Simple Summary
Survival rates among Non-Hodgkin Lymphoma patients have significantly improved in recent years. However, the impact of Non-Hodgkin Lymphoma persists in survivors. The aim of this population-based study was to describe Health-Related Quality of Life and to identify the association between sociodemographic, clinical and psychosocial factors, and self-reported Health-Related Quality of Life among Non-Hodgkin Lymphoma survivors. In total, 251 patients received the questionnaires, of whom 157 responded (63%). The main factors found to be associated with poor HRQoL of NHL survivors were age, sex, presence of anxiety, depression and economic problems. These findings suggest the need for supportive care to improve Health-Related Quality of Life and the consideration of these problems when developing care plans for Non-Hodgkin Lymphoma survivors.
Abstract
Purpose: To describe Health-Related Quality of Life (HRQoL) and to identify the association between sociodemographic, clinical and psychosocial factors, and self-reported HRQoL among NHL survivors. Methods: The data of the cancer registry specialized in hematological malignancies in Côte d’Or (France) were used to identify all patients diagnosed with follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL) from 2010 to 2017. Patients were invited to complete SF-12 and other questionnaires. Results: The HRQoL of NHL survivors was poorer than that of the French general population (p < 0.05) in vitality (48 vs. 56), general health (56 vs. 63), role physical scores (60 vs. 70), role emotional scores (64 vs. 72) and the Mental Component Scale (45 vs. 49). The mean difference in physical functioning decreased per unit increase in age (β = −1.1 (0.3); p < 0.001). Men had better vitality than women (β = 12.4 (6.1); p = 0.04) and the high education level was associated with greater role emotional scores (β = 14.1 (5.4); p = 0.01). Symptoms of anxiety and depression were associated with poorer HRQoL. The satisfaction of social support was associated with significantly greater scores on mental health (β = 17.3 (5.1); p = 0.001) and social functioning (β = 15.7 (7.8); p = 0.04). Socioeconomic deprivation was associated with poorer general health (β = −12.8 (5.2); p = 0.01). Conclusions: From 3 to 11 years post-diagnosis, the main factors found to be associated with poor HRQoL of NHL survivors were age, sex, presence of anxiety, depression and economic problems. These findings suggest the need for supportive care to improve HRQOL and the consideration of these problems when developing care plans for NHL survivors.
1. Introduction
Non-Hodgkin Lymphoma (NHL) is the largest group of hematological malignancies (63%) in France. Aggressive NHL such as diffuse large B-cell lymphoma (DLBCL) and indolent NHL such as follicular lymphoma (FL) are common subtypes of NHL—approximately 18% and 11%, respectively [1]. Survival rates of NHL patients have improved due to advances in treatment, with the introduction of the anti-CD20 monoclonal antibody (rituximab), added to the CHOP chemotherapy regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) [2,3,4]. The 5-year relative survival in Europe was reported to be 72% for FL and 51% for DLBCL (2000–2007), with a 5-year relative survival of 86% for FL and 61% for DLBCL in France (1989–2018) [5,6,7]. However, late physical effects due to the toxicity of treatment, including cardiac dysfunction, neuropathy and metabolic complications, have been identified [8,9]. Moreover, issues such as psychosocial well-being, socioeconomic status and sexual problems tend to persist in the long term and may impair the quality of life of NHL survivors [10,11,12,13,14,15].
In this context, the question of health-related quality of life (HRQoL) in NHL patients is garnering increasing interest. HRQoL is a multi-dimensional concept that encompasses subjective perceptions and symptoms of patients as assessed by psychometric instruments [16,17]. Some studies have investigated the HRQoL of NHL survivors. Smith et al. compared non-active NHL patients with NHL patients on active treatment, to assess HRQoL in a US population-based study using different tools including the 36-item Short Form health survey (SF-36). It was shown that survivors with active disease had worse physical and mental well-being due to a more negative impact of their NHL. However, in this study, the authors did not use the eight dimensions of the SF-36 to investigate HRQOL, but rather the summary scales [18]. In France, Ben Diane et al., using the 12-item Short Form health survey (SF-12), reported that five years after a cancer diagnosis, NHL patients had more impaired physical and mental HRQoL than the general population. However, the study was based on health insurance data, which are less representative than population-based data [19].
To the best of our knowledge, few data on NHL survivors are available from population-based surveys in France. To improve our knowledge of NHL survivors in real life, we conducted a population-based study of HRQoL among survivors of NHL using a French population-based cancer registry specialized in hematological malignancies. The aim of our study was to describe HRQoL and to assess the association between sociodemographic, clinical and psychosocial factors, and self-reported HRQoL among NHL survivors.
2. Materials and Methods
2.1. Study Participants
A population-based, cross-sectional survey was conducted at the Cancer Registry specialized in hematological malignancies in the Côte d’Or area (a French Department with a total of 532,901 residents in 2019). Patients diagnosed with DLBCL or FL between 2010 and 2017, who were still alive on 1 March 2021, with an updated address, were identified. The diagnosis of DLBCL and FL was defined according to the third edition of the International Classification of Diseases for Oncology (ICD-O-3) [20].
Vital status (dead or alive) was updated using the medical files and the administrative data on death certificates to complete missing data. Death status was updated in March 2021. Other forms of hematological malignancies at diagnosis and subjects aged under 18 years were not eligible for this study. Adults with a wrong postal address, unable to express their consent and those who refused to participate were not included in this study.
