Chemoprevention of Colon Cancer by DFMO, Sulindac, and NO-Sulindac Administered Individually or in Combinations in F344 Rats
Abstract
:Simple Summary
Abstract
1. Introduction
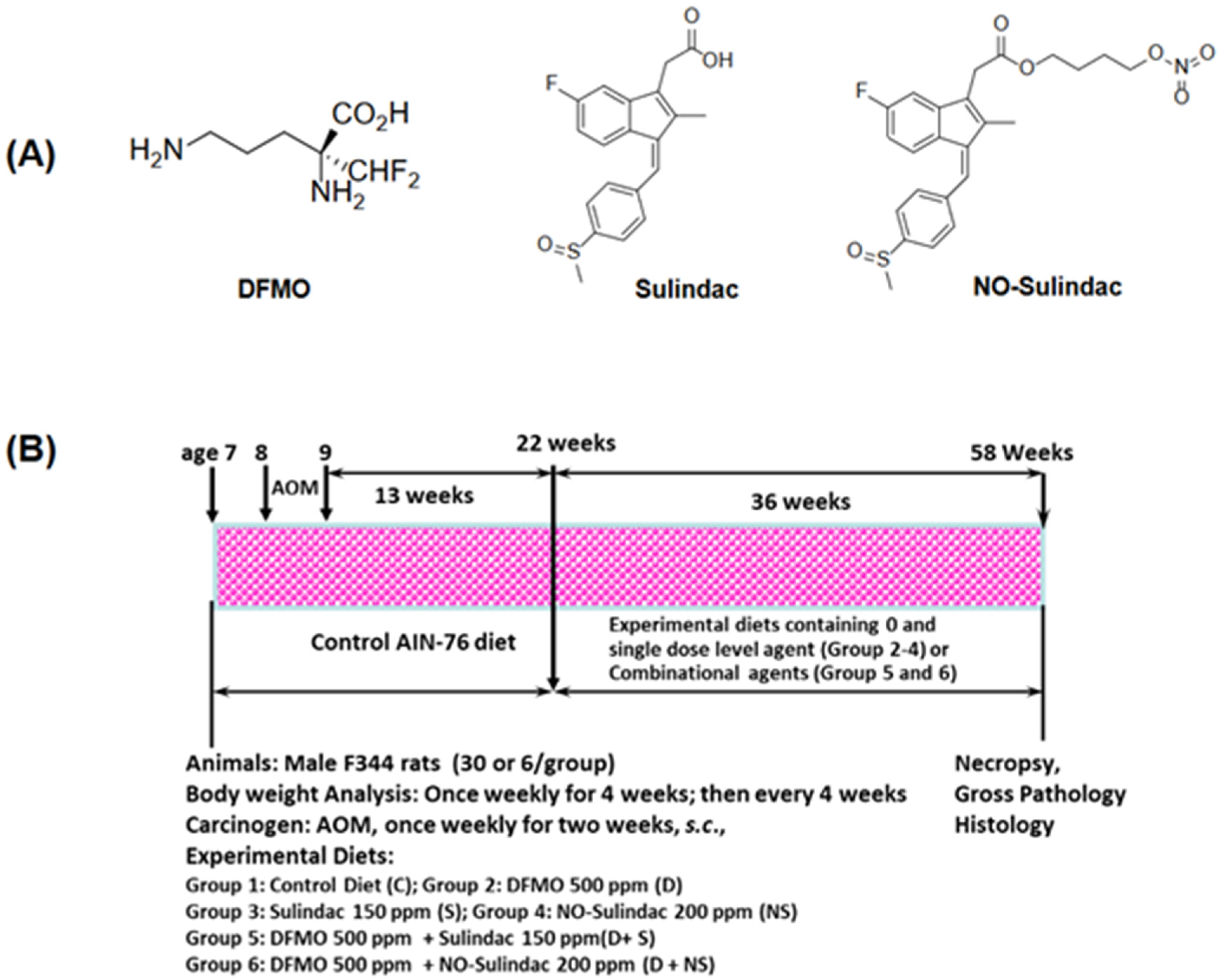
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.A. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon cancer chemoprevention. Lancet Oncol. 2002, 3, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Rivenson, A.; Simi, B.; Zang, E.; Kelloff, G.; Steele, V.; Reddy, B.S. Chemoprevention of colon carcinogenesis by sulindac a nonsteroidal anti-inflammatory agent. Cancer Res. 1995, 55, 1464–1472. [Google Scholar] [PubMed]
- Boolbol, S.K.; Dannenberg, A.J.; Chadburn, A.; Martucci, C.; Guo, X.J.; Ramonetti, J.T.; Abreu-Goris, M.; Newmark, H.L.; Lipkin, M.L.; Decosse, J.J.; et al. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996, 56, 2556–2560. [Google Scholar]
- Giardiello, F.M.; Yang, V.W.; Hylind, L.M.; Krush, A.J.; Petersen, G.M.; Trimbath, J.D.; Piantadosi, S.; Garrett, E.; Geiman, D.E.; Hubbard, W.; et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N. Engl. J. Med. 2002, 346, 1054–1059. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Triadafilopoulos, G. Epidemiology of NSAID-induced gastrointestinal complications. J. Rheumatol. 1999, 56 (Suppl. 26), 18–24. [Google Scholar]
- Lazzaroni, M.; Porro, G.B. Management of NSAID-induced gastrointestinal toxicity: Focus on proton pump inhibitors. Drugs 2009, 69, 51–69. [Google Scholar] [CrossRef]
- Williams, J.L.; Borgo, S.; Hasan, I.; Castillo, E.; Traganos, F.; Rigas, B. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NSAIDS) alter the kinetics of human colon cancer cell lines more effectively than traditional NSAIDS: Implications for colon cancer chemoprevention. Cancer Res. 2001, 61, 3285–3289. [Google Scholar] [PubMed]
- Yeh, R.K.; Chen, J.; Williams, J.L.; Baluch, M.; Hundley, T.R.; Rosenbaum, R.E.; Kalala, S.; Traganos, F.; Benardini, F.; del Soldato, P.; et al. NO-donating nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit colon cancer cell growth more potently than traditional NSAIDs: A general pharmacological property? Biochem. Pharmacol. 2004, 67, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Steele, V.E.; Rao, C.V.; Zhang, Y.; Patlolla, J.; Boring, D.; Kopelovich, L.; Juliana, M.M.; Grubbs, C.J.; Lubet, R.A. Chemopreventive efficacy of naproxen and nitric oxide-naproxen in rodent models of colon, urinary bladder, and mammary cancers. Cancer Prev. Res 2009, 11, 951–956. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, L.; Xie, Z.; Zhou, S.; Li, Y.; Zhou, Y.; Sun, M. Nitric Oxide (NO) and NO Synthases (NOS)-Based Targeted Therapy for Colon Cancer. Cancers 2020, 12, 1881. [Google Scholar] [CrossRef]
