MicroRNA-371a-3p—The Novel Serum Biomarker in Testicular Germ Cell Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Body
2.1. Rationale for Investigating microRNAs and First Clinical Studies
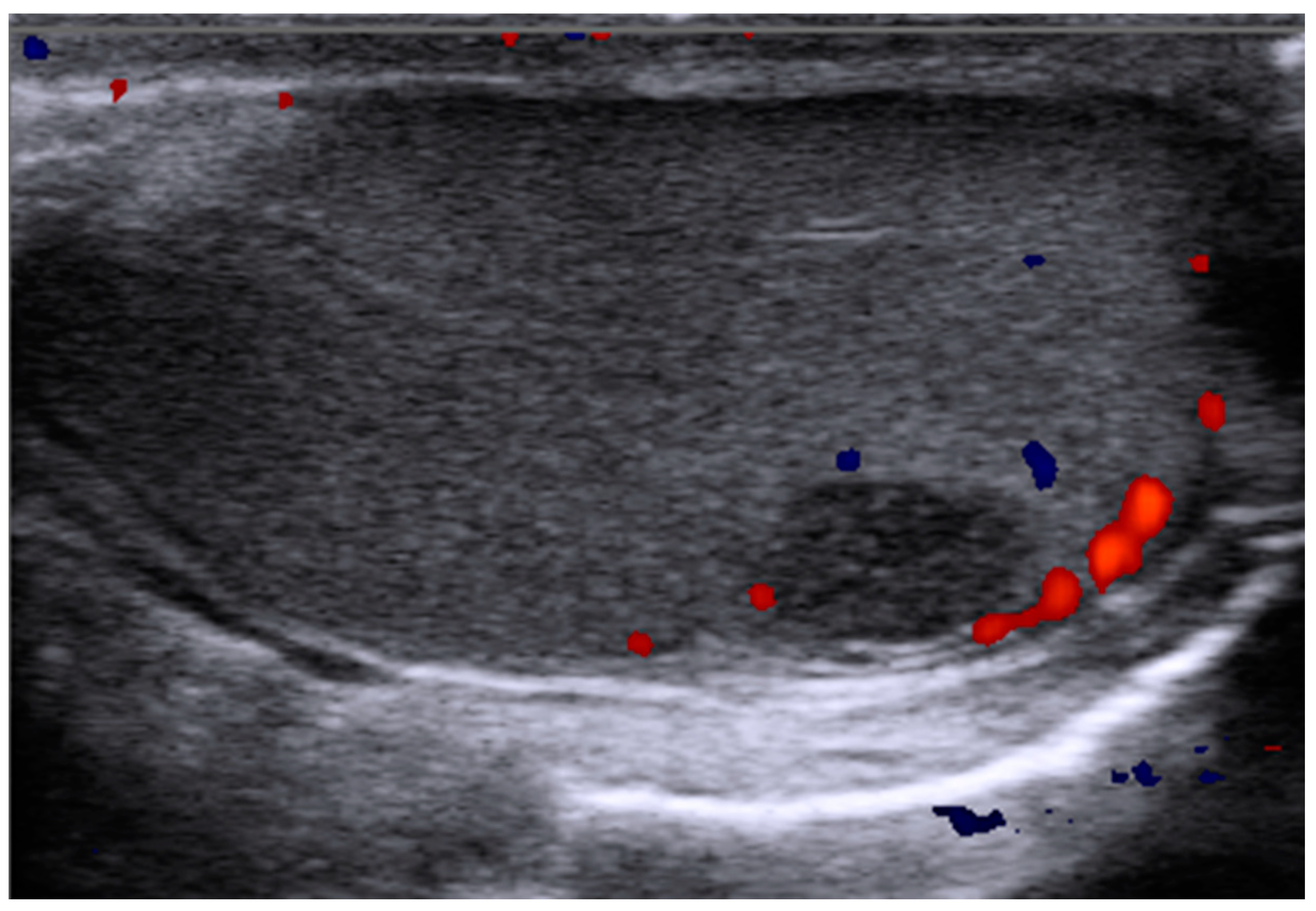
2.2. Non-Specific Small Testicular Masses
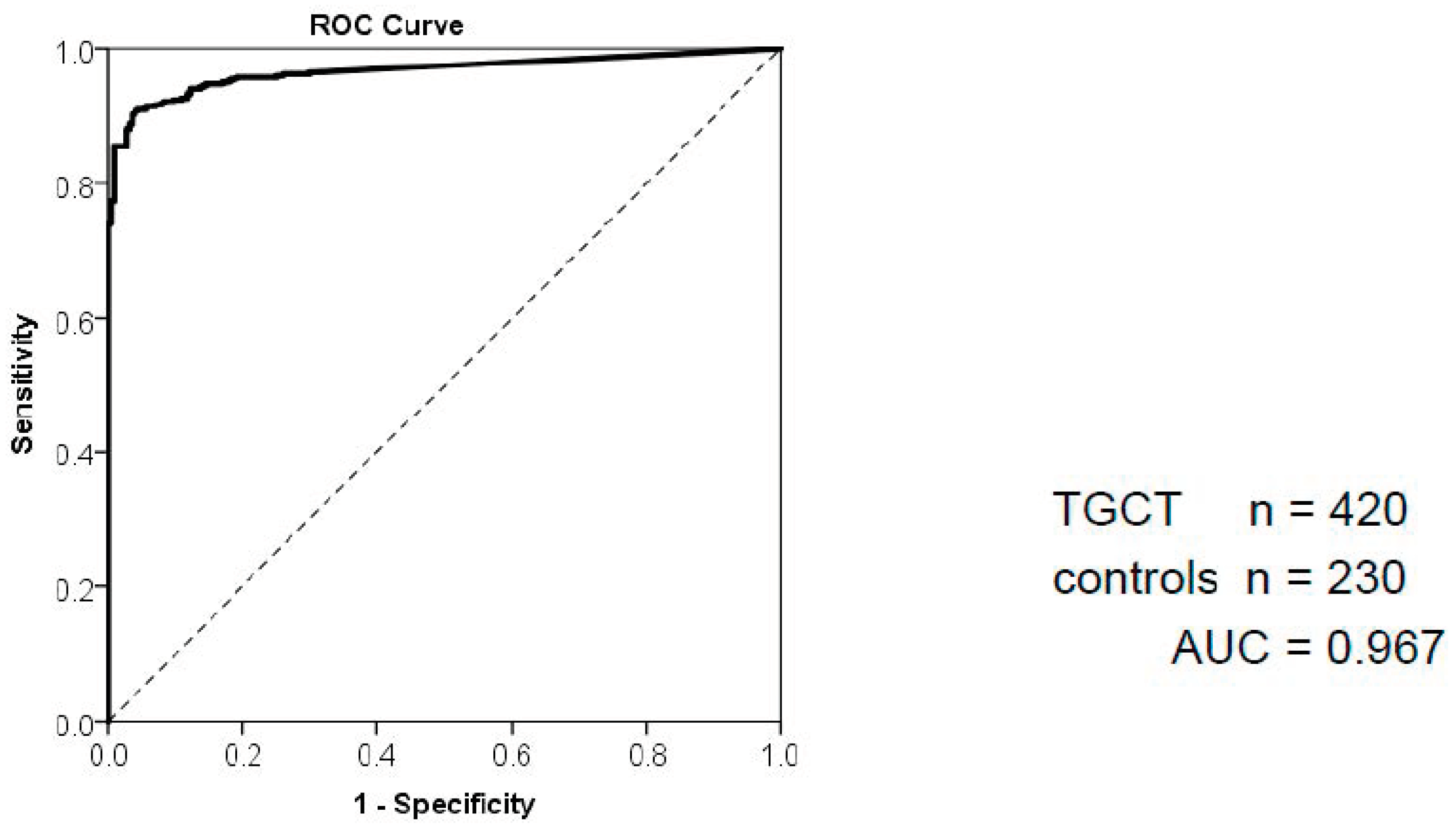
2.3. Use of miR371 in Primary Diagnosis of TGCT
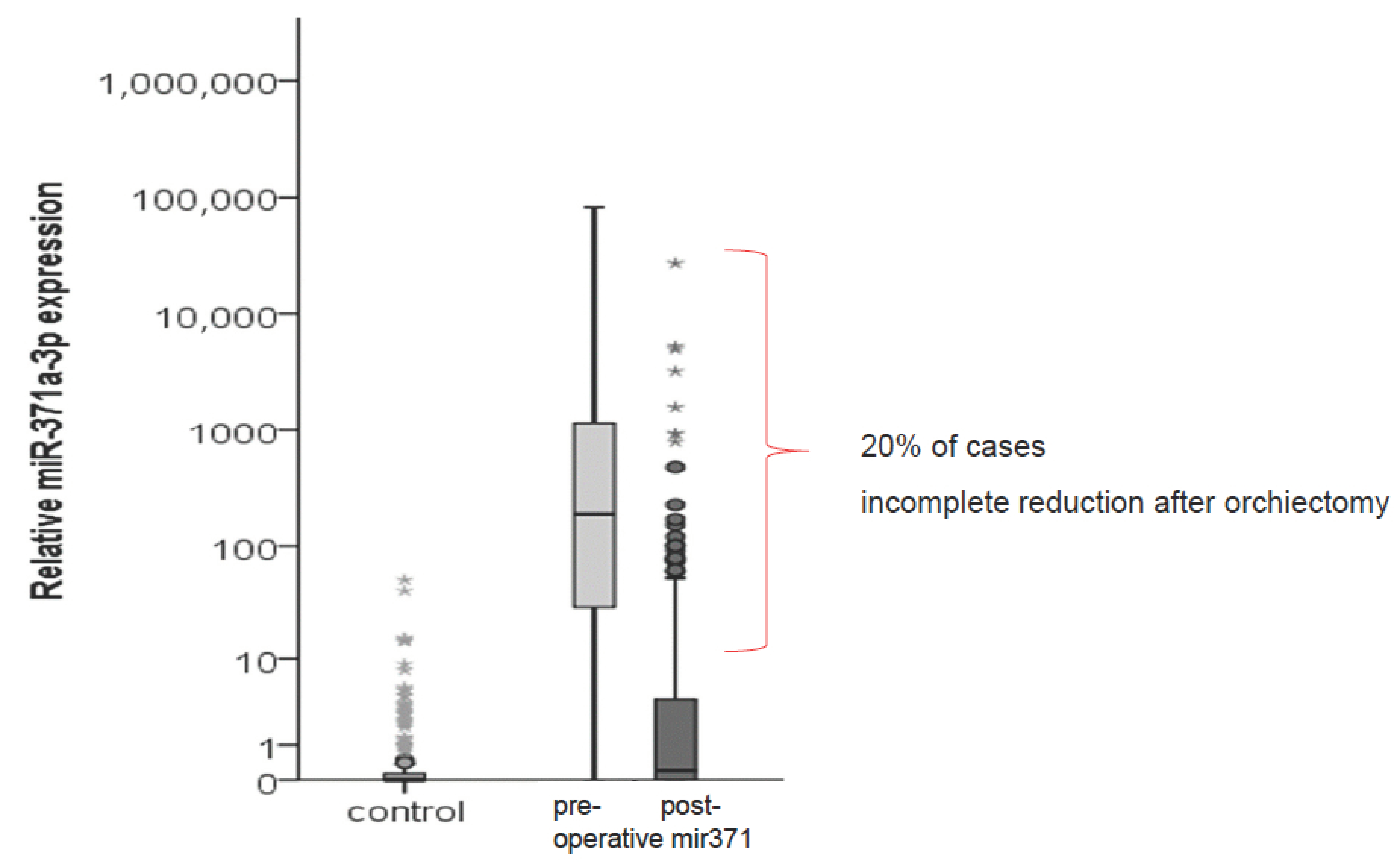
2.4. Use of miR371 in Management and Follow-Up of Early Stage TGCT
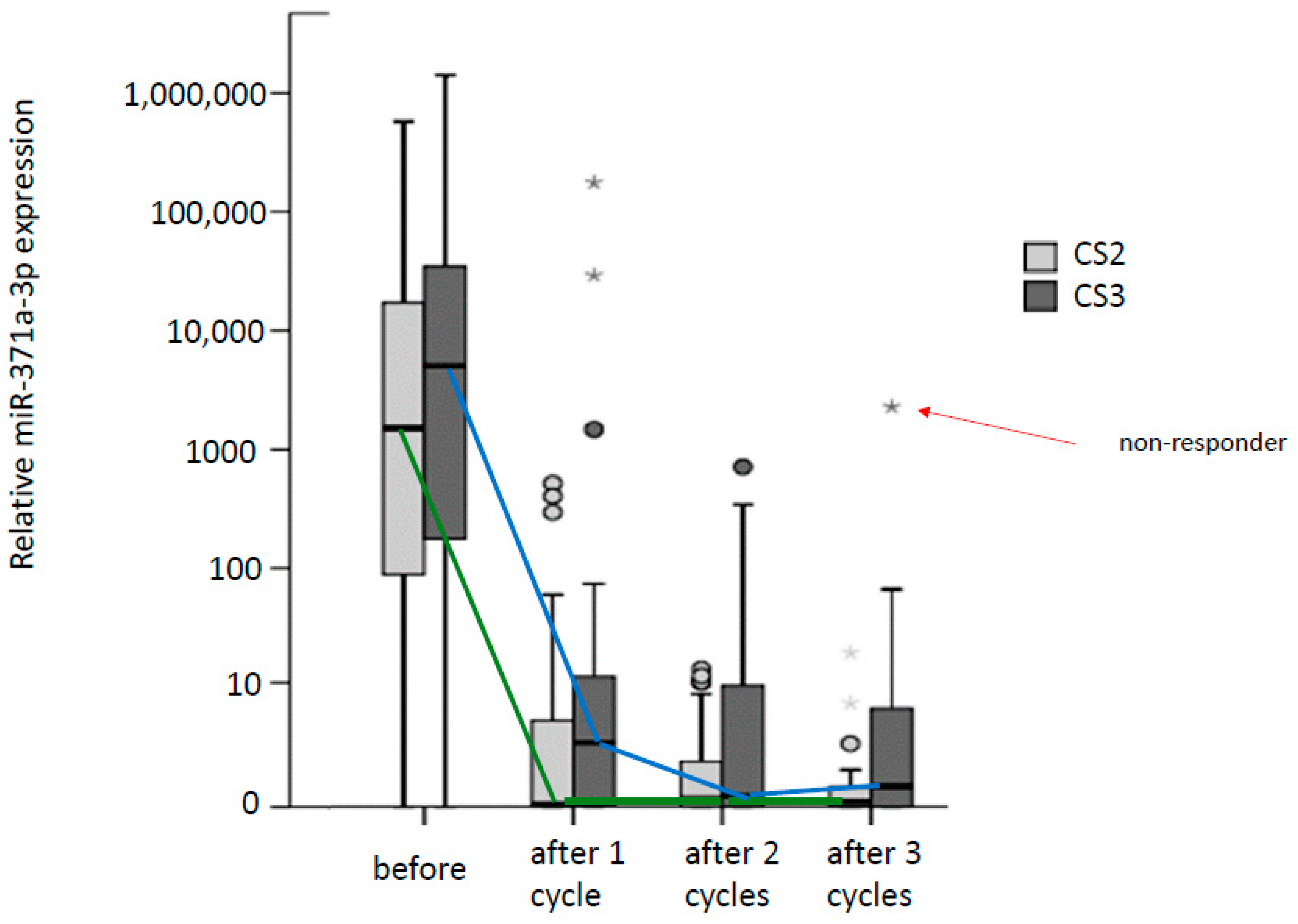
2.5. Monitoring Chemotherapy with miR371
2.6. Use of miR371 for Assessing Residual Masses after Chemotherapy
2.7. The Role of miR371 in Current Guideline Recommendations
2.8. Ongoing Studies
2.9. Certified Test Kit—Practical Handling
3. Conclusions
4. Expert Opinion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scott, A.R.; Stoltzfus, K.C.; Tchelebi, L.T.; Trifiletti, D.M.; Lehrer, E.J.; Rao, P.; Bleyer, A.; Zaorsky, N.G. Trends in Cancer Incidence in US Adolescents and Young Adults, 1973–2015. JAMA Netw. Open 2020, 3, e2027738. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, S.C.; Tin, M.S.; Liu, X.; Lok, V.T.; Ngai, C.H.; Zhang, L.; Lucero-Prisno, D.E.R.; Xu, W.; Zheng, Z.J.; et al. Worldwide Distribution, Risk Factors, and Temporal Trends of Testicular Cancer Incidence and Mortality: A Global Analysis. Eur. Urol. Oncol. 2022, 5, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Garner, M.J.; Turner, M.C.; Ghadirian, P.; Krewski, D. Epidemiology of testicular cancer: An overview. Int. J. Cancer 2005, 116, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, M.; Cheng, L. Advances in diagnosis and treatment of testicular cancer. BMJ 2022, 379, e070499. [Google Scholar] [CrossRef] [PubMed]
- Berney, D.M.; Cree, I.; Rao, V.; Moch, H.; Srigley, J.R.; Tsuzuki, T.; Amin, M.B.; Comperat, E.M.; Hartmann, A.; Menon, S.; et al. An Introduction to the WHO 5th Edition 2022 Classification of Testicular Tumours. Histopathology 2022, 81, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Laguna, M.P.; Albers, P.; Algaba, F.; Bokemeyer, C.; Boormans, J.L.; di Nardo, D.; Fischer, S.; Fizazi, K.; Gremmels, H.; Leao, R.; et al. EAU Guideleines on Testicular Cancer. Presented at the EAU Annual Congress, Amsterdam, The Netherlands, 1–4 July 2022; ISBN 978-994-92671-92616-92675. [Google Scholar]
- Gilligan, T.D.; Seidenfeld, J.; Basch, E.M.; Einhorn, L.H.; Fancher, T.; Smith, D.C.; Stephenson, A.J.; Vaughn, D.J.; Cosby, R.; Hayes, D.F. American Society of Clinical Oncology Clinical Practice Guideline on Uses of Serum Tumor Markers in Adult Males with Germ Cell Tumors. J. Clin. Oncol. 2010, 28, 3388–3404. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.P.; Simonsen-Richter, H.; Kulejewski, M.; Anheuser, P.; Zecha, H.; Isbarn, H.; Pichlmeier, U. Serum Tumour Markers in Testicular Germ Cell Tumours: Frequencies of Elevated Levels and Extents of Marker Elevation Are Significantly Associated with Clinical Parameters and with Response to Treatment. BioMed Res. Int. 2019, 2019, 5030349. [Google Scholar] [CrossRef] [PubMed]
