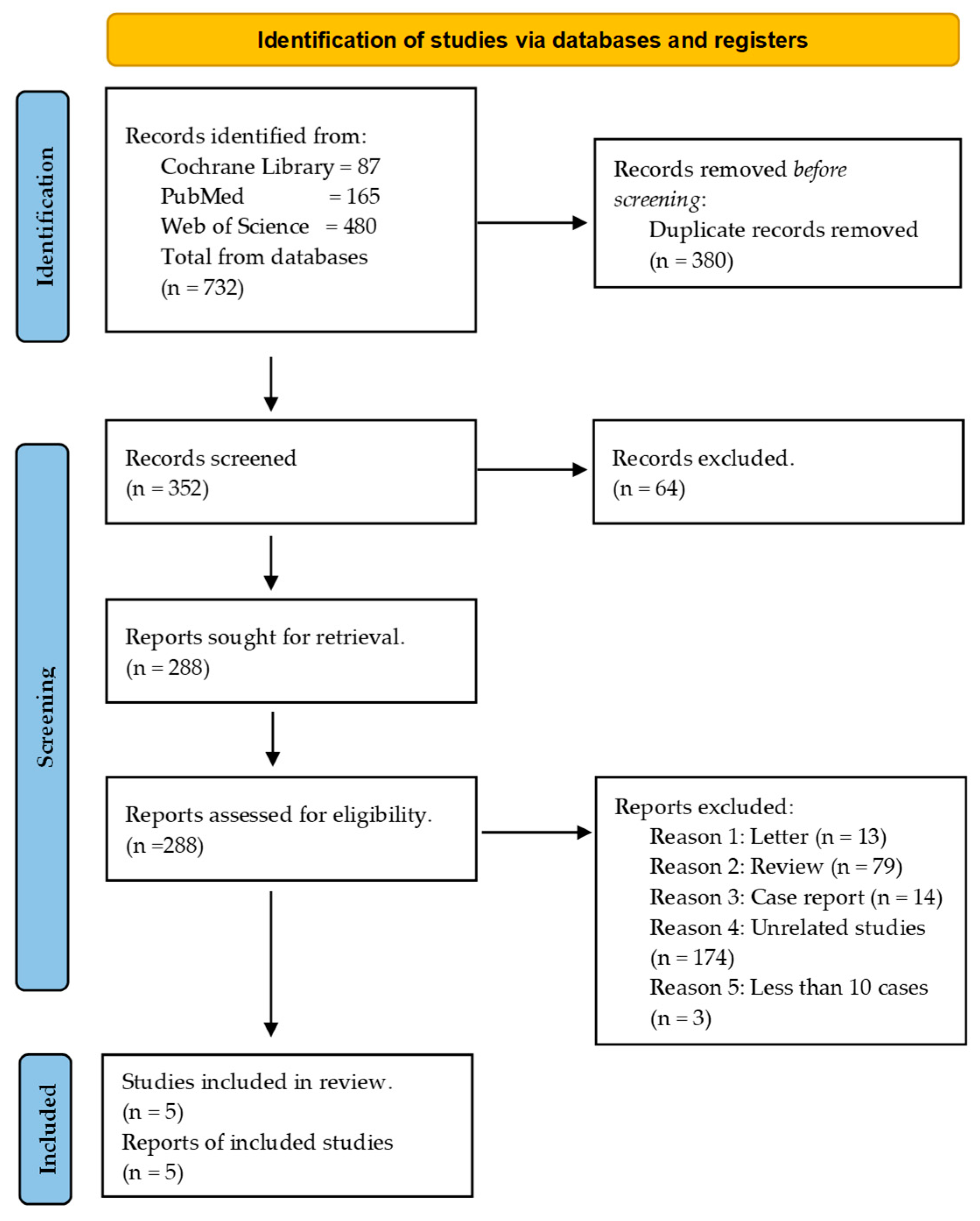
3.1. Search Sequence and Quality Assessment of Selected Publications
3.2. Study Characteristics
3.3. Quantitative Synthesis
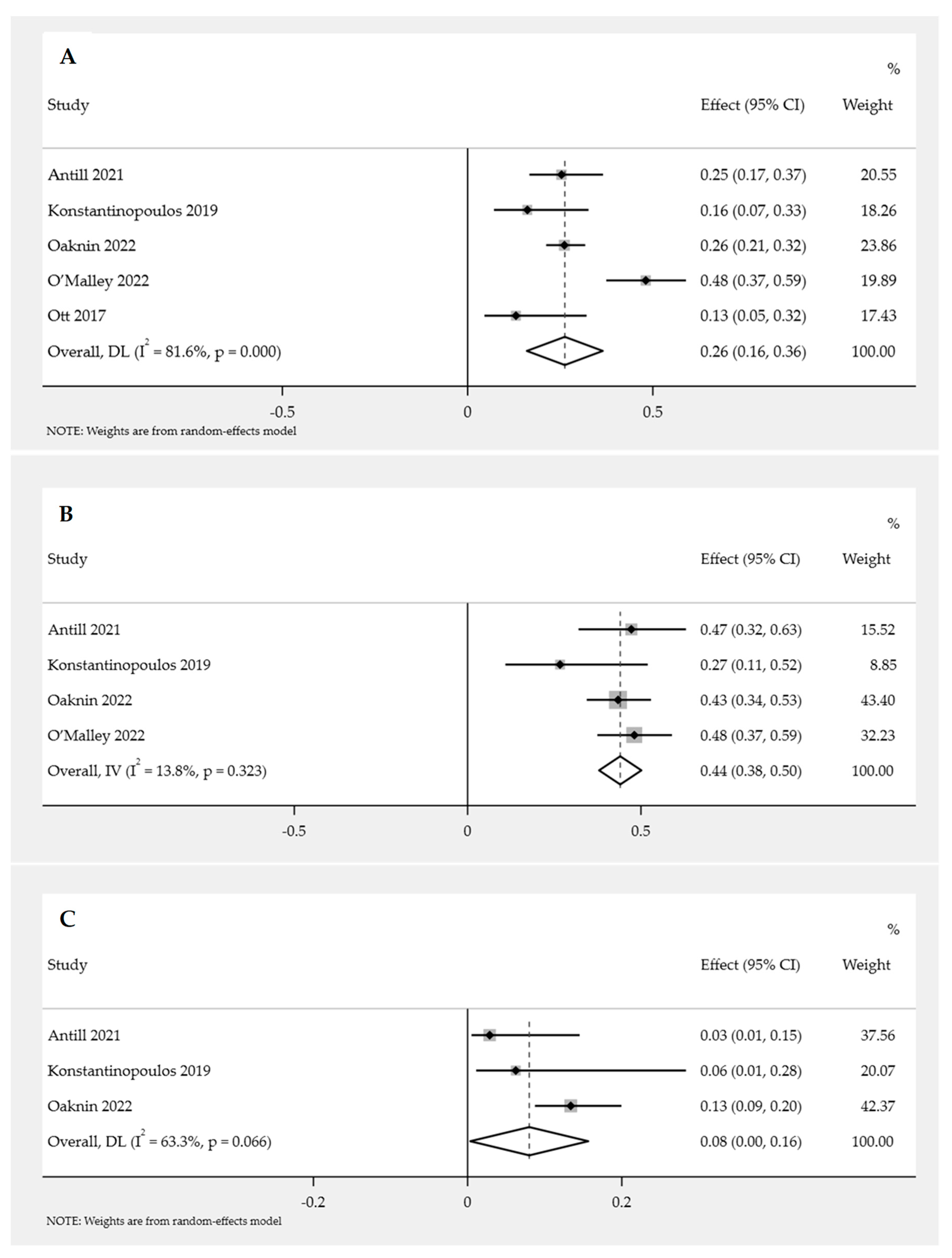
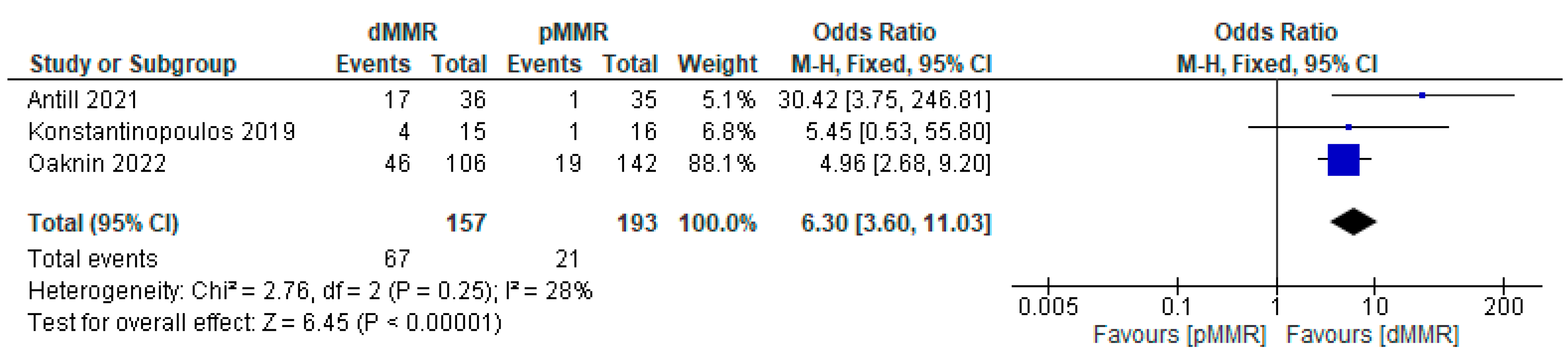
3.3.1. Efficacy of PD-1/PD-L1 Inhibitor Immunotherapy in Endometrial Cancer Based on Objective Response Rate (ORR)
