Interpretation of Tumor Response Grade following Preoperative Therapy for Gastric Cancer: An Overview
Abstract
Simple Summary
Abstract
1. Introduction
2. Gastric Cancer and Preoperative Therapies
3. Gastric Cancer Staging and Tumor Response Grade
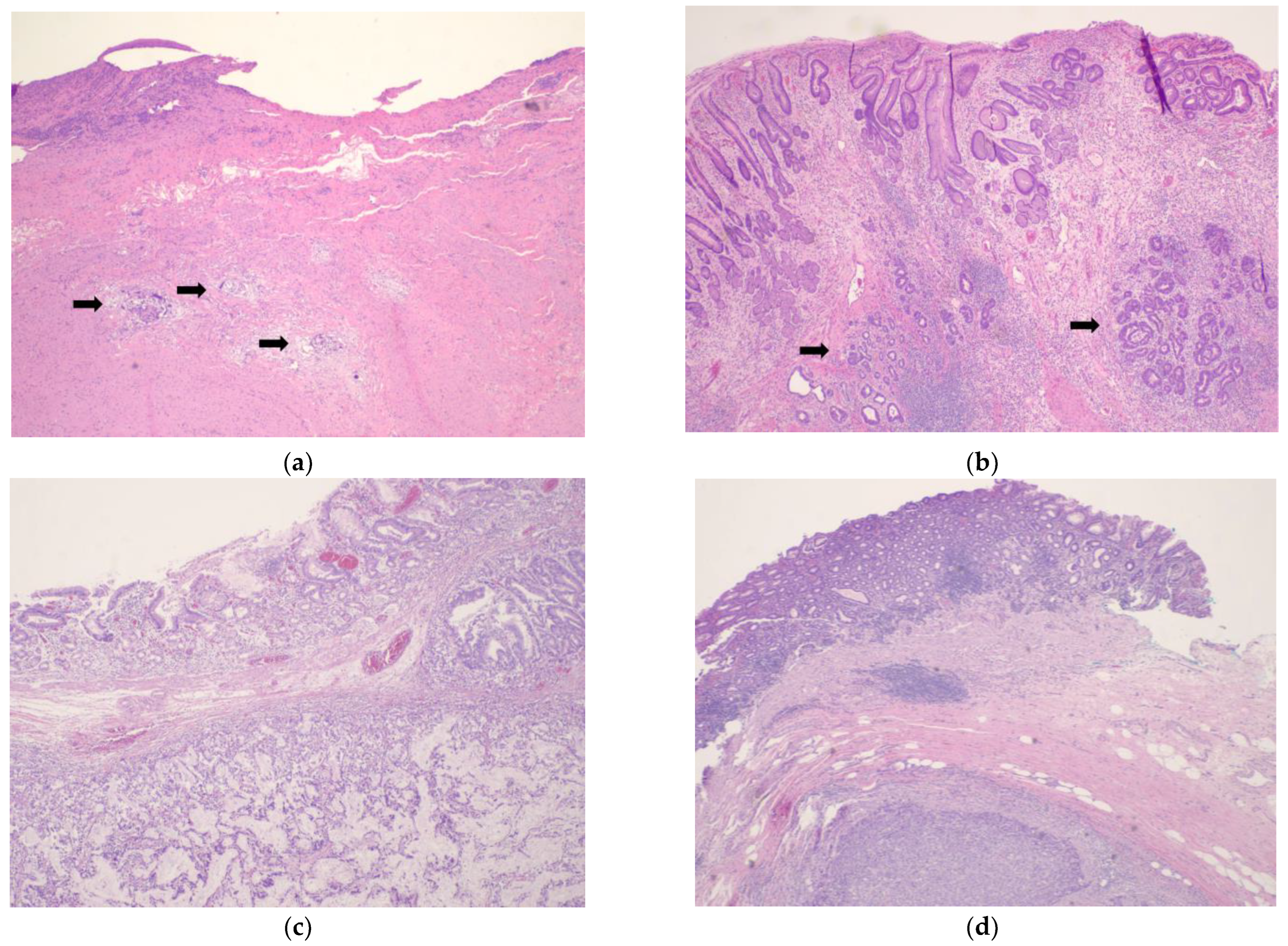
4. Tumor Response Grading Systems
5. Tumor Response Grade Prognostication
6. Radiographic and Endoscopic Response to Chemotherapy and Prognostication
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacques Ferlay, M.C.; Isabelle, S.; Donald, M.P.; Marion, P.; Ariana, Z.; Freddie, B. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. 2020. Available online: https://gco.iarc.fr/today/home (accessed on 23 February 2023).