- Kuzmits, R.; Schernthaner, G.; Krisch, K. Serum neuron-specific enolase. A marker for responses to therapy in seminoma. Cancer 1987, 60, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Lajer, H.; Daugaard, G.; Andersson, A.M.; Skakkebaek, N.E. Clinical use of serum TRA-1-60 as tumor marker in patients with germ cell cancer. Int. J. Cancer 2002, 100, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.S.; Munro, A.J.; Duncan, W.; Sturgeon, J.; Gospodarowicz, M.K.; Jewett, M.A.; Malkin, A.; Thomas, G.M. Is placental alkaline phosphatase (PLAP) a useful marker for seminoma? Eur. J. Cancer 1990, 26, 1049–1054. [Google Scholar] [CrossRef]
- Leão, R.; Ahmad, A.E.; Hamilton, R.J. Testicular Cancer Biomarkers: A Role for Precision Medicine in Testicular Cancer. Clin. Genitourin. Cancer 2018, 17, e176–e183. [Google Scholar]
- Zamore, P.D.; Haley, B. Ribo-gnome: The big world of small RNAs. Science 2005, 309, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Coleman, N. MicroRNA Dysregulation in Malignant Germ Cell Tumors: More Than a Biomarker? J. Clin. Oncol. 2019, 37, 1432–1435. [Google Scholar] [CrossRef]
- Lange, P.H.; Winfield, H.N. Biological markers in urologic cancer. Cancer 1987, 60, 464–472. [Google Scholar] [CrossRef]
- Egan, J.; Salari, K. Biomarkers in Testicular Cancer: Classic Tumor Markers and Beyond. Urol. Clin. N. Am. 2023, 50, 133–143. [Google Scholar]
- Mir, M.C.; Pavan, N.; Gonzalgo, M.L. Current Clinical Applications of Testicular Cancer Biomarkers. Urol. Clin. N. Am. 2016, 43, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; Gillis, A.J.M.; Jerónimo, C.; Henrique, R.; Looijenga, L.H.J. Human Germ Cell Tumors are Developmental Cancers: Impact of Epigenetics on Pathobiology and Clinic. Int. J. Mol. Sci. 2019, 20, E258. [Google Scholar] [CrossRef]
- Eini, R.; Dorssers, L.C.; Looijenga, L.H. Role of stem cell proteins and microRNAs in embryogenesis and germ cell cancer. Int. J. Dev. Biol. 2013, 57, 319–3132. [Google Scholar] [CrossRef]
- Suh, M.R.; Lee, Y.; Kim, J.Y.; Kim, S.K.; Moon, S.H.; Lee, J.Y.; Cha, K.Y.; Chung, H.M.; Yoon, H.S.; Moon, S.Y.; et al. Human embryonic stem cells express a unique set of microRNAs. Dev. Biol. 2004, 270, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.J.; Stoop, H.J.; Hersmus, R.; Oosterhuis, J.W.; Sun, Y.; Chen, C.; Guenther, S.; Sherlock, J.; Veltman, I.; Baeten, J.; et al. High-throughput microRNAome analysis in human germ cell tumours. J. Pathol. 2007, 213, 319–328. [Google Scholar] [CrossRef]
- Palmer, R.D.; Murray, M.J.; Saini, H.K.; van Dongen, S.; Abreu-Goodger, C.; Muralidhar, B.; Pett, M.R.; Thornton, C.M.; Nicholson, J.C.; Enright, A.J.; et al. Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res. 2010, 70, 2911–2923. [Google Scholar]
- Murray, M.J.; Halsall, D.J.; Hook, C.E.; Williams, D.M.; Nicholson, J.C.; Coleman, N. Identification of microRNAs from the miR-371~373 and miR-302 clusters as potential serum biomarkers of malignant germ cell tumors. Am. J. Clin. Pathol. 2011, 135, 119–125. [Google Scholar] [CrossRef]
- Belge, G.; Dieckmann, K.P.; Spiekermann, M.; Balks, T.; Bullerdiek, J. Serum Levels of MicroRNAs miR-371-3: A Novel Class of Serum Biomarkers for Testicular Germ Cell Tumors? Eur. Urol. 2012, 61, 1068–1069. [Google Scholar] [PubMed]
- Syring, I.; Bartels, J.; Holdenrieder, S.; Kristiansen, G.; Müller, S.C.; Ellinger, J. Circulating serum microRNA (miR-367-3p, miR-371a-3p, miR-372-3p, miR-373-3p) as biomarkers for patients with testicular germ cell cancers. J. Urol. 2015, 193, 331–337. [Google Scholar]
