Clock-like Mutation Signature May Be Prognostic for Worse Survival Than Signatures of UV Damage in Cutaneous Melanoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. NGS Analysis Using MelArray
2.3. Statistical Analysis
3. Results
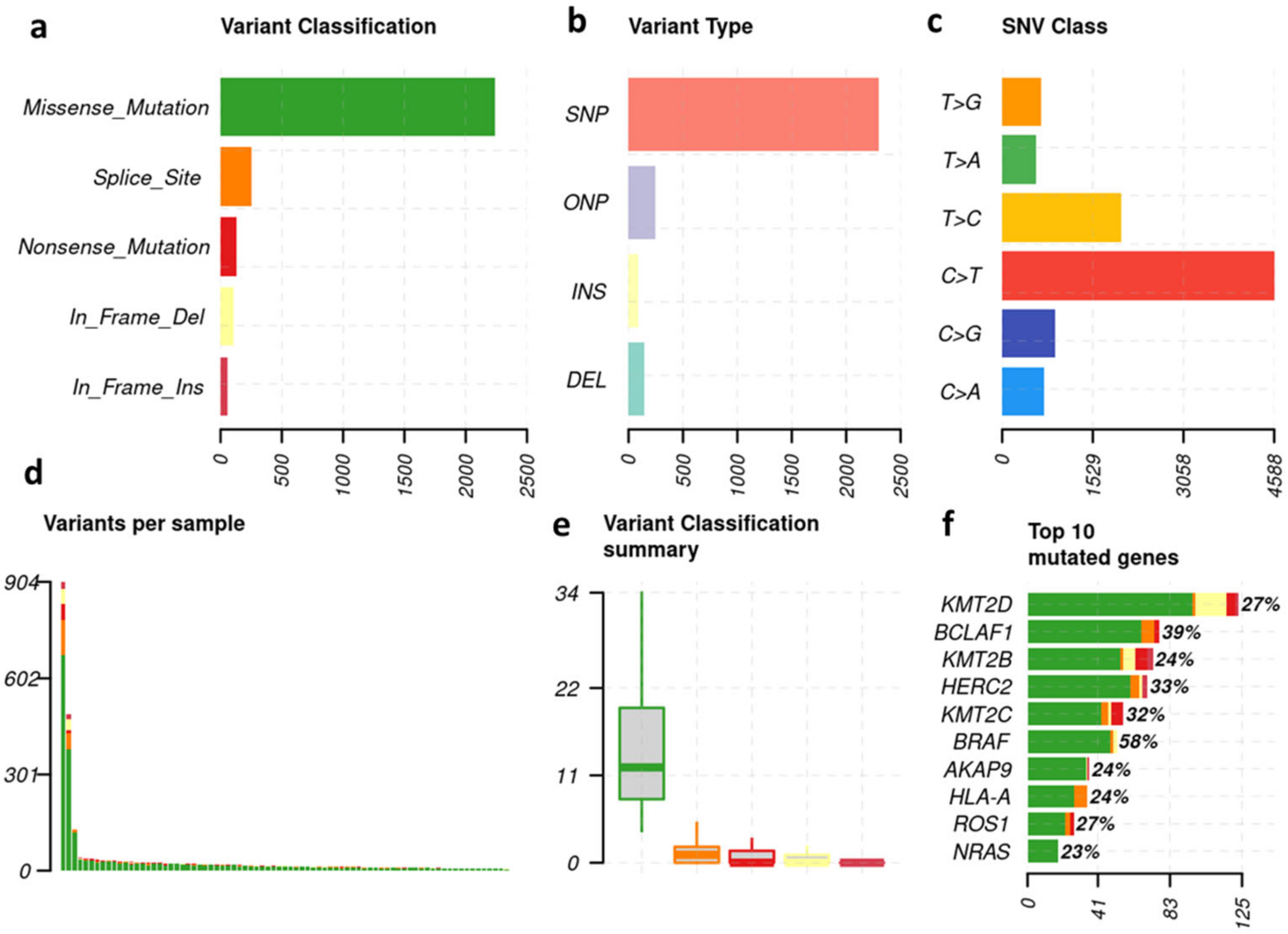
3.1. Melanoma-Typical Mutations Are Represented in Patient Cohort
3.2. The 30 Most Frequent Mutations—Overall and Subgroup Analysis
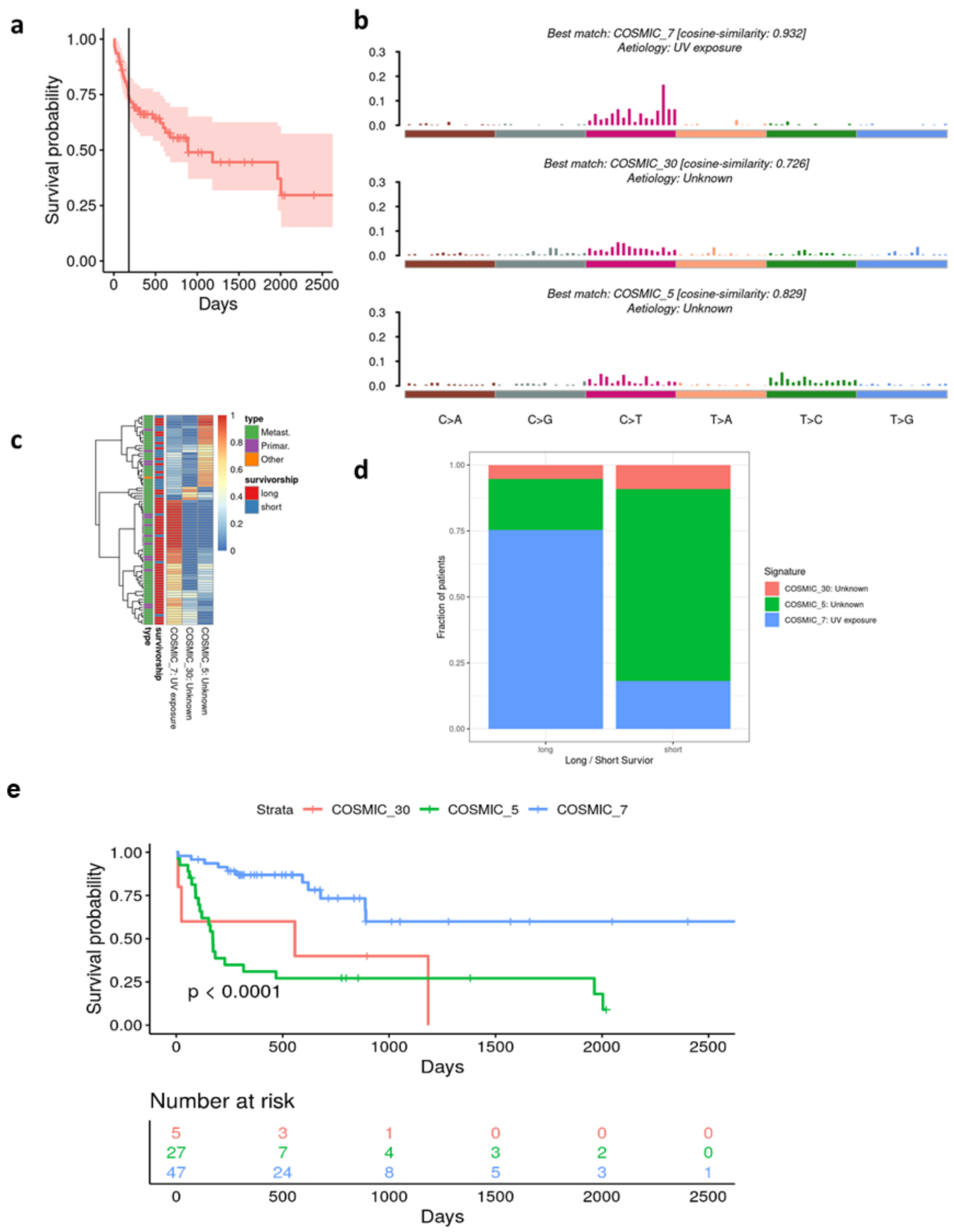
3.3. Survivorship Analysis
3.4. Long vs. Short Survivors
3.5. Alignment of the Cohorts’ Mutational Profile with COSMIC Signatures
4. Discussion
5. Conclusions
6. Statement of Translational Relevance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thielmann, C.M.; Matull, J.; Zaremba, A.; Murali, R.; Chorti, E.; Lodde, G.; Jansen, P.; Herbst, R.; Terheyden, P.; Utikal, J.; et al. TERT Promoter Mutations Are Associated with Longer Progression-Free and Overall Survival in Patients with BRAF-Mutant Melanoma Receiving BRAF and MEK Inhibitor Therapy. Eur. J. Cancer 2022, 161, 99–107. [Google Scholar] [CrossRef]
- Kraehenbuehl, L.; Weng, C.-H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing Immunotherapy in Cancer by Targeting Emerging Immunomodulatory Pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus Investigator-Choice Chemotherapy for Ipilimumab-Refractory Melanoma (KEYNOTE-002): A Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Hersh, E.M.; O’Day, S.J.; Powderly, J.; Khan, K.D.; Pavlick, A.C.; Cranmer, L.D.; Samlowski, W.E.; Nichol, G.M.; Yellin, M.J.; Weber, J.S. A Phase II Multicenter Study of Ipilimumab with or without Dacarbazine in Chemotherapy-Naïve Patients with Advanced Melanoma. Investig. New Drugs 2011, 29, 489–498. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant Ipilimumab versus Placebo after Complete Resection of High-Risk Stage III Melanoma (EORTC 18071): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H.; Lao, C.D.; et al. Nivolumab versus Chemotherapy in Patients with Advanced Melanoma Who Progressed after Anti-CTLA-4 Treatment (CheckMate 037): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Franklin, C.; Mohr, P.; Bluhm, L.; Grimmelmann, I.; Gutzmer, R.; Meier, F.; Garzarolli, M.; Weichenthal, M.; Pfoehler, C.; Herbst, R.; et al. Impact of Radiotherapy and Sequencing of Systemic Therapy on Survival Outcomes in Melanoma Patients with Previously Untreated Brain Metastasis: A Multicenter DeCOG Study on 450 Patients from the Prospective Skin Cancer Registry ADOREG. J. Immunother. Cancer 2022, 10, e004509. Available online: https://jitc.bmj.com/content/10/6/e004509 (accessed on 6 January 2023). [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Mandalà, M.; Ferrucci, P.F.; Guidoboni, M.; Rutkowski, P.; Ferraresi, V.; Arance, A.; Guida, M.; Maiello, E.; Gogas, H.; et al. Sequencing of Ipilimumab Plus Nivolumab and Encorafenib Plus Binimetinib for Untreated BRAF-Mutated Metastatic Melanoma (SECOMBIT): A Randomized, Three-Arm, Open-Label Phase II Trial. J. Clin. Oncol. 2023, 41, 212–221. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Ribas, A.; Larkin, J.; McArthur, G.A.; Lewis, K.D.; Hauschild, A.; Flaherty, K.T.; McKenna, E.; Zhu, Q.; Mun, Y.; et al. Impact of Initial Treatment and Prognostic Factors on Postprogression Survival in BRAF-Mutated Metastatic Melanoma Treated with Dacarbazine or Vemurafenib ± Cobimetinib: A Pooled Analysis of Four Clinical Trials. J. Transl. Med. 2020, 18, 294. [Google Scholar] [CrossRef]