- Mohammed, A.; Yarla, N.S.; Madka, V.; Rao, C.V. Clinically Relevant Anti-Inflammatory Agents for Chemoprevention of Colorectal Cancer: New Perspectives. Int. J. Mol. Sci. 2018, 19, 2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, C.V.; Reddy, B.S.; Steele, V.E.; Wang, C.X.; Liu, X.; Ouyang, N.; Patlolla, J.M.; Simi, B.; Kopelovich, L.; Rigas, B. Nitric oxide-releasing aspirin and indomethacin are potent inhibitors against colon cancer in azoxymethane-treated rats: Effects on molecular targets. Mol. Cancer Ther. 2006, 5, 1530–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozharisski, K.M. Tumors of intestine. In Pathology of Tumors in Laboratory Animals; Turcusov, M.V., Mohr, V., Eds.; IARC. Publications: Lyon, France, 1990; Volume 1, p. 159. [Google Scholar]
- Sjoblom, T.; Jones, S.; Wood, L.D.; Parsons, D.W.; Lin, J.; Barber, T.D.; Mandelker, D.; Leary, R.J.; Ptak, J.; Silliman, N.; et al. The consensus coding sequences of human breast and colorectal cancers. Science 2006, 314, 268–274. [Google Scholar] [CrossRef]
- Takahashi, M.; Wakabayashi, K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004, 95, 475–480. [Google Scholar] [CrossRef]
- Gerner, E.W.; Meyskens, F.L., Jr.; Goldschmid, S.; Lance, P.; Pelot, D. Polyamines and cancer: Old molecules, new understanding. Nat. Rev. Cancer 2004, 4, 781–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, C.V.; Simi, B.; Reddy, B.S. Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis 1993, 14, 2219–2225. [Google Scholar] [CrossRef]
- Hexson, L.J.; Garewal, H.S.; McGee, D.L.; Sloan, D.; Fennerty, M.B.; Sampliner, R.E.; Gerner, E.W. Ornithine decarboxylase and polyamines in colorectal neoplasia and mucosa. Cancer Epidemiol. Biomark. Prev. 1993, 2, 369–374. [Google Scholar]
- Sporn, M.B. Combination chemoprevention of cancer. Nature 1980, 287, 107–108. [Google Scholar] [CrossRef]
- Ignatenko, N.A.; Besselsen, D.G.; Stringer, D.E.; Blohm-Mangone, K.A.; Cui, H.; Gerner, E.W. Combination chemoprevention of intestinal carcinogenesis in a murine model of familial adenomatous polyposis. Nutr. Cancer 2008, 60 (Suppl. S1), 30–35. [Google Scholar] [CrossRef]
- Rial, N.S.; Meyskens, F.L.; Gerner, E.W. Polyamines as mediators of APC-dependent intestinal carcinogenesis and cancer chemoprevention. Essays Biochem. 2009, 46, 111–124. [Google Scholar]
