Mapping the Potential of Microfluidics in Early Diagnosis and Personalized Treatment of Head and Neck Cancers
Abstract
Simple Summary
Abstract
1. Introduction
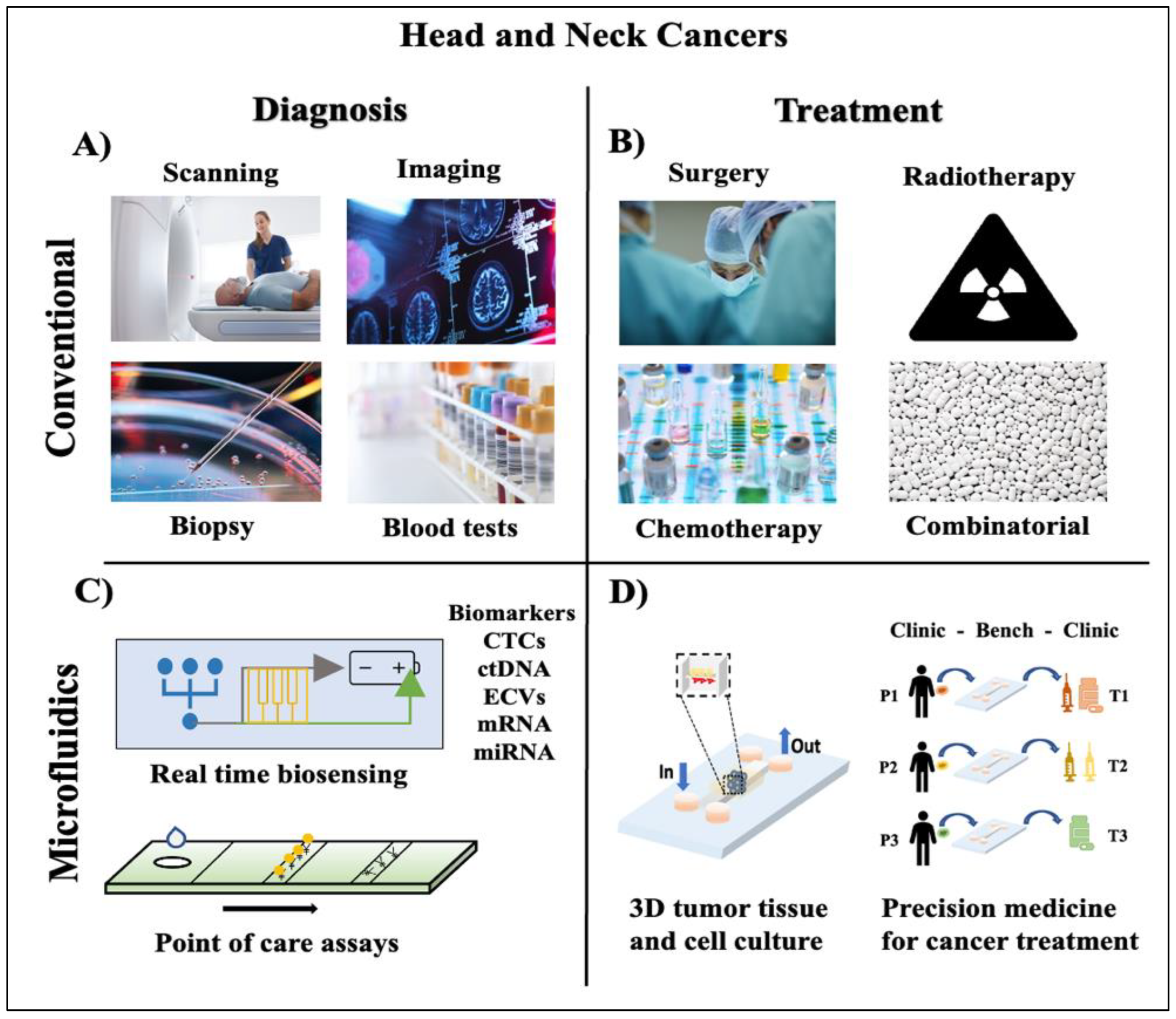
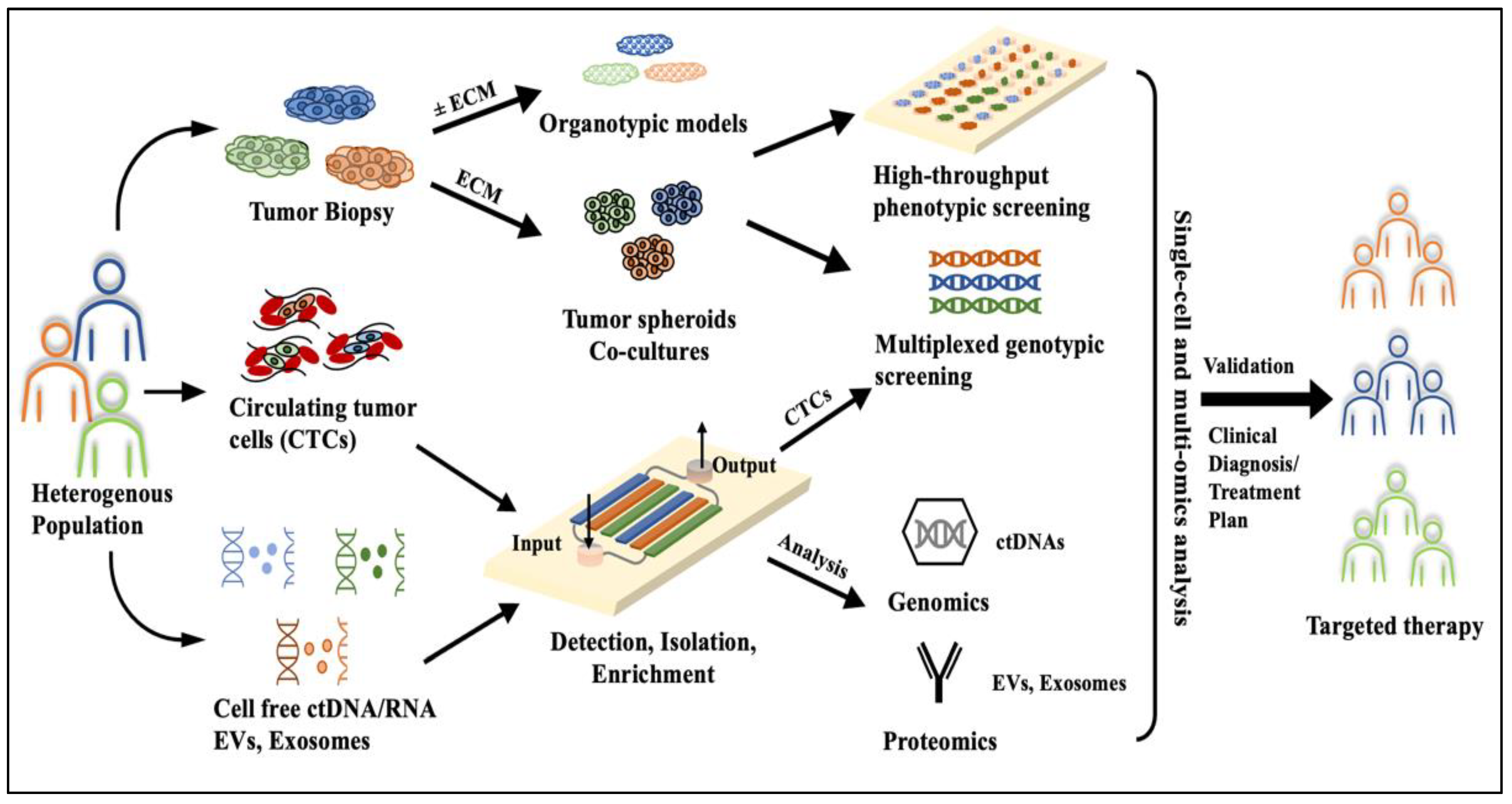
2. Microfluidics, a Peephole into Precision Oncology
3. Microfluidic Systems for HNC Diagnosis and Screening
4. Microfluidic Tumor-on-a-Chip Platforms for HNC Therapy

5. Pros and Cons towards Clinical Translation
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Chow, L.Q. Head and neck cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Ha, P.K.; Chang, S.S.; Glazer, C.A.; Califano, J.A.; Sidransky, D. Molecular techniques and genetic alterations in head and neck cancer. Oral Oncol. 2009, 45, 335–339. [Google Scholar] [CrossRef]
- Suh, Y.; Amelio, I.; Urbano, T.G.; Tavassoli, M. Clinical update on cancer: Molecular oncology of head and neck cancer. Cell Death Dis. 2014, 5, e1018. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Sullivan, K.D.; Galbraith, M.D.; Andrysik, Z.; Espinosa, J.M. Mechanisms of transcriptional regulation by p53. Cell Death Differ. 2018, 25, 133–143. [Google Scholar] [CrossRef]
- Network, C.G.A. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576. [Google Scholar] [CrossRef]
- Bykov, V.J.N.; Eriksson, S.E.; Bianchi, J.; Wiman, K.G. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer 2018, 18, 89–102. [Google Scholar] [CrossRef]
- Gu, W.; Meng, F.; Haag, R.; Zhong, Z. Actively targeted nanomedicines for precision cancer therapy: Concept, construction, challenges and clinical translation. J. Control. Release 2021, 329, 676–695. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Head and Neck Cancers. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437 (accessed on 26 July 2023).
- Nigro, C.L.; Denaro, N.; Merlotti, A.; Merlano, M. Head and neck cancer: Improving outcomes with a multidisciplinary approach. Cancer Manag. Res. 2017, 9, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner, H., Jr.; Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. 2003, 21, 92–98. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J. Clin. Oncol. 2013, 31, 845. [Google Scholar] [CrossRef] [PubMed]
- Cocks, H.; Ah-See, K.; Capel, M.; Taylor, P. Palliative and supportive care in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S198–S207. [Google Scholar] [CrossRef]
- Seliger, B. Combinatorial approaches with checkpoint inhibitors to enhance anti-tumor immunity. Front. Immunol. 2019, 10, 999. [Google Scholar] [CrossRef]
- Seliger, B.; Al-Samadi, A.; Yang, B.; Salo, T.; Wickenhauser, C. In vitro models as tools for screening treatment options of head and neck cancer. Front. Med. 2022, 9, 971726. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast cancer models: Engineering the tumor microenvironment. Acta Biomater. 2020, 106, 1–21. [Google Scholar] [CrossRef]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.-J.; Chun, S.-M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.A.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer–derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef]
- Moya-Garcia, C.R.; Okuyama, H.; Sadeghi, N.; Li, J.; Tabrizian, M.; Li-Jessen, N.Y.K. In vitro models for head and neck cancer: Current status and future perspective. Front. Oncol. 2022, 12, 960340. [Google Scholar] [CrossRef]
