Clinical Trials of Cellular Therapies in Solid Tumors
Abstract
Simple Summary
Abstract
1. Introduction
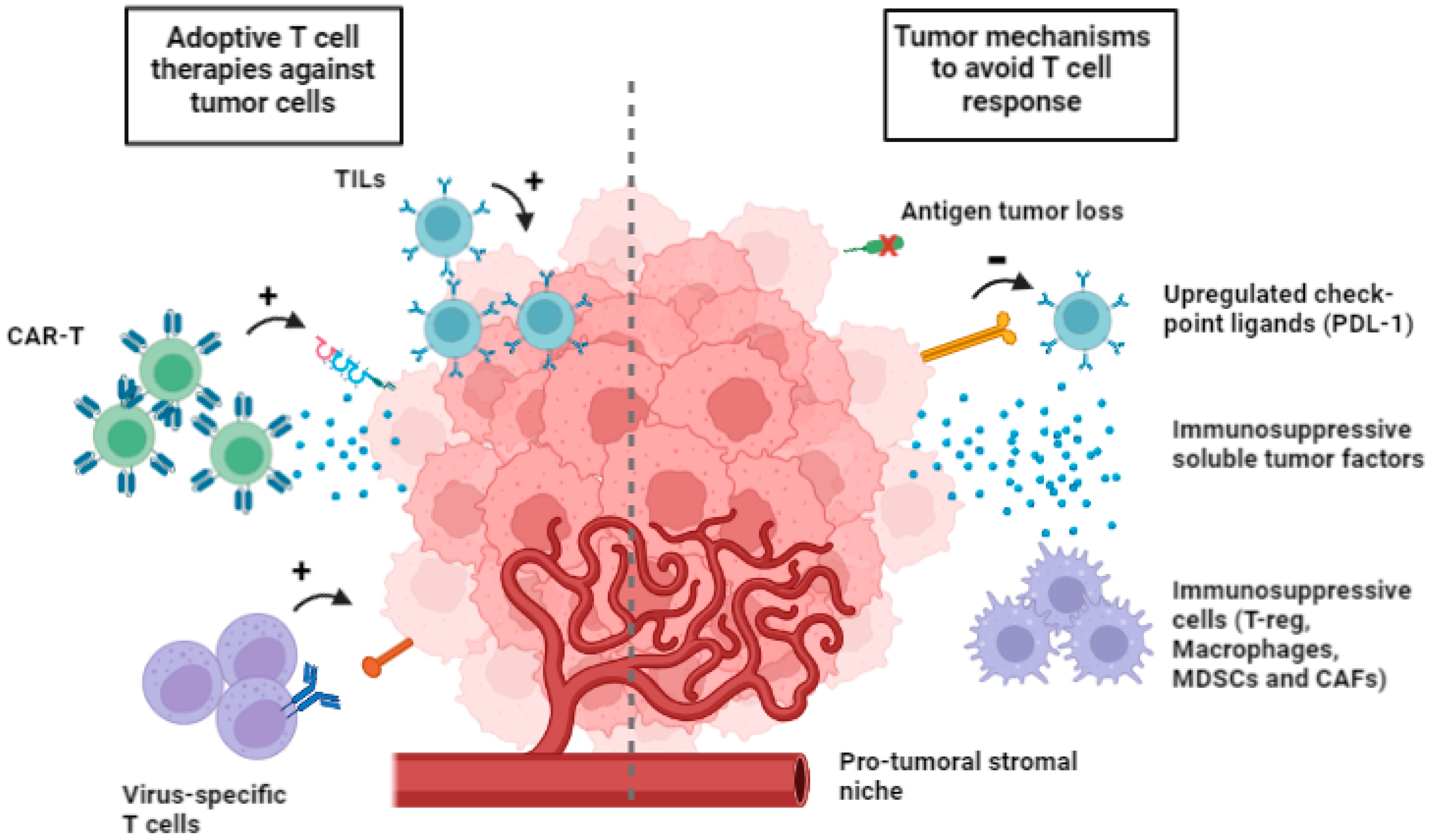
2. Adaptive Cell Therapy with T Cells Generated and Expanded In Vitro
2.1. Tumor-Infiltrating Lymphocytes (TILs)
2.2. Tumor-Infiltrating Lymphocytes (TILs) in Combination with Immune Checkpoint Inhibitors (ICIs)
2.3. Virus-Specific T Cells as ACT
2.4. T Cells Targeting Tumor Neoantigens
3. Tumor Mutational Burden
4. Adaptive Cell Therapy with CAR-T: Progress and Pitfalls
4.1. CAR-T Interactions with Tumor Microenvironment (TME)
4.2. CAR-T: How to Increase Endogenous Immune Response
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukine-2 or high-dose interleukin-2 alone. N. Engl. J. Med. 1987, 316, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Rosenberg, S.A. Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. J. Exp. Med. 1984, 159, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Gajewski, T.F. Tumor-intrinsic oncogene pathways mediating immune avoidance. Oncoimmunology 2015, 5, e1086862. [Google Scholar] [CrossRef] [PubMed]
- Comoli, P.; Chabannon, C.; Koehl, U.; Lanza, F.; Urbano-Ispizua, A.; Hudecek, M.; Ruggeri, A.; Secondino, S.; Bonini, C.; Pedrazzoli, P. Development of adaptive immune effector therapies in solid tumors. Ann. Oncol. 2019, 30, 1740–1750. [Google Scholar] [CrossRef]
- Eggermont, A.M.M. Advances in systemic treatment of melanoma. Ann. Oncol. 2010, 21 (Suppl. 7), vi339–vi344. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Topalian, S.L.; Weiner, G.P.; Pardoll, D.M. Cancer immunotherapy comes of age. J. Clin. Oncol. 2011, 29, 4828–4836. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 317, 1507–1517. [Google Scholar] [CrossRef]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell-receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef]
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.M.; Hamid, M. scFv antibody: Principles and clinical application. Clin. Dev. Immunol. 2012, 2012, 980250. [Google Scholar] [CrossRef]
- Dotti, G.; Savoldo, B.; Brenner, M. Fifteen years of gene therapy based on chimeric antigen receptors: “are we nearly there yet?”. Hum. Gene Ther. 2009, 20, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Apetoh, L.; Ladoire, S.; Coukos, G.; Ghiringhelli, F. Combining immunotherapy and anticancer agents: The right path to achieve cancer cure? Ann. Oncol. 2015, 26, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Packard, B.S.; Aebersold, P.M.; Solomon, D.; Topalian, S.L.; Toy, S.T.; Simon, P.; Lotze, M.T.; Yang, J.C.; Seipp, C.A.; et al. Use of tumor infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 1988, 319, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.E.; Yang, J.C.; Sherry, R.; Hughes, M.S.; Royal, R.; Kammula, U.; Robbins, P.F.; Huang, J.; Citrin, D.E.; Leitman, S.F.; et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008, 26, 5233–5239. [Google Scholar] [CrossRef]