- Meyskens, F.L., Jr.; McLaren, C.E.; Pelot, D.; Fujikawa-Brooks, S.; Carpenter, P.M.; Hawk, E.; Kelloff, G.; Lawson, M.J.; Kidao, J.; McCracken, J.; et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo-controlled, double-blind trial. Cancer Prev. Res. 2008, 1, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, C.V.; Tokumo, K.; Rigotty, J.; Zang, E.; Kelloff, G.; Reddy, B.S. Chemoprevention of colon carcinogenesis by dietary administration of piroxicam, alpha-difluoromethylornithine, 16 alpha-fluoro-5-androsten-17-one, and ellagic acid individually and in combination. Cancer Res. 1991, 51, 4528–4534. [Google Scholar] [PubMed]
- Madka, V.; Kumar, G.; Pathuri, G.; Panneerselvam, J.; Zhang, Y.; Ganta, V.; Lightfoot, S.; Lubet, R.; Suen, C.S.; Steele, V.E.; et al. Proton Pump Inhibitor Omeprazole Suppresses Carcinogen-induced Colonic Adenoma Progression to Adenocarcinoma in F344 Rat. Cancer Prev. Res. 2021, 14, 1009–1020. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Crawford, S.E.; Radosevich, A.; Wali, R.K.; Stypula, Y.; Kunte, D.P.; Mutyal, N.; Ruderman, S.; Gomes, A.; Cornwell, M.L.; et al. Neo-angiogenesis and the premalignant micro-circulatory augmentation of early colon carcinogenesis. Cancer Lett. 2011, 306, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, C.V.; Wang, C.Q.; Simi, B.; Rodriguez, J.G.; Cooma, I.; El-Bayoumy, K.; Reddy, B.S. Chemoprevention of colon cancer by a glutathione conjugate of 1,4-phenylenebis(methylene) selenocyanate, a novel organoselenium compound with low toxicity. Cancer Res. 2001, 61, 3452–3647. [Google Scholar] [PubMed]
- DuBois, R.N.; Radhika, A.; Reddy, B.S.; Entingh, A.J. Increased cyclooxygenase-2 levels in carcinogen-induced rat colonic tumors. Gastroenterology 1996, 110, 1259–1262. [Google Scholar] [CrossRef]
- Shah, V.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004, 126, 903–913. [Google Scholar] [CrossRef]
- Wallace, J.L.; Del Soldato, P. The therapeutic potential of NO-NSAIDs. Fundam. Clin. Pharmacol. 2003, 17, 11–20. [Google Scholar] [CrossRef]
- Iconomou, G.; Kalofonos, H.P.; AK, K.; Vagenakis, A.G.; Rigas, B. A pilot study of NO-donating aspirin in patients with pancreatic cancer pain. J. Support Oncol. 2006, 4, 168. [Google Scholar] [PubMed]
- Pegg, A.E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988, 48, 759–774. [Google Scholar] [PubMed]