- Dieckmann, K.P.; Spiekermann, M.; Balks, T.; Flor, I.; Löning, T.; Bullerdiek, J.; Belge, G. MicroRNAs miR-371-3 in serum as diagnostic tools in the management of testicular germ cell tumours. Br. J. Cancer 2012, 107, 1754–1760. [Google Scholar]
- Van Agthoven, T.; Looijenga, L.H. Accurate primary germ cell cancer diagnosis using serum based microRNA detection (ampTSmiR test). Oncotarget 2017, 8, 58037–58049. [Google Scholar] [PubMed]
- Dieckmann, K.P.; Radke, A.; Spiekermann, M.; Balks, T.; Matthies, C.; Becker, P.; Ruf, C.; Oing, C.; Oechsle, K.; Bokemeyer, C.; et al. Serum Levels of MicroRNA 371a-3p: A Sensitive and Specific New Biomarker for Germ Cell Tumors. Eur. Urol. 2017, 71, 213–220. [Google Scholar]
- Radtke, A.; Cremers, J.F.; Kliesch, S.; Riek, S.; Junker, K.; Mohamed, S.A.; Anheuser, P.; Belge, G.; Dieckmann, K.P. Can germ cell neoplasia in situ be diagnosed by measuring serum levels of microRNA371a-3p? J. Cancer Res. Clin. Oncol. 2017, 143, 2383–2392. [Google Scholar] [PubMed]
- Sharbidre, K.G.; Lockhart, M.E. Imaging of scrotal masses. Abdom. Radiol. 2020, 45, 2087–2108. [Google Scholar] [CrossRef]
- Wardak, S.; Pang, K.H.; Castiglione, F.; Lindsay, J.; Walkden, M.; Heffernan Ho, D.; Kirkham, A.; Hadway, P.; Nigam, R.; Rees, R.; et al. Management of small testicular masses: Outcomes from a single centre multidisciplinary team. BJU Int. 2023, 131, 73–81. [Google Scholar] [PubMed]
- Kliesch, S.; Schmidt, S.; Wilborn, D.; Aigner, C.; Albrecht, W.; Bedke, J.; Beintker, M.; Beyersdorff, D.; Bokemeyer, C.; Busch, J.; et al. Management of Germ Cell Tumours of the Testis in Adult Patients. German Clinical Practice Guideline Part I: Epidemiology, Classification, Diagnosis, Prognosis, Fertility Preservation, and Treatment Recommendations for Localized Stages. Urol. Int. 2021, 105, 169–180. [Google Scholar] [CrossRef]
- Paffenholz, P.; Held, L.; Loosen, S.H.; Pfister, D.; Heidenreich, A. Testis-sparing surgery for benign testicular masses—Diagnostics and therapeutic approaches. J. Urol. 2018, 200, 353–360. [Google Scholar] [PubMed]
- Dieckmann, K.P.; Radtke, A.; Geczi, L.; Matthies, C.; Anheuser, P.; Eckardt, U.; Sommer, J.; Zengerling, F.; Trenti, E.; Pichler, R.; et al. Serum levels of microRNA-371a-3p (M371 Test) as a new biomarker of testicular germ cell-tumors: Results of a prospective multicentric study. J. Clin. Oncol. 2019, 37, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.P.; Isbarn, H.; Grobelny, F.; Dumlupinar, C.; Utschig, J.; Wülfing, C.; Pichlmeier, U.; Belge, G. Testicular neoplasms: Primary tumour size is closely interrelated with histology, clinical staging, and tumour marker expression rates—A comprehensive statistical analysis. Cancers 2022, 14, 5447. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.J.; Rijlaarsdam, M.A.; Eini, R.; Dorssers, L.C.; Biermann, K.; Murray, M.J.; Nicholson, J.C.; Coleman, N.; Dieckmann, K.P.; Belge, G.; et al. Targeted serum miRNA (TSmiR) test for diagnosis and follow-up of (testicular) germ cell cancer patients: A proof of principle. Mol. Oncol. 2013, 7, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Nappi, L.; Thi, M.; Lum, A.; Huntsman, D.; Eigl, B.J.; Martin, C.; O’Neil, B.; Maughan, B.L.; Chi, K.; So, A.; et al. Developing a Highly Specific Biomarker for Germ Cell Malignancies: Plasma miR371 Expression Across the Germ Cell Malignancy Spectrum. J. Clin. Oncol. 2019, 37, 3090–3098. [Google Scholar] [PubMed]
