Predictive Power of the Albumin–Bilirubin Score for Hepatotoxicity in Stereotactic Ablative Radiation Therapy for Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. ALBI Score
2.3. Treatment
2.4. Evaluation
2.5. Statistics
3. Results
3.1. Patients and Treatment Characteristics
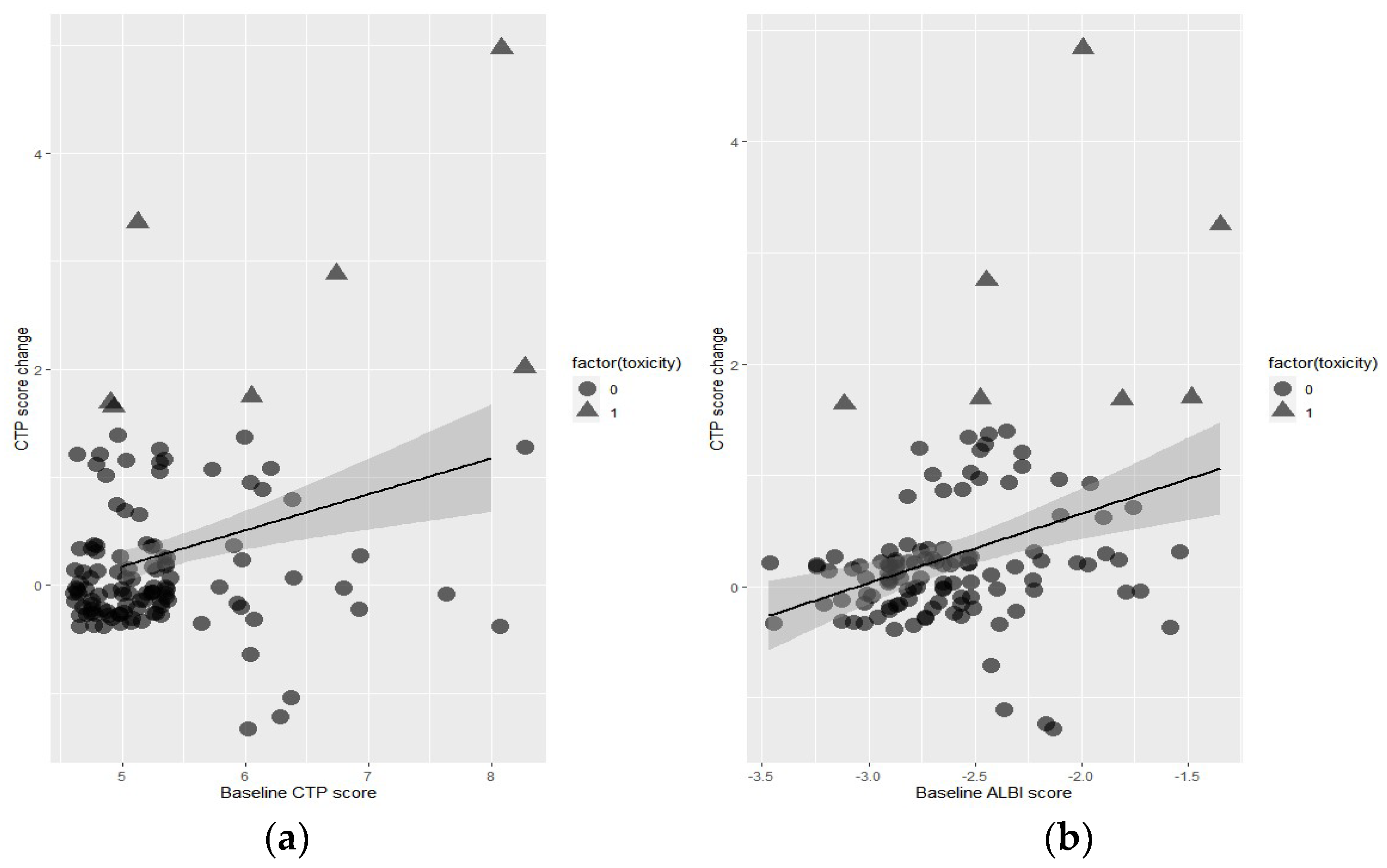
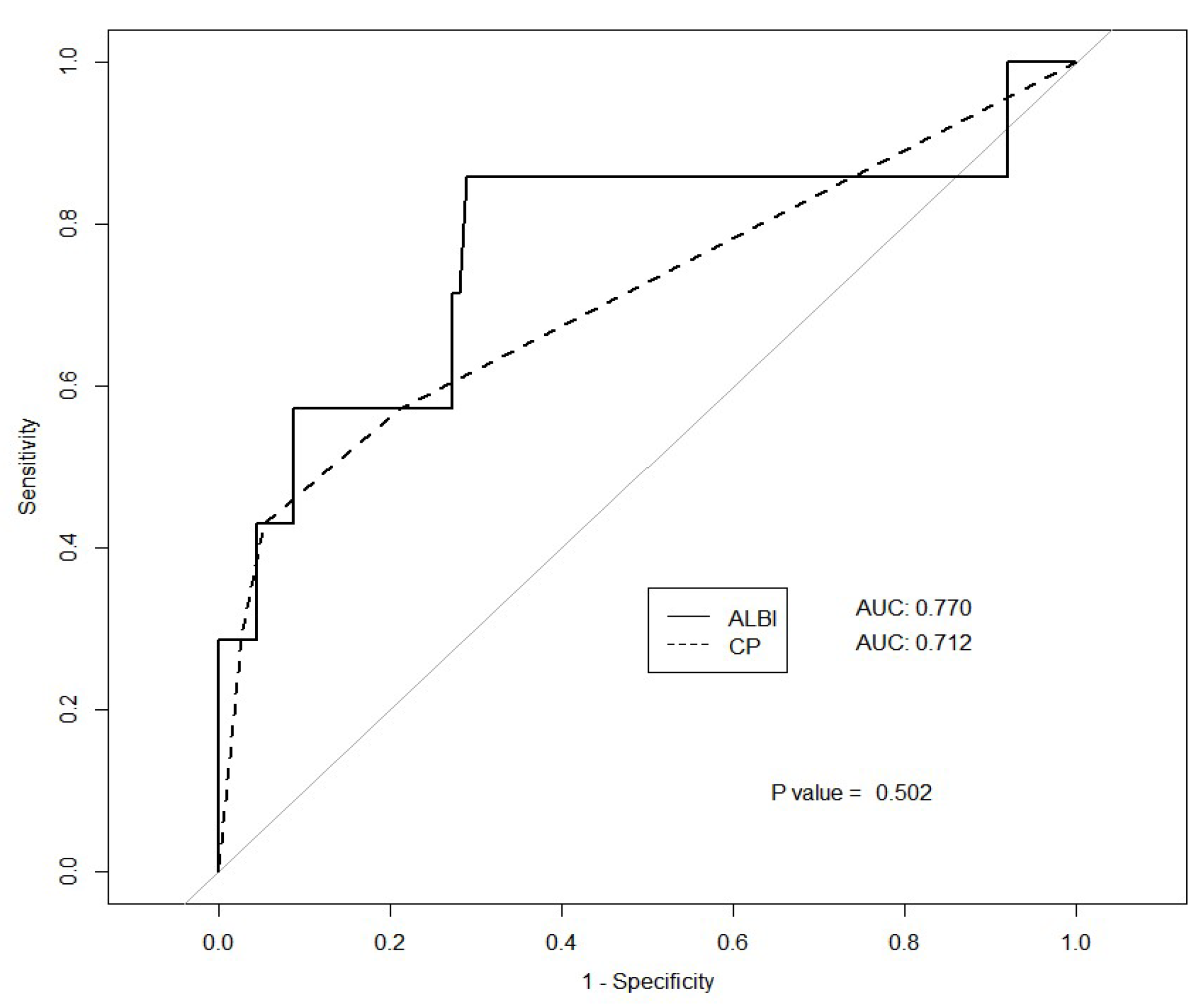
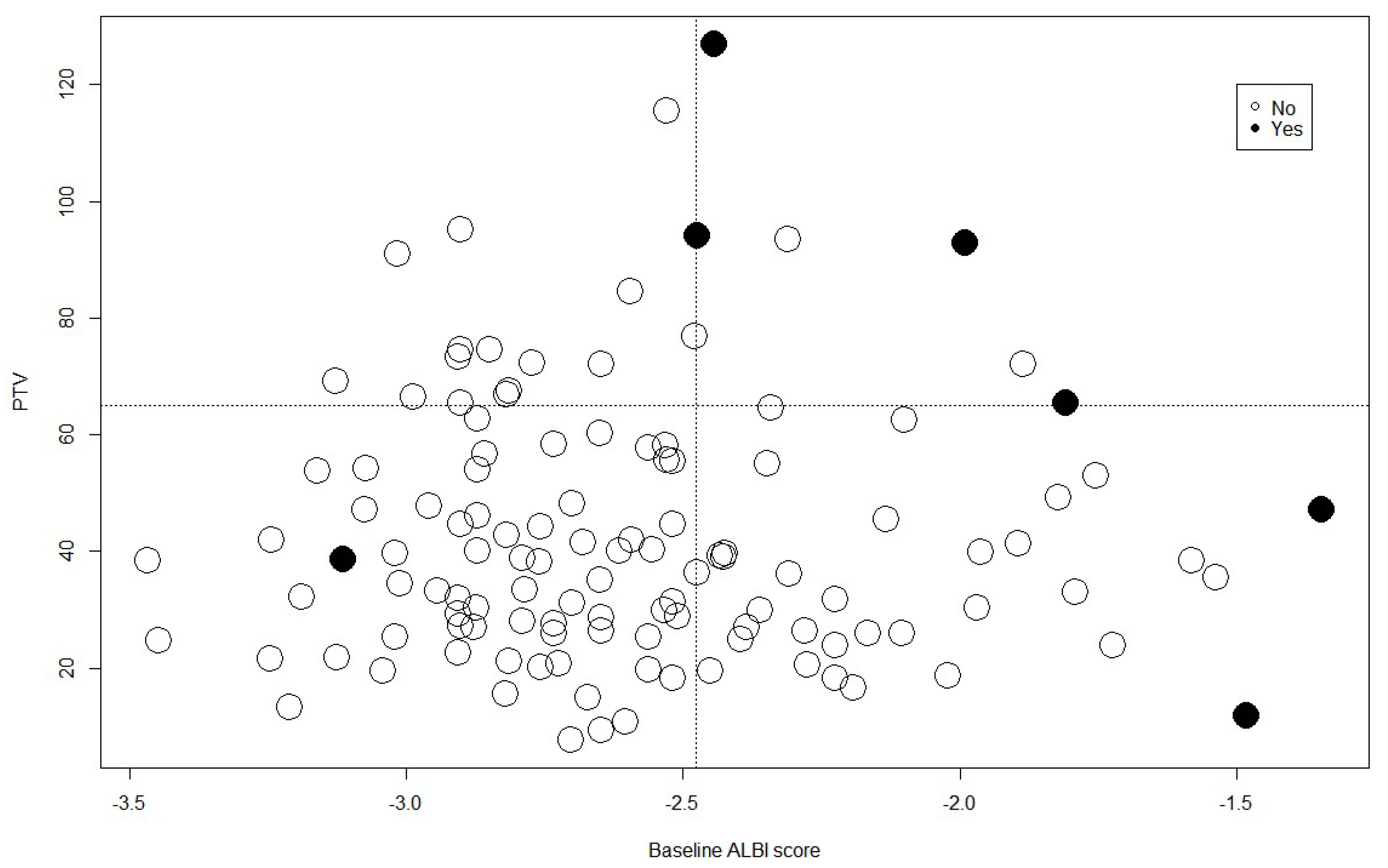
3.2. Predictors of Toxicity
3.3. Cutoff Value
3.4. Mean Liver Dose
3.5. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Zech, C.J.; Ricke, J.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, R.; Solà, E.; Moreau, R.; Ginès, P. Acute-on-chronic liver failure: An update. Gut 2017, 66, 541–553. [Google Scholar] [CrossRef]
- Cardenes, H.R.; Price, T.R.; Perkins, S.M.; Maluccio, M.; Kwo, P.; Breen, T.E.; Henderson, M.A.; Schefter, T.E.; Tudor, K.; Deluca, J.; et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin. Transl. Oncol. 2010, 12, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Tse, R.V.; Hawkins, M.; Lockwood, G.; Kim, J.J.; Cummings, B.; Knox, J.; Sherman, M.; Dawson, L.A. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Clin. Oncol. 2008, 26, 657–664. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhong, J.H.; Su, Z.Y.; Huang, J.F.; Lu, S.D.; Xiang, B.D.; Ma, L.; Qi, L.N.; Ou, B.N.; Li, L.Q. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br. J. Surg. 2016, 103, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.S.; Sinn, D.H.; Kang, T.W.; Lee, M.W.; Kang, W.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; et al. Liver Function Assessment Using Albumin-Bilirubin Grade for Patients with Very Early-Stage Hepatocellular Carcinoma Treated with Radiofrequency Ablation. Dig. Dis. Sci. 2017, 62, 3235–3242. [Google Scholar] [CrossRef]
