Characterization of DoTc2 4510—Identifying HPV16 Presence in a Cervical Carcinoma Cell Line Previously Considered to Be HPV-Negative
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Authentication, Contamination Testing, HLA Typing
2.3. Nested Multiplex PCR for Type-Specific HPV Detection
2.4. PCR Detection of HPV16 E6/E7
2.5. Gel Electrophoresis and Product Sequencing
2.6. Western Blot
2.7. siRNA Transfection
2.8. Colony Formation Assay
2.9. Senescence Induction Assay
3. Results
3.1. Authentication, Contamination Testing, HLA Typing
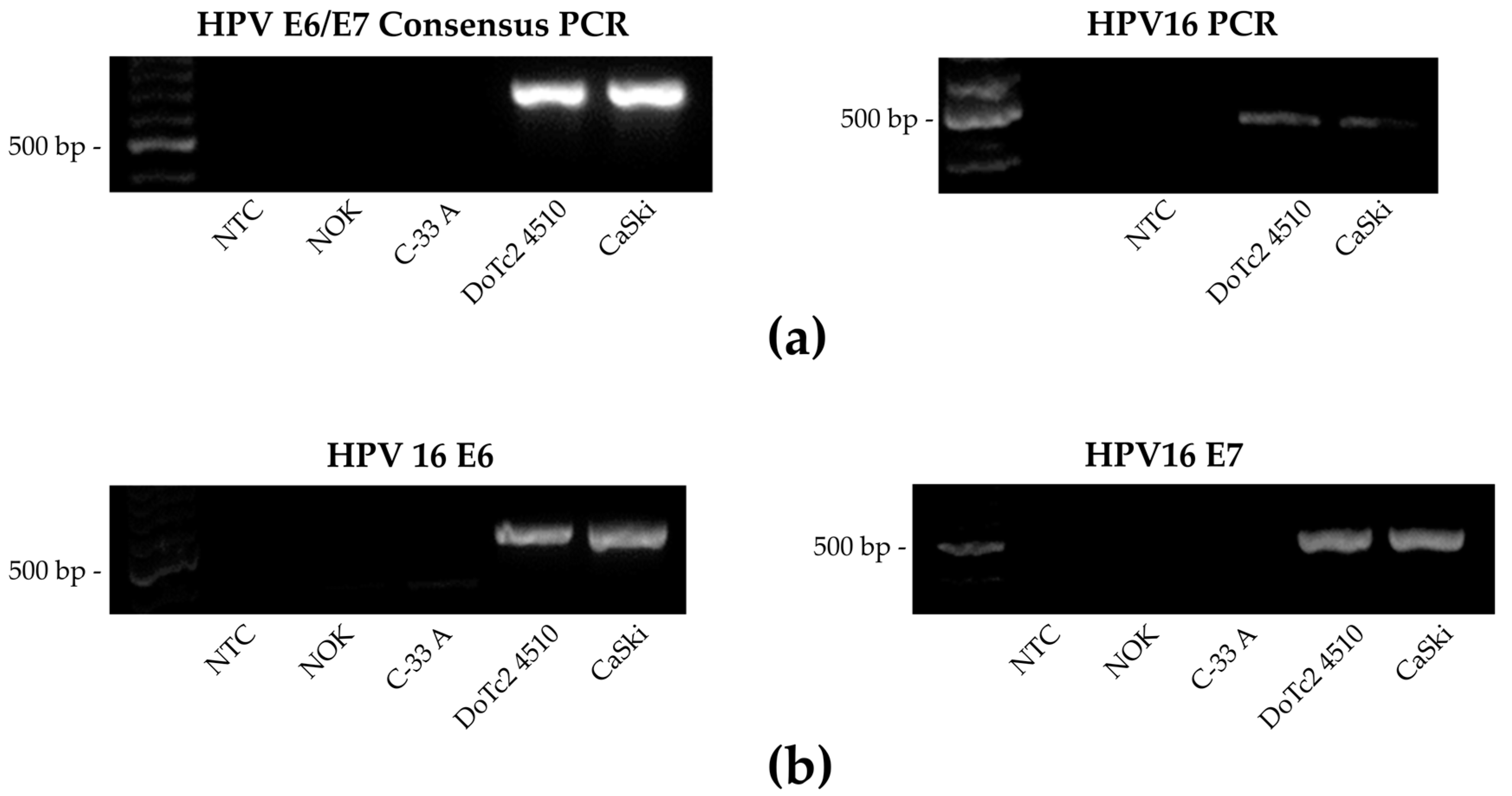
3.2. HPV Detection and Genotype Identification by Nested Multiplex PCR and HPV16 E6/E7 Specific PCR
3.3. Expression of HPV16 E6 and E7 Oncoproteins and Affected Downstream Tumor Suppressor Proteins
3.4. Assessment of DoTc 2 4510 Cells’ Dependence on HPV16 E6 and E7 Oncoproteins by siRNA Knock-Down
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| HPV Type | Amplicon (bp) | Sequence (5′-3′) |
|---|---|---|
| 16 | 457 | CAC AGT TAT GCA CAG AGC TGC |
| CAT ATA TTC ATG CAA TGT AGG TGT A | ||
| 18 | 322 | CAC TTC ACT GCA AGA CAT AGA |
| GTT GTG AAA TCG TCG TTT TTC A | ||
| 31 | 263 | GAA ATT GCA TGA ACT AAG CTC G |
| CAC ATA TAC CTT TGT TTG TCA A | ||
| 33 | 398 | ACT ATA CAC AAC ATT GAA CTA |
| GTT TTT ACA CGT CAC AGT GCA | ||
| 35 | 358 | CAA CGA GGT AGA AGA AAG CAT C |
| CCG ACC TGT CCA CCG TCC ACC G | ||
| 39 | 280 | GAC GAC CAC TAC AGC AAA CC |
| TTA TGA AAT CTT CGT TTG CT | ||
| 45 | 151 | GTG GAA AAG TGC ATT ACA GG |
| ACC TCT GTG CGT TCC AAT GT | ||
| 51 | 223 | GAG TAT AGA CGT TAT AGC AGG |
| TTT CGT TAC GTT GTC GTG TAC G | ||
| 52 | 229 | TAA GGC TGC AGT GTG TGC AG |
| CTA ATA GTT ATT TCA CTT AAT GGT | ||
| 56 | 181 | GTG TGC AGA GTA TGT TTA TTG |
| TTT CTG TCA CAA TGC AAT TGC | ||
| 58 | 274 | GTA AAG TGT GCT TAC GAT TGC |
| GTT GTT ACA GGT TAC ACT TGT | ||
| 59 | 215 | CAA AGG GGA ACT GCA AGA AAG |
| TAT AAC AGC GTA TCA GCA GC |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Spaans, V.M.; Trietsch, M.D.; Peters, A.A.; Osse, M.; Ter Haar, N.; Fleuren, G.J.; Jordanova, E.K. Precise Classification of Cervical Carcinomas Combined with Somatic Mutation Profiling Contributes to Predicting Disease Outcome. PLoS ONE 2015, 10, e0133670. [Google Scholar] [CrossRef]
- Xing, B.; Guo, J.; Sheng, Y.; Wu, G.; Zhao, Y. Human Papillomavirus-Negative Cervical Cancer: A Comprehensive Review. Front. Oncol. 2021, 10, 606335. [Google Scholar] [CrossRef]