- Mørup, N.; Rajpert-De Meyts, E.; Juul, A.; Daugaard, G.; Almstrup, K. Evaluation of Circulating miRNA Biomarkers of Testicular Germ Cell Tumors during Therapy and Follow-up-A Copenhagen Experience. Cancers 2020, 12, 759. [Google Scholar] [CrossRef]
- Badia, R.R.; Abe, D.; Wong, D.; Singla, N.; Savelyeva, A.; Chertack, N.; Woldu, S.L.; Lotan, Y.; Mauck, R.; Ouyang, D.; et al. Real-World Application of Pre-orchiectomy miR-371a-3p Test in Testicular Germ Cell Tumor Management. J. Urol. 2021, 205, 137–144. [Google Scholar] [CrossRef]
- Myklebust, M.P.; Thor, A.; Rosenlund, B.; Gjengstø, P.; Karlsdottir, Á.; Brydøy, M.; Bercea, B.S.; Olsen, C.; Johnson, I.; Berg, M.I.; et al. Serum miR371 in testicular germ cell cancer before and after orchiectomy, assessed by digital-droplet PCR in a prospective study. Sci. Rep. 2021, 11, 15582. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Feldman, D.R.; Valentino, A.; So, R.; Bromberg, M.; Khan, S.; Funt, S.A.; Sheinfeld, J.; Solit, D.B.; Pessin, M.S.; et al. Analytical Validation and Performance Characteristics of Molecular Serum Biomarkers, miR-371a-3p and miR-372-3p, for Male Germ Cell Tumors, in a Clinical Laboratory Setting. J. Mol. Diagn. 2022, 24, 867–877. [Google Scholar] [CrossRef]
- Sequeira, J.P.; Lobo, J.; Constâncio, V.; Brito-Rocha, T.; Carvalho-Maia, C.; Braga, I.; Maurício, J.; Henrique, R.; Jerónimo, C. DigiMir Test: Establishing a Novel Pipeline for MiR-371a Quantification Using Droplet Digital PCR in Liquid Biopsies from Testicular Germ Cell Tumor Patients. Front. Oncol. 2022, 12, 876732. [Google Scholar] [CrossRef] [PubMed]
- Myklebust, M.P.; Rosenlund, B.; Gjengstø, P.; Bercea, B.S.; Karlsdottir, Á.; Brydøy, M.; Dahl, O. Quantitative PCR Measurement of miR-371a-3p and miR-372-p Is Influenced by Hemolysis. Front. Genet. 2019, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Blok, J.M.; Pluim, I.; Daugaard, G.; Wagner, T.; Jóźwiak, K.; Wilthagen, E.A.; Looijenga, L.H.J.; Meijer, R.P.; Bosch, J.L.H.R.; Horenblas, S. Lymphovascular invasion and presence of embryonal carcinoma as risk factors for occult metastatic disease in clinical stage I nonseminomatous germ cell tumour: A systematic review and meta-analysis. BJU Int. 2019, 124, 424–430. [Google Scholar] [PubMed]
- Boormans, J.L.; Mayor de Castro, J.; Marconi, L.; Yuan, Y.; Laguna Pes, M.P.; Bokemeyer, C.; Nicolai, N.; Algaba, F.; Oldenburg, J.; Albers, P. Testicular Tumour Size and Rete Testis Invasion as Prognostic Factors for the Risk of Relapse of Clinical Stage I Seminoma Testis Patients Under Surveillance: A Systematic Review by the Testicular Cancer Guidelines Panel. Eur. Urol. 2018, 73, 394–405. [Google Scholar] [CrossRef]
- Bagrodia, A.; Savelyeva, A.; Lafin, J.T.; Speir, R.W.; Chesnut, G.T.; Frazier, A.L.; Woldu, S.L.; Margulis, V.; Murray, M.J.; Amatruda, J.F.; et al. Impact of Circulating miRNA-371a-3p Test on Appropriateness of Treatment and Cost Outcomes in Patients with Stage I Nonseminomatous Germ Cell Tumors. BJU Int. 2021, 128, 57–64. [Google Scholar] [CrossRef]