Overall ORR for PD-1/PD-L1 Inhibitor Immunotherapy in Endometrial Cancer
Subgroup Analysis of ORR for PD-1/PD-L1 Inhibitor Immunotherapy in Endometrial Cancer
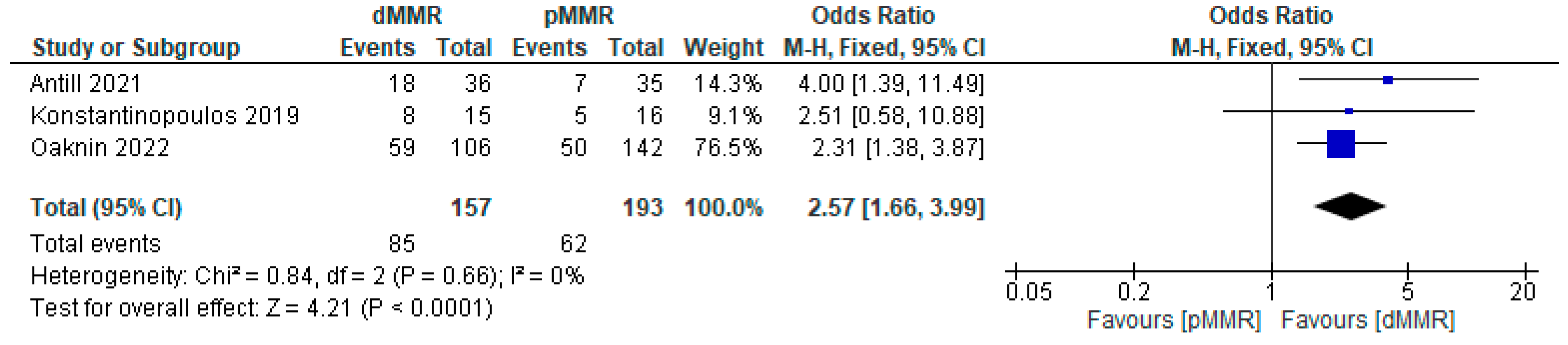
3.3.2. Efficacy of PD-1/PD-L1 Inhibitor Immunotherapy in Endometrial Cancer Based on the Disease Control Rate (DCR)
