A Machine Learning Approach Using FDG PET-Based Radiomics for Prediction of Tumor Mutational Burden and Prognosis in Stage IV Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Subjects and Clinical Data
2.2. Tumor Mutational Burden
2.3. FDG PET/CT Imaging
2.4. PET Radiomic Features
2.5. Machine Learning Approach
2.6. Statistical Analysis
3. Results
3.1. Patients
3.2. PET-Based Radiomics and Tumor Mutation Burden
3.3. Prognostic Validation of PET Radiomic Score
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 1 September 2022).
- Glimelius, B.; Hoffman, K.; Graf, W.; Haglund, U.; Nyrén, O.; Páhlman, L.; Sjödén, P.-O. Cost-effectiveness of palliative chemotherapy in advanced gastrointestinal cancer. Ann. Oncol. 1995, 6, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Colorectal Cancer Collaborative Group. Palliative chemotherapy for advanced colorectal cancer: Systematic review and meta-analysis. BMJ 2000, 321, 531–535. [Google Scholar] [PubMed] [Green Version]
- Biller, L.H.; Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Ooki, A.; Shinozaki, E.; Yamaguchi, K. Immunotherapy in Colorectal Cancer: Current and Future Strategies. J. Anus Rectum Colon 2021, 5, 11–24. [Google Scholar] [CrossRef]
- Almuhaideb, A.; Papathanasiou, N.; Bomanji, J. 18F-FDG PET/CT imaging in oncology. Ann. Saudi Med. 2011, 31, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Ben-Haim, S.; Ell, P. 18F-FDG PET and PET/CT in the Evaluation of Cancer Treatment Response. J. Nucl. Med. 2009, 50, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.S.; Jeong, Y.Y.; Min, J.J.; Kim, H.R.; Chung, T.W.; Kang, H.K. Preoperative staging of colorectal cancer: CT vs. integrated FDG PET/CT. Abdom. Imaging 2007, 33, 270–277. [Google Scholar] [CrossRef]
- Park, I.; Kim, H.; Yu, C.; Ryu, M.; Chang, H.; Ryu, J.; Yeo, J.; Kim, J. Efficacy of PET/CT in the accurate evaluation of primary colorectal carcinoma. Eur. J. Surg. Oncol. 2006, 32, 941–947. [Google Scholar] [CrossRef]
- Kido, H.; Kato, S.; Funahashi, K.; Shibuya, K.; Sasaki, Y.; Urita, Y.; Hori, M.; Mizumura, S. The metabolic parameters based on volume in PET/CT are associated with clinicopathological N stage of colorectal cancer and can predict prognosis. EJNMMI Res. 2021, 11, 87. [Google Scholar] [CrossRef]
