Prognostic Factors for Postoperative Bleeding Complications and Prolonged Intensive Care after Percutaneous Hepatic Chemosaturation Procedures with Melphalan
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Cohort
3.2. Postoperative Bleeding Complications
3.3. Prolonged Intensive Care Unit Length of Stay
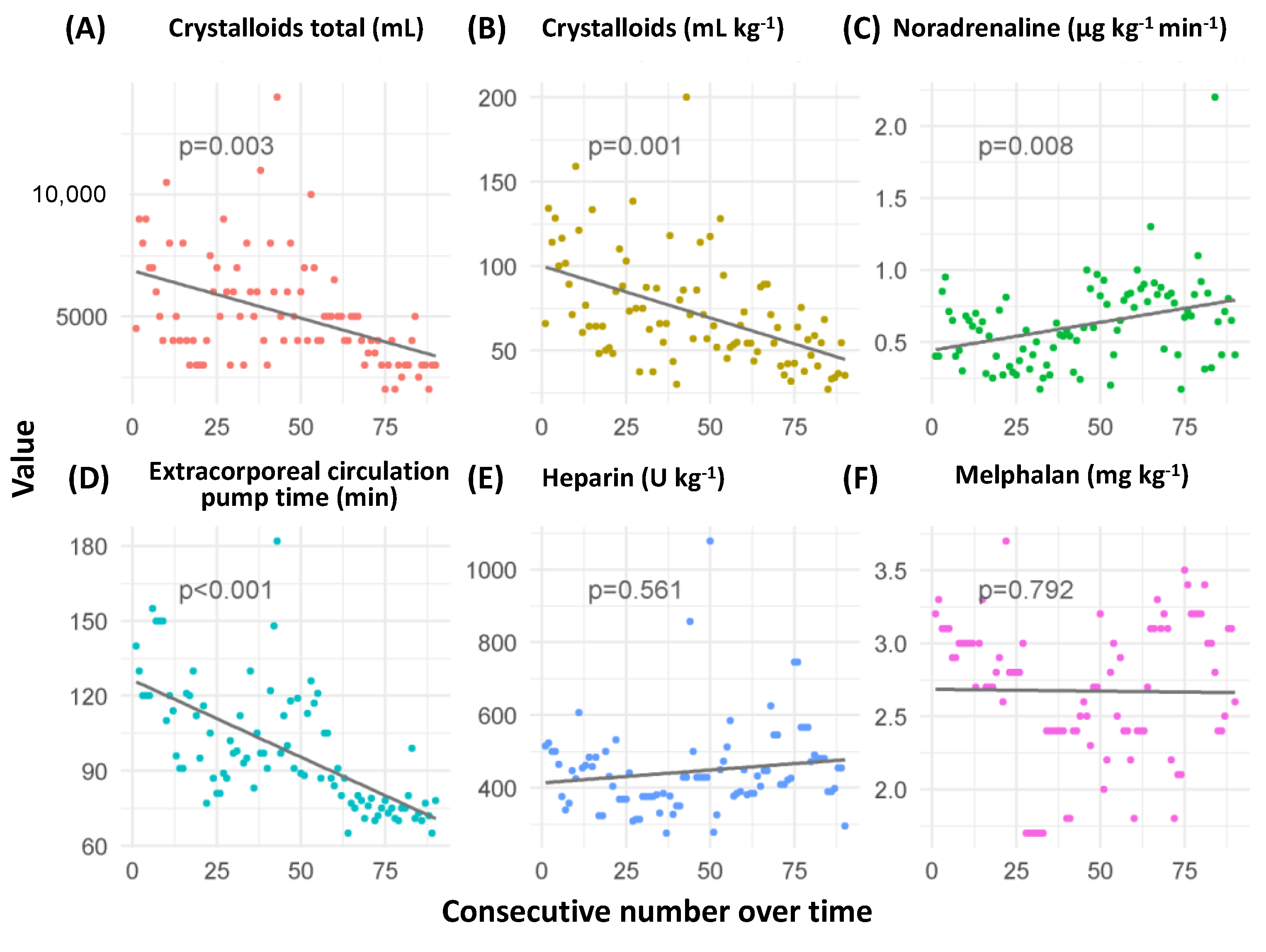
3.4. Development of Predictors across the Observation Period
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pingpank, J.F.; Libutti, S.K.; Chang, R.; Wood, B.J.; Neeman, Z.; Kam, A.W.; Figg, W.D.; Zhai, S.; Beresneva, T.; Seidel, G.D.; et al. Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J. Clin. Oncol. 2005, 23, 3465–3474. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.S.; Zager, J.; Faries, M.; Alexander, H.R.; Royal, R.E.; Wood, B.; Choi, J.; McCluskey, K.; Whitman, E.; Agarwala, S.; et al. Results of a Randomized Controlled Multicenter Phase III Trial of Percutaneous Hepatic Perfusion Compared with Best Available Care for Patients with Melanoma Liver Metastases. Ann. Surg. Oncol. 2016, 23, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Meijer, T.S.; Burgmans, M.C.; Fiocco, M.; de Geus-Oei, L.F.; Kapiteijn, E.; de Leede, E.M.; Martini, C.H.; van der Meer, R.W.; Tijl, F.G.J.; Vahrmeijer, A.L. Safety of Percutaneous Hepatic Perfusion with Melphalan in Patients with Unresectable Liver Metastases from Ocular Melanoma Using the Delcath Systems’ Second-Generation Hemofiltration System: A Prospective Non-randomized Phase II Trial. Cardiovasc. Intervent. Radiol. 2019, 42, 841–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schönfeld, L.; Hinrichs, J.B.; Marquardt, S.; Voigtländer, T.; Dewald, C.; Koppert, W.; Manns, M.P.; Wacker, F.; Vogel, A.; Kirstein, M.M. Chemosaturation with percutaneous hepatic perfusion is effective in patients with ocular melanoma and cholangiocarcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 3003–3012. [Google Scholar] [CrossRef]
- Delcath CHEMOSAT®. Hepatic Delivery System for Melphalan Hydrochloride for Injection. Instructions for Use. Version 120054 Rev E. 04.04.2022; Delcath Systems Inc.: Queensbury, NY, USA, 2022. [Google Scholar]
- Karydis, I.; Gangi, A.; Wheater, M.J.; Choi, J.; Wilson, I.; Thomas, K.; Pearce, N.; Takhar, A.; Gupta, S.; Hardman, D.; et al. Percutaneous hepatic perfusion with melphalan in uveal melanoma: A safe and effective treatment modality in an orphan disease. J. Surg. Oncol. 2018, 117, 1170–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artzner, C.; Mossakowski, O.; Hefferman, G.; Grosse, U.; Hoffmann, R.; Forschner, A.; Eigentler, T.; Syha, R.; Grözinger, G. Chemosaturation with percutaneous hepatic perfusion of melphalan for liver-dominant metastatic uveal melanoma: A single center experience. Cancer Imaging 2019, 19, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüning, R.; Tiede, M.; Schneider, M.; Wohlmuth, P.; Weilert, H.; Oldhafer, K.; Stang, A. Unresectable Hepatic Metastasis of Uveal Melanoma: Hepatic Chemosaturation with High-Dose Melphalan-Long-Term Overall Survival Negatively Correlates with Tumor Burden. Radiol. Res. Pract. 2020, 2020, 5672048. [Google Scholar] [CrossRef]