- Lafin, J.T.; Singla, N.; Woldu, S.L.; Lotan, Y.; Lewis, C.M.; Majmudar, K.; Savelyeva, A.; Kapur, P.; Margulis, V.; Strand, D.W.; et al. Serum MicroRNA-371a-3p Levels Predict Viable Germ Cell Tumor in Chemotherapy-naïve Patients Undergoing Retroperitoneal Lymph Node Dissection. Eur. Urol. 2020, 77, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; Leão, R.; Gillis, A.J.M.; van den Berg, A.; Anson-Cartwright, L.; Atenafu, E.G.; Kuhathaas, K.; Chung, P.; Hansen, A.; Bedard, P.L.; et al. Utility of Serum miR-371a-3p in Predicting Relapse on Surveillance in Patients with Clinical Stage I Testicular Germ Cell Cancer. Eur. Urol. Oncol. 2021, 4, 483–491. [Google Scholar] [CrossRef]
- Nappi, L.; Saxena, N.; Pautasso, S.; Mazurek, S.; Ozgun, G.; Kollmannsberger, C.; Trottier Chi, M.; Coulombe, A.M.; Soleimani, M.; Chi, K.N.; et al. Long term follow-up analysis of plasma miR371 expression to detect early relapse in patients with clinical stage I testicular germ cell tumors on surveillance. J. Clin. Oncol. 2023, 41, 5006. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Dumlupinar, C.; Radtke, A.; Matthies, C.; Pichler, R.; Paffenholz, P.; Sommer, J.; Winter, A.; Zengerling, F.; Hennig, F.; et al. Associations of serum levels of microRNA-371a-3p (M371) with risk factors for progression in nonseminomatous testicular germ cell tumours clinical stage 1. World J. Urol. 2022, 40, 317–326. [Google Scholar] [CrossRef]
- Smith-Bindman, R. Is computed tomography safe? N. Engl. J. Med. 2010, 363, 1–4. [Google Scholar] [CrossRef]
- Charytonowicz, D.; Aubrey, H.; Bell, C.; Ferret, M.; Tsui, K.; Atfield, R.; Coleman, N.; Murray, M.J.; Wilson, E.C.F. A cost analysis of non-invasive blood-based microRNA testing versus CT scans for follow-up in patients with testicular germ cell tumors. Clin. Genitourin. Cancer 2019, 17, e733–e744. [Google Scholar] [CrossRef] [PubMed]
- Terbuch, A.; Adiprasito, J.B.; Stiegelbauer, V.; Seles, M.; Klec, C.; Pichler, G.P.; Resel, M.; Posch, F.; Lembeck, A.L.; Stöger, H.; et al. MiR-371a-3p Serum Levels Are Increased in Recurrence of Testicular Germ Cell Tumor Patients. Int. J. Mol. Sci. 2018, 19, 3130. [Google Scholar] [CrossRef]
- Fankhauser, C.D.; Christiansen, A.J.; Rothermundt, C.; Cathomas, R.; Wettstein, M.S.; Grossmann, N.C.; Grogg, J.B.; Templeton, A.J.; Hirschi-Blickenstorfer, A.; Lorch, A.; et al. Detection of recurrences using serum miR-371a-3p during active surveillance in men with stage I testicular germ cell tumours. Br. J. Cancer 2022, 126, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, T.; Lin, D.W.; Aggarwal, R.; Chism, D.; Cost, N.; Derweesh, I.H.; Emamekhoo, H.; Feldman, D.R.; Geynisman, D.M.; Hancock, S.L.; et al. Testicular Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 1529–1554. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Hennig, F.; Ikogho, R.; Hammel, J.; Anheuser, P.; Wülfing, C.; Belge, G.; Dieckmann, K.P. The Novel Biomarker of Germ Cell Tumours, Micro-RNA-371a-3p, has a Very Rapid Decay in Patients with Clinical Stage 1. Urol. int. 2018, 100, 470–475. [Google Scholar] [CrossRef]
- Rosas Plaza, X.; van Agthoven, T.; Meijer, C.; van Vugt, M.A.T.M.; de Jong, S.; Gietema, J.A.; Looijenga, L.H.J. miR-371a-3p, miR-373-3p and miR-367-3p as Serum Biomarkers in Metastatic Testicular Germ Cell Cancers Before, During and After Chemotherapy. Cells 2019, 8, 1221. [Google Scholar] [CrossRef]
- Mego, M.; van Agthoven, T.; Gronesova, P.; Chovanec, M.; Miskovska, V.; Mardiak, J.; Looijenga, L.H.J. Clinical utility of plasma miR-371a-3p in germ cell tumors. J. Cell. Mol. Med. 2019, 23, 1128–1136. [Google Scholar] [CrossRef]