This study was performed in accordance with the declaration of Helsinki. It was approved by the French national data protection authority (CNIL-MR003 N°2210227-V0) and by the Ethics committee (CPP) South-East III under the number 2020-A03479-30.
2.2. Data Collection
In March 2021, a letter containing the study information leaflet, the study questionnaires and a prepaid return envelope was sent to all patients identified as eligible. In the letter, it was explained that, by returning a completed questionnaire, the patient agreed to participate. Patients were reassured that non-participation did not have any consequence for their follow-up care. For patients who did not respond within one month, a reminder letter was sent together with an additional copy of the questionnaire. The questionnaire responses were anonymous, and coded a random number on each questionnaire to link to the Cancer Registry database.
2.3. Measurements
The Cancer Registry specialized in hematological malignancies in the Côte d’Or area routinely collects data on patient characteristics (gender, date of birth), tumor characteristics (histology, date of diagnosis, Ann arbor stage), treatment and relapse after treatment. The main outcome of this study was HRQoL, as assessed by the French-language version of the 12-item Short Form health survey (SF-12). The SF-12 is a generic questionnaire which generates eight scales, namely: physical functioning, role physical, bodily pain, role emotional, vitality, social functioning, mental health and general health, and two summary scores, the physical component summary (PCS) and the mental component summary (MCS). All scales were scored according to the standard method of the SF-12 scoring manual. Each score ranges from 0 to 100, with higher scores representing a better level of HRQoL. A score of 50 or less on the PCS has been recommended as a cut-off to determine a physical condition; while a score of 42 or less on the MCS may be indicative of clinical depression. The test–retest reliability of the PCS summary measures was 0.890 in the US and 0.864 in the UK, Coefficients of 0.760 in US and 0.774 in UK were observed for the MCS [21,22]. Psychosocial and economics factors were assessed by validated instruments, namely the Hospital Anxiety and Depression Scale (HADS), the six-item Sarason social support (SSQ6) questionnaire, and the Assessment of Precariousness and Health Inequalities for Health Examination Centers (EPICES) score.
The HADS questionnaire, validated and adapted in French in 1989 by Lepine et al., was used to determine the presence of anxiety and depressive disorders. This scale has 14 items, 7 for anxiety and 7 for depression, all rated from 0 to 3. Total scores range from 0 to 21. A score greater than 11 indicates the presence of anxiety or depression [23].
The SSQ6 questionnaire, validated and adapted in French by Rascle et al. in 2005, measures the availability of social support and the individual’s satisfaction with the perceived support. Availability scores range from 0 to 54, and satisfaction scores range from 6 to 36. A higher satisfaction score represents better perceived social support [24].
The EPICES score (“Assessment of Precariousness and Health Inequalities for Health Examination Centers”) was used to determine the level of social deprivation. This questionnaire, validated in France, comprises 11 items, each with 2 possible answers (yes/no), generating an individual deprivation score. The score varies from 0 to 100. A score > 30 constitutes a high level of social and/or material deprivation [25].
The study questionnaires also included questions on marital status, educational level, weight and height and current comorbidity. Sexuality was described on the basis of the questionnaire used in the French national study [19].
2.4. Statistical Analysis
To assess potential selection bias, we compared respondents and non-respondents from routinely collected data of the Cancer Registry specialized in hematological malignancies in the Côte d’Or area. Data from respondents are described using mean (SD) and median (IQR) for quantitative variables, and number (percentage) for categorical variables. We used Fisher’s exact test for categorical variables and the Mann–Whitney test for continuous variables. A p-value < 0.05 was considered statistically significant.
HRQoL, anxiety and depression, social support and deprivation scores were calculated, and described. We also recorded BMI into 4 categories according to the WHO recommendations [26], SSQ6 score (categorized as <median and ≥median), and lymphoma Ann Arbor stage (categorized as stage I–II and stage III–IV) [27]. HRQoL is described in the overall population, and separately in the FL and DLBCL groups. The Mann–Whitney test was used to compare continuous variables and the chi-square test was used to compare categorical variables between FL and DLBCL.A multivariate linear regression model was built to identify independent associations between sociodemographic (age, gender), clinical (time since diagnosis, Ann Arbor stage, comorbidity, BMI) and psychosocial factors (anxiety, depression, social support, economic deprivation) and each scale of the SF-12 questionnaire. Independent variables were selected based on a priori knowledge on risk factors of HRQoL [28]. The backward elimination method with a p-value criterion of 0.157 was used to select the predictors to be included in the multivariable models [29]. Correlations were tested between candidate covariates, at a significance level fixed at 0.05. Additionally, a complementary analysis after multiple imputation was performed to account for missing data. We imputed missing data 20 times to produce 20 complete datasets. We chose variables of theoretical interest: age, sex, time since diagnosis and histology type as predictors. A linear regression model was fitted to these variables. Proc mi- analyze was used to combine the results. The significance level for the multivariable analysis was fixed at p-value < 0.05 for each scale of the SF-12. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Characteristics of the Study Population
Among 436 patients diagnosed with FL and DLBCL, questionnaires were sent to 251 survivors, of whom 157 completed the questionnaires, yielding a response rate of 63% (Figure 1). There was no significant difference in age between respondents and non-respondents. Non-respondents were more often women (62% vs. 45%; p = 0.01) and DLBCL survivors (62% vs. 49%) than respondents (Table 1).