- Struck, M.F.; Kliem, P.; Ebel, S.; Bauer, A.; Gössmann, H.; Veelken, R.; van Bömmel, F.; Dennecke, T.; Stehr, S.N.; Girrbach, F.F. Percutaneous hepatic melphalan perfusion: Single center experience of procedural characteristics, hemodynamic response, complications, and postoperative recovery. PLoS ONE 2021, 16, e0254817. [Google Scholar] [CrossRef]
- Facchetti, N.; Hinrichs, J.B.; Becker, L.S.; Schneider, M.A.; Brüning, R.; Rademacher, J.; Lenz, J.; Kudrass, K.; Vogel, A.; Wacker, F.K.; et al. Heparin reversal with protamine sulfate after Percutaneous Hepatic Perfusion (PHP): Is less more? Cancer Imaging 2023, 23, 68. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sakakura, K.; Taniguchi, Y.; Yamamoto, K.; Wada, H.; Momomura, S.I.; Fujita, H. Safety of Reversing Anticoagulation by Protamine Following Elective Transfemoral Percutaneous Coronary Intervention in the Drug-Eluting Stent Era. Int. Heart J. 2018, 59, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Chun, K.J.; Jung, S.M.; Lee, S.Y.; Chon, M.K.; Lee, S.H.; Hwang, K.W.; Kim, J.S.; Park, Y.H.; Kim, J.H. Safety and efficacy of immediate heparin reversal with protamine after complex percutaneous coronary intervention. BMC Cardiovasc. Disord. 2022, 22, 207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Y.; Jia, X.; Xin, W.; Wang, H. The emerging role of adopting protamine for reducing the risk of bleeding complications during the percutaneous coronary intervention: A meta-analysis. J. Card. Surg. 2022, 37, 5341–5350. [Google Scholar] [CrossRef] [PubMed]
- Al-Kassou, B.; Kandt, J.; Lohde, L.; Shamekhi, J.; Sedaghat, A.; Tabata, N.; Weber, M.; Sugiura, A.; Fimmers, R.; Werner, N.; et al. Safety and Efficacy of Protamine Administration for Prevention of Bleeding Complications in Patients Undergoing TAVR. JACC Cardiovasc. Interv. 2020, 13, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.H.; Giles, K.A.; Kubilis, P.; Suckow, B.D.; Goodney, P.P.; Huber, T.S.; Powell, R.J.; Cronenwett, J.L.; Scali, S.T. Editor’s Choice—Protamine Reduces Serious Bleeding Complications Associated with Carotid Endarterectomy in Asymptomatic Patients without Increasing the Risk of Stroke, Myocardial Infarction, or Death in a Large National Analysis. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 800–807. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Z.; Yang, T.; Jiao, Q.; Wei, W.; Ji, J.; Xin, W. A Meta-Analysis of Using Protamine for Reducing the Risk of Hemorrhage During Carotid Recanalization: Direct Comparisons of Post-operative Complications. Front. Pharmacol. 2022, 13, 796329. [Google Scholar] [CrossRef]
- Boer, C.; Meesters, M.I.; Veerhoek, D.; Vonk, A.B.A. Anticoagulant and side-effects of protamine in cardiac surgery: A narrative review. Br. J. Anaesth. 2018, 120, 914–927. [Google Scholar] [CrossRef] [Green Version]