- Mazumdar, M.; Bajorin, D.F.; Bacik, J.; Higgins, G.; Motzer, R.J.; Bosl, G.J. Predicting outcome to chemotherapy in patients with germ cell tumors: The value of the rate of decline of human chorionic gonadotrophin and alpha-fetoprotein during therapy. J. Clin. Oncol. 2001, 19, 2534–2541. [Google Scholar] [CrossRef]
- Fizazi, K.; Pagliaro, L.; Laplanche, A.; Fléchon, A.; Mardiak, J.; Geoffrois, L.; Kerbrat, P.; Chevreau, C.; Delva, R.; Rolland, F.; et al. Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): A phase 3, multicentre, randomised trial. Lancet Oncol. 2014, 15, 1442–1450. [Google Scholar]
- Kollmannsberger, C.; Daneshmand, S.; So, A.; Chi, K.N.; Murray, N.; Moore, C.; Hayes-Lattin, B.; Nichols, C. Management of disseminated nonseminomatous germ cell tumors with risk-based chemotherapy followed by response-guided postchemotherapy surgery. J. Clin. Oncol. 2010, 28, 537–542. [Google Scholar] [CrossRef]
- Daneshmand, S.; Albers, P.; Fosså, S.D.; Heidenreich, A.; Kollmannsberger, C.; Krege, S.; Nichols, C.; Oldenburg, J.; Wood, L. Contemporary management of postchemotherapy testis cancer. Eur. Urol. 2012, 62, 867–876. [Google Scholar] [PubMed]
- Paffenholz, P.; Nestler, T.; Hoier, S.; Pfister, D.; Hellmich, M.; Heidenreich, A. External validation of 2 models to predict necrosis/fibrosis in postchemotherapy residual retroperitoneal masses of patients with advanced testicular cancer. Urol. Oncol. 2019, 37, 809.e9–809.e18. [Google Scholar] [CrossRef] [PubMed]
- Baessler, B.; Nestler, T.; Pinto Dos Santos, D.; Paffenholz, P.; Zeuch, V.; Pfister, D.; Maintz, D.; Heidenreich, A. Radiomics allows for detection of benign and malignant histopathology in patients with metastatic testicular germ cell tumors prior to post-chemotherapy retroperitoneal lymph node dissection. Eur. Radiol. 2020, 30, 2334–2345. [Google Scholar] [CrossRef]
- Leão, R.; van Agthoven, T.; Figueiredo, A.; Jewett, M.A.S.; Fadaak, K.; Sweet, J.; Ahmad, A.E.; Anson-Cartwright, L.; Chung, P.; Hansen, A.; et al. Serum miRNA predicts viable disease post-chemotherapy in testicular non-seminoma germ cell tumor patients. J. Urol. 2018, 200, 126–135. [Google Scholar] [CrossRef]
- Myklebust, M.P.; Søviknes, A.M.; Halvorsen, O.J.; Thor, A.; Dahl, O.; Ræder, H. MicroRNAs in Differentiation of Embryoid Bodies and the Teratoma Subtype of Testicular Cancer. Cancer Genomics Proteomics 2022, 19, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Shih, J.; Hollern, D.P.; Wang, L.; Bowlby, R.; Tickoo, S.K.; Thorsson, V.; Mungall, A.J.; Newton, Y.; Hegde, A.M.; et al. Integrated Molecular Characterization of Testicular Germ Cell Tumors. Cell Rep. 2018, 23, 3392–3406. [Google Scholar]
- Kremer, L.; von Brandenstein, M.; Wittersheim, M.; Koeditz, B.; Paffenholz, P.; Hellmich, M.; Pfister, D.; Heidenreich, A.; Nestler, T. The combination of microRNA-371a-3p and 375-5p can distinguish viable germ cell tumor and teratoma from necrosis in postchemotherapy retroperitoneal lymph node dissection specimens. Transl. Androl. Urol. 2021, 10, 1647–1655. [Google Scholar] [CrossRef]
- Lafin, J.T.; Kenigsberg, A.P.; Meng, X.; Abe, D.; Savelyeva, A.; Singla, N.; Woldu, S.L.; Lotan, Y.; Mauck, R.J.; Lewis, C.M.; et al. Serum Small RNA Sequencing and miR-375 Assay Do Not Identify the Presence of Pure Teratoma at Postchemotherapy Retroperitoneal Lymph Node Dissection. Eur. Urol. Open Sci. 2021, 26, 83–87. [Google Scholar] [CrossRef]