Overall DCR for PD-1/PD-L1 Inhibitor Immunotherapy in Endometrial Cancer
Subgroup Analysis of DCR for PD-1/PD-L1 Inhibitor Immunotherapy in Endometrial Cancer
3.3.3. Safety of PD-1/PD-L1 Inhibitor Immunotherapy in Endometrial Cancer
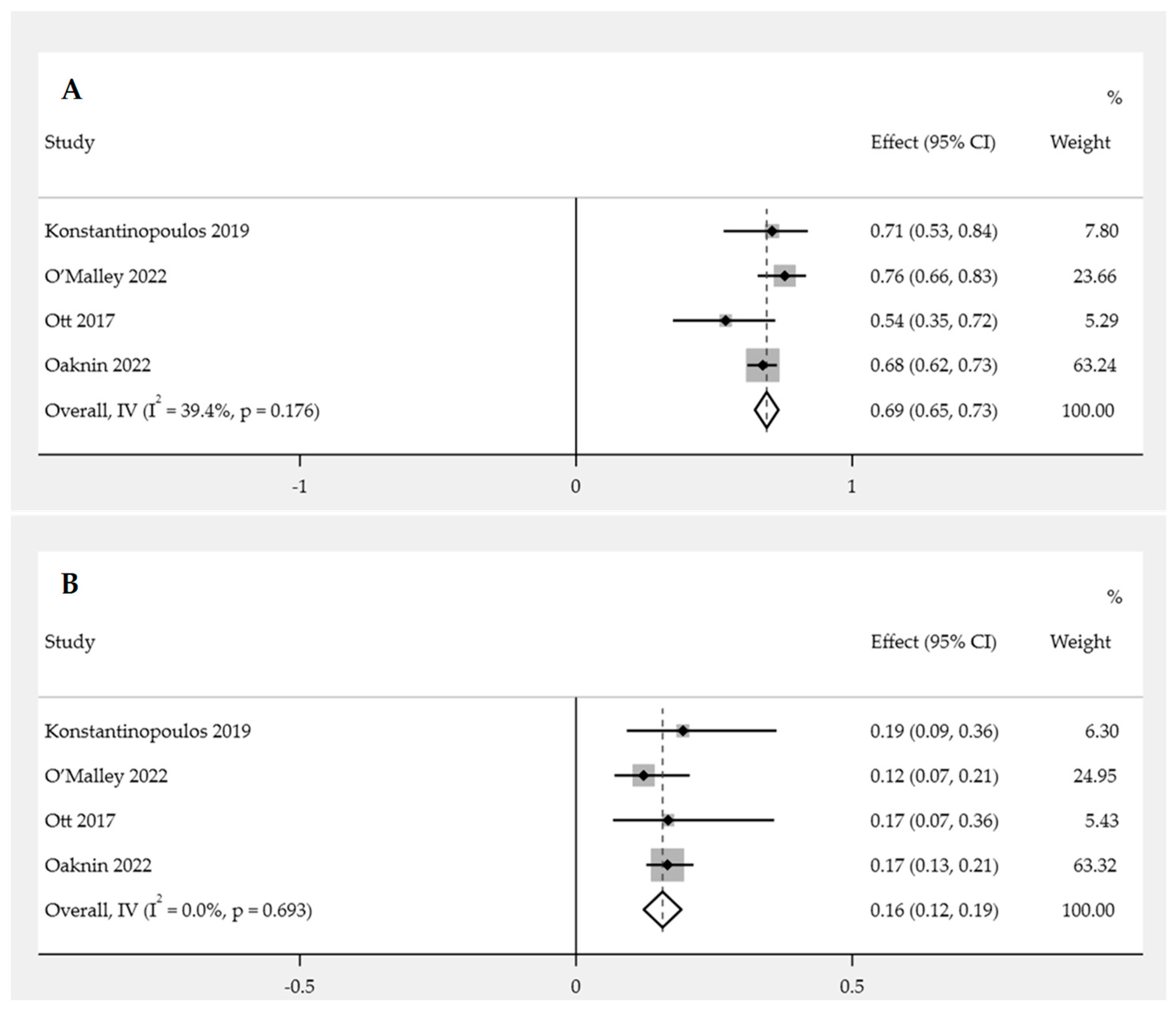
Overall Incidence of Adverse Events (AEs)
Incidence of Grade 3 or Higher AEs
Treatment-Related AEs Observed in Patients
3.3.4. Publication Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uterine Cancer—Cancer Stat Facts. Cancer Stat Facts 2020. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 23 May 2023).