- Pirog, E.C. Cervical Adenocarcinoma: Diagnosis of Human Papillomavirus-Positive and Human Papillomavirus-Negative Tumors. Arch. Pathol. Lab. Med. 2017, 141, 1653–1667. [Google Scholar] [CrossRef] [Green Version]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Milde-Langosch, K.; Riethdorf, S.; Kraus-Pöppinghaus, A.; Riethdorf, L.; Löning, T. Expression of cyclin-dependent kinase inhibitors p16MTS1, p21WAF1, and p27KIP1 in HPV-positive and HPV-negative cervical adenocarcinomas. Virchows Arch. 2001, 439, 55–61. [Google Scholar] [CrossRef]
- Lee, J.E.; Chung, Y.; Rhee, S.; Kim, T.H. Untold story of human cervical cancers: HPV-negative cervical cancer. BMB Rep. 2022, 55, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Auersperg, N. Long-term cultivation of hypodiploid human tumor cells. J. Natl. Cancer Inst. 1964, 32, 135–163. [Google Scholar] [PubMed]
- Yee, C.; Krishnan-Hewlett, I.; Baker, C.C.; Schlegel, R.; Howley, P.M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am. J. Pathol. 1985, 119, 361–366. [Google Scholar] [PubMed]
- Scheffner, M.; Münger, K.; Byrne, J.C.; Howley, P.M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc. Natl. Acad. Sci. USA 1991, 88, 5523–5527. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.J.; Capes-Davis, A.; Davis, J.M.; Downward, J.; Freshney, R.I.; Knezevic, I.; Lovell-Badge, R.; Masters, J.R.W.; Meredith, J.; Stacey, G.N.; et al. Guidelines for the use of cell lines in biomedical research. Br. J. Cancer 2014, 111, 1021–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotlar, K.; Diemer, D.; Dethleffs, A.; Hack, Y.; Stubner, A.; Vollmer, N.; Menton, S.; Menton, M.; Dietz, K.; Wallwiener, D.; et al. Detection and typing of human papillomavirus by E6 nested multiplex PCR. J. Clin. Microbiol. 2004, 42, 3176–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitz, J.; Reuschenbach, M.; Lohrey, C.; Honegger, A.; Accardi, R.; Tommasino, M.; Llano, M.; von Knebel Doeberitz, M.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Oncogenic human papillomaviruses activate the tumor-associated lens epithelial-derived growth factor (LEDGF) gene. PLoS Pathog. 2014, 10, 1003957. [Google Scholar] [CrossRef]
- Honegger, A.; Schilling, D.; Bastian, S.; Sponagel, J.; Kuryshev, V.; Sültmann, H.; Scheffner, M.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015, 11, 1004712. [Google Scholar] [CrossRef]