- Dickson, P.V.; Gershenwald, J.E. Staging and Prognosis of Cutaneous Melanoma. Surg. Oncol. Clin. N. Am. 2011, 20, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final Version of 2009 AJCC Melanoma Staging and Classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schadendorf, D.; Long, G.V.; Stroiakovski, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion-Sileni, V.; Schachter, J.; Garbe, C.; Dutriaux, C.; et al. Three-Year Pooled Analysis of Factors Associated with Clinical Outcomes across Dabrafenib and Trametinib Combination Therapy Phase 3 Randomised Trials. Eur. J. Cancer 2017, 82, 45–55. [Google Scholar] [CrossRef]
- Long, G.V.; Blank, C.; Ribas, A.; Mortier, L.; Carlino, M.S.; Lotem, M.; Lorigan, P.; Neyns, B.; Petrella, T.M.; Puzanov, I.; et al. 1141—Impact of Baseline Serum Lactate Dehydrogenase Concentration on the Efficacy of Pembrolizumab and Ipilimumab in Patients with Advanced Melanoma: Data from KEYNOTE-006. Eur. J. Cancer 2017, 72, S122–S123. [Google Scholar] [CrossRef]
- Yang, J.; Lian, J.W.; Chin, Y.-P.; Wang, L.; Lian, A.; Murphy, G.F.; Zhou, L. Assessing the Prognostic Significance of Tumor-Infiltrating Lymphocytes in Patients with Melanoma Using Pathologic Features Identified by Natural Language Processing. JAMA Netw. Open 2021, 4, e2126337. [Google Scholar] [CrossRef] [PubMed]
- Gide, T.N.; Pires da Silva, I.; Quek, C.; Ferguson, P.M.; Batten, M.; Shang, P.; Ahmed, T.; Menzies, A.M.; Carlino, M.S.; Saw, R.P.M.; et al. Clinical and Molecular Heterogeneity in Patients with Innate Resistance to Anti-PD-1 +/− Anti-CTLA-4 Immunotherapy in Metastatic Melanoma Reveals Distinct Therapeutic Targets. Cancers 2021, 13, 3186. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 2: Treatment—Update 2022. Eur. J. Cancer 2022, 170, 256–284. [Google Scholar] [CrossRef]