- Melissaridou, S.; Wiechec, E.; Magan, M.; Jain, M.V.; Chung, M.K.; Farnebo, L.; Roberg, K. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Eke, I.; Schneider, L.; Förster, C.; Zips, D.; Kunz-Schughart, L.A.; Cordes, N. EGFR/JIP-4/JNK2 Signaling Attenuates Cetuximab-Mediated Radiosensitization of Squamous Cell Carcinoma CellsEGFR/JIP-4 Interaction. Cancer Res. 2013, 73, 297–306. [Google Scholar] [CrossRef]
- Hagemann, J.; Jacobi, C.; Hahn, M.; Schmid, V.; Welz, C.; Schwenk-Zieger, S.; Stauber, R.; Baumeister, P.; Becker, S. Spheroid-based 3D cell cultures enable personalized therapy testing and drug discovery in head and neck cancer. Anticancer Res. 2017, 37, 2201–2210. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; De Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef]
- Magan, M.; Wiechec, E.; Roberg, K. CAFs affect the proliferation and treatment response of head and neck cancer spheroids during co-culturing in a unique in vitro model. Cancer Cell Int. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Thorat, N.D.; Pricl, S.; Patil, R.M.; Rohiwal, S.; Townley, H. Bioink: A 3D-bioprinting tool for anticancer drug discovery and cancer management. Drug Discov. Today 2021, 26, 1574–1590. [Google Scholar] [CrossRef]
- Tuomainen, K.; Al-Samadi, A.; Potdar, S.; Turunen, L.; Turunen, M.; Karhemo, P.-R.; Bergman, P.; Risteli, M.; Åström, P.; Tiikkaja, R.; et al. Human tumor–derived matrix improves the predictability of head and neck cancer drug testing. Cancers 2019, 12, 92. [Google Scholar] [CrossRef]
- Tanaka, N.; Osman, A.A.; Takahashi, Y.; Lindemann, A.; Patel, A.A.; Zhao, M.; Takahashi, H.; Myers, J.N. Head and neck cancer organoids established by modification of the CTOS method can be used to predict in vivo drug sensitivity. Oral Oncol. 2018, 87, 49–57. [Google Scholar] [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Quake, S.R. Microfluidics in structural biology: Smaller, faster… better. Curr. Opin. Struct. Biol. 2003, 13, 538–544. [Google Scholar] [CrossRef] [PubMed]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Sun, Y. Microfluidic approaches for cancer cell detection, characterization, and separation. Lab A Chip 2012, 12, 1753–1767. [Google Scholar] [CrossRef]
- Garcia-Cordero, J.L.; Maerkl, S.J. Microfluidic systems for cancer diagnostics. Curr. Opin. Biotechnol. 2020, 65, 37–44. [Google Scholar] [CrossRef]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Virumbrales-Muñoz, M.; Lang, J.M.; Beebe, D.J. A role for microfluidic systems in precision medicine. Nat. Commun. 2022, 13, 3086. [Google Scholar] [CrossRef]
- Sawyers, C.L.; Hochhaus, A.; Feldman, E.; Goldman, J.M.; Miller, C.B.; Ottmann, O.G.; Schiffer, C.A.; Talpaz, M.; Guilhot, F.; Deininger, M.W. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: Results of a phase II study: Presented in part at the 43rd Annual Meeting of The American Society of Hematology, Orlando, FL, December 11 2001. Blood 2002, 99, 3530–3539. [Google Scholar]
- Prasad, V. Perspective: The precision-oncology illusion. Nature 2016, 537, S63. [Google Scholar] [CrossRef]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. A Review on Biomaterials in Dental Implantology. Int. J. Biomed. Sci. 2015, 11, 113–120. [Google Scholar]
- Friedman, A.A.; Letai, A.; Fisher, D.E.; Flaherty, K.T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 2015, 15, 747–756. [Google Scholar] [CrossRef]