- Khandelwal, A.; Phua, C.W.; Chaudhry, H.R.; Tsui, H.; Rivard, G.E.; Teitel, J.M.; Sholzberg, M. Confounding effect of therapeutic protamine and heparin levels on routine and special coagulation testing. Blood Coagul. Fibrinolysis 2020, 31, 60–64. [Google Scholar] [CrossRef]
- McRae, H.L.; Militello, L.; Refaai, M.A. Updates in Anticoagulation Therapy Monitoring. Biomedicines 2021, 9, 262. [Google Scholar] [CrossRef]
- Holzmacher, J.L.; Sarani, B. Indications and Methods of Anticoagulation Reversal. Surg. Clin. N. Am. 2017, 97, 1291–1305. [Google Scholar] [CrossRef]
- Pai, M.; Crowther, M.A. Neutralization of heparin activity. Handb. Exp. Pharmacol. 2012, 207, 265–277. [Google Scholar]
- Aldhaeefi, M.; Badreldin, H.A.; Alsuwayyid, F.; Alqahtani, T.; Alshaya, O.; Al Yami, M.S.; Bin Saleh, K.; Al Harbi, S.A.; Alshaya, A.I. Practical Guide for Anticoagulant and Antiplatelet Reversal in Clinical Practice. Pharmacy 2023, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Blann, A.D.; Landray, M.; Lip, G.Y.H. ABC of antithrombotic therapy: An overview of antithrombotic therapy. BMJ 2002, 325, 762–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterworth, J.; Lin, Y.A.; Prielipp, R.C.; Bennett, J.; Hammon, J.W.; James, R.L. Rapid disappearance of protamine in adults undergoing cardiac operation with cardiopulmonary bypass. Ann. Thorac. Surg. 2002, 74, 1589–1595. [Google Scholar] [CrossRef]
- Butterworth, J.; Lin, Y.A.; Prielipp, R.; Bennett, J.; James, R. The pharmacokinetics and cardiovascular effects of a single intravenous dose of protamine in normal volunteers. Anesth. Analg. 2002, 94, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Miles, L.F.; Burt, C.; Arrowsmith, J.; McKie, M.A.; Villar, S.S.; Govender, P.; Shaylor, R.; Tan, Z.; De Silva, R.; Falter, F. Optimal protamine dosing after cardiopulmonary bypass: The PRODOSE adaptive randomised controlled trial. PLoS Med. 2021, 18, e1003658. [Google Scholar] [CrossRef] [PubMed]
- Taneja, R.; Szoke, D.J.; Hynes, Z.; Jones, P.M. Minimum protamine dose required to neutralize heparin in cardiac surgery: A single-centre, prospective, observational cohort study. Can. J. Anaesth. 2023, 70, 219–227. [Google Scholar] [CrossRef]
- Freundlich, R.E.; Duggal, N.M.; Housey, M.; Tremper, T.T.; Engoren, M.C.; Kheterpal, S. Intraoperative medications associated with hemodynamically significant anaphylaxis. J. Clin. Anesth. 2016, 35, 415–423. [Google Scholar] [CrossRef]
- Lott, C.; Truhlář, A.; Alfonzo, A.; Barelli, A.; González-Salvado, V.; Hinkelbein, J.; Nolan, J.P.; Paal, P.; Perkins, G.D.; Thies, K.C.; et al. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation 2021, 161, 152–219. [Google Scholar]
- Giustiniano, E.; Nisi, F.; Rocchi, L.; Zito, P.C.; Ruggieri, N.; Cimino, M.M.; Torzilli, G.; Cecconi, M. Perioperative Management of Complex Hepatectomy for Colorectal Liver Metastases: The Alliance between the Surgeon and the Anesthetist. Cancers 2021, 13, 2203. [Google Scholar] [CrossRef]