- Bafaloukos, D.; Gazouli, I.; Koutserimpas, C.; Samonis, G. Evolution and Progress of MRNA Vaccines in the Treatment of Melanoma: Future Prospects. Vaccines 2023, 11, 636. [Google Scholar] [CrossRef]
- Jiang, H.; Lei, R.; Ding, S.-W.; Zhu, S. Skewer: A Fast and Accurate Adapter Trimmer for next-Generation Sequencing Paired-End Reads. BMC Bioinform. 2014, 15, 182. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Van der Auwera, G.; O’Connor, B.D. Genomics in the Cloud: Using Docker, GATK, and WDL in Terra, 1st ed.; O’Reilly Media: Sebastopol, CA, USA, 2020. [Google Scholar]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ Data to High Confidence Variant Calls: The Genome Analysis Toolkit Best Practices Pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.1–11.10.33. [Google Scholar] [CrossRef] [Green Version]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, L.B.; Kim, J.; Haradhvala, N.J.; Huang, M.N.; Tian Ng, A.W.; Wu, Y.; Boot, A.; Covington, K.R.; Gordenin, D.A.; Bergstrom, E.N.; et al. The Repertoire of Mutational Signatures in Human Cancer. Nature 2020, 578, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayakonda, A.; Lin, D.-C.; Assenov, Y.; Plass, C.; Koeffler, H.P. Maftools: Efficient and Comprehensive Analysis of Somatic Variants in Cancer. Genome Res. 2018, 28, 1747–1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brash, D.E. UV Signature Mutations. Photochem. Photobiol. 2015, 91, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, S.A.; Bindal, N.; Bamford, S.; Cole, C.; Kok, C.Y.; Beare, D.; Jia, M.; Shepherd, R.; Leung, K.; Menzies, A.; et al. COSMIC: Mining Complete Cancer Genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011, 39, D945–D950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- COSMIC|Mutational Signatures. Available online: https://cancer.sanger.ac.uk/signatures/ (accessed on 1 April 2022).