- Lo, C.H.; Liu, M.Y.; Lee, M.S.; Yang, J.F.; Jen, Y.M.; Lin, C.S.; Chao, H.L.; Shen, P.C.; Huang, W.Y. Comparison between Child-Turcotte-Pugh and Albumin-Bilirubin Scores in Assessing the Prognosis of Hepatocellular Carcinoma After Stereotactic Ablative Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 145–152. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Michitaka, K.; Kudo, M. Newly Proposed ALBI Grade and ALBI-T Score as Tools for Assessment of Hepatic Function and Prognosis in Hepatocellular Carcinoma Patients. Liver Cancer 2019, 8, 312–325. [Google Scholar] [CrossRef]
- Hickey, R.; Mouli, S.; Kulik, L.; Desai, K.; Thornburg, B.; Ganger, D.; Baker, T.; Abecassis, M.; Ralph Kallini, J.; Gabr, A.; et al. Independent Analysis of Albumin-Bilirubin Grade in a 765-Patient Cohort Treated with Transarterial Locoregional Therapy for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2016, 27, 795–802. [Google Scholar] [CrossRef]
- Gui, B.; Weiner, A.A.; Nosher, J.; Lu, S.E.; Foltz, G.M.; Hasan, O.; Kim, S.K.; Gendel, V.; Mani, N.B.; Carpizo, D.R.; et al. Assessment of the Albumin-Bilirubin (ALBI) Grade as a Prognostic Indicator for Hepatocellular Carcinoma Patients Treated With Radioembolization. Am. J. Clin. Oncol. 2018, 41, 861–866. [Google Scholar] [CrossRef]
- Gkika, E.; Bettinger, D.; Krafft, L.; Schultheiss, M.; Neeff, H.P.; Maruschke, L.; Schulenburg, M.; Adebahr, S.; Kirste, S.; Nestle, U.; et al. The role of albumin-bilirubin grade and inflammation-based index in patients with hepatocellular carcinoma treated with stereotactic body radiotherapy. Strahlenther. Onkol. 2018, 194, 403–413. [Google Scholar] [CrossRef]
