Long-Term Outcomes after Incomplete Resection of Intramedullary Grade II Ependymomas: Is Adjuvant Radiotherapy Justified?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
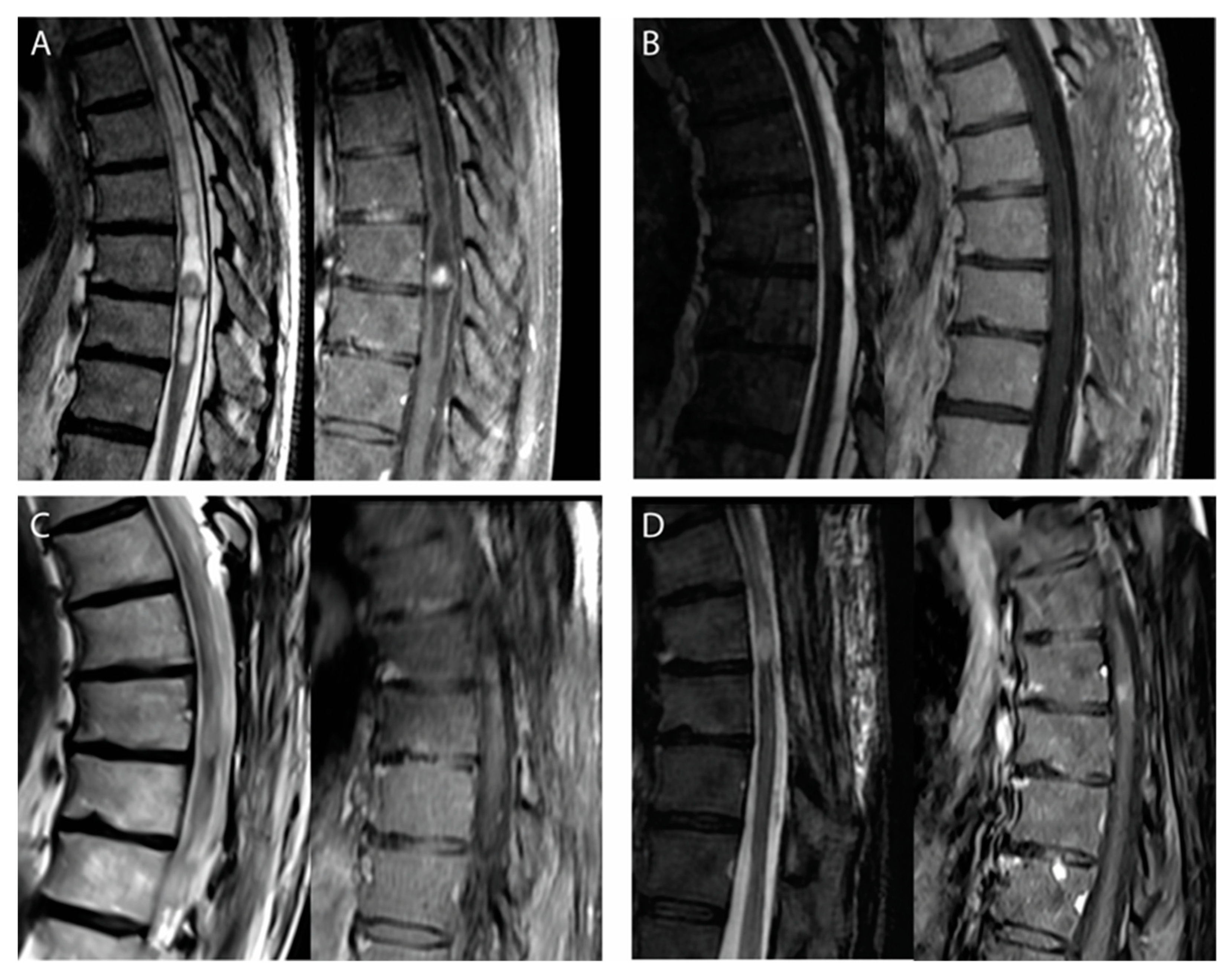
3.1. Patients
3.2. Surgery
3.3. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aghakhani, N.; David, P.; Parker, F.; Lacroix, C.; Benoudiba, F.; Tadie, M. Intramedullary Spinal Ependymomas: Analysis of a Consecutive Series of 82 Adult Cases with Particular Attention to Patients with No Preoperative Neurological Deficit. Neurosurgery 2008, 62, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, N.; Bradac, O.; de Lacy, P.; Benes, V. Intramedullary Ependymoma: Long-Term Outcome after Surgery. Acta Neurochir. 2018, 160, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, S.; Tebha, S.S.; Qamar, M.A.; Singh, S.; Alfawares, Y.; Ramanathan, V.; Haider, A.S.; Ferini, G.; Sharma, M.; Umana, G.E.; et al. Clinical Characteristics, Management, and Outcomes of Intramedullary Spinal Cord Ependymomas in Adults: A Systematic Review. World Neurosurg. 2023, 173, 237–250.e8. [Google Scholar] [CrossRef]
- Klekamp, J. Spinal Ependymomas. Part 1: Intramedullary Ependymomas. Neurosurg. Focus 2015, 39, E6. [Google Scholar] [CrossRef]
- Wostrack, M.; Ringel, F.; Eicker, S.O.; Jägersberg, M.; Schaller, K.; Kerschbaumer, J.; Thomé, C.; Shiban, E.; Stoffel, M.; Friedrich, B.; et al. Spinal Ependymoma in Adults: A Multicenter Investigation of Surgical Outcome and Progression-Free Survival. J. Neurosurg. Spine 2018, 28, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Savoor, R.; Sita, T.L.; Dahdaleh, N.S.; Helenowski, I.; Kalapurakal, J.A.; Marymont, M.H.; Lukas, R.; Kruser, T.J.; Smith, Z.A.; Koski, T.; et al. Long-Term Outcomes of Spinal Ependymomas: An Institutional Experience of More than 60 Cases. J. Neuro-Oncol. 2021, 151, 241–247. [Google Scholar] [CrossRef]
- Boström, A.; von Lehe, M.; Hartmann, W.; Pietsch, T.; Feuss, M.; Boström, J.P.; Schramm, J.; Simon, M. Surgery for Spinal Cord EpendymomasOutcome and Prognostic Factors. Neurosurgery 2011, 68, 302–309. [Google Scholar] [CrossRef]
- Giammattei, L.; Penet, N.; Parker, F.; Messerer, M. Intramedullary Ependymoma: Microsurgical Resection Technique. Neuro-Chirurgie 2016, 63, 398–401. [Google Scholar] [CrossRef]
- Klekamp, J. Treatment of Intramedullary Tumors: Analysis of Surgical Morbidity and Long-Term Results. J. Neurosurg. Spine 2013, 19, 12–26. [Google Scholar] [CrossRef]