- Hoppe-Seyler, K.; Herrmann, A.L.; Däschle, A.; Kuhn, B.J.; Strobel, T.D.; Lohrey, C.; Bulkescher, J.; Krijgsveld, J.; Hoppe-Seyler, F. Effects of Metformin on the virus/host cell crosstalk in human papillomavirus-positive cancer cells. Int. J. Cancer 2021, 149, 1137–1149. [Google Scholar] [CrossRef]
- Peng, X.; Woodhouse, I.; Hancock, G.; Parker, R.; Marx, K.; Müller, J.; Salatino, S.; Partridge, T.; Nicastri, A.; Liao, H.; et al. Novel canonical and non-canonical viral antigens extend current targets for immunotherapy of HPV-driven cervical cancer. iScience 2023, 26, 106101. [Google Scholar] [CrossRef]
- Iden, M.; Fye, S.; Li, K.; Chowdhury, T.; Ramchandran, R.; Rader, J.S. The lncRNA PVT1 Contributes to the Cervical Cancer Phenotype and Associates with Poor Patient Prognosis. PLoS ONE 2016, 11, 0156274. [Google Scholar] [CrossRef] [Green Version]
- Sergazy, S.; Vetrova, A.; Orhan, I.E.; Senol Deniz, F.S.; Kahraman, A.; Zhang, J.Y.; Aljofan, M. Antiproliferative and cytotoxic activity of Geraniaceae plant extracts against five tumor cell lines. Future Sci. OA 2021, 8, FSO775. [Google Scholar] [CrossRef]
- Luo, A.; Lan, X.; Qiu, Q.; Zhou, Q.; Li, J.; Wu, M.; Liu, P.; Zhang, H.; Lu, B.; Lu, Y.; et al. LncRNA SFTA1P promotes cervical cancer progression by interaction with PTBP1 to facilitate TPM4 mRNA degradation. Cell Death Dis. 2022, 13, 936. [Google Scholar] [CrossRef]
- Morgan, E.L.; Macdonald, A. Autocrine STAT3 activation in HPV positive cervical cancer through a virus-driven Rac1-NFκB-IL-6 signalling axis. PLoS Pathog. 2019, 15, 1007835. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.L.; Macdonald, A. JAK2 Inhibition Impairs Proliferation and Sensitises Cervical Cancer Cells to Cisplatin-Induced Cell Death. Cancers 2019, 11, 1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feil, L.; Koch, A.; Utz, R.; Ackermann, M.; Barz, J.; Stope, M.; Krämer, B.; Wallwiener, D.; Brucker, S.Y.; Weiss, M. Cancer-Selective Treatment of Cancerous and Non-Cancerous Human Cervical Cell Models by a Non-Thermally Operated Electrosurgical Argon Plasma Device. Cancers 2020, 12, 1037. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.L.; Scarth, J.A.; Patterson, M.R.; Wasson, C.W.; Hemingway, G.C.; Barba-Moreno, D.; Macdonald, A. E6-mediated activation of JNK drives EGFR signalling to promote proliferation and viral oncoprotein expression in cervical cancer. Cell Death Differ. 2021, 28, 1669–1687. [Google Scholar] [CrossRef]