- Nappi, L.; Thi, M.; Adra, N.; Hamilton, R.J.; Leao, R.; Lavoie, J.M.; Soleimani, M.; Eigl, B.J.; Chi, K.; Gleave, M.; et al. Integrated Expression of Circulating miR375 and miR371 to Identify Teratoma and Active Germ Cell Malignancy Components in Malignant Germ Cell Tumors. Eur. Urol. 2021, 79, 16–19. [Google Scholar] [CrossRef]
- Belge, G.; Grobelny, F.; Matthies, C.; Radtke, A.; Dieckmann, K.P. Serum Level of microRNA-375-3p Is Not a Reliable Biomarker of Teratoma. In Vivo 2020, 34, 163–168. [Google Scholar] [CrossRef]
- Lobo, J.; van Zogchel, L.M.J.; Nuru, M.G.; Gillis, A.J.M.; van der Schoot, C.E.; Tytgat, G.A.M.; Looijenga, L.H.J. Combining hypermethylated RASSF1A detection using ddPCR withmiR-371-a3 testing: An improved panel of liquid biopsy biomarkers for testicular germ cell tumor patients. Cancers 2021, 13, 5228. [Google Scholar] [CrossRef] [PubMed]
- Nestler, T.; Kremer, L.; von Brandenstein, M.; Wittersheim, M.; Paffenholz, P.; Wagener-Ryczek, S.; Quaas, A.; Hellmich, M.; Müller, S.; Pfister, D.; et al. Differentially expressed messenger RNA/proteins can distinguish teratoma from necrosis in postchemotherapy retroperitoneal lymph node dissection (pcRPLND) tissue. Cancer 2022, 129, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.R.; Beck, S.D.; Bihrle, R.; Cary, K.C.; Einhorn, L.H.; Foster, R.S. Survival Analysis of Pure Seminoma at Postchemotherapy Retroperitoneal Lymph Node Dissection. J. Urol. 2014, 192, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Flechon, A.; Bompas, E.; Biron, P.; Droz, J.P. Management of post-chemotherapy residual masses in advanced seminoma. J. Urol. 2002, 168, 1975–1979. [Google Scholar] [CrossRef]
- Cathomas, R.; Klingbiel, D.; Bernard, B.; Lorch, A.; Garcia Del Muro, X.; Morelli, F.; De Giorgi, U.; Fedyanin, M.; Oing, C.; Haugnes, H.S.; et al. Questioning the Value of Fluorodeoxyglucose Positron Emission Tomography for Residual Lesions After Chemotherapy for Metastatic Seminoma: Results of an International Global Germ Cell Cancer Group Registry. J. Clin. Oncol. 2018, 36, 3381–3387. [Google Scholar]
- Dieckmann, K.P.; Klemke, M.; Grobelny, F.; Radtke, A.; Dralle-Filiz, I.; Wülfing, C.; Belge, G. Serum levels of microRNA 371a-3p (M371) can predict absence or presence of vital disease in residual masses after chemotherapy of metatstatic seminoma. Front. Oncol. 2022, 12, 889624. [Google Scholar] [CrossRef]
- Konneh, B.; Lafin, J.T.; Howard, J.; Gerald, T.; Amini, A.; Savelyeva, A.; Woldu, S.L.; Lewis, C.M.; Jia, L.; Margulis, V.; et al. Evaluation of miR-371a-3p to predict viable germ cell tumor in patients with pure seminoma receiving retroperitoneal lymph node dissection. Andrology 2022, 11, 634–640. [Google Scholar] [CrossRef]
- Oldenburg, J.; Berney, D.M.; Bokemeyer, C.; Climent, M.A.; Daugaard, G.; Gietema, J.A.; De Giorgi, U.; Haugnes, H.S.; Huddart, R.A.; Leão, R.; et al. Testicular seminoma and non-seminoma: ESMO-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 362–375. [Google Scholar]
- Conduit, C.; Grimison, P.S.; Lewin, J.H.; Hansen, A.R.; Lynam, J.F.; Weickhardt, A.J.; Parente, P.; Campbell, D.C.; Hong, W.; Marx, G.M.; et al. CLIMATE: Assessing the clinical utility of miR-371a-3p as a marker of residual disease in stage 1 testicular germ cell tumour (TGCT) following orchiectomy: ANZUP 1906. J. Clin. Oncol. 2023, 41, TPS5100. [Google Scholar] [CrossRef]