- Byun, H.K.; Yi, S.; Yoon, H.I.; Kim, S.H.; Cho, J.; Suh, C.-O. Clinical Outcomes of Radiotherapy for Spinal Cord Ependymoma with Adverse Prognostic Features: A Single-Center Study. J. Neuro-Oncol. 2018, 140, 649–657. [Google Scholar] [CrossRef]
- Oh, M.C.; Ivan, M.E.; Sun, M.Z.; Kaur, G.; Safaee, M.; Kim, J.M.; Sayegh, E.T.; Aranda, D.; Parsa, A.T. Adjuvant Radiotherapy Delays Recurrence Following Subtotal Resection of Spinal Cord Ependymomas. Neuro-Oncology 2013, 15, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Chung, C.K.; Kim, C.H.; Yoon, S.H.; Hyun, S.-J.; Kim, K.-J.; Kim, E.-S.; Eoh, W.; Kim, H.-J. Long-Term Outcomes of Surgical Resection with or without Adjuvant Radiation Therapy for Treatment of Spinal Ependymoma: A Retrospective Multicenter Study by the Korea Spinal Oncology Research Group. Neuro-Oncology 2013, 15, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Goyal, A.; Takami, H.; Graffeo, C.S.; Mahajan, A.; Krauss, W.E.; Bydon, M. Radiotherapy in Addition to Surgical Resection May Not Improve Overall Survival in WHO Grade II Spinal Ependymomas. Clin. Neurol. Neurosurg. 2020, 189, 105632. [Google Scholar] [CrossRef] [PubMed]
- Rudà, R.; Reifenberger, G.; Frappaz, D.; Pfister, S.M.; Laprie, A.; Santarius, T.; Roth, P.; Tonn, J.C.; Soffietti, R.; Weller, M.; et al. EANO Guidelines for the Diagnosis and Treatment of Ependymal Tumors. Neuro-Oncology 2017, 20, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Knafo, S.; Aghakhani, N.; David, P.; Parker, F. Management of Intramedullary Spinal Cord Tumors: A Single-Center Experience of 247 Patients. Rev. Neurol. 2021, 177, 508–514. [Google Scholar] [CrossRef] [PubMed]
- McCormick, P.C.; Torres, R.; Post, K.D.; Stein, B.M. Intramedullary Ependymoma of the Spinal Cord. J. Neurosurg. 1990, 72, 523–532. [Google Scholar] [CrossRef]
- Kucia, E.J.; Bambakidis, N.C.; Chang, S.W.; Spetzler, R.F. Surgical Technique and Outcomes in the Treatment of Spinal Cord Ependymomas: Part II: Myxopapillary Ependymoma. Oper. Neurosurg. 2011, 68, ons57–ons63. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Corso, C.D.; Ward, M.C.; Heinzerling, J.H.; Dhakal, R.; Buchwald, Z.S.; Patel, K.R.; Asher, A.L.; Sumrall, A.L.; Burri, S.H. The Effect of Adjuvant Radiotherapy on Overall Survival in Adults with Intracranial Ependymoma. Neuro-Oncol. Pract. 2019, 7, 391–399. [Google Scholar] [CrossRef]
- Sahgal, A.; Chang, J.H.; Ma, L.; Marks, L.B.; Milano, M.T.; Medin, P.; Niemierko, A.; Soltys, S.G.; Tomé, W.A.; Wong, C.S.; et al. Spinal Cord Dose Tolerance to Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 124–136. [Google Scholar] [CrossRef]