- Carrier, F.M.; Chassé, M.; Wang, H.T.; Aslanian, P.; Iorio, S.; Bilodeau, M.; Turgeon, A.F. Restrictive fluid management strategies and outcomes in liver transplantation: A systematic review. Can. J. Anaesth. 2020, 67, 109–127. [Google Scholar] [CrossRef]
- Morkane, C.M.; Sapisochin, G.; Mukhtar, A.M.; Reyntjens, K.M.E.M.; Wagener, G.; Spiro, M.; Raptis, D.A.; Klinck, J.R.; ERAS4OLT.org Working Group. Perioperative fluid management and outcomes in adult deceased donor liver transplantation—A systematic review of the literature and expert panel recommendations. Clin. Transplant. 2022, 36, e14651. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Robba, C.; Calabrò, L.; Zambelli, D.; Iannuzzi, F.; Molinari, E.; Scarano, S.; Battaglini, D.; Baggiani, M.; De Mattei, G.; et al. Association between perioperative fluid administration and postoperative outcomes: A 20-year systematic review and a meta-analysis of randomized goal-directed trials in major visceral/noncardiac surgery. Crit. Care 2021, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Dushianthan, A.; Knight, M.; Russell, P.; Grocott, M.P. Goal-directed haemodynamic therapy (GDHT) in surgical patients: Systematic review and meta-analysis of the impact of GDHT on post-operative pulmonary complications. Perioper. Med. 2020, 9, 30. [Google Scholar] [CrossRef]
- Madhavan, S.; Chan, S.P.; Tan, W.C.; Eng, J.; Li, B.; Luo, H.D.; Teoh, L.K. Cardiopulmonary bypass time: Every minute counts. J. Cardiovasc. Surg. 2018, 59, 274–281. [Google Scholar] [CrossRef]
- Chalmers, J.; Pullan, M.; Mediratta, N.; Poullis, M. A need for speed? Bypass time and outcomes after isolated aortic valve replacement surgery. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Salis, S.; Mazzanti, V.V.; Merli, G.; Salvi, L.; Tedesco, C.C.; Veglia, F.; Sisillo, E. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2008, 22, 814–822. [Google Scholar] [CrossRef] [PubMed]

| Value | |
|---|---|
| ECCP time, min; median (IQR) | 92 (78–115.5) |
| Melphalan, mg kg−1; median (IQR) | 3 (2.4–3.1) |
| Noradrenaline, µg kg−1 min−1; median (IQR) | 0.62 (0.4–0.82) |
| Crystalloid fluid volume total, mL; median (IQR) | 5000 (3500–6000) |
| Crystalloid fluid volume, mL kg−1; median (IQR) | 73 (49.8–87.8) |
| Heparin, U kg−1; median (IQR) | 429 (375–484) |
| Heparin reversal with protamine, n (%) | 68 (75.5) |
| Postoperative bleeding complication a, n (%) | 13 (14.4) |
| Intensive care unit length of stay > 1 d, n (%) | 9 (10) |
| Heparin-to-Protamine Ratio | Crystalloids (mL) | Bleeding Site | Treatment | Transfusion | ICU LOS (d) |
|---|---|---|---|---|---|
| 4:3 | 10,500 | Neck | Compression, ventilation | None | 1 |
| 3:2 | 8000 | Face | Tamponade, ventilation | 1PLT | 1 |
| No protamine | 7500 | Neck | Re-ETI, CT, angiography | 3FFP, 1 PLT, 2TXA | 3 |
| 1:1 | 9000 | Neck | Compression, ventilation | None | 1 |
| No protamine | 7000 | Face | Compression, ventilation | None | 1 |
| No protamine | 11,000 | Femoral | Compression, ventilation | 1PLT, 1FFP | 1 |
| No protamine | 8000 | Femoral | Compression | 1RBC | 1 |