- Ramos-Santillan, V.; Friedmann, P.; Eskander, M.; Chuy, J.; Parides, M.; In, H. The order of surgery and chemotherapy matters: Multimodality therapy and stage-specific differences in survival in gastric cancer. J. Surg. Oncol. 2023, 127, 56–65. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Gastric Cancer (Version 1.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 11 April 2023).
- Petrillo, A.; Smyth, E.C. Multimodality treatment for localized gastric cancer: State of the art and new insights. Curr. Opin. Oncol. 2020, 32, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouche, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar]
- Ajani, J.A.; In, H.; Sano, T.; Gaspar, L.E.; Erasmus, J.J.; Tang, L.H.; Washington, M.K.; Gerdes, H.; Wittekind, C.W.; Mansfield, P.F.; et al. AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S.B., Greene, F.L., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer: New York, NY, USA, 2017; pp. 203–220. [Google Scholar]
- In, H.; Ravetch, E.; Langdon-Embry, M.; Palis, B.; Ajani, J.A.; Hofstetter, W.L.; Kelsen, D.P.; Sano, T. The newly proposed clinical and post-neoadjuvant treatment staging classifications for gastric adenocarcinoma for the American Joint Committee on Cancer (AJCC) staging. Gastric Cancer 2018, 21, 1–9. [Google Scholar] [CrossRef]
- Kim, G.; Friedmann, P.; Solsky, I.; Muscarella, P.; McAuliffe, J.; In, H. Providing Reliable Prognosis to Patients with Gastric Cancer in the Era of Neoadjuvant Therapies: Comparison of AJCC Staging Schemata. J. Gastric Cancer 2020, 20, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Chung, Y.R.; Woo, J.W.; Ahn, S.; Kang, E.; Kim, E.K.; Jang, M.; Kim, S.M.; Kim, S.H.; Kim, J.H.; Park, S.Y. Prognostic implications of regression of metastatic axillary lymph nodes after neoadjuvant chemotherapy in patients with breast cancer. Sci. Rep. 2021, 11, 12128. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, J.C.; Kim, J.; Kim, Y.H.; Yoon, Y.S.; Han, H.S.; Kim, H.; Hwang, J.H. Four-Tier Pathologic Tumor Regression Grading System Predicts the Clinical Outcome in Patients Who Undergo Surgical Resection for Locally Advanced Pancreatic Cancer after Neoadjuvant Chemotherapy. Gut Liver 2022, 16, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.F.; Yu, W.D.; Pan, H.D.; Wang, L.; Li, M.; Yao, Y.F.; Zhao, J.; Gu, J. Tumor regression grades: Potential outcome predictor of locally advanced rectal adenocarcinoma after preoperative radiotherapy. World J. Gastroenterol. 2015, 21, 1851–1856. [Google Scholar] [CrossRef]
- Li, J.Y.; Huang, X.Z.; Gao, P.; Song, Y.X.; Chen, X.W.; Lv, X.E.; Fu, Y.; Xiao, Q.; Ye, S.Y.; Wang, Z.N. Survival landscape of different tumor regression grades and pathologic complete response in rectal cancer after neoadjuvant therapy based on reconstructed individual patient data. BMC Cancer 2021, 21, 1214. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; American Joint Commission on Cancer; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Becker, K.; Mueller, J.D.; Schulmacher, C.; Ott, K.; Fink, U.; Busch, R.; Bottcher, K.; Siewert, J.R.; Hofler, H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003, 98, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Mandard, A.M.; Dalibard, F.; Mandard, J.C.; Marnay, J.; Henry-Amar, M.; Petiot, J.F.; Roussel, A.; Jacob, J.H.; Segol, P.; Samama, G.; et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994, 73, 2680–2686. [Google Scholar] [CrossRef]