- Lee, S.-W.; Park, H.L.; Yoon, N.; Kim, J.H.; Oh, J.K.; Buyn, J.H.; Choi, E.K.; Hong, J.H. Prognostic Impact of Total Lesion Glycolysis (TLG) from Preoperative 18F-FDG PET/CT in Stage II/III Colorectal Adenocarcinoma: Extending the Value of PET/CT for Resectable Disease. Cancers 2022, 14, 582. [Google Scholar] [CrossRef]
- Lam, L.H.T.; Chu, N.T.; Tran, T.-O.; Do, D.T.; Le, N.Q.K. A Radiomics-Based Machine Learning Model for Prediction of Tumor Mutational Burden in Lower-Grade Gliomas. Cancers 2022, 14, 3492. [Google Scholar] [CrossRef]
- Le, V.H.; Kha, Q.H.; Minh, T.N.T.; Nguyen, V.H.; Le, V.L.; Le, N.Q.K. Development and Validation of CT-Based Radiomics Signature for Overall Survival Prediction in Multi-organ Cancer. J. Digit. Imaging 2023, 36, 911–922. [Google Scholar] [CrossRef]
- Nakajo, M.; Kajiya, Y.; Tani, A.; Jinguji, M.; Nakajo, M.; Kitazono, M.; Yoshiura, T. A pilot study for texture analysis of 18F-FDG and 18F-FLT-PET/CT to predict tumor recurrence of patients with colorectal cancer who received surgery. Eur. J. Nucl. Med. 2017, 44, 2158–2168. [Google Scholar] [CrossRef]
- Hotta, M.; Minamimoto, R.; Gohda, Y.; Miwa, K.; Otani, K.; Kiyomatsu, T.; Yano, H. Prognostic value of 18F-FDG PET/CT with texture analysis in patients with rectal cancer treated by surgery. Ann. Nucl. Med. 2021, 35, 843–852. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Lee, D.-W.; Han, S.-W.; Bae, J.M.; Jang, H.; Han, H.; Kim, H.; Kim, T.Y. Tumor Mutation Burden and Prognosis in Patients with Colorectal Cancer Treated with Adjuvant Fluoropyrimidine and OxaliplatinTumor Mutation Burden in Colorectal Cancer. Clin. Cancer Res. 2019, 25, 6141–6147. [Google Scholar] [CrossRef] [Green Version]
- Fabrizio, D.A.; George, T.J., Jr.; Dunne, R.F.; Frampton, G.; Sun, J.; Gowen, K.; Klempner, S.J. Beyond microsatellite testing: Assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J. Gastrointest. Oncol. 2018, 9, 610–617. [Google Scholar] [CrossRef]
- Moon, S.H.; Kim, J.; Joung, J.-G.; Cha, H.; Park, W.-Y.; Ahn, J.S.; Ahn, M.-J.; Park, K.; Choi, J.Y.; Lee, K.-H.; et al. Correlations between metabolic texture features, genetic heterogeneity, and mutation burden in patients with lung cancer. Eur. J. Nucl. Med. 2018, 46, 446–454. [Google Scholar] [CrossRef]