- Trucco, L.D.; Mundra, P.A.; Hogan, K.; Garcia-Martinez, P.; Viros, A.; Mandal, A.K.; Macagno, N.; Gaudy-Marqueste, C.; Allan, D.; Baenke, F.; et al. Ultraviolet Radiation-Induced DNA Damage Is Prognostic for Outcome in Melanoma. Nat. Med. 2019, 25, 221–224. [Google Scholar] [CrossRef]
- Chong, W.; Wang, Z.; Shang, L.; Jia, S.; Liu, J.; Fang, Z.; Du, F.; Wu, H.; Liu, Y.; Chen, Y.; et al. Association of Clock-like Mutational Signature with Immune Checkpoint Inhibitor Outcome in Patients with Melanoma and NSCLC. Mol. Ther. Nucleic Acids 2021, 23, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Haanen, J.B.; Ribas, A.; Schumacher, T.N. CANCER IMMUNOLOGY. The “Cancer Immunogram”. Science 2016, 352, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Hugo, W.; Shi, H.; Sun, L.; Piva, M.; Song, C.; Kong, X.; Moriceau, G.; Hong, A.; Dahlman, K.B.; Johnson, D.B.; et al. Non-Genomic and Immune Evolution of Melanoma Acquiring MAPKi Resistance. Cell 2015, 162, 1271–1285. [Google Scholar] [CrossRef] [Green Version]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016, 165, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Dousset, L.; Poizeau, F.; Robert, C.; Mansard, S.; Mortier, L.; Caumont, C.; Routier, É.; Dupuy, A.; Rouanet, J.; Battistella, M.; et al. Positive Association Between Location of Melanoma, Ultraviolet Signature, Tumor Mutational Burden, and Response to Anti–PD-1 Therapy. JCO Precis. Oncol. 2021, 5, 1821–1829. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-A.; Wojtowicz, D.; Sarto Basso, R.; Sason, I.; Robinson, W.; Hochbaum, D.S.; Leiserson, M.D.M.; Sharan, R.; Vadin, F.; Przytycka, T.M. Network-Based Approaches Elucidate Differences within APOBEC and Clock-like Signatures in Breast Cancer. Genome Med. 2020, 12, 52. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröhlich, F.; Ramelyte, E.; Turko, P.; Dzung, A.; Freiberger, S.N.; Mangana, J.; Levesque, M.P.; Dummer, R. Clock-like Mutation Signature May Be Prognostic for Worse Survival Than Signatures of UV Damage in Cutaneous Melanoma. Cancers 2023, 15, 3818. https://doi.org/10.3390/cancers15153818
Fröhlich F, Ramelyte E, Turko P, Dzung A, Freiberger SN, Mangana J, Levesque MP, Dummer R. Clock-like Mutation Signature May Be Prognostic for Worse Survival Than Signatures of UV Damage in Cutaneous Melanoma. Cancers. 2023; 15(15):3818. https://doi.org/10.3390/cancers15153818
Chicago/Turabian StyleFröhlich, Fabienne, Egle Ramelyte, Patrick Turko, Andreas Dzung, Sandra N. Freiberger, Joanna Mangana, Mitchell P. Levesque, and Reinhard Dummer. 2023. "Clock-like Mutation Signature May Be Prognostic for Worse Survival Than Signatures of UV Damage in Cutaneous Melanoma" Cancers 15, no. 15: 3818. https://doi.org/10.3390/cancers15153818
APA StyleFröhlich, F., Ramelyte, E., Turko, P., Dzung, A., Freiberger, S. N., Mangana, J., Levesque, M. P., & Dummer, R. (2023). Clock-like Mutation Signature May Be Prognostic for Worse Survival Than Signatures of UV Damage in Cutaneous Melanoma. Cancers, 15(15), 3818. https://doi.org/10.3390/cancers15153818