- Mamat @ Yusof, M.N.; Chew, K.T.; Kampan, N.; Nor, N.H.; Md Zin, R.R.; Tan, G.C.; Shafiee, M.N. PD-L1 Expression in Endometrial Cancer and Its Association with Clinicopathological Features: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3911. [Google Scholar] [CrossRef]
- Fujimura, T.; Muto, Y.; Asano, Y. Immunotherapy for Melanoma: The Significance of Immune Checkpoint Inhibitors for the Treatment of Advanced Melanoma. Int. J. Mol. Sci. 2022, 23, 15720. [Google Scholar] [CrossRef]
- Kalampokas, E.; Giannis, G.; Kalampokas, T.; Papathanasiou, A.A.; Mitsopoulou, D.; Tsironi, E.; Triantafyllidou, O.; Gurumurthy, M.; Parkin, D.E.; Cairns, M.; et al. Current Approaches to the Management of Patients with Endometrial Cancer. Cancers 2022, 14, 4500. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Balega, J.; Buckley, L.; Clamp, A.; Crosbie, E.; Drew, Y.; Durrant, L.; Forrest, J.; Fotopoulou, C.; Gajjar, K.; et al. British Gynaecological Cancer Society (BGCS) uterine cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, 50–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Aguilera, J.V.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front. Oncol. 2018, 8, 330851. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Antill, Y.; Kok, P.S.; Robledo, K.; Yip, S.; Cummins, M.; Smith, D.; Spurdle, A.; Barnes, E.; Lee, Y.C.; Friedlander, M.; et al. Clinical activity of durvalumab for patients with advanced mismatch repair-deficient and repair-proficient endometrial cancer. A nonrandomized phase 2 clinical trial. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Luo, W.; Liu, J.F.; Gulhan, D.C.; Krasner, C.; Ishizuka, J.J.; Gockley, A.A.; Buss, M.; Growdon, W.B.; Crowe, H.; et al. Phase II Study of Avelumab in Patients With Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer. J. Clin. Oncol. 2019, 37, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Gilbert, L.; Tinker, A.V.; Brown, J.; Mathews, C.; Press, J.; Sabatier, R.; O’Malley, D.M.; Samouelian, V.; Boni, V.; et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: Interim results from GARNET—A phase I, sin. J. Immunother. Cancer 2022, 10, e003777. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Bariani, G.M.; Cassier, P.A.; Marabelle, A.; Hansen, A.R.; De Jesus Acosta, A.; Miller, W.H.; Safra, T.; Italiano, A.; Mileshkin, L.; et al. Pembrolizumab in Patients With Microsatellite Instability–High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J. Clin. Oncol. 2022, 40, 752–761. [Google Scholar] [CrossRef]
- Ott, P.A.; Bang, Y.-J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Mehnert, J.M.; Aung, K.L.; Lopez, J.; et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1–Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. Obstet. Gynecol. Surv. 2017, 73, 26–27. [Google Scholar] [CrossRef]
- Wang, D.R.; Wu, X.L.; Sun, Y.L. Therapeutic targets and biomarkers of tumor immunotherapy: Response versus non-response. Signal Transduct. Target. Ther. 2022, 7, 1–27. [Google Scholar] [CrossRef]
- Khan, M.; Maker, A.V.; Jain, S. The evolution of cancer immunotherapy. Vaccines 2021, 9, 614. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Kluetz, P.G.; Schneider, J.; Goldberg, K.B.; McKee, A.E.; Pazdur, R. Oncology Drug Approvals: Evaluating Endpoints and Evidence in an Era of Breakthrough Therapies. Oncologist 2017, 22, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Wilcox, K.H.; Gonen, M.; Polotsky, M.; Hirsch, B.R.; Schwartz, L.H. Response rate as a regulatory end point in single-arm studies of advanced solid tumors. JAMA Oncol. 2016, 2, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Guddati, A.K. Clinical endpoints in oncology—A primer. Am. J. Cancer Res. 2021, 11, 1121. [Google Scholar] [PubMed]