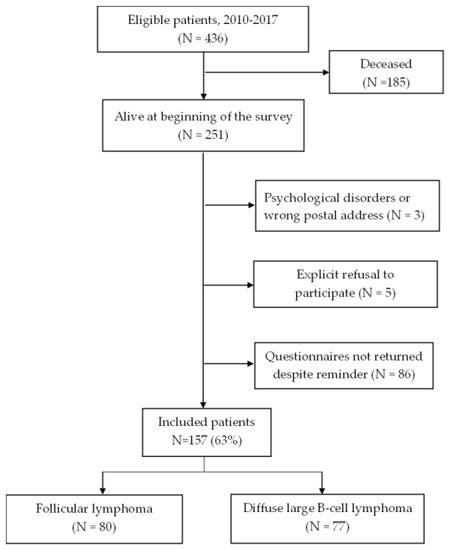
Figure 1.
Flow chart of the study population.

Table 1.
Comparison of sociodemographic and clinical characteristics between respondents and non-respondents.
Table 2 shows that most respondents were married or living maritally (73%) and 48% had a university level of education. The median time since diagnosis was 6 years [4,5,6,7,8], more than half were at Ann Arbor stage III–IV (61%) and did not have a relapse after treatment (92%). Overall, 32% of patients had economic problems, 46% had decreased sexual desire, 14% had symptoms of anxiety and 6% had depressive symptoms as assessed by the HADS. There was no difference between FL and DLBCL survivors, except for treatment by R-CHOP chemotherapy (57% vs. 99%; p < 0.0001).

Table 2.
Sociodemographic, psychological and clinical characteristics of Non-Hodgkin Lymphoma Survivors.
Overall, the highest mean HRQoL score was on the physical functioning scale (72) and the lowest was on the vitality scale (47.6). All subscale scores were higher in FL than DLBCL survivors (Table 3). The comparison of SF-12 scales of NHL survivors with those of the normative sample from the French general population showed a significant difference (p < 0.05) in favor of the general population in vitality (48 vs. 56), general health (56 vs. 63), role physical (60 vs. 70), role emotional (64 vs. 72) and MCS (45 vs. 49). FL survivors had better HRQoL than the general population, the difference was significant (p < 0.05) for bodily pain (73 vs. 66) and physical functioning (76 vs. 71). DLBCL survivors had poorer HRQoL than the general population, the difference was significant (p < 0.05) for general health (55 vs. 62), role physical (55 vs. 71) and MCS (43 vs. 47) (Figure 2a–c).

Table 3.
Characteristics of SF-12 subscale scores in Non-Hodgkin Lymphoma survivors.
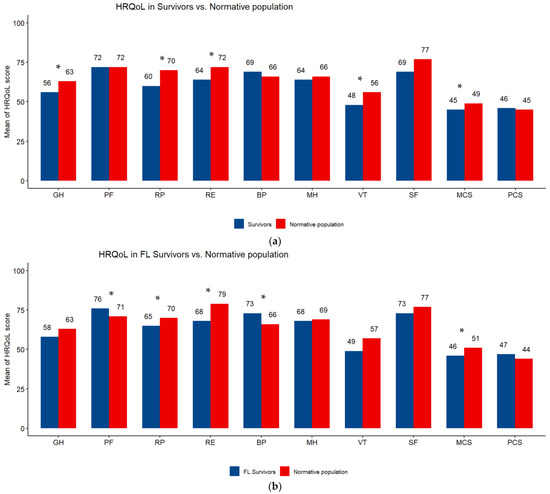
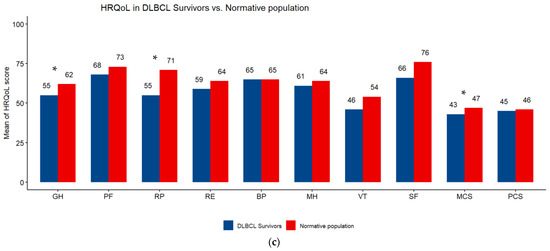
Figure 2.
(a) Subscale scores on the SF-12 questionnaire. Differences between survivors of Non-Hodgkin Lymphoma and a sex- and age-matched normative population. HRQoL: health-related quality of life; PF: physical functioning; RP: role limitations/physical health; RE role limitations/emotional problems; VT: vitality; MH: Mental Health; SF: social functioning; BP: bodily pain; GH: general health; MCS: Mental Component Scale; PCS: Physical Component Scale. Higher scores are indicative of better HRQoL. * p < 0.05. (b) Subscale scores on the SF-12 questionnaire. Differences between survivors of follicular lymphoma (FL) and a sex- and age-matched normative population. HRQoL: health-related quality of life; PF: physical functioning; RP: role limitations/physical health; RE role limitations/emotional problems; VT: vitality; MH: Mental Health; SF: social functioning; BP: bodily pain; GH: general health; MCS: Mental Component Scale; PCS: Physical Component Scale. Higher scores are indicative of better HRQoL. * p < 0.05. (c) Subscale scores on the SF-12 questionnaire. Differences between survivors of diffuse large B-cell lymphoma (DLBCL) and a sex- and age-matched normative population. HRQoL: health-related quality of life; PF: physical functioning; RP: role limitations/physical health; RE role limitations/emotional problems; VT: vitality; MH: Mental Health; SF: social functioning; BP: bodily pain; GH: general health; MCS: Mental Component Scale; PCS: Physical Component Scale. Higher scores are indicative of better HRQoL. * p < 0.05.