- Ryan, R.; Gibbons, D.; Hyland, J.M.; Treanor, D.; White, A.; Mulcahy, H.E.; O’Donoghue, D.P.; Moriarty, M.; Fennelly, D.; Sheahan, K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005, 47, 141–146. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Martin-Romano, P.; Sola, J.J.; Diaz-Gonzalez, J.A.; Chopitea, A.; Iragorri, Y.; Martinez-Regueira, F.; Ponz-Sarvise, M.; Arbea, L.; Subtil, J.C.; Cano, D.; et al. Role of histological regression grade after two neoadjuvant approaches with or without radiotherapy in locally advanced gastric cancer. Br. J. Cancer 2016, 115, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Tsekrekos, A.; Vieth, M.; Ndegwa, N.; Bateman, A.; Flejou, J.F.; Grabsch, H.I.; Mastracci, L.; Meijer, S.L.; Saragoni, L.; Sheahan, K.; et al. Interobserver agreement of a gastric adenocarcinoma tumor regression grading system that incorporates assessment of lymph nodes. Hum. Pathol. 2021, 116, 94–101. [Google Scholar] [CrossRef]
- Tsekrekos, A.; Detlefsen, S.; Riddell, R.; Conner, J.; Mastracci, L.; Sheahan, K.; Shetye, J.; Lundell, L.; Vieth, M. Histopathologic tumor regression grading in patients with gastric carcinoma submitted to neoadjuvant treatment: Results of a Delphi survey. Hum. Pathol. 2019, 84, 26–34. [Google Scholar] [CrossRef]
- Burgart, J.J.; Chopp, W.V.; Jain, D. Protocol for the Examination of Specimens from Patients with Carcinoma of the Stomach; College of American Pathologists: Northfield, IL, USA, 2022. [Google Scholar]
- Mansour, J.C.; Tang, L.; Shah, M.; Bentrem, D.; Klimstra, D.S.; Gonen, M.; Kelsen, D.P.; Brennan, M.F.; Coit, D.G. Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann. Surg. Oncol. 2007, 14, 3412–3418. [Google Scholar] [CrossRef]
- Saliba, G.; Detlefsen, S.; Carneiro, F.; Conner, J.; Dorer, R.; Flejou, J.F.; Hahn, H.; Kamaradova, K.; Mastracci, L.; Meijer, S.L.; et al. Tumor regression grading after neoadjuvant treatment of esophageal and gastroesophageal junction adenocarcinoma: Results of an international Delphi consensus survey. Hum. Pathol. 2021, 108, 60–67. [Google Scholar] [CrossRef]
- West, C.; Mirza, A.; Naveed, A.; Hayes, S.; Formela, L.; Welch, I.; West, C.M.; Pritchard, S. Assessment of Histopathological Response in Gastric and Gastro-Oesophageal Junction Adenocarcinoma following Neoadjuvant Chemotherapy: Which Scoring System to Use? Int. Sch. Res. Not. 2012, 2012, 519351. [Google Scholar] [CrossRef]
- Lowy, A.M.; Mansfield, P.F.; Leach, S.D.; Pazdur, R.; Dumas, P.; Ajani, J.A. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann. Surg. 1999, 229, 303–308. [Google Scholar] [CrossRef]
- Becker, K.; Langer, R.; Reim, D.; Novotny, A.; Meyer zum Buschenfelde, C.; Engel, J.; Friess, H.; Hofler, H. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: A summary of 480 cases. Ann. Surg. 2011, 253, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.W.; Lu, J.; Xu, B.B.; Zheng, C.H.; Li, P.; Wang, J.B.; Lin, J.X.; Chen, Q.Y.; Cao, L.L.; Lin, M.; et al. Prognostic Value of Tumor Regression Grading in Patients Treated with Neoadjuvant Chemotherapy Plus Surgery for Gastric Cancer. Front. Oncol. 2021, 11, 587856. [Google Scholar] [CrossRef]