- Gembruch, O.; Chihi, M.; Haarmann, M.; Parlak, A.; Oppong, M.D.; Rauschenbach, L.; Michel, A.; Jabbarli, R.; Ahmadipour, Y.; Sure, U.; et al. Surgical Outcome and Prognostic Factors in Spinal Cord Ependymoma: A Single-Center, Long-Term Follow-up Study. Ther. Adv. Neurol. Diso 2021, 14, 17562864211055694. [Google Scholar] [CrossRef]
- Ellison, D.W.; Aldape, K.D.; Capper, D.; Fouladi, M.; Gilbert, M.R.; Gilbertson, R.J.; Hawkins, C.; Merchant, T.E.; Pajtler, K.; Venneti, S.; et al. CIMPACT-NOW Update 7: Advancing the Molecular Classification of Ependymal Tumors. Brain Pathol. 2020, 30, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, D.R.; Sill, M.; Okonechnikov, K.; Korshunov, A.; Yip, S.; Schutz, P.W.; Scheie, D.; Kruse, A.; Harter, P.N.; Kastelan, M.; et al. MYCN Amplification Drives an Aggressive Form of Spinal Ependymoma. Acta Neuropathol. 2019, 138, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Witt, H.; Gramatzki, D.; Hentschel, B.; Pajtler, K.W.; Felsberg, J.; Schackert, G.; Löffler, M.; Capper, D.; Sahm, F.; Sill, M.; et al. DNA Methylation-Based Classification of Ependymomas in Adulthood: Implications for Diagnosis and Treatment. Neuro-Oncology 2018, 20, 1616–1624. [Google Scholar] [CrossRef] [PubMed]


| Clinical Features | ||
|---|---|---|
| Mean age (years, [min–max]) | 45.5 [24–69] | |
| Sex ratio (M/F) | 24/22 (52.2%) | |
| Mean delay between symptom onset and surgery (months, [min–max]) | 30.3 [1–204] | |
| Preoperative McCormick grade | I | 25 (54.4%) |
| II | 19 (41.3%) | |
| III | 2 (4.4%) | |
| IV | 0 | |
| Preoperative symptoms | pain | 38 (82.6%) |
| motor | 19 (41.3%) | |
| sensory | 43 (93.5%) | |
| sphincter | 14 (30.4%) | |
| Radiological Features | ||
| Vertebral levels involved | mean [min–max] | 3 [1–6] |
| cervical | 20 (43.5%) | |
| cervicodorsal | 8 (17.4%) | |
| dorsal | 18 (39.1%) | |
| T1-weighted sequence signal (%) | hypo | 54.8% |
| iso | 25.0% | |
| hyper | 16.1% | |
| T2-weighted sequence signal (%) | hypo | 17.1% |
| iso | 25.7% | |
| hyper | 57.1% | |
| Gadolinium enhancement | 100% | |
| Peritumoral cyst | 86.7% | |
| Peritumoral hemorrhage | 58.6% | |
| Surgical Outcome | |||
|---|---|---|---|
| Clear cleavage plane throughout surgery | 21/43 (48.8%) | ||
| Residue according to surgeon | 27 (58.7%) | ||
| Residue visible on first postop MRI | 22 (47.8%) | ||
| Extent of resection | gross total resection | 19 (41.3%) | |
| subtotal resection | 21 (45.7%) | ||
| partial resection | 6 (13%) | ||
| WHO grade | II | 46 (100%) | |
| III * | 0 | ||
| Ki-67 levels | <5% | 40 (90.9%) | |
| ≥5% | 4 (9.1%) | ||
| Postoperative Outcome | |||