- Affolter, A.; Lammert, A.; Kern, J.; Scherl, C.; Rotter, N. Precision medicine gains momentum: Novel 3D models and stem cell-based approaches in head and neck cancer. Front. Cell Dev. Biol. 2021, 9, 666515. [Google Scholar] [CrossRef]
- Licitra, L.; Mesia, R.; Rivera, F.; Remenár, E.; Hitt, R.; Erfán, J.; Rottey, S.; Kawecki, A.; Zabolotnyy, D.; Benasso, M.; et al. Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann. Oncol. 2011, 22, 1078–1087. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Malone, E.; Siu, L.L. Precision medicine in head and neck cancer: Myth or reality? Clin. Med. Insights Oncol. 2018, 12, 1179554918779581. [Google Scholar] [CrossRef]
- Wang, Y.; Navin, N.E. Advances and applications of single-cell sequencing technologies. Mol. Cell 2015, 58, 598–609. [Google Scholar] [CrossRef]
- Lewis, S.M.; Asselin-Labat, M.-L.; Nguyen, Q.; Berthelet, J.; Tan, X.; Wimmer, V.C.; Merino, D.; Rogers, K.L.; Naik, S.H. Spatial omics and multiplexed imaging to explore cancer biology. Nat. Methods 2021, 18, 997–1012. [Google Scholar] [CrossRef]
- Krol, I.; Castro-Giner, F.; Maurer, M.; Gkountela, S.; Szczerba, B.M.; Scherrer, R.; Coleman, N.; Carreira, S.; Bachmann, F.; Anderson, S.; et al. Detection of circulating tumour cell clusters in human glioblastoma. Br. J. Cancer 2018, 119, 487–491. [Google Scholar] [CrossRef]
- Ozkumur, E.; Shah, A.M.; Ciciliano, J.C.; Emmink, B.L.; Miyamoto, D.T.; Brachtel, E.; Yu, M.; Chen, P.-i.; Morgan, B.; Trautwein, J. Inertial focusing for tumor antigen–dependent and–independent sorting of rare circulating tumor cells. Sci. Transl. Med. 2013, 5, 179ra47. [Google Scholar] [CrossRef]
- Zhang, Z.; Shiratsuchi, H.; Lin, J.; Chen, G.; Reddy, R.M.; Azizi, E.; Fouladdel, S.; Chang, A.C.; Lin, L.; Jiang, H.; et al. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget 2014, 5, 12383. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Tran, T.H.P.; Blick, T.; O’byrne, K.; Thompson, E.W.; Warkiani, M.E.; Nelson, C.; Kenny, L.; Punyadeera, C. Enrichment of circulating head and neck tumour cells using spiral microfluidic technology. Sci. Rep. 2017, 7, 42517. [Google Scholar] [CrossRef]
- Mannino, M.C.; Kerr, C.P.; Schehr, J.L.; Zhao, S.G.; Morris, Z.S.; Lang, J.M. Microfluidic characterization of circulating tumor cells from mouse models and patients with head and neck cancer. Cancer Res. 2022, 82, 5121. [Google Scholar] [CrossRef]
- Stiefel, J.; Freese, C.; Sriram, A.; Alebrand, S.; Srinivas, N.; Sproll, C.; Wandrey, M.; Gül, D.; Hagemann, J.; Becker, J.C.; et al. Characterization of a novel microfluidic platform for the isolation of rare single cells to enable CTC analysis from head and neck squamous cell carcinoma patients. Eng. Life Sci. 2022, 22, 391–406. [Google Scholar] [CrossRef]
- Mishra, V.; Singh, A.; Chen, X.; Rosenberg, A.J.; Pearson, A.T.; Zhavoronkov, A.; Savage, P.A.; Lingen, M.W.; Agrawal, N.; Izumchenko, E. Application of liquid biopsy as multi-functional biomarkers in head and neck cancer. Br. J. Cancer 2022, 126, 361–370. [Google Scholar] [CrossRef]
- Meng, Y.; Bian, L.; Zhang, M.; Bo, F.; Lu, X.; Li, D. Liquid biopsy and their application progress in head and neck cancer: Focus on biomarkers CTCs, cfDNA, ctDNA and EVs. Biomark. Med. 2020, 14, 1393–1404. [Google Scholar] [CrossRef]
- Gwak, H.; Kim, J.; Cha, S.; Cheon, Y.; Kim, S.-I.; Kwak, B.; Hyun, K.-A.; Jung, H.-I. On-chip isolation and enrichment of circulating cell-free DNA using microfluidic device. Biomicrofluidics 2019, 13, 024113. [Google Scholar] [CrossRef]