- Jackson, W.C.; Hartman, H.E.; Gharzai, L.A.; Maurino, C.; Karnak, D.M.; Mendiratta-Lala, M.; Parikh, N.D.; Mayo, C.S.; Haken, R.K.T.; Schipper, M.J.; et al. The Potential for Midtreatment Albumin-Bilirubin (ALBI) Score to Individualize Liver Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.J.; Sykes, J.; Brierley, J.; Kim, J.J.; Wong, R.K.S.; Ringash, J.; Craig, T.; Velec, M.; Lindsay, P.; Knox, J.J.; et al. Baseline Albumin-Bilirubin (ALBI) Score in Western Patients With Hepatocellular Carcinoma Treated with Stereotactic Body Radiation Therapy (SBRT). Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Toesca, D.A.S.; Osmundson, E.C.; von Eyben, R.; Shaffer, J.L.; Koong, A.C.; Chang, D.T. Assessment of hepatic function decline after stereotactic body radiation therapy for primary liver cancer. Pract. Radiat. Oncol. 2017, 7, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Song, M.J.; Kim, S.H.; Park, M. Comparing various scoring system for predicting overall survival according to treatment modalities in hepatocellular carcinoma focused on Platelet-albumin-bilirubin (PALBI) and albumin-bilirubin (ALBI) grade: A nationwide cohort study. PLoS ONE 2019, 14, e0216173. [Google Scholar] [CrossRef]
- Sugimoto, H.; Okochi, O.; Hirota, M.; Kanazumi, N.; Nomoto, S.; Inoue, S.; Takeda, S.; Nakao, A. Early detection of liver failure after hepatectomy by indocyanine green elimination rate measured by pulse dye-densitometry. J. Hepatobiliary Pancreat Surg. 2006, 13, 543–548. [Google Scholar] [CrossRef]
- Lee, C.F.; Yu, M.C.; Kuo, L.M.; Chan, K.M.; Jan, Y.Y.; Chen, M.F.; Lee, W.C. Using indocyanine green test to avoid post-hepatectomy liver dysfunction. Chang. Gung Med. J. 2007, 30, 333–338. [Google Scholar]