- Goff, S.L.; Dudley, M.E.; Citrin, D.E.; Somerville, R.P.; Wunderlich, J.R.; Danforth, D.N.; Zlott, D.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J. Clin. Oncol. 2016, 34, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Sekine, T.; Makuuchi, M.; Yamasaki, S.; Kosuge, T.; Yamamoto, J.; Shimada, K.; Sakamoto, M.; Hirohashi, S.; Ohashi, Y.; et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: A randomized trial. Lancet 2000, 356, 802–807. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, J.; Li, H.Y.; Wang, Z.H.; Wu, J. Immunotherapy for advanced hepatocellular carcinoma, where are we? Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188441. [Google Scholar] [CrossRef]
- Stevanović, S.; Draper, L.M.; Langhan, M.M.; Campbell, T.E.; Kwong, M.L.; Wunderlich, J.R.; Dudley, M.E.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus targeted tumor-infiltratin T cells. J. Clin. Oncol. 2015, 33, 1543–1550. [Google Scholar] [CrossRef]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.-C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer immunotherapy based in mutation-specific CD4+T cells in a patient with epithelial cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Creelan, B.C.; Wang, C.; Teer, J.K.; Toloza, E.M.; Yao, J.; Kim, S.; Landin, A.M.; Mullinax, J.E.; Saller, J.J.; Saltos, A.N.; et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistent metatstatic lung cancer: A phase 1 trial. Nat. Med. 2021, 27, 1410–1418. [Google Scholar] [CrossRef]
- Bobisse, S.; Genolet, R.; Roberti, A.; Tanyi, J.L.; Racle, J.; Stevenson, B.J.; Iseli, C.; Michel, A.; Le Bitoux, M.-A.; Guillaume, P.; et al. Sensitive and frequent identification of high avidity neo-epitope specific CD8 (+) T cells in immunotherapy naïve ovarian cancer. Nat. Commun. 2018, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Robbins, P.F.; Lu, Y.-C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. Cell transfer therapy targeting mutant KRAS in cancer. N. Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Zacharakis, N.; Chinnasamy, H.; Black, M.; Xu, H.; Lu, Y.-C.; Zheng, Z.; Pasetto, A.; Langhan, M.; Shelton, T.; Prickett, T.; et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 2018, 24, 724–730. [Google Scholar] [CrossRef]
- Tran, E.; Ahmadzadeh, M.; Lu, Y.C.; Gros, A.; Turcotte, S.; Robbins, P.F. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 2015, 350, 1387–1390. [Google Scholar] [CrossRef]
- Kumar, A.; Watkins, R.; Vilgelm, A.E. Cell therapy with TILs: Training and taming T cells to fight cancer. Front. Imuunol. 2021, 12, 690499. [Google Scholar] [CrossRef]
- Huang, J.; El-Gamil, M.; Dudley, M.E.; Li, Y.F.; Rosenberg, S.A.; Robbins, P.F. T cells associated with tumor regression recognize frameshifted products of the CDKN2A tumor suppror gene locus and a mutated HLA Class I gene product. J. Immunol. 2004, 172, 6057–6064. [Google Scholar] [CrossRef]
- Zhou, J.; Dudley, M.E.; Rosenberg, S.A.; Robbins, P.F. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J. Immunother. 2005, 28, 53–62. [Google Scholar] [CrossRef]
- Li, Q.; Din, Z.Y. The ways of isolating neoantigens-specific T cells. Front. Oncol. 2020, 10, 1347. [Google Scholar] [CrossRef]