- Jahromi, A.H.; Barkauskas, D.A.; Zabel, M.; Goodman, A.M.; Frampton, G.; Nikanjam, M.; Hoh, C.K.; Kurzrock, R. Relationship between tumor mutational burden and maximum standardized uptake value in 2-[18F]FDG PET (positron emission tomography) scan in cancer patients. EJNMMI Res. 2020, 10, 150. [Google Scholar] [CrossRef]
- Kim, H.R.; Ahn, S.; Jo, H.; Kim, H.; Hong, J.; Lee, J.; Kim, S.T. The impact of tumor mutation burden (TMB) on the effect of frontline trastuzumab plus chemotherapy in human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancers. Front. Oncol. 2021, 11, 792340. [Google Scholar] [CrossRef] [PubMed]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Buvat, I. LIFEx: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortin, J.-P.; Parker, D.; Tunç, B.; Watanabe, T.; Elliott, M.A.; Ruparel, K.; Roalf, D.R.; Satterthwaite, T.D.; Gur, R.C.; Gur, R.E.; et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 2017, 161, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Beham, A.; Rentsch, M.; Püllmann, K.; Mantouvalou, L.; Spatz, H.; Schlitt, H.J.; Obed, A. Survival benefit in patients after palliative resection vs non-resection colon cancer surgery. World J. Gastroenterol. 2006, 12, 6634–6638. [Google Scholar] [PubMed]
- Brouwer, N.P.; Bos, A.C.; Lemmens, V.E.; Tanis, P.J.; Hugen, N.; Nagtegaal, I.D.; de Wilt, J.H.; Verhoeven, R.H. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int. J. Cancer 2018, 143, 2758–2766. [Google Scholar] [CrossRef]
- Riaz, N.; Morris, L.; Havel, J.J.; Makarov, V.; Desrichard, A.; Chan, T.A. The role of neoantigens in response to immune checkpoint blockade. Int. Immunol. 2016, 28, 411–419. [Google Scholar] [CrossRef] [Green Version]
- El Naqa, I.; Grigsby, P.; Apte, A.; Kidd, E.; Donnelly, E.; Khullar, D.; Chaudhari, S.; Yang, D.; Schmitt, M.; Laforest, R.; et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit. 2009, 42, 1162–1171. [Google Scholar] [CrossRef] [Green Version]