3.2. Factors Associated with the Health-Related Quality of Life
The results of the multivariable analysis reporting the factors significantly associated with HRQoL are presented in Table 4. Age was associated with poorer physical functioning (β = −1.1 (0.3); p < 0.001), PCS (β = −0.3 (0.08); p = 0.002) and greater MCS (β = 0.2 (0.09); p = 0.01). Men had better vitality than women (β = 12.4 (6.1); p = 0.04) and the high education level was associated with greater role emotional scores (β = 14.1 (5.4); p = 0.01). Patients with symptoms of anxiety were more likely to have poor general health (β = −14.3 (6.3); p = 0.02), mental health (β = −27.1 (6.1); p < 0.0001) and MCS scales (β = −12.1 (3.4); p = 0.001). Presence of depressive symptoms was associated with significantly poorer scores on the vitality (β = −36.6 (11.1); p = 0.002), social functioning (β = −32.1 (13.8); p = 0.02) and MCS scales (β = −16.3 (4.9); p = 0.001). The satisfaction of social support was associated with significantly greater scores on mental health (β = 17.3 (5.1); p = 0.001) and social functioning (β = 15.7 (7.8); p = 0.04). Socioeconomic deprivation was associated with poorer general health (β = −12.8 (5.2); p = 0.01).

Table 4.
Multivariate linear regression model evaluating independent factors for the SF-12 subscale scores.
A complementary multivariable analysis was carried out after multiple imputation on missing data. The results were almost identical to the main analysis (Appendix A Table A1).
4. Discussion
This population-based study provides a snapshot of HRQoL and the association between sociodemographic, psychosocial and clinical factors and HRQoL in NHL survivors at 3 to 11 years post-diagnosis.
Our study found that NHL survivors had a poorer physical condition and did not have clinical depression. In comparison to the French general population, NHL survivors had poorer general health, vitality and mental HRQoL. Similar findings have been reported in France. Ben Diane et al. reported that five years after a cancer diagnosis, NHL patients had more impaired physical and mental HRQoL than the general population [19]. In the Netherlands, Mols et al. showed that from 5 to 15 years after diagnosis, the general health perceptions and vitality levels of NHL survivors remained significantly lower than those of their peers in the general population [30]. Our study also provides further insights into the HRQoL of FL and DLBCL survivors. We had hypothesized that patients with DLBCL, which is an aggressive form of NHL, would have poorer HRQoL compared to patients FL, which is an indolent form of NHL. The indolent progression of FL may not require aggressive treatment by chemotherapy and immunotherapy, depending on the case. Indeed, the “watch-and-wait” approach established by the Stanford group in the early 1980s is a conservative approach to the treatment of a select group of patients with indolent NHL [31]. In contrast, those with aggressive NHL such as DLBCL require more aggressive treatment. Our results partially support our hypothesis. Indeed, we found that all subscale HRQoL scores were higher in FL than DLBCL survivors. This may be explained by the fact that 99% of our patients with DLBCL had required chemotherapy and immunotherapy compared to 57% of FL patients.
Several factors may explain the poor HRQoL observed such a long time after diagnosis, including the impact of sociodemographic, clinical and psycho-social factors. Indeed, Immanuel et al. found that age was significantly negatively correlated with global QoL, physical and role functioning [32]. Furthermore, time since diagnosis was associated with quality of life after cancer. Wang et al. reported that compared with individuals without cancer, cancer survivors in remission of some types of cancer, such as breast cancer, colorectal cancer and melanoma, may have a similar level of HRQOL after 10 years, while survivors of prostate or cervical cancer still had lower levels of HRQOL. Survivors of hematologic malignancies had a significantly lower physical health between 2 and 9 years. [33]. Chronic conditions may be associated with increased healthcare use, medical expenditure, and lost productivity in cancer survivors. Guy et al. demonstrated that survivors of cancer are more likely than individuals without a history of cancer to have other chronic conditions, with 12.7% reporting four or more chronic conditions in addition to cancer [34]. Moreover, Vissers et al. found that among NHL survivors, comorbidities were significantly associated with lower physical and emotional function, all estimates were in the same direction whereby more comorbidities resulted in lower physical and emotional function [35]. Pettengell et al. showed that patients with relapsed disease had the lowest scores on several HRQoL dimensions. Furthermore, they compared patients on and off chemotherapy, and found that participants receiving chemotherapy reported worse scores on the overall HRQoL scale [36]. Psychosocial effects must be considered when exploring the factors associated with HRQoL, Indeed Lekdamrongkul et al. showed that when NHL survivors had lower anxiety and depression, the HRQoL score was higher [37]. Moreover, low incomes may be associated with poor HRQoL. This view was supported by a cross-sectional study that identified predictors of HRQoL in NHL survivors, finding that financial difficulties related to the disease and its treatment were clinically meaningful problems for NHL survivors and were strongly predictive of deteriorated functioning and global HRQoL [38].