- Villaruz, L.C.; Socinski, M.A. The clinical viewpoint: Definitions, limitations of RECIST, practical considerations of measurement. Clin. Cancer Res. 2013, 19, 2629–2636. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.N.; Moon, J.; Redman, M.W.; Semrad, T.J.; Kelly, K.; Allen, J.; Gitlitz, B.; Mack, P.C.; Gandara, D.R. Disease control rate at 8 weeks predicts subsequent survival in platinum-treated extensive stage small cell lung cancer: Results from the Southwest Oncology Group (SWOG) database. Clin. Lung Cancer 2016, 17, 113. [Google Scholar] [CrossRef]
- Antill, Y.; Buchanan, D.D.; Scott, C.L. Mismatch repair and clinical response to immune checkpoint inhibitors in endometrial cancer. Cancer 2022, 128, 1157. [Google Scholar] [CrossRef]
- Yamashita, H.; Nakayama, K.; Ishikawa, M.; Nakamura, K.; Ishibashi, T.; Sanuki, K.; Ono, R.; Sasamori, H.; Minamoto, T.; Iida, K.; et al. Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget 2018, 9, 5652–5664. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Pirš, B.; Škof, E.; Smrkolj, V.; Smrkolj, Š. Overview of Immune Checkpoint Inhibitors in Gynecological Cancer Treatment. Cancers 2022, 14, 631. [Google Scholar] [CrossRef]
- Granier, C.; De Guillebon, E.; Blanc, C.; Roussel, H.; Badoual, C.; Colin, E.; Saldmann, A.; Gey, A.; Oudard, S.; Tartour, E. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2017, 2, E000213. [Google Scholar] [CrossRef] [PubMed]
- Galienne, M.; Rodrigues, M. New drug approval: Dostarlimab—Second line in advanced MSI endometrial cancer. Bull. Cancer 2021, 108, 675–676. [Google Scholar] [CrossRef] [PubMed]
- Redondo, A.; Gallego, A.; Mendiola, M. Dostarlimab for the treatment of advanced endometrial cancer. Expert Rev. Clin. Pharmacol. 2022, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gao, F.; Yang, J.; Fan, H.; Xie, Q.; Jiang, K.; Gong, J.; Gao, B.; Yang, Q.; Lei, Z. Risk of Adverse Events in Cancer Patients Receiving Nivolumab With Ipilimumab: A Meta-Analysis. Front. Oncol. 2022, 12, 2950. [Google Scholar] [CrossRef]
- Cuccu, I.; D’Oria, O.; Sgamba, L.; De Angelis, E.; Golia D’Augè, T.; Turetta, C.; Di Dio, C.; Scudo, M.; Bogani, G.; Di Donato, V.; et al. Role of Genomic and Molecular Biology in the Modulation of the Treatment of Endometrial Cancer: Narrative Review and Perspectives. Healthcare 2023, 11, 571. [Google Scholar] [CrossRef]
- Golia D’Augè, T.; Cuccu, I.; Santangelo, G.; Muzii, L.; Giannini, A.; Bogani, G.; Di Donato, V. Novel Insights into Molecular Mechanisms of Endometrial Diseases. Biomolecules 2023, 13, 499. [Google Scholar] [CrossRef]
- Getz, G.; Gabriel, S.B.; Cibulskis, K.; Lander, E.; Sivachenko, A.; Sougnez, C.; Lawrence, M.; Kandoth, C.; Dooling, D.; Fulton, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Mittica, G.; Ghisoni, E.; Giannone, G.; Aglietta, M.; Genta, S.; Valabrega, G. Checkpoint inhibitors in endometrial cancer: Preclinical rationale and clinical activity. Oncotarget 2017, 8, 90532–90544. [Google Scholar] [CrossRef]
- Loukovaara, M.; Pasanen, A.; Bützow, R. Mismatch repair deficiency as a predictive and prognostic biomarker in molecularly classified endometrial carcinoma. Cancers 2021, 13, 3124. [Google Scholar] [CrossRef]
- Cao, W.; Ma, X.; Fischer, J.V.; Sun, C.; Kong, B.; Zhang, Q. Immunotherapy in endometrial cancer: Rationale, practice and perspectives. Biomark. Res. 2021, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef]