- Thibault, G.F.B.; Navarro, C.; Pereira, S.; Cau, P.; Levy, N.; Sequeira, J.; Mari, J.L. Texture Indexes and Gray Level Size Zone Matrix Application to Cell Nuclei Classification. In Proceedings of the 10th International Conference on Pattern Recognition and Information Processing, Minsk, Belarus, 19–21 May 2009; pp. 140–145. [Google Scholar]
- Zhang, Y.; Chang, L.; Yang, Y.; Fang, W.; Guan, Y.; Wu, A.; Hong, S.; Zhou, H.; Chen, G.; Chen, X.; et al. The correlations of tumor mutational burden among single-region tissue, multi-region tissues and blood in non-small cell lung cancer. J. Immunother. Cancer 2019, 7, 98. [Google Scholar] [CrossRef]
- Ozaki, Y.; Muto, S.; Takagi, H.; Watanabe, M.; Inoue, T.; Fukuhara, M.; Yamaura, T.; Okabe, N.; Matsumura, Y.; Hasegawa, T.; et al. Tumor mutation burden and immunological, genomic, and clinicopathological factors as biomarkers for checkpoint inhibitor treatment of patients with non-small-cell lung cancer. Cancer Immunol. Immunother. 2019, 69, 127–134. [Google Scholar] [CrossRef]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden–High Solid Tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef]
- Alborelli, I.; Leonards, K.; Rothschild, S.I.; Leuenberger, L.P.; Prince, S.S.; Mertz, K.; Poechtrager, S.; Buess, M.; Zippelius, A.; Läubli, H.; et al. Tumor mutational burden assessed by targeted NGS predicts clinical benefit from immune checkpoint inhibitors in non-small cell lung cancer. J. Pathol. 2019, 250, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.; Xie, F.; Mao, J.; Bai, Y.; Wang, X. Significance of Tumor Mutation Burden in Immune Infiltration and Prognosis in Cutaneous Melanoma. Front. Oncol. 2020, 10, 573141. [Google Scholar] [CrossRef]
- Bundschuh, R.A.; Dinges, J.; Neumann, L.; Seyfried, M.; Zsótér, N.; Papp, L.; Rosenberg, R.; Becker, K.; Astner, S.T.; Henninger, M.; et al. Textural Parameters of Tumor Heterogeneity in 18F-FDG PET/CT for Therapy Response Assessment and Prognosis in Patients with Locally Advanced Rectal Cancer. J. Nucl. Med. 2014, 55, 891–897. [Google Scholar] [CrossRef] [Green Version]



| Characteristics | Overall | Low TMB | High TMB | p |
|---|---|---|---|---|
| (n = 91) | (n = 70) | (n = 21) | ||
| Age (range, years) | 58.0 (21–88) | 56.8 (33–88) | 62.1 (21–84) | 0.154 |