- Scorcioni, F.; Corti, A.; Davalli, P.; Astancolle, S.; Bettuzzi, S. Manipulation of the expression of regulatory genes of polyamine metabolism results in specific alterations of the cell-cycle progression. Biochem. J. 2001, 354, 217–223. [Google Scholar] [CrossRef]
- Li, L.; Rao, J.N.; Bass, B.L.; Wang, J.Y. NF-kappaB activation and susceptibility to apoptosis after polyamine depletion in intestinal epithelial cells. Am. J. Physiol. 2001, 280, G992–G1004. [Google Scholar]
- Vujcic, S.; Halmekyto, M.; Diegelman, P.; Gan, G.; Kramer, D.L.; Janne, J.; Porter, C.W. Effects of conditional overexpression of spermidine/spermine N1-acetyltransferase on polyamine pool dynamics, cell growth, and sensitivity to polyamine analogs. J. Biol. Chem. 2000, 275, 38319–38328. [Google Scholar] [CrossRef] [Green Version]
- Babbar, N.; Ignatenko, N.A.; Casero, R.A., Jr.; Gerner, E.W. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J. Biol. Chem. 2003, 278, 47762–47775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, C.V. Nitric oxide signaling in colon cancer chemoprevention. Mutat. Res. 2004, 555, 107–119. [Google Scholar] [CrossRef]
- Marnett, L.J.; DuBois, R.N. COX-2: A target for colon cancer prevention. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 55–80. [Google Scholar] [CrossRef]
- Reddy, B.S.; Rao, C.V. Novel approaches for colon cancer prevention by cyclooxygenase-2 inhibitors. J. Environ. Pathol. Toxicol. Oncol. 2002, 21, 155–164. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Landino, L.M.; Crews, B.C.; Timmons, M.D.; Morrow, J.D.; Marnett, L.J. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc. Natl. Acad. Sci. USA 1996, 93, 15069–15074. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.A.; Wertheim, B.C.; Zell, J.A.; Chen, W.P.; McLaren, C.E.; LaFleur, B.J.; Meyskens, F.L.; Gerner, E.W. Levels of rectal mucosal polyamines and prostaglandin E2 predict ability of DFMO and sulindac to prevent colorectal adenoma. Gasteroenterology 2010, 139, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, M.T.; Gerner, E.W. Polyamine-mediated post-transcriptional regulation of COX-2. Biochimie 2002, 84, 815–819. [Google Scholar] [CrossRef]
- Hao, X.P.; Pretlow, T.G.; Rao, J.S.; Pretlow, T.P. Beta-catenin expression is altered in human colonic aberrant crypt foci. Cancer Res. 2001, 61, 8085–8088. [Google Scholar]
- Moon, R.T.; Bowerman, B.; Boutros, M.; Perrimon, N. The promise and perils of Wnt signaling through beta-catenin. Science 2002, 296, 1644–1646. [Google Scholar] [CrossRef]