Our multivariable regression analyses revealed that among NHL survivors, sociodemographic and psychosocial factors were significantly associated with HRQoL. The mean difference in physical functioning decreased per unit increase in age. This may be related to the high age of NHL survivors, as the median age in our study was 69 years. Men had better vitality than women. Our findings suggest that physicians should be aware of possible sex difference in NHL survivors. There may be a need for support services that focus on women’s needs. Patients with symptoms of anxiety were more likely to have poor general health and mental health. The presence of depressive symptoms was associated with significantly poorer scores on the vitality, social functioning and MCS scales. Similar findings have been reported, notably indicating that NHL survivors who had more psychological problems also experienced lower HRQoL [39]. This suggests that comprehensive mood disorder management and improved guidelines for appropriate referral to psychological services could enhance HRQOL. Satisfaction with social support was associated with significantly greater scores on role emotional, mental health and social functioning. These findings suggest that more social support and supportive care are warranted during the follow-up of NHL survivors. [40] Socioeconomic deprivation was associated with poorer general health. Thus, to improve the HRQoL of NHL survivors, healthcare providers should consider social inequalities due to low financial capacity when planning for their care.
Clinical factors were not significantly associated with HRQoL except advanced Ann Arbor stages III and IV, which were associated with higher bodily pain. Similar results were found in another study that showed that BMI, type of lymphoma, systemic therapy and radiotherapy were not statistically significantly different for NHL survivors [41].
The strengths of our study are the use of validated instruments to assess HRQoL and psychosocial outcomes and the use of a specialized registry database, which had the advantage of being representative of regionally treated patients and avoiding potential selection bias. Furthermore, the response rate was high (63%). Moreover, to avoid non-response bias, we sent out reminders to increase the response rate. Lastly, independent variables were selected based on a priori knowledge of the risk factors for HRQoL and the backward elimination method with a p-value criterion of 0.157 was used to select the predictors to be included in the multivariable model. Additionally, complementary analysis was performed after multiple imputation and the results were almost identical to the main analysis, with lower R-square values than the analysis on complete data. However, this study had some limitations; notably, the cross-sectional design precluded documenting changes in HRQoL over time, and the study population was relatively small. In a later step, we will conduct a study including all registries specialized in hematological malignancies in France to consolidate the findings of our study. HRQoL two years later will also be investigated in Côte d’Or to assess changes in quality of life over time.
5. Conclusions
Our study found that, from 3 to 11 years post-diagnosis, the HRQoL of NHL survivors was poorer than in the French general population. Overall, NHL survivors had better mental HRQoL and poorer physical HRQoL. The main factors found to be associated with poor HRQoL of NHL survivors were age, sex, presence of anxiety, depression and economic problems. These findings suggest a need for supportive care to improve HRQoL, and consideration of these problems when developing care plans for NHL survivors.
Author Contributions
Conceptualization, M.M. (Marc Maynadie), T.S.D.-Y., M.M. (Morgane Mounier) and S.K.W.; methodology, M.M. (Marc Maynadie), T.S.D.-Y., M.M. (Morgane Mounier); software, M.M. (Morgane Mounier) and J.A.; validation, M.M. (Marc Maynadie), T.S.D.-Y., M.M. (Morgane Mounier); formal analysis, S.K.W., T.S.D.-Y., E.A. and K.M.A.; investigation, S.K.W. and E.A.; resources, M.M. (Marc Maynadie); data curation, S.K.W., S.G. (Sophie Gauthier), S.G. (Stephanie Girard) and J.A.; writing—original draft preparation, S.K.W.; writing—review and editing, S.K.W., M.M. (Marc Maynadie), T.S.D.-Y., C.R. and M.M. (Morgane Mounier); visualization, S.K.W., M.M. (Marc Maynadie), T.S.D.-Y.; supervision, M.M. (Marc Maynadie), T.S.D.-Y. and M.M. (Morgane Mounier); project administration, S.K.W., M.M. (Marc Maynadie), T.S.D.-Y. and M.M. (Morgane Mounier); funding acquisition, M.M. (Marc Maynadie). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the hematological malignancy registry of the Côte d’or which is funded by the French National Cancer Institute.
Institutional Review Board Statement
This study is registered with ClinicalTrials.gov under the identifier NCT04859920 and was performed in accordance with the declaration of Helsinki. This study was approved by the French national data protection authority (CNIL-MR003 N°2210227-V0) and by the Ethics Committee “Committee for the Protection of Persons South-East III” (2020-A03479-30).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The dataset in this study is available from the first author upon reasonable request.
Acknowledgments
We thank Fiona Ecarnot (EA3920, University of Franche-Comté, Besancon, France) for editorial assistance and Adrien Guilloteau (Dijon-Bourgogne University Hospital) for reviewing.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Multivariate Linear Regression Model Evaluating Independent Factors for the SF-12 Subscale Scores after multiple imputation.
Table A1.