- Robbins, P.F.; Lu, Y.C.; El-Gamil, M.; Li, Y.F.; Gross, C.; Gartner, J.; Lin, J.C.; Teer, J.K.; Cliften, P.; Tycksen, E.; et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 2013, 19, 747–752. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Lee, S.J.; Hodi, F.S.; Rao, U.N.M.; Cohen, G.I.; Hamid, O.; Hutchins, L.F.; Sosman, J.A.; Kluger, H.M.; Eroglu, Z.; et al. Phase III study of adjuvant Ipilimumab (3 or 10 mg/kg) versus high-dose Interferon alfa-2B for resected high-risk melanoma: North American Itergroup E1609. J. Clin. Oncol. 2010, 38, 567–576. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Five-year survival with combined Nivolumab and Ipilimumab in advanced melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for recurrent squamous-cell carcinoma of head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Pardol, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Kverneland, A.H.; Pedersen, M.; Westergaard, M.C.W.; Nielsen, M.; Borch, T.H.; Olsen, L.R.; Aasbjerg, G.; Santegoets, S.J.; van der Burg, S.H.; Milne, K.; et al. Adoptive cell therapy in combination with checkpoint inhibitors in ovarian cancer. Oncotarget 2020, 11, 2092–2105. [Google Scholar] [CrossRef]
- Caushi, J.X.; Zhang, J.; Ji, Z.; Vaghasia, A.; Zhang, B.; Hsiue, E.H.-C.; Mog, B.J.; Hou, W.; Justesen, S.; Blosser, R.; et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature 2021, 596, 126–132. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, J.; Duan, C.; Liu, Y. Retrospective analysis of adoptive TIL therapy plus anti-PD1 therapy in patients with chemotherapy-resistant metastatic osteosarcoma. J. Immunol. Res. 2020, 7890985. [Google Scholar] [CrossRef]
- Wang, C.; Li, M.; Wei, R.; Wu, J. Adoptive transfer of TILs plus anti-PD1 therapy: An alternative combination therapy for treating metastatic osteosarcoma. J. Bone Oncol. 2020, 25, 100332. [Google Scholar] [CrossRef]
- Chou, A.J.; Gorlick, R. Chemotherapy resistance in osteosarcoma: Current challenges and future directions. Expert Rev. Anticancer Ther. 2006, 6, 1075–1085. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcomas (SARC028): A multicenter, two-cohort, single arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Gao, P.; Lazare, C.; Cao, C.; Meng, Y.; Wu, P.; Zhi, W.; Lin, S.; Wei, J.; Huang, X.; Xi, L.; et al. Immune checkpoint inhibitors in the treatment of virus-associated cancers. J. Hematol. Oncol. 2019, 12, 58. [Google Scholar] [CrossRef]
- Rooney, C.M.; Smith, C.A.; Ng, C.Y.; Loftin, S.; Li, C.; Krance, R.A.; Brenner, M.K.; Heslop, H.E. Use of gene-modified virus-specific T lymphocytes Epstein-Barr-virus-related lymphoproliferation. Lancet 1995, 345, 9–13. [Google Scholar] [CrossRef]
- Heslop, H.E.; Slobod, K.S.; Pule, M.A.; Hale, G.A.; Rousseau, A.; Smith, C.A.; Bollard, C.M.; Liu, H.; Wu, M.-F.; Rochester, R.J.; et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010, 115, 925–936. [Google Scholar] [CrossRef]
- Comoli, P.; Basso, S.; Zecca, M.; Pagliara, D.; Baldanti, F.; Bernardo, M.E.; Barberi, W.; Moretta, A.; Labirio, M.; Paulli, M.; et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am. J. Transplant. 2007, 7, 1648–1655. [Google Scholar] [CrossRef]
- Straathof, K.C.M.; Bollard, C.M.; Popat, U.; Huls, M.H.; Lopez, T.; Morriss, M.C.; Gresik, M.V.; Gee, A.P.; Russell, H.V.; Brenner, M.K.; et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus-specific T Lymphocytes. Blood 2005, 105, 1898–1905. [Google Scholar] [CrossRef]
- Comoli, P.; Pedrazzoli, P.; Maccario, R.; Basso, S.; Carminati, O.; Labirio, M.; Schiavo, R.; Secondino, S.; Frasson, C.; Perotti, C.; et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J. Clin. Oncol. 2005, 23, 8942–8949. [Google Scholar] [CrossRef]