| Sex, male | 53 (57.6%) | 38 (54.3%) | 15 (71.4%) | 0.162 |
| TMB | 9.4 ± 12.1 | 5.7 ± 2.3 | 22.0 ± 2.3 | 0.002 |
| Histological type | 0.570 | |||
| Adenocarcinoma | 88 (96.7%) | 68 (97.1%) | 20 (95.2%) | |
| Small cell carcinoma | 1 (1.1%) | 1 (1.4%) | 0 (0%) | |
| Unclassified carcinoma | 2 (2.2%) | 1 (1.4%) | 1 (4.8%) | |
| Histological grade | 0.430 | |||
| Unknown | 14 (15.2%) | 9 (12.9%) | 5 (23.8%) | |
| Well-differentiated | 12 (13.0%) | 11 (15.7%) | 1 (4.8%) | |
| Moderately differentiated | 53 (57.6) | 41 (58.6%) | 12 (57.1%) | |
| Poorly differentiated | 12 (13.0%) | 9 (12.9%) | 3 (14.3%) | |
| Location | 0.012 | |||
| Colon | 39 (42.9%) | 25 (35.7%) | 14 (66.7%) | |
| Rectum | 52 (57.1%) | 45 (64.3%) | 7 (33.3%) | |
| Chemotherapy regimen | 0.760 | |||
| FOLFOX | 58 (63.7%) | 46 (65.7%) | 12 (57.1%) | |
| FOLFIRI | 25 (27.5%) | 18 (25.7%) | 6 (28.6%) | |
| Others | 6 (6.6%) | 5 (7.1%) | 2 (9.5%) | |
| None | 2 (2.2%) | 1 (1.4%) | 1 (4.8%) | |
| Palliative surgery | 0.157 | |||
| Yes | 44 (48.4%) | 31 (44.3%) | 13 (61.9%) | |
| No | 47 (51.6%) | 39 (55.7%) | 8 (38.1%) |
| Features | Log (OR) | p | FDR |
|---|---|---|---|
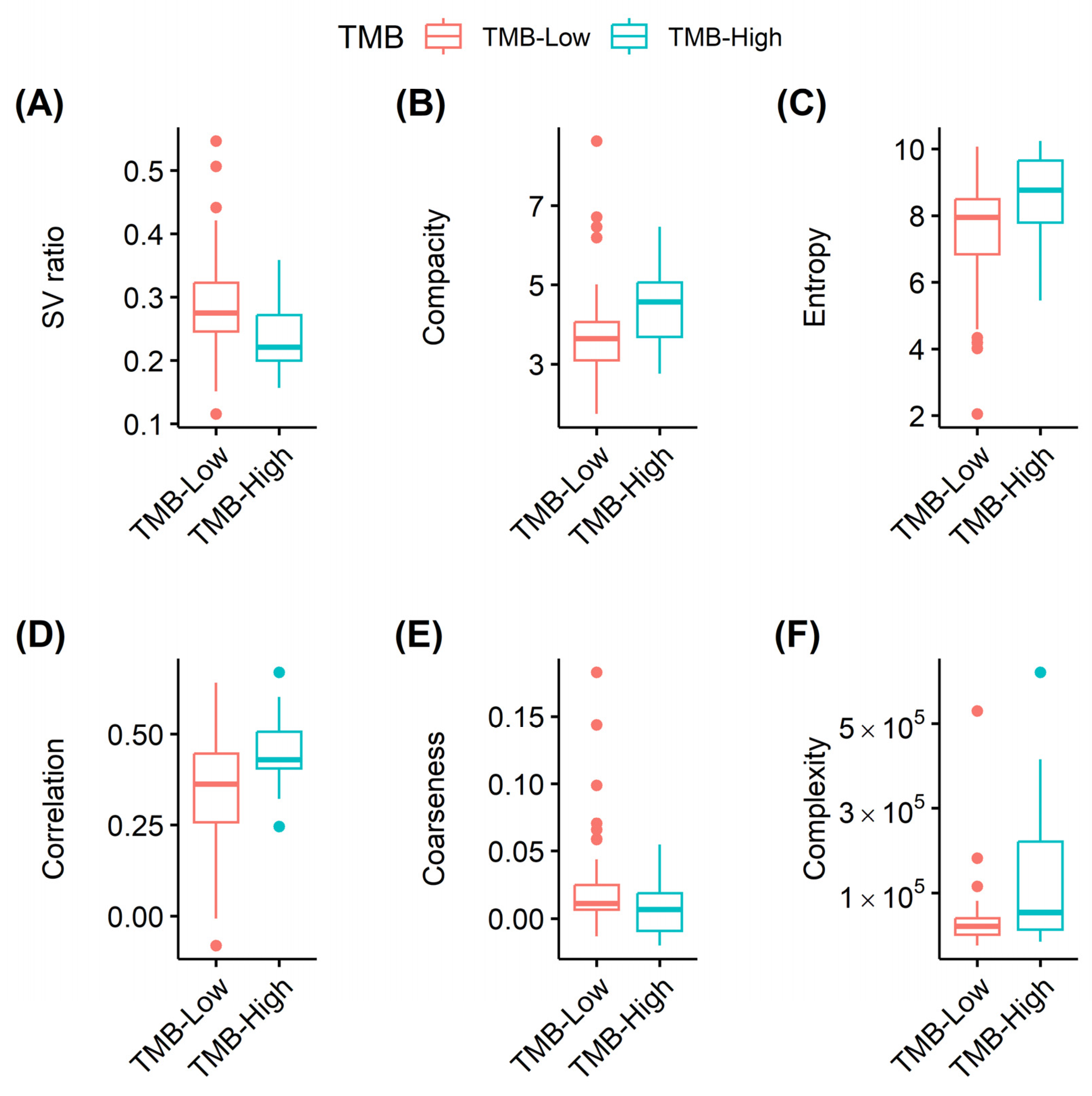
| Surface-to-volume ratio | −13.33 | 0.006 | 0.036 |
| Total lesion glycolysis | 2.02 × 10−6 | 0.007 | 0.036 |
| Volume | 1.86 × 10−5 | 0.010 | 0.036 |
| Area | 9.93 × 10−5 | 0.012 | 0.036 |
| Compacity | 0.58 | 0.014 | 0.036 |
| Complexity | 8.48 × 10−6 | 0.004 | 0.019 |
| Entropy | 0.627 | 0.011 | 0.051 |
| Correlation | 6.20 | 0.006 | 0.051 |
| Coarseness | −33.64 | 0.037 | 0.092 |
| Zone size non-uniformity | 0.003 | 0.002 | 0.037 |
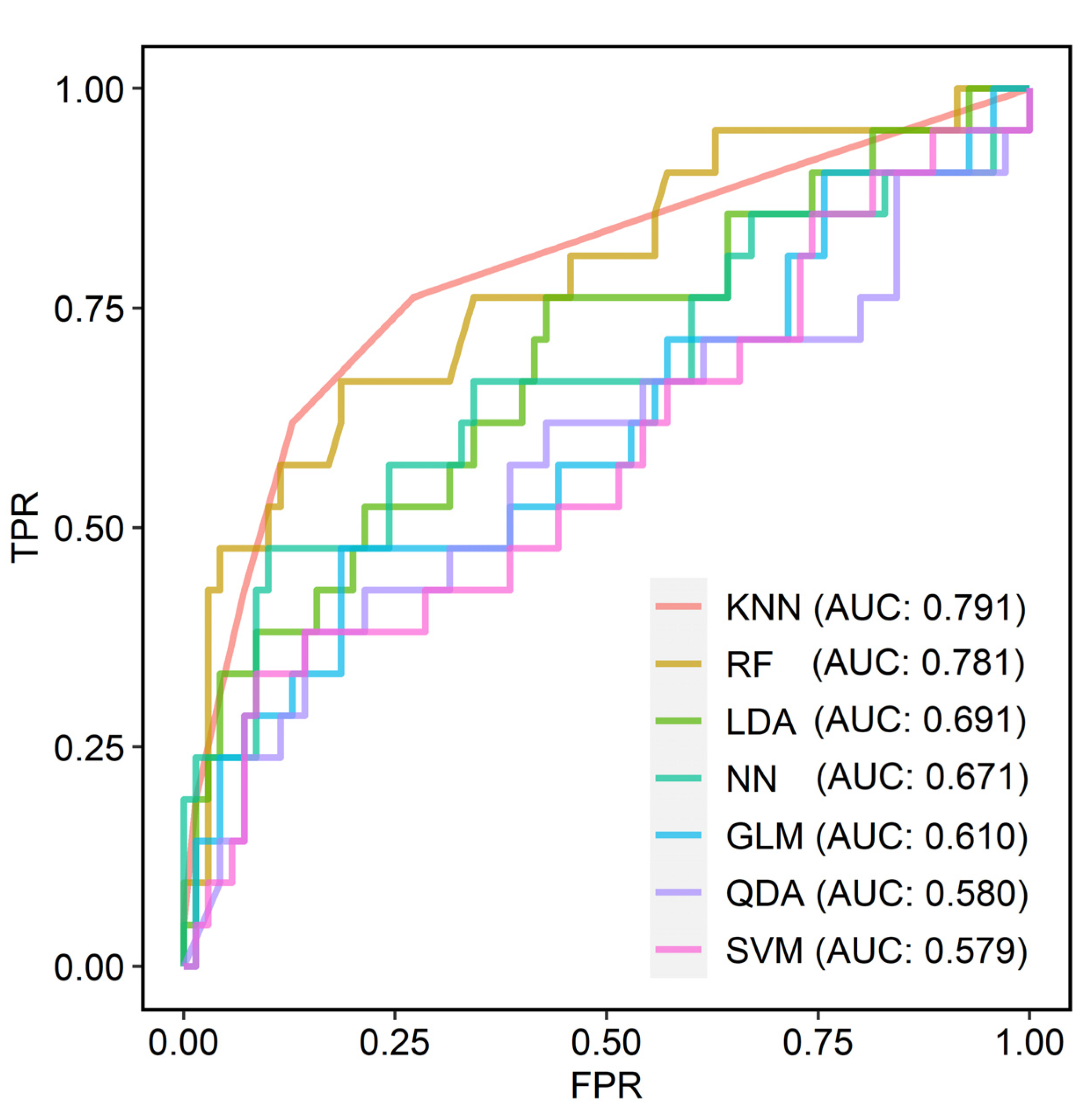
| Models | AUC | Accuracy | Sensitivity | Specificity | F1 Score | Precision |
|---|---|---|---|---|---|---|
| GLM | 0.610 | 0.769 | 0.476 | 0.814 | 0.455 | 0.435 |
| LDA | 0.691 | 0.802 | 0.762 | 0.571 | 0.478 | 0.348 |
| QDA | 0.580 | 0.747 | 0.381 | 0.857 | 0.410 | 0.444 |
| KNN | 0.791 | 0.814 | 0.619 | 0.871 | 0.605 | 0.591 |
| SVM | 0.579 | 0.758 | 0.333 | 0.914 | 0.412 | 0.538 |
| RF | 0.781 | 0.836 | 0.667 | 0.814 | 0.583 | 0.519 |
| NN | 0.671 | 0.780 | 0.476 | 0.900 | 0.526 | 0.588 |
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Sex | ||||||