- Saiki, A.Y.; Caenepeel, S.; Cosgrove, E.; Su, C.; Boedigheimer, M.; Oliner, J.D. Identifying the determinants of response to MDM2 inhibition. Oncotarget 2015, 10, 7701–7712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DoTc2 4510–CRL-7920 ATCC. Available online: https://www.atcc.org/products/crl-7920 (accessed on 20 July 2023).
- Korch, C.T.; Capes-Davis, A. The Extensive and Expensive Impacts of HEp-2 [HeLa], Intestine 407 [HeLa], and Other False Cell Lines in Journal Publications. SLAS Discov. 2021, 26, 1268–1279. [Google Scholar] [CrossRef]
- Drexler, H.G.; Dirks, W.G.; MacLeod, R.A.; Uphoff, C.C. False and mycoplasma-contaminated leukemia-lymphoma cell lines: Time for a reappraisal. Int. J. Cancer 2017, 140, 1209–1214. [Google Scholar] [CrossRef] [Green Version]
- Capes-Davis, A.; Neve, R.M. Authentication: A Standard Problem or a Problem of Standards? PLoS Biol. 2016, 14, 1002477. [Google Scholar] [CrossRef] [Green Version]
- Souren, N.Y.; Fusenig, N.E.; Heck, S.; Dirks, W.G.; Capes-Davis, A.; Bianchini, F.; Plass, C. Cell line authentication: A necessity for reproducible biomedical research. EMBO J. 2022, 41, 111307. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vučković, N.; Hoppe-Seyler, K.; Riemer, A.B. Characterization of DoTc2 4510—Identifying HPV16 Presence in a Cervical Carcinoma Cell Line Previously Considered to Be HPV-Negative. Cancers 2023, 15, 3810. https://doi.org/10.3390/cancers15153810
Vučković N, Hoppe-Seyler K, Riemer AB. Characterization of DoTc2 4510—Identifying HPV16 Presence in a Cervical Carcinoma Cell Line Previously Considered to Be HPV-Negative. Cancers. 2023; 15(15):3810. https://doi.org/10.3390/cancers15153810
Chicago/Turabian StyleVučković, Nika, Karin Hoppe-Seyler, and Angelika B. Riemer. 2023. "Characterization of DoTc2 4510—Identifying HPV16 Presence in a Cervical Carcinoma Cell Line Previously Considered to Be HPV-Negative" Cancers 15, no. 15: 3810. https://doi.org/10.3390/cancers15153810
APA StyleVučković, N., Hoppe-Seyler, K., & Riemer, A. B. (2023). Characterization of DoTc2 4510—Identifying HPV16 Presence in a Cervical Carcinoma Cell Line Previously Considered to Be HPV-Negative. Cancers, 15(15), 3810. https://doi.org/10.3390/cancers15153810