- Louis, C.U.; Straathof, K.; Bollard, C.M.; Gerken, C.; Huls, M.H.; Gresik, M.V.; Wu, M.-F.; Weiss, H.L.; Gee, A.P.; Brenner, M.K.; et al. Enhancing the in-vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood 2009, 113, 2422–2450. [Google Scholar] [CrossRef]
- Secondino, S.; Zecca, M.; Licitra, L.; Gurrado, A.; Schiavetto, I.; Bossi, P.; Locati, L.; Schiavo, R.; Basso, S.; Baldanti, F.; et al. T-cell theapy for EBV-associated nasopharyngeal carcinoma: Preparative lymphodepleting chemotherapy does not improve clinical results. Ann. Oncol. 2012, 23, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.M.; Somerville, R.P.; Klebanoff, C.A.; Kammula, U.S.; Sherry, R.M.; et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin. Cancer Res. 2019, 25, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, L.; Tong, R.; Yang, F.; Yin, L.; Li, M.; You, L.; Xue, J.; Lu, Y. PD-1/PD-L1 inhibitors in cervical cancer. Front. Pharmacol. 2019, 10, 65. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- Yin, H.; Guo, W.; Sun, X.; Li, R.; Feng, C.; Tan, Y. TILs and anti-PD1 therapy: An alternative combination therapy for PDL1 negative metastatic cervical cancer. J. Immunol. Res. 2020, 2020, 8345235. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer ant the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef]
- Leko, V.; Rosenberg, S.A. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumor. Cancer Cell 2020, 38, 454–472. [Google Scholar] [CrossRef]
- Schumaker, T.N.; Scheper, W.; Kvistbong, P. Cancer neoantigens. Ann. Rev. Immunol. 2019, 37, 173–200. [Google Scholar] [CrossRef]
- Schumaker, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumor mutational burden with outcomes in patients with advanced solid tumor treated with Pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Pecina-Slaus, N.; Kafka, A.; Salamon, I.; Bukovac, A. Mismatch repair pathway, genome instability and cancer. Front. Mol. Biosci. 2020, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in microsatellite instability-high advanced colorectal cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Thistlethwaite, F.C.; Gilham, D.E.; Guest, R.D.; Rothwell, D.G.; Pillai, M.; Burt, D.J.; Byatte, A.J.; Kirillova, N.; Valle, J.W.; Sharma, S.K.; et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 2017, 66, 1425–1436. [Google Scholar] [CrossRef]
- Gardner, R.A.; Finney, O.; Annesley, C.; Brakke, H.; Summers, C.; Leger, K.; Bleakley, M.; Brown, C.; Mgebroff, S.; Kelly-Spratt, K.S.; et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017, 129, 3322–3331. [Google Scholar] [CrossRef]
- Fielding, A.K.; Richards, S.M.; Chopra, R.; Lazarus, H.M.; Litzow, M.R.; Buck, G.; Durrant, I.J.; Luger, S.M.; Marks, D.I.; Franklin, I.M.; et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007, 109, 944–950. [Google Scholar] [CrossRef]
- Kershaw, M.H.; Westwood, J.A.; Parker, L.L.; Wang, G.; Eshhar, Z.; Mavroukakis, S.A.; White, D.E.; Wunderlich, J.R.; Canevari, S.; Rogers-Freezer, L.; et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006, 12, 6106–6115. [Google Scholar] [CrossRef]