- Reim, D.; Novotny, A.; Friess, H.; Slotta-Huspenina, J.; Weichert, W.; Ott, K.; Dislich, B.; Lorenzen, S.; Becker, K.; Langer, R. Significance of tumour regression in lymph node metastases of gastric and gastro-oesophageal junction adenocarcinomas. J. Pathol. Clin. Res. 2020, 6, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Fassan, M.; Cunningham, D.; Allum, W.H.; Okines, A.F.; Lampis, A.; Hahne, J.C.; Rugge, M.; Peckitt, C.; Nankivell, M.; et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J. Clin. Oncol. 2016, 34, 2721–2727. [Google Scholar] [CrossRef]
- Achilli, P.; De Martini, P.; Ceresoli, M.; Mari, G.M.; Costanzi, A.; Maggioni, D.; Pugliese, R.; Ferrari, G. Tumor response evaluation after neoadjuvant chemotherapy in locally advanced gastric adenocarcinoma: A prospective, multi-center cohort study. J. Gastrointest. Oncol. 2017, 8, 1018–1025. [Google Scholar] [CrossRef]
- Ott, K.; Sendler, A.; Becker, K.; Dittler, H.J.; Helmberger, H.; Busch, R.; Kollmannsberger, C.; Siewert, J.R.; Fink, U. Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: A prospective phase II study. Gastric Cancer 2003, 6, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, D.; Karpeh, M.; Schwartz, G.; Gerdes, H.; Lightdale, C.; Botet, J.; Lauers, G.; Klimstra, D.; Huang, Y.; Saltz, L.; et al. Neoadjuvant therapy of high-risk gastric cancer: A phase II trial of preoperative FAMTX and postoperative intraperitoneal fluorouracil-cisplatin plus intravenous fluorouracil. J. Clin. Oncol. 1996, 14, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Mansfield, P.F.; Lynch, P.M.; Pisters, P.W.; Feig, B.; Dumas, P.; Evans, D.B.; Raijman, I.; Hargraves, K.; Curley, S.; et al. Enhanced staging and all chemotherapy preoperatively in patients with potentially resectable gastric carcinoma. J. Clin. Oncol. 1999, 17, 2403–2411. [Google Scholar] [CrossRef]
- Park, S.R.; Lee, J.S.; Kim, C.G.; Kim, H.K.; Kook, M.C.; Kim, Y.W.; Ryu, K.W.; Lee, J.H.; Bae, J.M.; Choi, I.J. Endoscopic ultrasound and computed tomography in restaging and predicting prognosis after neoadjuvant chemotherapy in patients with locally advanced gastric cancer. Cancer 2008, 112, 2368–2376. [Google Scholar] [CrossRef]
- Goodman, K.A.; Ou, F.S.; Hall, N.C.; Bekaii-Saab, T.; Fruth, B.; Twohy, E.; Meyers, M.O.; Boffa, D.J.; Mitchell, K.; Frankel, W.L.; et al. Randomized Phase II Study of PET Response-Adapted Combined Modality Therapy for Esophageal Cancer: Mature Results of the CALGB 80803 (Alliance) Trial. J. Clin. Oncol. 2021, 39, 2803–2815. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Ott, K.; Krause, B.J.; Weber, W.A.; Becker, K.; Stein, H.J.; Lorenzen, S.; Schuster, T.; Wieder, H.; Herrmann, K.; et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial. Lancet Oncol. 2007, 8, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Berenato, R.; Morano, F.; Pietrantonio, F.; Cotsoglou, C.; Caporale, M.; Infante, G.; Pellegrinelli, A.; Alessi, A.; Battiston, C.; Coppa, J.; et al. Preoperative Capecitabine, Oxaliplatin, and Irinotecan in Resectable Gastric or Gastroesophageal Junction Cancer: Pathological Response as Primary Endpoint and FDG-PET Predictions. Oncology 2017, 93, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Vallbohmer, D.; Holscher, A.H.; Schneider, P.M.; Schmidt, M.; Dietlein, M.; Bollschweiler, E.; Baldus, S.; Alakus, H.; Brabender, J.; Metzger, R.; et al. [18F]-fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemotherapy in gastric cancer. J. Surg. Oncol. 2010, 102, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Ott, K.; Herrmann, K.; Lordick, F.; Wieder, H.; Weber, W.A.; Becker, K.; Buck, A.K.; Dobritz, M.; Fink, U.; Ulm, K.; et al. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: Long-term results of a prospective study. Clin. Cancer Res. 2008, 14, 2012–2018. [Google Scholar] [CrossRef]