- Timmerman, D.M.; Gillis, A.J.M.; Mego, M.; Looijenga, L.H.J. Comparative Analyses of Liquid-Biopsy MicroRNA371a-3p Isolation Protocols for Serum and Plasma. Cancers 2021, 13, 4260. [Google Scholar] [CrossRef]
- Lobo, J.; Gillis, A.J.M.; van den Berg, A.; Dorssers, L.C.J.; Belge, G.; Dieckmann, K.P.; Roest, H.P.; van der Laan, L.J.W.; Gietema, J.; Hamilton, R.J.; et al. Identification and Validation Model for Informative Liquid Biopsy-Based microRNA Biomarkers: Insights from Germ Cell Tumor In Vitro, In Vivo and Patient-Derived Data. Cells 2019, 8, 1637. [Google Scholar] [CrossRef] [PubMed]
| Criteria | |
|---|---|
| 1 | The marker is exclusively produced by the malignancy. |
| 2 | The marker is secreted into body fluids. |
| 3 | The measurement is reproducible. |
| 4 | Marker levels in body fluids correlate with actual tumor amount. |
| 5 | The marker is detectable in early disease stages. |
| 6 | Marker levels correlate with treatment response. |
| 7 | The half-life of the marker is short. |
| Author, Year | Number of Patients | Sensitivity (%) | Specificity (%) | AUC | PPV (%) | NPV (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Dieckmann, 2012 | 20 | 70.8 | n/a | n/a | n/a | n/a | [26] |
| Gillis, 2013 | 80 | >90 | 61 | 0.89 | n/a | n/a | [36] |
| Syring, 2015 | 89 | 84.7 | 99 | 0.93 | n/a | n/a | [25] |
| Dieckmann, 2017 * | 166 | 92 | 84.7 | 0.94 | n/a | n/a | [28] |
| van Agthoven, 2017 | 250 | 89 | 90 | 0.95 | 94 | 79 | [27] |
| Dieckmann, 2019 * | 616 | 91.8 | 96.1 | 0.97 | 97.2 | 82.7 | [34] |
| Nappi, 2019 * | 110 | 96 | 100 | 0.97 | 100 | 98 | [37] |
| Mørup, 2020 * | 52 | 67.5 | n/a | n/a | n/a | n/a | [38] |
| Badia, 2021 | 69 | 93.1 | 100 | 0.98 | 100 | 73.3 | [39] |
| Myklebust, 2021 * | 180 | 89 | 100 | n/a | 100 | 55 | [40] |
| Ye, 2022 | 51 | 81.8 | 100 | 0.93 | n/a | n/a | [41] |
| Sequeira, 2022 | 82 | 93.6 | 100 | 0.98 | 100 | 96 | [42] |
| Author, Year | Number of Patients (Total) | Number of Patients with Relapse | Sensitivity (%) | Specificity (%) | AUC | Ref. |
|---|---|---|---|---|---|---|
| Terbuch, 2018 | 10 | 10 | n/a | n/a | n/a | [53] |
| Dieckmann, 2019 * | 616 | 46 | 82.6 | 96.1 | 0.92 | [34] |
| Lobo, 2020 | 151 | 34 | 94.1 | n/a | n/a | [48] |
| Fankhauser, 2022 * | 33 | 10 | 100 | 100 | 1.0 | [54] |
| Nappi 2023 | 101 | 35 | 62.8 | 100 | 0.81 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nestler, T.; Schoch, J.; Belge, G.; Dieckmann, K.-P. MicroRNA-371a-3p—The Novel Serum Biomarker in Testicular Germ Cell Tumors. Cancers 2023, 15, 3944. https://doi.org/10.3390/cancers15153944
Nestler T, Schoch J, Belge G, Dieckmann K-P. MicroRNA-371a-3p—The Novel Serum Biomarker in Testicular Germ Cell Tumors. Cancers. 2023; 15(15):3944. https://doi.org/10.3390/cancers15153944
Chicago/Turabian StyleNestler, Tim, Justine Schoch, Gazanfer Belge, and Klaus-Peter Dieckmann. 2023. "MicroRNA-371a-3p—The Novel Serum Biomarker in Testicular Germ Cell Tumors" Cancers 15, no. 15: 3944. https://doi.org/10.3390/cancers15153944
APA StyleNestler, T., Schoch, J., Belge, G., & Dieckmann, K.-P. (2023). MicroRNA-371a-3p—The Novel Serum Biomarker in Testicular Germ Cell Tumors. Cancers, 15(15), 3944. https://doi.org/10.3390/cancers15153944