- Lamers, C.H.J.; Sleijfer, S.; Vulto, A.G.; Kruit, W.H.J.; Kliffen, M.; Debets, R.; Gratama, J.W.; Stoter, G.; Oosterwijk, E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: First clinical experience. J. Clin. Oncol. 2006, 24, 22–24. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 2019, 25, 1341–1355. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, A.; Maus, M.V. Making CAR T cells a solid option for solid tumors. Front. Immunol. 2018, 9, 2593. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Filies, D.B. Molecular mechanism of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Haynes, N.M.; Trapani, J.A.; Teng, M.W.L.; Jackson, J.T.; Cerruti, L.; Jane, S.M.; Kershaw, M.H.; Smyth, M.J.; Darcy, P.K. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood 2002, 100, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Clubb, J.D.; Chen, Y.Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 2020, 4, 473–488. [Google Scholar] [CrossRef]
- Milone, M.C.; Fish, J.D.; Carpenito, C.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B.; Danet-Desnoyers, G.; et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009, 17, 1453–1464. [Google Scholar] [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef]
- Del Bufalo, F.; De Angelis, B.; Caruana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N. Engl. J. Med. 2023, 388, 1284–1295. [Google Scholar] [CrossRef]
- Srivastava, S.; Riddell, S.R. Engineering CAR-T cells: Design concepts. Trends Immunol. 2015, 36, 494–502. [Google Scholar] [CrossRef]
- Newick, K.; O’Brien, S.; Moon, E.; Abdela, S.M. CART T cell therapy for solid tumors. Annu. Rev. Med. 2017, 68, 139–152. [Google Scholar] [CrossRef]
- Craddock, J.A.; Lu, A.; Bear, A.; Pule, M.; Brenner, M.K.; Rooney, C.M.; Foster, A.E. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 2010, 33, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.K.; Carpenito, C.; Sun, J.; Wang, L.C.; Kapoor, V.; Predina, J.; Powell, D.J., Jr.; Riley, J.L.; June, C.H.; Albelda, S.M. Expression of a functional CCR2 receptor enhanced tumor localization and tumor eradication by targeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 2011, 17, 4719–4730. [Google Scholar] [CrossRef] [PubMed]
- Caruana, I.; Savoldo, B.; Hoyos, V.; Weber, G.; Liu, H.; Kim, E.S.; Ittmann, M.M.; Marchetti, D.; Dotti, G. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 2015, 21, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013, 138, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Muranski, P.; Boni, A.; Wrzesinski, C.; Citrin, D.E.; Rosenberg, S.A.; Childs, R.; Restifo, N.P. Increased intensity lymphodepletion and adoptive immunotherapy—How far can we go? Nat. Clin. Pract. Oncol. 2006, 3, 668–681. [Google Scholar] [CrossRef]
- Pegram, H.J.; Lee, J.C.; Hayman, E.G.; Imperato, G.H.; Tedder, T.F.; Sadelain, M.; Brentjens, R.J. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 2012, 119, 4133–4141. [Google Scholar] [CrossRef]
- Boccalatte, F.; Mina, R.; Aroldi, A.; Leone, S.; Suryadevara, C.M.; Placantonakis, D.G.; Bruno, B. Advances and hurdles in CAR T cell immune therapy for solid tumors. Cancers 2022, 14, 5108. [Google Scholar] [CrossRef]
- Juillerat, A.; Marechal, A.; Filhol, J.M.; Valogne, Y.; Valton, J.; Duclert, A.; Duchateau, P.; Poirot, L. An oxygen sensitive self-decision making engineered CAR T-cell. Sci. Rep. 2017, 7, 39833. [Google Scholar] [CrossRef]