- Ezaki, T.; Yamamoto, K.; Yamaguchi, H.; Sasaki, Y.; Ishida, T.; Mori, M.; Aimitsu, S. Hepatic resection for hepatocellular carcinoma existing with liver cirrhosis. Hepatogastroenterology 2002, 49, 1363–1368. [Google Scholar] [PubMed]
- Cherrick, G.R.; Stein, S.W.; Leevy, C.M.; Davidson, C.S. Indocyanine green: Observations on its physical properties, plasma decay, and hepatic extraction. J. Clin. Investig. 1960, 39, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.I.; Koom, W.S.; Lee, I.J.; Jeong, K.; Chung, Y.; Kim, J.K.; Lee, K.S.; Han, K.H.; Seong, J. The significance of ICG-R15 in predicting hepatic toxicity in patients receiving radiotherapy for hepatocellular carcinoma. Liver Int. 2012, 32, 1165–1171. [Google Scholar] [CrossRef]
- Kawashima, M.; Furuse, J.; Nishio, T.; Konishi, M.; Ishii, H.; Kinoshita, T.; Nagase, M.; Nihei, K.; Ogino, T. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J. Clin. Oncol. 2005, 23, 1839–1846. [Google Scholar] [CrossRef]
- Cheng, S.H.; Lin, Y.M.; Chuang, V.P.; Yang, P.S.; Cheng, J.C.; Huang, A.T.; Sung, J.L. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J. Gastroenterol. Hepatol. 1999, 14, 1025–1033. [Google Scholar] [CrossRef]
- Toyoda, H.; Lai, P.B.; O’Beirne, J.; Chong, C.C.; Berhane, S.; Reeves, H.; Manas, D.; Fox, R.P.; Yeo, W.; Mo, F.; et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: Application of the ALBI grade. Br. J. Cancer 2016, 114, 744–750. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kudo, M.; Hirooka, M.; Tsuji, K.; Itobayashi, E.; Kariyama, K.; Ishikawa, T.; Tajiri, K.; Ochi, H.; et al. Real-Life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics). Albumin-Bilirubin (ALBI) Grade as Part of the Evidence-Based Clinical Practice Guideline for HCC of the Japan Society of Hepatology: A Comparison with the Liver Damage and Child-Pugh Classifications. Liver Cancer 2017, 6, 204–215. [Google Scholar] [CrossRef]
- Pan, C.C.; Kavanagh, B.D.; Dawson, L.A.; Li, X.A.; Das, S.K.; Miften, M.; Ten Haken, R.K. Radiation-Associated Liver Injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S94–S100. [Google Scholar] [CrossRef]
- Velec, M.; Haddad, C.R.; Craig, T.; Wang, L.; Lindsay, P.; Brierley, J.; Brade, A.; Ringash, J.; Wong, R.; Kim, J.; et al. Predictors of Liver Toxicity Following Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.d.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]

| Variables | n | (%) | |
|---|---|---|---|
| Sex | Male | 100 | (82.6) |
| Female | 21 | (17.4) | |
| Age | mean, year (range) | 67.9 | (60–76) |
| Underlying liver disease | HBV | 60 | (49.6) |
| HCV | 31 | (25.6) | |
| Others | 30 | (24.8) | |
| Initial platelet count | Median, k | 129.1 ± 54.7 | |
| Child–Pugh Class | A | 112 | (92.6) |
| B | 9 | (7.4) | |
| Child–Pugh Score | 5 | 93 | (76.9) |
| 6 | 19 | (15.7) | |
| 7 | 4 | (3.3) | |
| 8 | 5 | (4.1) | |
| ALBI score | −2.6 ± 0.4 | ||
| ALBI grade | 1 | 66 | (54.5) |
| 2 | 55 | (44.7) | |
| 3 | 1 | (0.8) | |
| Tumor size | median, cm3 | 2.7 ± 1.1 | |
| GTV | median, cm3 | 8.7 ± 8.0 | |
| PTV | median, cm3 | 42.8 ± 22.7 | |
| Radiation dose | 36 Gy | 2 | (1.7) |
| 40 Gy | 16 | (13.2) | |
| 44 Gy | 4 | (3.3) | |
| 48 Gy | 99 | (81.8) | |
| Normal liver volume | median, cm3 | 1244.9 ± 262.2 | |
| Mean liver dose | median, Gy | 8.0 ± 2.9 | |