| TRG System | Grade—Interpretation |
|---|---|
| Mandard et al. (1994) [19] | 1—No residual tumor, presence of fibrosis 2—Rare residual tumor cells scattered through fibrosis 3—Increased residual tumor cells but fibrosis is predominant 4—Residual tumor cells outgrowing fibrosis 5—Absence of regression |
| Becker et al. (2003) [18] | 1a—Complete response; no residual tumor per tumor bed 1b—Subtotal response; <10% residual tumor per tumor bed 2—Partial response; 10–50% residual tumor per tumor bed 3—Minimal or no response; >50% residual tumor per tumor bed |
| Ryan et al. (2005) [20] | 1—No viable tumor cells or single cells or small groups of tumor cells 2—Residual tumor outgrown by fibrosis 3—No fibrosis with extensive residual tumor or significant fibrosis outgrown by tumor |
| NCCN-CAP (Modified Ryan et al.) [3,25] | 0—Complete response; no viable tumor cells, including lymph nodes 1—Near complete response; single cells or rare small groups of tumor cells 2—Partial response; residual tumor with evident regression but more than single cells or rare small groups of tumor cells 3—Poor or no response; no evidence of tumor regression |
| JGCA (2011) [11] | 0—No effect seen 1a—Very slight effect; More than 2/3 of viable tumor present 1b—Slight effect; Between 1/3 and 2/3 of viable tumor 2—Considerable effect; Less than 1/3 of viable tumor 3—Complete response; no tumor cells seen |
| Martin-Romano et al. (2016) [22] | Primary tumor Use Becker TRG Lymph nodes A—Negative lymph nodes with no evidence of treatment effect (true negative) B—Positive lymph nodes with no evidence of treatment effect C—Positive lymph nodes with evidence of tumor regression/treatment effect D—Negative lymph nodes with complete pathologic response |
| Tsekrekos et al. (Modified Becker et al.) 2019 [24] | Primary tumor 1—Complete response; no residual tumor cells, only signs of regression 2—Less than 10% residual tumor cells 3—Between 10–50% residual tumor cells 4—More than 50% residual tumor cells Lymph nodes a—Complete regression; all lymph nodes are negative, with signs of regression b—Partial regression; lymph nodes with tumor cells and signs of regression c—No regression; lymph nodes with tumor cells and no signs of regression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsagkalidis, V.; Blaszczyk, M.B.; In, H. Interpretation of Tumor Response Grade following Preoperative Therapy for Gastric Cancer: An Overview. Cancers 2023, 15, 3662. https://doi.org/10.3390/cancers15143662
Tsagkalidis V, Blaszczyk MB, In H. Interpretation of Tumor Response Grade following Preoperative Therapy for Gastric Cancer: An Overview. Cancers. 2023; 15(14):3662. https://doi.org/10.3390/cancers15143662
Chicago/Turabian StyleTsagkalidis, Vasileios, Maryjka B. Blaszczyk, and Haejin In. 2023. "Interpretation of Tumor Response Grade following Preoperative Therapy for Gastric Cancer: An Overview" Cancers 15, no. 14: 3662. https://doi.org/10.3390/cancers15143662
APA StyleTsagkalidis, V., Blaszczyk, M. B., & In, H. (2023). Interpretation of Tumor Response Grade following Preoperative Therapy for Gastric Cancer: An Overview. Cancers, 15(14), 3662. https://doi.org/10.3390/cancers15143662