| No protamine | 14,000 | Neck, femoral | Compression, ventilation | 2RBC, 2FFP, 1PLT, 2FIB | 3 |
| No protamine | 7000 | Neck | Compression, ventilation | 3FFP, 4FIB, 2TXA | 2 |
| No protamine | 10,000 | Face | Re-ETI, tamponade, ventilation | 3FFP, 1TXA | 2 |
| No protamine | 7000 | Femoral | Compression, ventilation | 1RBC, 2FIB | 2 |
| No protamine | 6500 | Neck | Re-ETI, CPR, Surgery, TT, ventilation | 4RBC, 4FFP, 2PLT, 4FIB, 2TXA | 7 |
| 1:1 | 3000 | Femoral | Compression | 1RBC | 1 |
| Predictor | Coefficient (SE) | Z-Value | p-Value |
|---|---|---|---|
| ECCP time | 0.65 (0.47) | 1.40 | 0.167 |
| Melphalan dosage | 0.096 (0.41) | 0.23 | 0.815 |
| Noradrenaline dosage | −0.27 (0.43) | −0.62 | 0.535 |
| Crystalloid fluid volume | 4.1 (2.8) | 1.40 | 0.151 |
| Heparin dosage | 0.082 (0.39) | 0.21 | 0.833 |
| Protamine use | −2.7 (0.93) | −3.00 | 0.003 |
| Predictor | Odds Ratio (95% CI) | Z-Value | p-Value |
|---|---|---|---|
| Crystalloid fluid volume | 12 (2.3–60) | 2.9 | 0.003 |
| Protamine use | 0.065 (0.007–0.55) | −2.5 | 0.012 |
| Predictor | Coefficient (SE) | Z-Value | p-Value |
|---|---|---|---|
| ECCP time | 1 (0.55) | 1.90 | 0.062 |
| Melphalan dosage | 0.47 (0.64) | 0.74 | 0.459 |
| Noradrenaline dosage | 0.15 (0.58) | 0.26 | 0.797 |
| Crystalloid fluid volume | 1.6 (0.62) | 2.70 | 0.008 |
| Heparin dosage | −0.21 (0.63) | −0.34 | 0.735 |
| Protamine use | −3 (1.4) | −2.1 | 0.032 |
| Predictor | Odds Ratio (95% CI) | Z-Value | p-Value |
|---|---|---|---|
| Crystalloid fluid volume | 5.2 (1.5–18) | 2.7 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Struck, M.F.; Werdehausen, R.; Kirsten, H.; Gössmann, H.; Veelken, R.; van Bömmel, F.; Stehr, S.; Denecke, T.; Ebel, S. Prognostic Factors for Postoperative Bleeding Complications and Prolonged Intensive Care after Percutaneous Hepatic Chemosaturation Procedures with Melphalan. Cancers 2023, 15, 3776. https://doi.org/10.3390/cancers15153776
Struck MF, Werdehausen R, Kirsten H, Gössmann H, Veelken R, van Bömmel F, Stehr S, Denecke T, Ebel S. Prognostic Factors for Postoperative Bleeding Complications and Prolonged Intensive Care after Percutaneous Hepatic Chemosaturation Procedures with Melphalan. Cancers. 2023; 15(15):3776. https://doi.org/10.3390/cancers15153776
Chicago/Turabian StyleStruck, Manuel Florian, Robert Werdehausen, Holger Kirsten, Holger Gössmann, Rhea Veelken, Florian van Bömmel, Sebastian Stehr, Timm Denecke, and Sebastian Ebel. 2023. "Prognostic Factors for Postoperative Bleeding Complications and Prolonged Intensive Care after Percutaneous Hepatic Chemosaturation Procedures with Melphalan" Cancers 15, no. 15: 3776. https://doi.org/10.3390/cancers15153776
APA StyleStruck, M. F., Werdehausen, R., Kirsten, H., Gössmann, H., Veelken, R., van Bömmel, F., Stehr, S., Denecke, T., & Ebel, S. (2023). Prognostic Factors for Postoperative Bleeding Complications and Prolonged Intensive Care after Percutaneous Hepatic Chemosaturation Procedures with Melphalan. Cancers, 15(15), 3776. https://doi.org/10.3390/cancers15153776