- Park, J.; Han, D.H.; Park, J.-K. Towards practical sample preparation in point-of-care testing: User-friendly microfluidic devices. Lab A Chip 2020, 20, 1191–1203. [Google Scholar] [CrossRef]
- Malhotra, R.; Patel, V.; Chikkaveeraiah, B.V.; Munge, B.S.; Cheong, S.C.; Zain, R.B.; Abraham, M.T.; Dey, D.K.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive detection of cancer biomarkers in the clinic by use of a nanostructured microfluidic array. Anal. Chem. 2012, 84, 6249–6255. [Google Scholar] [CrossRef]
- Soares, A.C.; Soares, J.C.; Rodrigues, V.C.; Follmann, H.D.M.; Arantes, L.M.R.B.; Carvalho, A.C.; Melendez, M.E.; Fregnani, J.H.T.G.; Reis, R.M.; Carvalho, A.L.; et al. Microfluidic-based genosensor to detect human papillomavirus (HPV16) for head and neck cancer. ACS Appl. Mater. Interfaces 2018, 10, 36757–36763. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wu, C.-C.; Su, W.-T.; Tseng, P.-C.; Hsueh, Y.-Y.; Hsiao, Y.-C.; Chang, K.-P.; Yu, J.-S.; Chuang, Y.-J. Target peptide enrichment microfluidic chip for rapid detection of oral squamous cell carcinoma using stable isotope standards and capture by anti-peptide antibodies. Sens. Actuators B Chem. 2020, 322, 128607. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Chen, T.; Ozkaya, G.U.; Choudhary, D.; Molinolo, A.A.; Gutkind, J.S.; Rusling, J.F. Detecting cancer metastasis and accompanying protein biomarkers at single cell levels using a 3D-printed microfluidic immunoarray. Biosens. Bioelectron. 2021, 171, 112681. [Google Scholar] [CrossRef]
- Yin, B.; Qian, C.; Wan, X.; Sohan, A.M.F.; Lin, X. Tape integrated self-designed microfluidic chip for point-of-care immunoassays simultaneous detection of disease biomarkers with tunable detection range. Biosens. Bioelectron. 2022, 212, 114429. [Google Scholar] [CrossRef]
- Zeng, S.; Sun, X.; Wan, X.; Qian, C.; Yue, W.; Sohan, A.M.F.; Lin, X.; Yin, B. A cascade Fermat spiral microfluidic mixer chip for accurate detection and logic discrimination of cancer cells. Analyst 2022, 147, 3424–3433. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Sayson, D.; Nguyen, B.H.; Tran, S.D. Advances in medical wearable biosensors: Design, fabrication and materials strategies in healthcare monitoring. Molecules 2021, 27, 165. [Google Scholar] [CrossRef]
- Qin, J.; Wang, W.; Gao, L.; Yao, S.Q. Emerging biosensing and transducing techniques for potential applications in point-of-care diagnostics. Chem. Sci. 2022, 13, 2857–2876. [Google Scholar] [CrossRef]
- Chu, H.; Liu, C.; Liu, J.; Yang, J.; Li, Y.; Zhang, X. Recent advances and challenges of biosensing in point-of-care molecular diagnosis. Sens. Actuators B Chem. 2021, 348, 130708. [Google Scholar] [CrossRef]
- Capture and Harvest Live, Intact Circulating Tumor Cells (CTCs). Available online: https://angleplc.com/parsortix-technology/?_gl=1*12e4q97*_up*MQ..&gclid=Cj0KCQjwzdOlBhCNARIsAPMwjbzOj30jCu9PV1H0HKjI9L_4Yqpaay18dLHzjveoJeRRVre9yiWIQxsaArm0EALw_wcB (accessed on 17 July 2023).
- CIRCULATING TUMOR CELL (CTC) CHIP. Available online: https://azar-innovations.com/portfolio/circulating-tumor-cell-ctc-chip-azar-innovations/ (accessed on 17 July 2023).
- The Gold Standard. The First and Only Actionable Test for Detecting CTCs in Cancer Patients with Metastatic Breast, Prostate* or Colorectal Cancer. Available online: https://www.cellsearchctc.com/ (accessed on 17 July 2023).