Multivariate Linear Regression Model Evaluating Independent Factors for the SF-12 Subscale Scores after multiple imputation.
| GH | PF | RP | RE | BP | MH | VT | SF | MCS | PCS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent Variable | β(SE) | p * | β(SE) | p * | β(SE) | p * | β(SE) | p * | β(SE) | p * | β(SE) | p * | β(SE) | p * | β(SE) | p * | β(SE) | p * | β(SE) | p * |
| Age at time of survey (years) | −0.9 (0.2) | <0.001 | 0.2 (0.06) | 0.003 | −0.2 (0.07) | 0.001 | ||||||||||||||
| Time since diagnosis (years) | 1.2 (0.8) | 0.13 | ||||||||||||||||||
| Sex | 0.09 | |||||||||||||||||||
| Men | 2.2 (5.3) | |||||||||||||||||||
| Women | Ref | |||||||||||||||||||
| Education level | 0.01 | |||||||||||||||||||
| University or higher | 14.1 (5.4) | |||||||||||||||||||
| Primary or Secondary | Ref | |||||||||||||||||||
| Relapse post-treatment | 0.12 | |||||||||||||||||||
| Yes | −8.5 (8.6) | |||||||||||||||||||
| No | ||||||||||||||||||||
| Lymphoma Ann Arbor stage | 0.11 | |||||||||||||||||||
| III–IV | 12.7 (8.1) | |||||||||||||||||||
| I–II | Ref | |||||||||||||||||||
| Anxiety | 0.001 | 0.01 | <0.0001 | 0.005 | ||||||||||||||||
| ≥11 | −17.8 (4.7) | −18.7 (7.5) | −30.1 (5.5) | −9.1 (2.6) | ||||||||||||||||
| <11 | Ref | Ref | Ref | Ref | ||||||||||||||||
| Depression | <0.001 | <0.001 | 0.008 | <0.001 | <0.001 | |||||||||||||||
| ≥11 | −44.2 (9.1) | −48.8 (11.2) | −27.8 (10.5) | −35.7 (8.2) | −16.8 (3.2) | |||||||||||||||
| <11 | Ref | Ref | Ref | Ref | Ref | |||||||||||||||
| Social support satisfaction | <0.001 | 0.002 | 0.003 | <0.001 | ||||||||||||||||
| ≥30 | 23.9 (5.6) | 12.2 (4.1) | 16.6 (4.6) | 10.3 (1.8) | ||||||||||||||||
| <30 | Ref | Ref | Ref | Ref | ||||||||||||||||
| EPICES deprivation score | 0.002 | 0.006 | 0.02 | |||||||||||||||||
| ≥30 | −10.7 (3.5) | −23.2 (8.4) | −4.4 (1.9) | |||||||||||||||||
| <30 | Ref | Ref | Ref | |||||||||||||||||
PF: physical functioning; RP: role limitations/physical health; RE role limitations/emotional problems; VT: vitality; MH: Mental Health; SF: social functioning; BP: bodily pain; GH: general health; MCS: Mental Component Scale; PCS: Physical Component Scale. Variables included in the multivariable model for each scale of SF-12: GH: age at time of survey, anxiety, EPICES deprivation score; PF: age at time of survey; RP: depression; RE: age at time of survey, time since diagnosis, education level, lymphoma Ann Arbor stage, anxiety, depression, BMI at time of survey, social support satisfaction; BP: time since diagnosis, marital status, lymphoma Ann Arbor stage, relapse post-treatment, EPICES deprivation score; MH: time since diagnosis, lymphoma Ann Arbor stage, anxiety, social support satisfaction; VT: sex, depression, relapse post-treatment, EPICES deprivation score; SF: anxiety, depression, social support satisfaction; MCS: age at time of survey, anxiety, depression, social support satisfaction; PCS: age at time of survey, lymphoma Ann Arbor stage, EPICES deprivation score. * p-value significant at <0.05; SE: Standard error.
References
- Le Guyader-Peyrou, S.; Defossez, G.; Dantony, E.; Mounier, M.; Cornet, E.; Uhry, Z.; Cowppli-Bony, A.; Maynadie, M.; Troussard, X.; Delafosse, P.; et al. National Cancer Incidence and Mortality Estimates in France, 1990-2018; Hematologic malignancies. Study based on Francim cancer registries; The French National Cancer Institute: Paris, France, 2019; pp. 37–44, Volume 2. (In French) [Google Scholar]
- Borel, C.; Lamy, S.; Compaci, G.; Récher, C.; Jeanneau, P.; Nogaro, J.C.; Bauvin, E.; Despas, F.; Delpierre, C.; Laurent, G. A longitudinal study of non-medical determinants of adherence to R-CHOP therapy for diffuse large B-cell lymphoma: Implication for survival. BMC Cancer 2015, 15, 288. [Google Scholar] [CrossRef]
- Maloney, D.G. Anti-CD20 Antibody Therapy for B-Cell Lymphomas. N. Engl. J. Med. 2012, 366, 2008–2016. [Google Scholar] [CrossRef]
- Matasar, M.J.; Luminari, S.; Barr, P.M.; Barta, S.K.; Danilov, A.V.; Hill, B.T.; Phillips, T.J.; Jerkeman, M.; Magagnoli, M.; Nastoupil, L.J.; et al. Follicular Lymphoma: Recent and Emerging Therapies, Treatment Strategies, and Remaining Unmet Needs. Oncologist 2019, 24, e1236-50. [Google Scholar] [CrossRef]