| V15 | median, cm3 | 1023.5 ± 249.6 | |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | OR | (95% CI) | p | OR | (95% CI) | p |
| Sex | ||||||
| Male | 0.503 | (0.0752–5.656) | 0.350 | |||
| Age | 0.965 | (0.900–1.088) | 0.840 | |||
| Platelet | 0.996 | (0.982–1.011) | 0.650 | |||
| Child–Pugh Score | 2.790 | (1.399–5.564) | 0.004 | |||
| Child–Pugh Class | ||||||
| A | ||||||
| B | 12.828 | (1.540–97.824) | 0.009 | |||
| ALBI Score | 13.186 | (2.270–76.579) | 0.004 | 29.51 | (3.860–418.400) | 0.003 |
| ALBI Grade | ||||||
| 1 | ||||||
| 2 | 7.842 | (0.907–370.904) | 0.046 | |||
| Tumor size | 1.422 | (0.737–2.742) | 0.294 | |||
| GTV | 1.058 | (0.987–1.135) | 0.115 | |||
| PTV | 1.040 | (1.011–1.070) | 0.007 | 1.05 | (1.020–1.090) | 0.004 |
| Radiation Dose | 0.851 | (0.706–1.025) | 0.090 | |||
| Normal liver volume | 1.000 | (0.997–1.003) | 0.856 | |||
| Mean Liver dose | 1.151 | (0.892–1.485) | 0.281 | |||
| V15 | 1.000 | (0.996–1.003) | 0.756 | |||
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | (95% CI) | p | HR | (95% CI) | p |
| Sex | ||||||
| Male | 0.33 | (0.1–1.1) | 0.072 | |||
| Age | 0.99 | (0.92–1.06) | 0.723 | |||
| Platelet | 1 | (0.99–1.01) | 0.667 | |||
| Child–Pugh Score | 1.95 | (1.08–3.53) | 0.026 | 2.793 | (1.37–5.71) | 0.005 |
| Child–Pugh Class | ||||||
| A | ||||||
| B | 4.39 | (0.91–21.22) | 0.066 | |||
| ALBI Score | 2.69 | (0.7–10.34) | 0.149 | |||
| ALBI Grade | ||||||
| 1 | ||||||
| 2 | 1.61 | (0.51–5.11) | 0.417 | |||
| Tumor size | 1.82 | (1.11–2.98) | 0.017 | 2.184 | (1.23–3.87) | 0.007 |
| GTV | 1.03 | (0.98–1.08) | 0.319 | |||
| PTV | 1.01 | (0.99–1.03) | 0.286 | |||
| Underlying liver disease | ||||||