- Caballero, D.; Kaushik, S.; Correlo, V.M.; Oliveira, J.M.; Reis, R.L.; Kundu, S.C. Organ-on-chip models of cancer metastasis for future personalized medicine: From chip to the patient. Biomaterials 2017, 149, 98–115. [Google Scholar] [CrossRef]
- Saha, B.; Mathur, T.; Tronolone, J.J.; Chokshi, M.; Lokhande, G.K.; Selahi, A.; Gaharwar, A.K.; Afshar-Kharghan, V.; Sood, A.K.; Bao, G.; et al. Human tumor microenvironment chip evaluates the consequences of platelet extravasation and combinatorial antitumor-antiplatelet therapy in ovarian cancer. Sci. Adv. 2021, 7, eabg5283. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.-E.; Moon, D.; Doh, J. A multilayered blood vessel/tumor tissue chip to investigate T cell infiltration into solid tumor tissues. Lab A Chip 2021, 21, 2142–2152. [Google Scholar] [CrossRef]
- Jin, D.; Ma, X.; Luo, Y.; Fang, S.; Xie, Z.; Li, X.; Qi, D.; Zhang, F.; Kong, J.; Li, J.; et al. Application of a microfluidic-based perivascular tumor model for testing drug sensitivity in head and neck cancers and toxicity in endothelium. Rsc Adv. 2016, 6, 29598–29607. [Google Scholar] [CrossRef]
- Yu, J.; Lee, S.; Song, J.; Lee, S.-R.; Kim, S.; Choi, H.; Kang, H.; Hwang, Y.; Hong, Y.-K.; Jeon, N.L. Perfusable micro-vascularized 3D tissue array for high-throughput vascular phenotypic screening. Nano Converg. 2022, 9, 16. [Google Scholar] [CrossRef]
- Mencattini, A.; De Ninno, A.; Mancini, J.; Businaro, L.; Martinelli, E.; Schiavoni, G.; Mattei, F. High-throughput analysis of cell-cell crosstalk in ad hoc designed microfluidic chips for oncoimmunology applications. Methods Enzymol. 2020, 632, 479–502. [Google Scholar]
- Liu, W.; Wang, J.; Qi, H.; Jiao, Q.; Wu, L.; Wang, Y.; Liang, Q. The latest advances in high content screening in microfluidic devices. Expert Opin. Drug Discov. 2023, 18, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, S.; Feng, H.; Liao, X.; Xing, X.-H.; Bai, Z.; Liu, X.; Zhang, C. CRISPRi-microfluidics screening enables genome-scale target identification for high-titer protein production and secretion. Metab. Eng. 2023, 75, 192–204. [Google Scholar] [CrossRef]
- Abdrabou, A.M.; Duong, B.T.V.; Chen, K.; Atwal, R.S.; Labib, M.; Lin, S.; Angers, S.; Kelley, S.O. nuPRISM: Microfluidic Genome-Wide Phenotypic Screening Platform for Cellular Nuclei. ACS Cent. Sci. 2022, 8, 1618–1626. [Google Scholar] [CrossRef]
- Xia, F. Phenotypic Screening of Rare Tumor Cells Using Microfluidic Platforms. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2021. [Google Scholar]
- Liu, H.E.; Triboulet, M.; Zia, A.; Vuppalapaty, M.; Kidess-Sigal, E.; Coller, J.; Natu, V.S.; Shokoohi, V.; Che, J.; Renier, C.; et al. Workflow optimization of whole genome amplification and targeted panel sequencing for CTC mutation detection. NPJ Genom. Med. 2017, 2, 34. [Google Scholar] [CrossRef]
- Ebrahimi, G.; Pakchin, P.S.; Mota, A.; Omidian, H.; Omidi, Y. Electrochemical microfluidic paper-based analytical devices for cancer biomarker detection: From 2D to 3D sensing systems. Talanta 2023, 257, 124370. [Google Scholar] [CrossRef]
- Fernández-Baldo, M.A.; Ortega, F.G.; Pereira, S.V.; Bertolino, F.A.; Serrano, M.J.; Lorente, J.A.; Raba, J.; Messina, G.A. Nanostructured platform integrated into a microfluidic immunosensor coupled to laser-induced fluorescence for the epithelial cancer biomarker determination. Microchem. J. 2016, 128, 18–25. [Google Scholar] [CrossRef]
- Otieno, B.A.; Krause, C.E.; Latus, A.; Chikkaveeraiah, B.V.; Faria, R.C.; Rusling, J.F. On-line protein capture on magnetic beads for ultrasensitive microfluidic immunoassays of cancer biomarkers. Biosens. Bioelectron. 2014, 53, 268–274. [Google Scholar] [CrossRef]
- Peng, A.; Mao, X.; Zhong, J.; Fan, S.; Hu, Y. Single-cell multi-omics and its prospective application in cancer biology. Proteomics 2020, 20, 1900271. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.S.; Chaligne, R.; Landau, D.A. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat. Rev. Genet. 2021, 22, 3–18. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef]
- Batuwitage, B.; Hanlon, R.; Charters, P. Imaging in head and neck cancers. BJA Educ. 2021, 21, 2. [Google Scholar] [CrossRef]
- Akhavan-Moghadam, J.; Afaaghi, M.; Maleki, A.R.; Saburi, A. Fine needle aspiration: An atraumatic method to diagnose head and neck masses. Trauma Mon. 2013, 18, 117. [Google Scholar] [CrossRef]
- Kong, L.; Birkeland, A.C. Liquid biopsies in head and neck cancer: Current state and future challenges. Cancers 2021, 13, 1874. [Google Scholar] [CrossRef]
- Castellsagué, X.; Alemany, L.; Quer, M.; Halec, G.; Quirós, B.; Tous, S.; Clavero, O.; Alòs, L.; Biegner, T.; Szafarowski, T. HPV involvement in head and neck cancers: Comprehensive assessment of biomarkers in 3680 patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsagué, X.; Laporte, L.; Bosch, F.X.; de Sanjosé, S.; Trottier, H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Gioacchini, F.M.; Alicandri-Ciufelli, M.; Kaleci, S.; Magliulo, G.; Presutti, L.; Re, M. The prognostic value of cyclin D1 expression in head and neck squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 801–809. [Google Scholar] [CrossRef]
- Amenábar, J.M.; Da Silva, B.M.; Punyadeera, C. Salivary protein biomarkers for head and neck cancer. Expert Rev. Mol. Diagn. 2020, 20, 305–313. [Google Scholar] [CrossRef]
- Guerra, E.N.S.; Acevedo, A.C.; Leite, A.F.; Gozal, D.; Chardin, H.; Canto, G.D.L. Diagnostic capability of salivary biomarkers in the assessment of head and neck cancer: A systematic review and meta-analysis. Oral Oncol. 2015, 51, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Righini, C.A.; de Fraipont, F.; Timsit, J.-F.; Faure, C.; Brambilla, E.; Reyt, E.; Favrot, M.-C. Tumor-specific methylation in saliva: A promising biomarker for early detection of head and neck cancer recurrence. Clin. Cancer Res. 2007, 13, 1179–1185. [Google Scholar] [CrossRef]