- Batra, S.A.; Rathi, P.; Guo, L.; Courtney, A.N.; Fleurence, J.; Balzeau, J.; Shaik, R.S.; Nguyen, T.P.; Wu, M.-F.; Bulsara, S.; et al. Glypican-3-specific CAR T cells co-expressing IL15 and IL21 have superior expansion and antitumor activity against hepatocellular carcinoma. Cancer Immunol. Res. 2020, 8, 309–320. [Google Scholar] [CrossRef]
- Morgan, M.A.; Schambach, A. Engineering CAR-T cells for improved function against solid tumors. Front. Immunol. 2018, 29, 2493. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Mardiana, S.; House, I.G.; Sek, K.; Henderson, M.A.; Giuffrida, L.; Chen, A.X.Y.; Todd, K.L.; Petley, E.V.; Chan, J.D.; et al. Adoptive cellular therapy with T. ells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity. Nat. Immunol. 2020, 21, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Curran, K.J.; Seinstra, B.A.; Nikhamin, Y.; Yeh, R.; Usachenko, Y.; van Leeuwen, D.G.; Purdon, T.; Pegram, H.J.; Brentjens, R.J. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol. Ther. 2015, 23, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Yu, X.; Castano, A.P.; Bouffard, A.A.; Schmidts, A.; Larson, R.C.; Bailey, S.R.; Boroughs, A.C.; Frigault, M.J.; Leick, M.B.; et al. CAR-T cells secreting Bites circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 2019, 37, 1049–1058. [Google Scholar] [CrossRef]
- Schietinger, A.; Philip, M.; Krisnawan, V.E.; Chiu, E.Y.; Delrow, J.J.; Basom, R.S.; Lauer, P.; Brockstedt, D.G.; Knoblaugh, S.E.; Hämmerling, G.J.; et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program intiated early during tumorigenesis. Immunology 2016, 45, 389–401. [Google Scholar] [CrossRef]
- Good, C.R.; Aznar, M.A.; Kuramitsu, S.; Samareh, P.; Agarwal, S.; Donahue, G.; Ishiyama, K.; Wellhausen, N.; Rennels, A.K.; Ma, Y.; et al. An NK-like CAR T cell transition in CAR T cell dysfunction. Cell 2021, 184, 6081–6100. [Google Scholar] [CrossRef]
| Interventions | Disease | Phase Clinical Trial | Treatments | Endpoints | NCT |
|---|---|---|---|---|---|
| Autologous TILs (LN-145) | Metastatic NSCLC | Phase II | Lymphodepleting chemotherapy with Flu and Cy, prior to TIL infusion | ORR | NCT04614103 |
| TILs in combination with immunotherapy | Metastatic OC | Phase I-II | Lymphodepleting chemotherapy with Flu and Cy, prior to TIL infusion; subsequent therapy with Nivolumab and Relatlimab; a separate group of patients will also receive Ipilimumab before lymphodepleting chemotherapy | Safety ORR | NCT04611126 |
| Adoptive T cell therapy | Metastatic urothelial carcinoma | Phase II | TILs after lymphodepleting chemotherapy; subsequently high-dose bolus IL2 | ORR | NCT04383067 |
| Autologous TILs (LN-145) | Metastatic TNBC | Phase II | TILs (LN-145) after lymphodepleting chemotherapy; subsequently IL2 | ORR | NCT04111510 |
| Adoptive T cell therapy | Recurrent OC | Phase I-II | TILs after 6 cycles of carboplatin/taxol; a separate group will also receive Interferon Alfa2A | Safety ORR | NCT04072263 |
| Adoptive T cell therapy | Solid tumors | Phase II | TILs after lymphodepleting chemotherapy with Flu and Cy | ORR | NCT03935893 |
| Adoptive T cell therapy | Biliary carcinoma; Cholangiocarcinoma | Phase II | TILs and Adesleukin | ORR | NCT03801083 |