| hepatitis B | ||||||
| hepatitis C | 0.96 | (0.25–3.74) | 0.953 | |||
| Others | 0.69 | (0.14–3.36) | 0.647 | |||
| Radiation Dose | 0.96 | (0.8–1.14) | 0.617 | |||
| Normal liver volume | 1.00 | (1.00–1.00) | 0.258 | |||
| Mean Liver dose | 1.03 | (0.84–1.26) | 0.776 | |||
| rV15 | 1.00 | (1.00–1.00) | 0.347 | |||
| AFP | 1.00 | (1.00–1.00) | 0.771 | |||
| PIVKA-II | 1.00 | (1.00–1.00) | 0.011 | 1.00 | (1.00–1.00) | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, J.; Jeon, H.; Kim, D.; Kim, W.; Nam, J.; Kim, D.; Park, D.; Kim, C.; Ki, Y. Predictive Power of the Albumin–Bilirubin Score for Hepatotoxicity in Stereotactic Ablative Radiation Therapy for Hepatocellular Carcinoma. Cancers 2023, 15, 3777. https://doi.org/10.3390/cancers15153777
Joo J, Jeon H, Kim D, Kim W, Nam J, Kim D, Park D, Kim C, Ki Y. Predictive Power of the Albumin–Bilirubin Score for Hepatotoxicity in Stereotactic Ablative Radiation Therapy for Hepatocellular Carcinoma. Cancers. 2023; 15(15):3777. https://doi.org/10.3390/cancers15153777
Chicago/Turabian StyleJoo, Jihyeon, Hosang Jeon, Dongwoon Kim, Wontaek Kim, Jiho Nam, Donghyun Kim, Dahl Park, Choongrak Kim, and Yongkan Ki. 2023. "Predictive Power of the Albumin–Bilirubin Score for Hepatotoxicity in Stereotactic Ablative Radiation Therapy for Hepatocellular Carcinoma" Cancers 15, no. 15: 3777. https://doi.org/10.3390/cancers15153777
APA StyleJoo, J., Jeon, H., Kim, D., Kim, W., Nam, J., Kim, D., Park, D., Kim, C., & Ki, Y. (2023). Predictive Power of the Albumin–Bilirubin Score for Hepatotoxicity in Stereotactic Ablative Radiation Therapy for Hepatocellular Carcinoma. Cancers, 15(15), 3777. https://doi.org/10.3390/cancers15153777