- Rizzo, A. Immune Checkpoint Inhibitors and Mismatch Repair Status in Advanced Endometrial Cancer: Elective Affinities. J. Clin. Med. 2022, 11, 3912. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.N.; Behera, M.; Owonikoko, T.K.; Kamphorst, A.O.; Pakkala, S.; Belani, C.P.; Khuri, F.R.; Ahmed, R.; Ramalingam, S.S. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non–small cell lung cancer: A systematic analysis of the literature. Cancer 2018, 124, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, A.; Gridelli, C. “Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer”: Is there a substantial difference or not? J. Thorac. Dis. 2018, 10, S4065–S4068. [Google Scholar] [CrossRef]


| Authors | Domains | Results | |||
|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Score | Risk | |
| Antill 2021 [13] | **** | ** | * | 7 | Low |
| Konstantinopoulos 2019 [14] | **** | ** | *** | 9 | Low |
| Oaknin 2022 [15] | **** | ** | ** | 8 | Low |
| O’Malley 2022 [16] | **** | * | * | 6 | Low |
| Ott 2017 [17] | **** | * | * | 6 | Low |
| Author/Year | Country | Study Registration no./ Name of Drug/Anti PD-1 or PD-L1 | Study Phase | No. of Sample | MMR Status (n) | Objective Response Rate (ORR) | Disease Control Rate (DCR) | Adverse Event Incidence | Median PFS (Month) | Median OS (Month) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | MMR | Overall | MMR | Overall | Grade 3 or Higher | ||||||||
| Antill 2021 [13] | Australia | ANZGOG1601, ACTRN12617000106336, and NCT03015129/ durvalumab/ anti-PD-L1 | Phase II | 71 | dMMR = 35 | 18/71 | dMMR= 17/36 | 25/71 | dMMR= 18/36 | NA | NA | dMMR = 8.3 | dMMR = not reached |
| pMMR = 36 | pMMR= 1/35 | pMMR= 7/35 | pMMR = 1.8 | pMMR = 12.1 | |||||||||
| Konstantinopoulos 2019 [14] | USA | NCT02912572/avelumab/ anti-PD-L1 | Phase II | 31 | dMMR = 15 | 5/31 | dMMR= 4/15 | 13/31 | dMMR= 8/15 | 22/31 | 6/31 | dMMR = 4.4 | dMMR = not reached |
| pMMR = 16 | pMMR= 1/16 | pMMR= 5/16 | pMMR = 1.9 | pMMR = 6.6 | |||||||||
| Oaknin 2022 [15] | Spain | NCT02715284/ dostarlimab/ anti-PD-1 | Phase I | 264 | dMMR = 106 | 69/264 | dMMR= 46/106 | 114/264 | dMMR= 59/106 | 196/290 | 48/920 | NA | NA |
| pMMR = 142 | pMMR= 19/142 | pMMR= 19/142 | |||||||||||
| O’Malley 2022 [16] | USA | NCT02628067/pembrolizumab/anti-PD-1 | Phase II | 90 | dMMR = 90 | 38/79 | dMMR= 38/79 | NA | NA | 68/90 | 11/90 | Overall = 13.1 | Overall = not reached |
| Ott 2017 [17] | USA | NCT02054806/pembrolizumab/anti-PD-1 | Phase Ib | 24 | NA | 3/23 | NA | NA | NA | 13/24 | 4/24 | Overall = 1.8 | Overall = not reached |
| Adverse Events | Konstantinopoulos, 2019 [14] | Oaknin, 2022 [15] | O’Malley, 2022 [16] | Ott, 2018 [17] | Overall AEs, n = 435 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any Grade (n = 31) | Grade 3 or Higher (n = 31) | Any Grade (n = 290) | Grade 3 or Higher (n = 290) | Any Grade (n = 90) | Grade 3 or Higher (n = 90) | Any Grade (n = 24) | Grade 3 or Higher (n = 24) | Any Grade (%) | Grade 3 or Higher (%) | |
| Anemia | 3 | 2 | 27 | 5 | 22 | - | - | 1 | 11.95 | 1.84 |
| Diarrhea | 3 | 3 | 40 | 4 | 14 | - | - | 1 | 13.10 | 1.84 |
| Fatigue | 11 | - | 51 | 4 | 19 | - | 5 | - | 19.77 | 0.92 |
| Hypothyroidism | 4 | 1 | 25 | - | 12 | - | - | - | 9.43 | 0.23 |
| Nausea | 5 | - | 40 | 2 | 13 | - | - | - | 13.33 | 0.46 |
| Arthralgia | - | - | 21 | - | 13 | - | - | - | 7.82 | - |
| Decreased appetite | - | - | 18 | - | 8 | - | 3 | - | 6.67 | - |
| Asthenia | - | - | 31 | - | - | - | - | 1 | 7.13 | 0.23 |
| Rash | - | - | 21 | - | 10 | 1 | - | - | 7.13 | 0.23 |
| Vomiting | - | - | 22 | - | 5 | - | - | - | 6.21 | - |
| Increased AST | - | - | 19 | 3 | - | - | - | - | 4.37 | 0.69 |
| Hypergycemia | - | - | - | 3 | - | - | - | 1 | - | 0.92 |
| Sinus bradycardia | 1 | 1 | - | - | - | - | - | - | 0.23 | 0.23 |