- Thomaidou, A.C.; Batsaki, P.; Adamaki, M.; Goulielmaki, M.; Baxevanis, C.N.; Zoumpourlis, V.; Fortis, S.P. Promising biomarkers in head and neck cancer: The most clinically important miRNAs. Int. J. Mol. Sci. 2022, 23, 8257. [Google Scholar] [CrossRef]
- Spector, M.E.; Farlow, J.L.; Haring, C.T.; Brenner, J.C.; Birkeland, A.C. The potential for liquid biopsies in head and neck cancer. Discov. Med. 2018, 25, 251. [Google Scholar]
- Aulakh, S.S.; Silverman, D.A.; Young, K.; Dennis, S.K.; Birkeland, A.C. The promise of circulating tumor DNA in head and neck cancer. Cancers 2022, 14, 2968. [Google Scholar] [CrossRef]
- Patel, A.; Patel, S.; Patel, P.; Tanavde, V. Saliva based liquid biopsies in head and neck cancer: How far are we from the clinic? Front. Oncol. 2022, 12, 828434. [Google Scholar] [CrossRef]
- van Ginkel, J.H.; Huibers, M.M.; van Es, R.J.; de Bree, R.; Willems, S.M. Droplet digital PCR for detection and quantification of circulating tumor DNA in plasma of head and neck cancer patients. BMC Cancer 2017, 17, 428. [Google Scholar] [CrossRef]
- Veyer, D.; Wack, M.; Mandavit, M.; Garrigou, S.; Hans, S.; Bonfils, P.; Tartour, E.; Bélec, L.; Wang-Renault, S.; Laurent-Puig, P.; et al. HPV circulating tumoral DNA quantification by droplet-based digital PCR: A promising predictive and prognostic biomarker for HPV-associated oropharyngeal cancers. Int. J. Cancer 2020, 147, 1222–1227. [Google Scholar] [CrossRef]
- Perdomo, S.; Avogbe, P.H.; Foll, M.; Abedi-Ardekani, B.; Facciolla, V.L.; Anantharaman, D.; Chopard, P.; Le Calvez-Kelm, F.; Vilensky, M.; Polesel, J.; et al. Circulating tumor DNA detection in head and neck cancer: Evaluation of two different detection approaches. Oncotarget 2017, 8, 72621. [Google Scholar] [CrossRef]
- Gao, M.; Callari, M.; Beddowes, E.; Sammut, S.-J.; Grzelak, M.; Biggs, H.; Jones, L.; Boumertit, A.; Linn, S.C.; Cortes, J.; et al. Next Generation-Targeted Amplicon Sequencing (NG-TAS): An optimised protocol and computational pipeline for cost-effective profiling of circulating tumour DNA. Genome Med. 2019, 11, 1. [Google Scholar] [CrossRef]
- Zavridou, M.; Mastoraki, S.; Strati, A.; Koutsodontis, G.; Klinakis, A.; Psyrri, A.; Lianidou, E. Direct comparison of size-dependent versus EpCAM-dependent CTC enrichment at the gene expression and DNA methylation level in head and neck squamous cell carcinoma. Sci. Rep. 2020, 10, 6551. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Kapeleris, J.; Kimberley, R.; Mattarollo, S.R.; Thompson, E.W.; Thiery, J.-P.; Kenny, L.; O’byrne, K.; Punyadeera, C. The prognostic significance of circulating tumor cells in head and neck and non-small-cell lung cancer. Cancer Med. 2018, 7, 5910–5919. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Kenny, L.; Perry, C.; Thiery, J.-P.; Jovanovic, L.; Vela, I.; Nelson, C.; Punyadeera, C. Impact of label-free technologies in head and neck cancer circulating tumour cells. Oncotarget 2016, 7, 71223. [Google Scholar] [CrossRef]
- Kwak, B.; Lee, J.; Lee, J.; Kim, H.S.; Kang, S.; Lee, Y. Spiral shape microfluidic channel for selective isolating of heterogenic circulating tumor cells. Biosens. Bioelectron. 2018, 101, 311–316. [Google Scholar] [CrossRef]
- Mira, J.M.; Sapre, A.A.; Walker, B.S.; Alvarez, J.B.; Gustafson, K.T.; Tu, E.; Fischer, J.M.; Wong, M.H.; Esener, S.; Chiu, Y.-J. Label-free enrichment of rare unconventional circulating neoplastic cells using a microfluidic dielectrophoretic sorting device. Commun. Biol. 2021, 4, 1130. [Google Scholar] [CrossRef]
- Hoshino, K.; Huang, Y.-Y.; Lane, N.; Huebschman, M.; Uhr, J.W.; Frenkel, E.P.; Zhang, X. Microchip-based immunomagnetic detection of circulating tumor cells. Lab A Chip 2011, 11, 3449–3457. [Google Scholar] [CrossRef]
- Theodoraki, M.; Hoffmann, T.K.; Jackson, E.K.; Whiteside, T.L. Exosomes in HNSCC plasma as surrogate markers of tumour progression and immune competence. Clin. Exp. Immunol. 2018, 194, 67–78. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1+ Exosomes in Plasma of Head and Neck Cancer PatientsPD-L1+ Exosomes in Plasma of HNC Patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Li, T.; Liu, Z.; Li, L. Comparison of ultracentrifugation and density gradient separation methods for isolating Tca8113 human tongue cancer cell line-derived exosomes. Oncol. Lett. 2014, 8, 1701–1706. [Google Scholar] [CrossRef]
- Contreras-Naranjo, J.C.; Wu, H.-J.; Ugaz, V.M. Microfluidics for exosome isolation and analysis: Enabling liquid biopsy for personalized medicine. Lab A Chip 2017, 17, 3558–3577. [Google Scholar] [CrossRef]
- Hudečková, M.; Koucký, V.; Rottenberg, J.; Gál, B. Gene Mutations in Circulating Tumour DNA as a Diagnostic and Prognostic Marker in Head and Neck Cancer—A Systematic Review. Biomedicines 2021, 9, 1548. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Zhang, C.; Ji, T.; Wan, T.; Liu, W. The diagnostic value and prospects of gene mutations in circulating tumor DNA for head and neck cancer monitoring. Oral Oncol. 2022, 128, 105846. [Google Scholar] [CrossRef]
- Chikuie, N.; Urabe, Y.; Ueda, T.; Hamamoto, T.; Taruya, T.; Kono, T.; Yumii, K.; Takeno, S. Utility of plasma circulating tumor DNA and tumor DNA profiles in head and neck squamous cell carcinoma. Sci. Rep. 2022, 12, 9316. [Google Scholar] [CrossRef]