- Iwamoto, M.; Ahnen, D.J.; Franklin, W.A. Expression of β-catenin and full-length APC protein in normal and neoplastic colonic tissues. Carcinogenesis 2000, 21, 1935–1940. [Google Scholar] [CrossRef] [Green Version]
- Rice, P.L.; Kelloff, J.; Sullivan, H.; Driggers, L.J.; Beard, K.S.; Kuwada, S.; Piazza, G.; Ahnen, D.J. Sulindac metabolites induce caspase- and proteasome-dependent degradation of beta-catenin protein in human colon cancer cells. Mol. Cancer Ther. 2003, 2, 885–892. [Google Scholar] [PubMed]
- Koornstra, J.J.; Rijcken, F.E.; Oldenhuis, C.N.; Zwart, N.; van der Sluis, T.; Hollema, H.; deVries, E.G.; Keller, J.J.; Offerhaus, J.A.; Giardiello, F.M.; et al. Sulindac inhibits beta-catenin expression in normal-appearing colon of hereditary nonpolyposis colorectal cancer and familial adenomatous polyposis patients. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1608–1612. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Guo, X.; Rao, J.N.; Zou, T.; Marasa, B.S.; Chen, J.; Greenspon, J.; Casero, R.A., Jr.; Wang, J.Y. Polyamine-modulated c-Myc expression in normal intestinal epithelial cells regulates p21Cip1 transcription through a proximal promoter region. Biochem. J. 2006, 398, 257–267. [Google Scholar] [CrossRef] [Green Version]





| Experimental Group | Colon Tumor Incidence # (Rats with Tumors/Total Rats) (% Rats with Colonic Tumors) | Colon Tumor Multiplicity $ (Mean ± SEM, N = 30) (Mean Colonic Tumors/rat) | |||||
|---|---|---|---|---|---|---|---|
| Adenoma | Adenocarcinoma | Total | Adenoma | Adenocarcinoma | Total Tumors | ||
| 1 | AOM/Control diet | 14/30 (46.7%) | 28/30 (93.3%) | 29/30 (96.7%) | 0.70 ± 0.18 | 2.80 ± 0.34 | 3.50 ± 0.52 |
| 2 | 500 ppm DFMO | 12/30 (40%) | 16/30 (53.3%) (p < 0.0009) | 24/30 (80%) | 0.70 ± 0.14 | 1.03 ± 0.22 (p < 0.0001) | 1.73 ± 0.31 (p < 0.0026) |
| 3 | 150 ppm Sulindac | 18/30 (60%) | 18/30 (60%) (p < 0.0048) | 27/30 (90%) | 0.90 ± 0.21 | 1.37 ± 0.29 (p < 0.0011) | 2.27 ± 0.40 (p < 0.033) |
| 4 | 200 ppm NO-Sulindac | 19/30 (63.3%) | 24/30 (80%) (p = 0.25) | 27/30 (90%) | 1.00 ± 0.22 | 2.16 ± 0.35 (p = 0.097) | 3.16 ± 0.57 (p = 0.3) |
| 5 | 500 ppm DFMO + 150 ppm Sulindac | 20/30 (66.6%) | 12/30 (40%) (p < 0.0001) | 17/30 (56.6%) (p < 0.0004) | 0.97 ± 0.22 | 0.53 ± 0.14 (p < 0.0001) | 1.50 ± 0.24 (p < 0.0006) |
| 6 | 500 ppm DFMO + 200 ppm NO-Sulindac | 12/30 (40%) | 15/30 (50%) (p < 0.0004) | 21/30 (70%) (p < 0.001) | 0.60 ± 0.16 | 1.06 ± 0.23 (p < 0.0001) | 1.66 ± 0.30 (p < 0.0018) |