| Decreased neutrophil count | 4 | - | - | - | - | - | - | - | 0.92 | - |
| Myositis | 1 | 1 | - | - | - | - | - | - | 0.23 | 0.23 |
| Rash acneiform | 2 | 1 | - | - | - | - | - | - | 0.46 | 0.23 |
| Pruritus | - | - | 18 | - | - | - | 4 | - | 5.06 | - |
| Increased ALT | - | - | 18 | - | - | - | - | - | 4.14 | - |
| Increased amylase | - | - | 16 | 4 | - | - | - | - | 3.68 | 0.92 |
| Increased lipase | - | - | - | 4 | - | - | - | - | - | 0.92 |
| Colitis | - | - | - | 2 | - | - | - | - | - | 0.46 |
| Constipation | - | - | - | 2 | - | - | - | - | - | 0.46 |
| Hypertension | - | - | - | 2 | - | - | - | - | - | 0.46 |
| Pulmonary embolism | - | - | - | 2 | - | - | - | - | - | 0.46 |
| Dry mouth | - | - | - | - | 6 | - | - | - | 1.38 | - |
| Hyperthyroidism | - | - | - | - | 6 | - | - | - | 1.38 | - |
| Myalgia | - | - | - | - | 6 | - | - | - | 1.38 | - |
| Maculopapular rash | - | - | - | - | 5 | - | - | - | 1.15 | - |
| Increased aspartate aminotransferase | - | - | - | - | 5 | - | - | - | 1.15 | - |
| Pyrexia | - | - | - | - | - | - | 3 | 1 | 0.69 | 0.23 |
| Back pain | - | - | - | - | - | - | - | 1 | - | 0.23 |
| Hyponatremia | - | - | - | - | - | - | - | 1 | - | 0.23 |
| Chills | - | - | - | - | - | - | - | 1 | - | 0.23 |
| Analysis | Overall ES | Leave-One-Out Result | |||
|---|---|---|---|---|---|
| Lowest Study Range | p-Value | Highest Study Range | p-Value | ||
| Efficacy | |||||
| Overall ORR | 0.26 | 0.14–0.34 | 0.000 | 0.13–0.47 | 0.001 |
| ORR with dMMR | 0.44 | 0.39–0.52 | 0.000 | 0.33–0.54 | 0.000 |
| ORR with pMMR | 0.08 | 0.05–0.14 | 0.000 | −0.03–0.10 | 0.262 |
| Association of ORR (dMMR vs. pMMR) | 6.30 | 2.85–7.08 | 0.000 | −20.27–33.62 | 0.627 |
| Overall DCR | 0.41 | 0.36–0.47 | 0.000 | 0.28–0.46 | 0.000 |
| DCR with dMMR | 0.54 | 0.38–0.71 | 0.000 | 0.24–0.78 | 0.000 |
| DCR with pMMR | 0.31 | 0.17–0.47 | 0.000 | −0.04–0.51 | 0.089 |
| Association of DCR (dMMR vs. pMMR) | 2.57 | 1.11–3.53 | 0.000 | −0.34–6.88 | 0.076 |
| Safety | |||||
| Overall incidence of AEs | 0.69 | 0.64–0.73 | 0.000 | 0.64–0.79 | 0.000 |
| AEs incidence in grade 3 or higher | 0.16 | 0.12–0.19 | 0.000 | 0.08–0.20 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamat @ Yusof, M.N.; Chew, K.T.; Hafizz, A.M.H.A.; Abd Azman, S.H.; Ab Razak, W.S.; Hamizan, M.R.; Kampan, N.C.; Shafiee, M.N. Efficacy and Safety of PD-1/PD-L1 Inhibitor as Single-Agent Immunotherapy in Endometrial Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 4032. https://doi.org/10.3390/cancers15164032
Mamat @ Yusof MN, Chew KT, Hafizz AMHA, Abd Azman SH, Ab Razak WS, Hamizan MR, Kampan NC, Shafiee MN. Efficacy and Safety of PD-1/PD-L1 Inhibitor as Single-Agent Immunotherapy in Endometrial Cancer: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(16):4032. https://doi.org/10.3390/cancers15164032
Chicago/Turabian StyleMamat @ Yusof, Mohd Nazzary, Kah Teik Chew, Abdul Muzhill Hannaan Abdul Hafizz, Siti Hajar Abd Azman, Wira Sofran Ab Razak, Muhammad Rafi’uddin Hamizan, Nirmala Chandralega Kampan, and Mohamad Nasir Shafiee. 2023. "Efficacy and Safety of PD-1/PD-L1 Inhibitor as Single-Agent Immunotherapy in Endometrial Cancer: A Systematic Review and Meta-Analysis" Cancers 15, no. 16: 4032. https://doi.org/10.3390/cancers15164032
APA StyleMamat @ Yusof, M. N., Chew, K. T., Hafizz, A. M. H. A., Abd Azman, S. H., Ab Razak, W. S., Hamizan, M. R., Kampan, N. C., & Shafiee, M. N. (2023). Efficacy and Safety of PD-1/PD-L1 Inhibitor as Single-Agent Immunotherapy in Endometrial Cancer: A Systematic Review and Meta-Analysis. Cancers, 15(16), 4032. https://doi.org/10.3390/cancers15164032