- Chan, H.T.; Chin, Y.M.; Low, S.-K. Circulating tumor DNA-based genomic profiling assays in adult solid tumors for precision oncology: Recent advancements and Future challenges. Cancers 2022, 14, 3275. [Google Scholar] [CrossRef]
- Garrel, R.; Mazel, M.; Perriard, F.; Vinches, M.; Cayrefourcq, L.; Guigay, J.; Digue, L.; Aubry, K.; Alfonsi, M.; Delord, J.-P.; et al. Circulating tumor cells as a prognostic factor in recurrent or metastatic head and neck squamous cell carcinoma: The CIRCUTEC prospective study. Clin. Chem. 2019, 65, 1267–1275. [Google Scholar] [CrossRef]
- Nichols, A.C.; Lowes, L.E.; Szeto, C.C.T.; Basmaji, J.; Dhaliwal, S.; Chapeskie, C.; Todorovic, B.; Read, N.; Venkatesan, V.; Hammond, A.; et al. Detection of circulating tumor cells in advanced head and neck cancer using the CellSearch system. Head Neck 2012, 34, 1440–1444. [Google Scholar] [CrossRef]
- Lee, J.; Sul, O.; Lee, S.-B. Enrichment of circulating tumor cells from whole blood using a microfluidic device for sequential physical and magnetophoretic separations. Micromachines 2020, 11, 481. [Google Scholar] [CrossRef]
- Bankó, P.; Lee, S.Y.; Nagygyörgy, V.; Zrínyi, M.; Chae, C.H.; Cho, D.H.; Telekes, A. Technologies for circulating tumor cell separation from whole blood. J. Hematol. Oncol. 2019, 12, 1–20. [Google Scholar] [CrossRef]
- McMullen, K.P.; Chalmers, J.J.; Lang, J.C.; Kumar, P.; Jatana, K.R. Circulating tumor cells in head and neck cancer: A review. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Palmirotta, R.; Lovero, D.; Cafforio, P.; Felici, C.; Mannavola, F.; Pellè, E.; Quaresmini, D.; Tucci, M.; Silvestris, F. Liquid biopsy of cancer: A multimodal diagnostic tool in clinical oncology. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794630. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.; Nitiyanandan, R.; Rege, K. Emerging applications of exosomes in cancer therapeutics and diagnostics. Bioeng. Transl. Med. 2017, 2, 70–80. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Wu, H.; Punyadeera, C.; Warkiani, M.E. The use of microfluidic technology for cancer applications and liquid biopsy. Micromachines 2018, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-F.; Trubelja, A.; Shen, A.Q.; Bao, G. Tumour-on-a-chip: Microfluidic models of tumour morphology, growth and microenvironment. J. R. Soc. Interface 2017, 14, 20170137. [Google Scholar] [CrossRef]
- Yang, S.-M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic point-of-care (POC) devices in early diagnosis: A review of opportunities and challenges. Sensors 2022, 22, 1620. [Google Scholar] [CrossRef]
- Descamps, L.; Le Roy, D.; Deman, A.-L. Microfluidic-based technologies for CTC isolation: A review of 10 years of intense efforts towards liquid biopsy. Int. J. Mol. Sci. 2022, 23, 1981. [Google Scholar] [CrossRef]
- Zoupanou, S.; Volpe, A.; Primiceri, E.; Gaudiuso, C.; Ancona, A.; Ferrara, F.; Chiriacò, M.S. SMILE platform: An innovative microfluidic approach for on-chip sample manipulation and analysis in oral cancer diagnosis. Micromachines 2021, 12, 885. [Google Scholar] [CrossRef]
- Carter, S.-S.D.; Atif, A.-R.; Kadekar, S.; Lanekoff, I.; Engqvist, H.; Varghese, O.P.; Tenje, M.; Mestres, G. PDMS leaching and its implications for on-chip studies focusing on bone regeneration applications. Organs Chip 2020, 2, 100004. [Google Scholar] [CrossRef]
- Keshmiri, K.; Huang, H.; Nazemifard, N. Compatibility of poly (dimethylsiloxane) microfluidic systems with high viscosity hydrocarbons. SN Appl. Sci. 2019, 1, 711. [Google Scholar] [CrossRef]
- Lamberti, A.; Marasso, S.L.; Cocuzza, M. PDMS membranes with tunable gas permeability for microfluidic applications. Rsc Adv. 2014, 4, 61415–61419. [Google Scholar] [CrossRef]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review. Anal. Methods 2016, 8, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in materials and their fabrication and functionalization. Anal. Chem. 2019, 92, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Bargahi, N.; Ghasemali, S.; Jahandar-Lashaki, S.; Nazari, A. Recent advances for cancer detection and treatment by microfluidic technology, review and update. Biol. Proced. Online 2022, 24, 1–20. [Google Scholar] [CrossRef]
- Wang, B.; He, B.-S.; Ruan, X.-L.; Zhu, J.; Hu, R.; Wang, J.; Li, Y.; Yang, Y.-H.; Liu, M.-L. An integrated microfluidics platform with high-throughput single-cell cloning array and concentration gradient generator for efficient cancer drug effect screening. Mil. Med. Res. 2022, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Hong, J. Microfluidic systems for high throughput screening. Biochip J. 2008, 2, 12–26. [Google Scholar]
- Wang, C.; Zhang, Y.; Tang, W.; Wang, C.; Han, Y.; Qiang, L.; Gao, J.; Liu, H.; Han, L. Ultrasensitive, high-throughput and multiple cancer biomarkers simultaneous detection in serum based on graphene oxide quantum dots integrated microfluidic biosensing platform. Anal. Chim. Acta 2021, 1178, 338791. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Wu, Y.; Gao, J.; Han, Y.; Chu, Y.; Qiang, L.; Qiu, J.; Gao, Y.; Wang, Y.; et al. High-Throughput, Living Single-Cell, Multiple Secreted Biomarker Profiling Using Microfluidic Chip and Machine Learning for Tumor Cell Classification. Adv. Healthc. Mater. 2022, 11, 2102800. [Google Scholar] [CrossRef]
- Bower, R.; Green, V.L.; Kuvshinova, E.; Kuvshinov, D.; Karsai, L.; Crank, S.T.; Stafford, N.D.; Greenman, J. Maintenance of head and neck tumor on-chip: Gateway to personalized treatment? Future Sci. OA 2017, 3, FSO174. [Google Scholar] [CrossRef] [PubMed]