| iNOS-Activity a | COX-2 Activity b | ODC Activity c | Polyamines Levels d (nmol/g Wet Tumor Tissue) | |||
|---|---|---|---|---|---|---|
| Putrescine | Spermidine | Spermine | ||||
| 1. Control diet | 88.9 ± 7.4 e | 271 ± 15.3 | 155 ± 12.3 | 66.9 ± 5.8 | 183 ± 14 | 123 ± 11 |
| 2. 500 ppm DFMO | 56.3 ± 5.8 f p < 0.005 | 196 ± 13.7 p = 0.051 | 82 ± 7.9 p < 0.001 | 36.3 ± 3.3 p < 0.001 | 96.3 ± 10 p < 0.001 | 67.3 ± 8.3 p < 0.005 |
| 3. 150 ppm Sulindac | 50.6 ± 4.7 p < 0.001 | 148 ± 9.9 p < 0.002 | 128 ± 8.9 p = 0.2 | 53.7 ± 4.1 p = 0.09 | 148 ± 8.7 p = 0.06 | 128 ± 14 p = 0.9 |
| 4. 200 ppm NO-Sulindac | 73.7 ± 5.3 p = 0.2 | 205 ± 15.5 p = 0.07 | 145 ± 13.2 p = 0.5 | ND | ND | ND |
| 5. 500 ppm DFMO + 150 ppm Sulindac | 42.8 ± 3.5 p < 0.0001 | 122 ± 8.8 p < 0.0001 | 68 ± 4.9 p < 0.0001 | 28.9 ± 3.1 p < 0.0001 | 63.5 ± 7.2 p < 0.0001 | 49.3 ± 5.1 p < 0.0001 |
| 6. 500 ppm DFMO + 200 ppm NO-Sulindac | 53.5 ± 4.8 p < 0.001 | 189 ± 13.1 p < 0.05 | 78 ± 9.2 p < 0.001 | 34.5 ± 3.8 p < 0.0005 | 88.5 ± 8.8 p < 0.0008 | 64.4 ± 6.8 p < 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madka, V.; Patlolla, J.M.R.; Venkatachalam, K.; Zhang, Y.; Pathuri, G.; Stratton, N.; Lightfoot, S.; Janakiram, N.B.; Mohammed, A.; Rao, C.V. Chemoprevention of Colon Cancer by DFMO, Sulindac, and NO-Sulindac Administered Individually or in Combinations in F344 Rats. Cancers 2023, 15, 4001. https://doi.org/10.3390/cancers15154001
Madka V, Patlolla JMR, Venkatachalam K, Zhang Y, Pathuri G, Stratton N, Lightfoot S, Janakiram NB, Mohammed A, Rao CV. Chemoprevention of Colon Cancer by DFMO, Sulindac, and NO-Sulindac Administered Individually or in Combinations in F344 Rats. Cancers. 2023; 15(15):4001. https://doi.org/10.3390/cancers15154001
Chicago/Turabian StyleMadka, Venkateshwar, Jagan M. R. Patlolla, Karthikkumar Venkatachalam, Yuting Zhang, Gopal Pathuri, Nicole Stratton, Stanley Lightfoot, Naveena B. Janakiram, Altaf Mohammed, and Chinthalapally V. Rao. 2023. "Chemoprevention of Colon Cancer by DFMO, Sulindac, and NO-Sulindac Administered Individually or in Combinations in F344 Rats" Cancers 15, no. 15: 4001. https://doi.org/10.3390/cancers15154001
APA StyleMadka, V., Patlolla, J. M. R., Venkatachalam, K., Zhang, Y., Pathuri, G., Stratton, N., Lightfoot, S., Janakiram, N. B., Mohammed, A., & Rao, C. V. (2023). Chemoprevention of Colon Cancer by DFMO, Sulindac, and NO-Sulindac Administered Individually or in Combinations in F344 Rats. Cancers, 15(15), 4001. https://doi.org/10.3390/cancers15154001