| TILs alone or in combination with immunotherapy | Metastatic Melanoma or NSCLC or SCC Head and Neck | Phase II | LN-144 or LN-145 or LN-145-S1 alone or in combination with Ipilimumab or with Nivolumab or with Pembrolizumab | ORR | NCT03645928 |
| Autologous TILs (LN-145 or LN-145-S1) | Solid Tumors | Phase II | TILs after lymphodepleting chemotherapy (Flu and Cy), plus Adesleukin, alone or in combination with Ipilimumab/Nivolumab (according to histology) | ORR | NCT03449108 |
| Adoptive T cell therapy | Cervical cancer | Phase II | TILs (LN-145) after lymphodepleting chemotherapy followed by IL2, alone or in combination with Pembrolizumab | ORR | NCT03108495 |
| Adoptive T cell therapy | NSCLC | Phase II | TILs (Young TIL) after lymphodepleting chemotherapy followed by high-dose Adesleukin or low-dose Adesleukin | ORR | NCT02133196 |
| Adoptive T cell therapy in combination with Pembrolizumab | Solid Tumors | Phase II | TILs (Young TIL) after lymphodepleting chemotherapy followed by high-dose Adesleukin plus Pembrolizumab | ORR | NCT01174121 |
| Interventions | Disease | Phase Clinical Trial | Treatments | Endpoints | NCT |
|---|---|---|---|---|---|
| TCR-engineered T cells targeting HPV-16 E7 | HPV+ refractory carcinoma | Phase I-II | Cy and Flu preparative regimen followed by TCR(E7) T cells; high-dose aldesleukin | Safety and ORR | NCT02858310 |
| E7 TCR-T cell induction therapy for advanced HPV-related tumors | HPV-related tumors | Phase I-II | E7 TCR-T cells; aldesleukin | Safety and ORR | NCT05639972 |
| TG4001 and Avelumab in HPV16positive R/M cancers | HPV-related tumors | Phase Ib/II | TG4001; Avelumab | Safety and ORR | NCT03260023 |
| A vaccine (PDS0101) alone or in combination with Pembrolizumab for locally advanced HPV-related oropharynx cancer | Oropharyngeal carcinoma (Stage III or IV); HPV-related disease | Phase I/II | Liposomal HPV-16 E6/E7 multipeptide vaccine PDS0101; Pembrolizumab | Pathologic and ctHPVDNA response; ORR | NCT05232851 |
| HPV-16/18 E6/E7 specific T lymphocytes relapsed HPV-associated cancers | HPV-related cancers | Phase I | Cy and Flu lymphodepleting regimen followed by HPV-specific T cells (Group A); if safe, additional group of patients (Group B) also receive Nivolumab | Safety; ORR | NCT02379520 |
| R/M HPV-16 related SCC | HPV-related SCC | Phase I/II | TheraT® Vectors expressing HPV 16+ specific antigens (drugs: HB-201 and HB-202) | Safety; ORR | NCT04180215 |
| HPV-16 vaccination and Pembrolizumab plus CTRT for HPV-16-related head and neck SCC | Locally advanced, intermediate risk, HPV-associated head and neck SCC | Phase II | Combination of Pembrolizumab, HPV-16 E6/E7 specific vaccination (ISA101b) and cisplatin-based chemoradiotherapy | PFS at 2 years | NCT04369937 |
| Interventions | Disease | Phase Clinical Trial | Treatments | End Points | NCT |
|---|---|---|---|---|---|
| HLA-G- targeted CAR-T cells IVS-3001 | Advanced HLA-G+ solid tumors | Phase I/IIa | Cy and Flu preparative regimen followed by IVS-3001 Anti-HLA-G CAR-T | Safety; clinical activity | NCT05672459 |
| HER2 targeted HypoSti.CAR-T cells | HER2+ advanced solid tumors | Phase I/II | Preconditioning regimen of albumin-bound paclitaxel and Cy, before HypoSti.CAR-HER2 T cells | Safety; ORR | NCT05681650 |