| Early postoperative McCormick grade | I | 14 (30.4%) | |
| II | 22 (47.8%) | ||
| III | 7 (15.2%) | ||
| IV | 3 (6.5%) | ||
| Complications | medical (PE, UTI) | 2/46 (4.3%) | |
| reoperation < M3 | 2/46 (4.3%) | ||
| Long-Term Outcome | |||
| Follow-up | Mean (in months) | 86.7 | |
| Median [min–max] | 79 [36–186] | ||
| McCormick grade at last follow-up | I | 32 (69.6%) | |
| II | 7 (15.2%) | ||
| III | 5 (10.9%) | ||
| IV | 2 (4.3%) | ||
| Tumor progression | Mean delay [min–max] | 50.9 [18–85] | |
| Radiological | 7 (15.2%) | ||
| Symptomatic | 2 (4.4%) | ||
| Progression free survival (PFS) | 3 years | 93.4% | |
| 5 years | 90.1% | ||
| 10 years | 76.8% | ||
| Sex-Age | Spinal Segments | Preop. MCG | Extent of Resection | Ki-67 | Delay of Progression | Symptoms | Delay of Retreatment | Management of Progression | Last FU (Months) | Last FU MCG |
|---|---|---|---|---|---|---|---|---|---|---|
| F-37 | C7-T3 | I | STR | 8% | 12 | No | 40 | Surgery (STR) | 86 | I |
| H-59 | T8-T9 | I | STR | 1% | 48 | No | NA | Conservative | 67 | I |
| F-68 | C3-C6 | II | STR | 1% | 18 | Yes | 22 | Surgery (PR) + radiotherapy | 51 | IV |
| F-48 | C1-C2 | I | STR | 1% | 66 | No | 68 | Radiotherapy | 107 | I |
| F-48 | T2-T4 | I | PR | 3% | 16 | Yes | 18 | Surgery (GTR) | 151 | I |
| F-33 | C4 | I | STR | 1% | 81 | No | NA | Conservative | 103 | I |
| F-39 | C2-C4 | I | STR | 1% | 35 | No | NA | Conservative | 186 | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaskis, E.; Bouchaala, M.; David, P.; Parker, F.; Aghakhani, N.; Knafo, S. Long-Term Outcomes after Incomplete Resection of Intramedullary Grade II Ependymomas: Is Adjuvant Radiotherapy Justified? Cancers 2023, 15, 3674. https://doi.org/10.3390/cancers15143674
Chaskis E, Bouchaala M, David P, Parker F, Aghakhani N, Knafo S. Long-Term Outcomes after Incomplete Resection of Intramedullary Grade II Ependymomas: Is Adjuvant Radiotherapy Justified? Cancers. 2023; 15(14):3674. https://doi.org/10.3390/cancers15143674
Chicago/Turabian StyleChaskis, Elly, Mohamed Bouchaala, Philippe David, Fabrice Parker, Nozar Aghakhani, and Steven Knafo. 2023. "Long-Term Outcomes after Incomplete Resection of Intramedullary Grade II Ependymomas: Is Adjuvant Radiotherapy Justified?" Cancers 15, no. 14: 3674. https://doi.org/10.3390/cancers15143674
APA StyleChaskis, E., Bouchaala, M., David, P., Parker, F., Aghakhani, N., & Knafo, S. (2023). Long-Term Outcomes after Incomplete Resection of Intramedullary Grade II Ependymomas: Is Adjuvant Radiotherapy Justified? Cancers, 15(14), 3674. https://doi.org/10.3390/cancers15143674