- De Angelis, R.; Minicozzi, P.; Sant, M.; Dal Maso, L.; Brewster, D.; Osca-Gelis, G.; Visser, O.; Maynadie, M.; Marcos-Gragera, R.; Troussard, X.; et al. Survival variations by country and age for lymphoid and myeloid malignancies in Europe 2000-2007: Results of EUROCARE-5 population-based study. Eur. J. Cancer 2015, 51, 2254–2268. [Google Scholar] [CrossRef] [PubMed]
- Monnereau, A.; Cornet, E.; Maynadié, M.; Mounier, M.; Troussard, X.; Orazio, S.; Lecoffre, C.; De Brauer, C.; Coureau, G.; Trétarre, B. Survival of People with Cancer in France 1989–2018, Diffuse Large B-cell Lymphoma; Study Based on Francim Cancer Registries; Public Health France, National Institute of Cancer: Paris, France, 2021; pp. 3–7. (In French) [Google Scholar]
- Monnereau, A.; Cornet, E.; Maynadié, M.; Mounier, M.; Troussard, X.; Orazio, S.; Lecoffre, C.; De Brauer, C.; Coureau, G.; Trétarre, B. Survival of People with Cancer in France 1989–2018, Follicular Lymphoma; Study Based on Francim Cancer Registries; Public Health France, National Institute of Cancer: Paris, France, 2021; pp. 3–8. (In French) [Google Scholar]
- Kim, B.-J.; Park, H.-R.; Roh, H.J.; Jeong, D.-S.; Kim, B.S.; Park, K.-W.; Cho, S.C.; So, Y.T.; Oh, S.J.; Kim, S.J. Chemotherapy-related polyneuropathy may deteriorate quality of life in patients with B-cell lymphoma. Qual. Life Res. 2010, 19, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C.; Yi, A.; Horick, N.; Amonoo, H.L.; Newcomb, R.A.; Lavoie, M.W.; Rice, J.; Reynolds, M.J.; Ritchie, C.S.; Nipp, R.D.; et al. Clinical Outcomes, Treatment Toxicity, and Health Care Utilization in Older Adults with Aggressive Non-Hodgkin Lymphoma. Oncologist 2021, 26, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Cho, J.; Kim, I.R.; Kim, M.K.; Kim, W.S.; Kim, S.J. Health-Related Quality of Life in Non-Hodgkin Lymphoma Survivors: A Prospective Cohort Study. Cancer Res Treat. 2018, 50, 1051–1063. [Google Scholar] [CrossRef]
- Allart, P.; Soubeyran, P.; Cousson-Gélie, F. Are psychosocial factors associated with quality of life in patients with haematological cancer? A critical review of the literature. Psychooncology 2013, 22, 241–249. [Google Scholar] [CrossRef]
- Kim, I.-R.; Kim, S.H.; Ok, O.N.; Kim, S.H.; Lee, S.; Choi, E.; Kim, S.J.; Yoon, D.H.; Lee, M.H. Sexual problems in male vs. female non-Hodgkin lymphoma survivors: Prevalence, correlates, and associations with health-related quality of life. Ann. Hematol. 2017, 96, 739–747. [Google Scholar] [CrossRef]
- Kent, E.E.; Sender, L.S.; Morris, R.A.; Grigsby, T.J.; Montoya, M.J.; Ziogas, A.; Anton-Culver, H. Multilevel socioeconomic effects on quality of life in adolescent and young adult survivors of leukemia and lymphoma. Qual. Life Res. 2013, 22, 1339–1351. [Google Scholar] [CrossRef]
- Kim, S.J.; Kang, D.; Kim, I.R.; Yoon, S.E.; Kim, W.S.; Butow, P.N.; Guallar, E.; Cho, J. Impact of fear of cancer recurrence on survival among lymphoma patients. Psychooncology 2020, 29, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Leak, A.; Smith, S.K.; Crandell, J.; Jenerette, C.; Bailey, D.E.; Zimmerman, S.; Mayer, D.K. Demographic and Disease Characteristics associated with non- Hodgkin Lymphoma Survivors’ Quality of Life. Oncol. Nurs. Forum 2013, 40, 157–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Husson, O.; de Rooij, B.H.; Kieffer, J.; Oerlemans, S.; Mols, F.; Aaronson, N.K.; Van Der Graaf, W.T.A.; Van De Poll-Franse, L.V. The EORTC QLQ-C30 Summary Score as Prognostic Factor for Survival of Patients with Cancer in the “Real-World”: Results from the Population-Based PROFILES Registry. Oncologist 2020, 25, e722–e732. [Google Scholar] [CrossRef] [PubMed]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.K.; Zimmerman, S.; Williams, C.S.; Zebrack, B.J. Health status and quality of life among non-Hodgkin lymphoma survivors. Cancer 2009, 115, 3312–3323. [Google Scholar] [CrossRef] [PubMed]
- Ben Diane, M.-K.; Bousquet, P.J.; Chauvet, C.; Gaillot-De-Saintignon, J.; Lafay, L.; Morin, C.; Philibert, V. Life 5 Years after a Cancer Diagnosis. A 2004 Survey on the Living Conditions of Patients. 2018. Available online: https://www.proinfoscancer.org/sites/default/files/2018-6-19-la_vie_cinq_ans_apres_un_diagnostic_de_cancer_rapport_mel_inserm-inca.pdf (accessed on 1 April 2021). (In French).