- Al-Samadi, A.; Poor, B.; Tuomainen, K.; Liu, V.; Hyytiäinen, A.; Suleymanova, I.; Mesimaki, K.; Wilkman, T.; Mäkitie, A.; Saavalainen, P.; et al. In vitro humanized 3D microfluidic chip for testing personalized immunotherapeutics for head and neck cancer patients. Exp. Cell Res. 2019, 383, 111508. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Cho, M.; Park, J.-K. Biomarker barcodes: Multiplexed microfluidic immunohistochemistry enables high-throughput analysis of tissue microarray. Lab A Chip 2021, 21, 3471–3482. [Google Scholar] [CrossRef]
- Kennedy, R.; Kuvshinov, D.; Sdrolia, A.; Kuvshinova, E.; Hilton, K.; Crank, S.; Beavis, A.W.; Green, V.; Greenman, J. A patient tumour-on-a-chip system for personalised investigation of radiotherapy based treatment regimens. Sci. Rep. 2019, 9, 6327. [Google Scholar] [CrossRef] [PubMed]
- Hattersley, S.M.; Sylvester, D.C.; Dyer, C.E.; Stafford, N.D.; Haswell, S.J.; Greenman, J. A microfluidic system for testing the responses of head and neck squamous cell carcinoma tissue biopsies to treatment with chemotherapy drugs. Ann. Biomed. Eng. 2012, 40, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.D.; Green, V.L.; Stafford, N.D.; Greenman, J. Analysis of radiation-induced cell death in head and neck squamous cell carcinoma and rat liver maintained in microfluidic devices. Otolaryngol. Head Neck Surg. 2014, 150, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Cheah, R.; Srivastava, R.; Stafford, N.D.; Beavis, A.W.; Green, V.; Greenman, J. Measuring the response of human head and neck squamous cell carcinoma to irradiation in a microfluidic model allowing customized therapy. Int. J. Oncol. 2017, 51, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, M.; Peant, B.; Lateef, M.A.; Rousset, N.; Kendall-Dupont, J.; Carmona, E.; Monet, F.; Saad, F.; Provencher, D.; Mes-Masson, A.-M.; et al. Micro-dissected tumor tissues on chip: An ex vivo method for drug testing and personalized therapy. Lab A Chip 2016, 16, 312–325. [Google Scholar] [CrossRef]
- Huang, S.-P.; Chuang, Y.-J.; Lee, W.-B.; Tsai, Y.-C.; Lin, C.-N.; Hsu, K.-F.; Lee, G.-B. An integrated microfluidic system for rapid, automatic and high-throughput staining of clinical tissue samples for diagnosis of ovarian cancer. Lab A Chip 2020, 20, 1103–1109. [Google Scholar] [CrossRef]
- Jain, K.K.; Jain, K.K. Personalized immuno-oncology. In Textbook of Personalized Medicine; Springer: Cham, Switzerland, 2021; pp. 479–508. [Google Scholar]
- Sierra, J.; Marrugo-Ramírez, J.; Rodriguez-Trujillo, R.; Mir, M.; Samitier, J. Sensor-integrated microfluidic approaches for liquid biopsies applications in early detection of cancer. Sensors 2020, 20, 1317. [Google Scholar] [CrossRef]
- Wang, C.; Senapati, S.; Chang, H. Liquid biopsy technologies based on membrane microfluidics: High-yield purification and selective quantification of biomarkers in nanocarriers. Electrophoresis 2020, 41, 1878–1892. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.G.; Beekman, P.; Lemay, S.G.; Zuilhof, H.; Le Gac, S.; Van Der Wiel, W.G. Electrochemical detection of tumor-derived extracellular vesicles on nanointerdigitated electrodes. Nano Lett. 2019, 20, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Wuethrich, A.; Rajkumar, A.R.; Shanmugasundaram, K.B.; Reza, K.K.; Dey, S.; Howard, C.B.; Ibn Sina, A.A.; Trau, M. Single droplet detection of immune checkpoints on a multiplexed electrohydrodynamic biosensor. Analyst 2019, 144, 6914–6921. [Google Scholar] [CrossRef]
- Chiadò, A.; Palmara, G.; Chiappone, A.; Tanzanu, C.; Pirri, C.F.; Roppolo, I.; Frascella, F. A modular 3D printed lab-on-a-chip for early cancer detection. Lab A Chip 2020, 20, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.; Hamilton, P.; Wang, Y.; Gorbet, M.; Li, Z.; Phillips, K.S. Medical devices on chips. Nat. Biomed. Eng. 2017, 1, 0045. [Google Scholar] [CrossRef]
- Stewart, A.; Denoyer, D.; Gao, X.; Toh, Y.-C. The FDA modernisation act 2.0: Bringing non-animal technologies to the regulatory table. Drug Discov. Today 2023, 28, 103496. [Google Scholar] [CrossRef]
| Company/Device | Analyte/Sample | Description | Ref. |
|---|---|---|---|
| ANGLE/Parsortix® (Surrey, UK) | CTCs/Blood |
| [71] |
| AZAR innovations (Utrecht, The Netherlands.), Motamed Breast Cancer Research Centre, Eindhoven University of Technology | CTCs/Blood |
| [72] |
| Menarini Silicon Biosystems (Bryn Athyn, PA, USA)/CELLSEARCH® CTC test | CTC/Blood |
| [73] |
| ctDNAs | CTCs | Exosomes | |
|---|---|---|---|
| Location | Blood or Saliva | Blood | Blood, Saliva, Urine |
| Method of detection/screening /isolation | Label dependent:
| Label dependent:
| |
| Sensitivity and specificity |
|
|
|
| Other relevant characteristics |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pillai, S.; Kwan, J.C.; Yaziji, F.; Yu, H.; Tran, S.D. Mapping the Potential of Microfluidics in Early Diagnosis and Personalized Treatment of Head and Neck Cancers. Cancers 2023, 15, 3894. https://doi.org/10.3390/cancers15153894
Pillai S, Kwan JC, Yaziji F, Yu H, Tran SD. Mapping the Potential of Microfluidics in Early Diagnosis and Personalized Treatment of Head and Neck Cancers. Cancers. 2023; 15(15):3894. https://doi.org/10.3390/cancers15153894
Chicago/Turabian StylePillai, Sangeeth, Jan C. Kwan, Fares Yaziji, Hanwen Yu, and Simon D. Tran. 2023. "Mapping the Potential of Microfluidics in Early Diagnosis and Personalized Treatment of Head and Neck Cancers" Cancers 15, no. 15: 3894. https://doi.org/10.3390/cancers15153894
APA StylePillai, S., Kwan, J. C., Yaziji, F., Yu, H., & Tran, S. D. (2023). Mapping the Potential of Microfluidics in Early Diagnosis and Personalized Treatment of Head and Neck Cancers. Cancers, 15(15), 3894. https://doi.org/10.3390/cancers15153894