| NKG2D-based CAR-T cells in patients with NKG2DL+ solid tumors | HCC; GBL; medulloblastoma; colon cancer | Phase I | NKG2D-based CAR-T cells | Safety | NCT05131763 |
| B7H3 CAR-T cells in advanced solid tumors in children and AYAs | STS; EWS; GCC; OS; pediatric solid tumors | Phase I | B7H3-EGFRt- DHFR | Safety; tolerability | NCT04483778 |
| EGFR806 CAR-T cells in advanced solid tumors in children and AYAs | STS; EWS; OS; GCC; pediatric solid tumors | Phase I | EGFR806 | Maximum tolerated dose; DLT | NCT03618381 |
| GD2/PSMA Bi-specific CAR-T cells in advanced solid tumors | GD2 and PSMA positive solid tumors | Phase I/II | bi-4SCAR GD2/PSMA T cells | Safety; ORR | NCT05437315 |
| CLDN 18.2 targeting CAR-T cells in advanced solid tumors | Gastric cancer, pancreatic cancer, and ovarian cancer with CLDN 18.2 expression | Phase I | Claudin 18.2 CAR-T | Safety; ORR | NCT05472857 |
| LYL797, ROR1-targeting CAR-T cells in adult refractory solid tumors | TNBC; NSCLC | Phase I | LYL797 | Safety; ORR | NCT05274451 |
| HER2- targeted dual switch CAR-T cells in advanced HER2-positive cancer | HER2+ gene amplification | Phase I/II | HER2-targeted dual-switch CAR-T cells | Safety; ORR | NCT04650451 |
| Binary Oncolytic Adenovirus in combination with HER2-specific CAR-T cells in patients with advanced HER2+ solid tumors | Bladder cancer; Breast cancer; Lung cancer; HNSCC; Gastric cancer; Colorectal cancer; Pancreatic cancer. | Phase I | CAdVEC | DLT; ORR | NCT03740256 |
| CAR-T-targeting CEA in the treatment of CEA positive advanced solid tumors | Gastric cancer; Colon cancer; Rectal cancer; Esophageal cancer; Pancreatic cancer. | Phase I/II | Cy and Flu preparative regimen followed by CEA-targeting CAR-T cells | Safety; ORR | NCT05538195 |
| IL-15 armored Glypican-3-specific CAR-T cells in pediatric solid tumors | Liver cancer; Liposarcoma; Wilms tumor; Yolk sac tumor; Rhabdomyosarcoma. | Phase I | Cy and Flu preparative regimen followed by AGAR T cells (GPC3-CAR and the IL15) | DLT; ORR | NCT04377932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secondino, S.; Canino, C.; Alaimo, D.; Muzzana, M.; Galli, G.; Borgetto, S.; Basso, S.; Bagnarino, J.; Pulvirenti, C.; Comoli, P.; et al. Clinical Trials of Cellular Therapies in Solid Tumors. Cancers 2023, 15, 3667. https://doi.org/10.3390/cancers15143667
Secondino S, Canino C, Alaimo D, Muzzana M, Galli G, Borgetto S, Basso S, Bagnarino J, Pulvirenti C, Comoli P, et al. Clinical Trials of Cellular Therapies in Solid Tumors. Cancers. 2023; 15(14):3667. https://doi.org/10.3390/cancers15143667
Chicago/Turabian StyleSecondino, Simona, Costanza Canino, Domiziana Alaimo, Marta Muzzana, Giulia Galli, Sabrina Borgetto, Sabrina Basso, Jessica Bagnarino, Chiara Pulvirenti, Patrizia Comoli, and et al. 2023. "Clinical Trials of Cellular Therapies in Solid Tumors" Cancers 15, no. 14: 3667. https://doi.org/10.3390/cancers15143667
APA StyleSecondino, S., Canino, C., Alaimo, D., Muzzana, M., Galli, G., Borgetto, S., Basso, S., Bagnarino, J., Pulvirenti, C., Comoli, P., & Pedrazzoli, P. (2023). Clinical Trials of Cellular Therapies in Solid Tumors. Cancers, 15(14), 3667. https://doi.org/10.3390/cancers15143667