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam KSobin LParkin, D.M.; Whelan, S. International Classification of diseases for Oncology, 3rd ed.; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Ware, J.E.; Kosinski, M.; Turner-Bowker, D.M.; Gandek, B. How to Score Version 2 of the SF-12 Health Survey: With a Supplement Documenting Version 1; Quality Metric: Lincoln, RI, USA, 2005. [Google Scholar]
- Ware, J.E.; Kosinski, M.; Keller, S.D. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales, 2nd ed.; The Health Institute: Boston, MA, USA; New England Medical Center: Boston, MA, USA, 1995. [Google Scholar]
- Lepine, J.P.; Godchau, M.; Brun, P. Anxiety and depression in patients. Lancet 1985, 2, 1425–1426. [Google Scholar] [CrossRef]
- Rascle, N.; Bruchon-Schweitzer, M.; Sarason, I.G. Short Form of Sarason’s Social Support Questionnaire: French Adaptation and Validation. Psychol. Rep. 2005, 97, 195–202. [Google Scholar] [CrossRef]
- Sass, C.; Moulin, J.J.; Gueguen, R.; Abric, L.; Dauphinot, V.; Dupre, C.; Giordanella, J.P.; Girard, F.; Guenot, C.; Labbe, E.; et al. The Epices score: An individual score of deprivation. Score construction and measurement of relationships with health data, in a population of 197389. Bull. Epidemiology Hebd. 2006, 14, 93–96. (In French) [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. 2000. Available online: https://apps.who.int/iris/handle/10665/42330 (accessed on 1 June 2023).
- Rosenberg, S.A. Validity of the Ann Arbor staging classification for the non-Hodgkin's lymphomas. Cancer Treat. Rep. 1977, 61, 1023–1027. [Google Scholar]
- Kaul, S.; Avila, J.C.; Jupiter, D.; Rodriguez, A.M.; Kirchhoff, A.C.; Kuo, Y.F. Modifiable health-related factors (smoking, physical activity and body mass index) and health care use and costs among adult cancer survivors. J. Cancer Res. Clin. Oncol. 2017, 143, 2469–2480. [Google Scholar] [CrossRef]
- Heinze, G.; Dunkler, D. Five myths about variable selection. Transpl. Int. 2017, 30, 6–10. [Google Scholar] [CrossRef]
- Mols, F.; Aaronson, N.; Vingerhoets, A. Quality of life among long-term non-Hodgkin lymphoma survivors. Cancer 2007, 109, 1659–1667. [Google Scholar] [CrossRef]
- Horning, S.J.; Rosenberg, S.A. The natural history of initially untreated low-grade non-Hodgkin’s lymphomas. N. Engl. J. Med. 1984, 311, 1471–1475. [Google Scholar] [CrossRef]
- Immanuel, A.; Hunt, J.; McCarthy, H.; van Teijlingen, E.; Sheppard, Z. Quality of life in survivors of adult haematological ma-lignancy. Eur. J. Cancer Care 2019, 28, e13067. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hsu, S.H.; Gross, C.P.; Sanft, T.; Davidoff, A.J.; Ma, X.; Yu, J.B. Association between time since cancer diagnosis and health-related quality of life: A population-level analysis. Value Health 2016, 19, 631–638. [Google Scholar] [CrossRef]
- Guy, G.P., Jr.; Yabroff, K.R.; Ekwueme, D.U.; Rim, S.H.; Li, R.; Richardson, L.C. Economic burden of chronic conditions among survivors of cancer in the United States. J. Clin. Oncol. 2017, 35, 2053–2061. [Google Scholar] [CrossRef]
- Vissers, P.A.J.; Thong, M.S.Y.; Pouwer, F.; Zanders, M.M.J.; Coebergh, J.W.W.; Van de Poll-Franse, L.V. The impact of comorbidity on Health-Related Quality of Life among cancer survivors: Analyses of data from the PROFILES registry. J. Cancer Surviv. 2013, 7, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Pettengell, R.; Donatti, C.; Hoskin, P.; Poynton, C.; Kettle, P.J.; Hancock, B.; Johnson, S.; Dyer, M.J.; Rule, S.; Walker, M.; et al. The impact of follicular lymphoma on health-related quality of life. Ann. Oncol. 2008, 19, 570–576. [Google Scholar] [CrossRef]
- Lekdamrongkul, P.; Pongthavornkamol, K.; Molassiotis, A.; Sriyuktasuth, A.; Siritanaratkul, N.; Chansatitporn, N. Exploring health-related quality of life among non-Hodgkin’s lymphoma survivors after completion of primary treatment: A cross-sectional study in Thailand. Support. Care Cancer 2021, 29, 6511–6522. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, I.R.; Kim, S.H.; Lee, S.; Ok, O.; Kim, W.S.; Suh, C.; Lee, M.H. Health-related quality of life in Korean lymphoma survivors compared with the general population. Ann. Hematol. 2014, 93, 1531–1540. [Google Scholar] [CrossRef]
- Geffen, D.; Blaustein, A.; Amir, M.; Cohen, Y. Post-traumatic stress disorder and quality of life in longterm survivors of Hodgkin’s disease and non-Hodgkin’s lymphoma in Israel. Leuk. Lymphoma 2003, 44, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.; Wurz, A.; Bradshaw, A.; Saunders, S.; West, M.A.; Brunet, J. Physical activity and quality of life in cancer survivors: A meta-synthesis of qualitative research. Cancers 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Vlooswijk, C.; Oerlemans, S.; Ezendam, P.M.; Schep, G.; Slot, S.; Thong, S.Y.; Vissers, A.J.; Beijer, S. Physical Activity is Associated with Health Related Quality of Life in Lymphoma Survivors Regardless of Body Mass Index; Results from the Profiles Registry. Nutr. Cancer 2022, 74, 158–167. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).