| Female vs. Male | 0.877 | 0.295–2.610 | 0.813 | 1.422 | 0.403–5.020 | 0.584 |
| Age | ||||||
| ≥56 | 1.097 | 0.378–3.185 | 0.865 | 0.407 | 0.106–1.557 | 0.189 |
| Location | ||||||
| Colon vs. Rectum | 1.023 | 0.352–2.974 | 0.966 | 1.050 | 0.282–3.904 | 0.942 |
| Histological grade | ||||||
| Well-differentiated | ||||||
| Moderately differentiated | 0.732 | 0.083–6.434 | 0.779 | 0 | 0.998 | |
| Poorly differentiated | 1.947 | 0.212–17.886 | 0.556 | 5.389 | 0.424–68.475 | 0.194 |
| Chemotherapy regimen | ||||||
| FOLFOX | ||||||
| FOLFIRI | 0.324 | 0.067–1.561 | 0.160 | 0.315 | 0.056–1.778 | 0.191 |
| Others | 3.037 | 0.348–26.501 | 0.315 | 4.303 | 0.367–50.483 | 0.245 |
| Palliative surgery | ||||||
| Yes | 0.210 | 0.057–0.772 | 0.019 | 0.123 | 0.026–0.594 | 0.009 |
| TMB (continuous) | 0.948 | 0.861–1.044 | 0.280 | |||
| TMB group | ||||||
| Low vs. High | 1.334 | 0.398–4.471 | 0.640 | 0.471 | 0.097–2.280 | 0.349 |
| PET radiomic score | ||||||
| Low vs. High | 3.081 | 1.078–8.810 | 0.036 | 4.684 | 1.069–20.526 | 0.040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Moon, S.H.; Hong, J.Y.; Lee, J.; Hyun, S.H. A Machine Learning Approach Using FDG PET-Based Radiomics for Prediction of Tumor Mutational Burden and Prognosis in Stage IV Colorectal Cancer. Cancers 2023, 15, 3841. https://doi.org/10.3390/cancers15153841
Lee H, Moon SH, Hong JY, Lee J, Hyun SH. A Machine Learning Approach Using FDG PET-Based Radiomics for Prediction of Tumor Mutational Burden and Prognosis in Stage IV Colorectal Cancer. Cancers. 2023; 15(15):3841. https://doi.org/10.3390/cancers15153841
Chicago/Turabian StyleLee, Hyunjong, Seung Hwan Moon, Jung Yong Hong, Jeeyun Lee, and Seung Hyup Hyun. 2023. "A Machine Learning Approach Using FDG PET-Based Radiomics for Prediction of Tumor Mutational Burden and Prognosis in Stage IV Colorectal Cancer" Cancers 15, no. 15: 3841. https://doi.org/10.3390/cancers15153841
APA StyleLee, H., Moon, S. H., Hong, J. Y., Lee, J., & Hyun, S. H. (2023). A Machine Learning Approach Using FDG PET-Based Radiomics for Prediction of Tumor Mutational Burden and Prognosis in Stage IV Colorectal Cancer. Cancers, 15(15), 3841. https://doi.org/10.3390/cancers15153841





