Non-Covalent Bruton’s Tyrosine Kinase Inhibitors in the Treatment of Chronic Lymphocytic Leukemia
Abstract
Simple Summary
Abstract
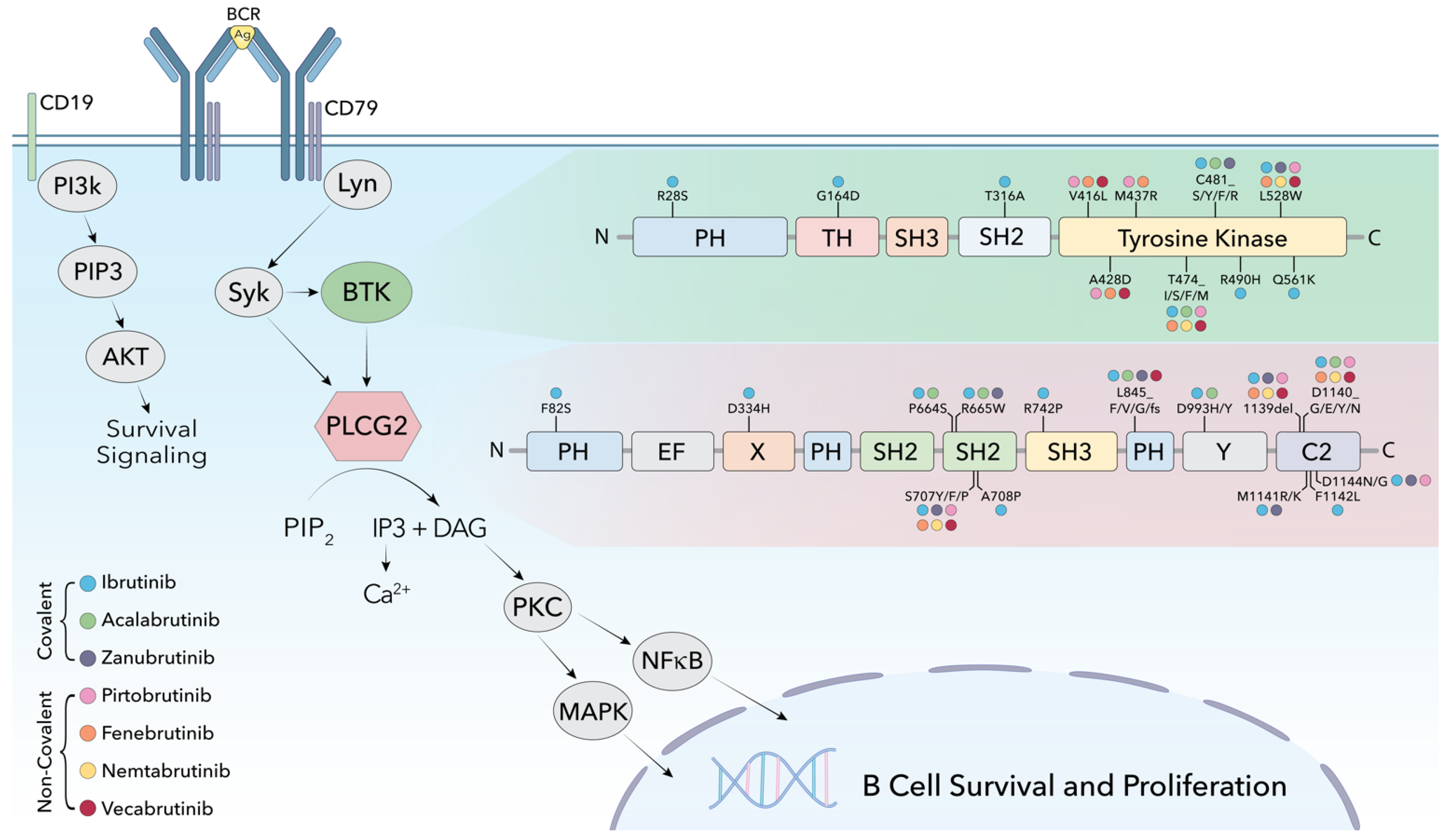
1. Background: BTK Inhibitors in CLL
2. Non-Covalent BTKis: Pre-Clinical and Clinical Data
2.1. Vecabrutinib
2.2. Fenebrutinib
2.3. Nemtabrutinib
2.4. Pirtobrutinib
3. Mechanisms of Resistance to Non-Covalent BTK Inhibitors
4. ncBTKi in CLL: Where Will These Agents Fit into the Treatment Paradigm?
5. Treatment of CLL after ncBTKi: An Emerging Challenge
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia 2022, 36, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Kay, N.E.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N. Engl. J. Med. 2014, 371, 213–223. [Google Scholar] [CrossRef]
- Byrd, J.C.; Harrington, B.; O’Brien, S.; Jones, J.A.; Schuh, A.; Devereux, S.; Chaves, J.; Wierda, W.G.; Awan, F.T.; Brown, J.R.; et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Barr, P.M.; Owen, C.; Robak, T.; Tedeschi, A.; Bairey, O.; Burger, J.A.; Hillmen, P.; Coutre, S.E.; Dearden, C.; Grosicki, S.; et al. Up to 8-year follow-up from RESONATE-2: First-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022, 6, 3440–3450. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kaźmierczak, M.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 319–332. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Hanson, C.A.; Paietta, E.M.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: Updated results of the E1912 trial. Blood 2022, 140, 112–120. [Google Scholar] [CrossRef]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.A.; Grant, B.; Sharman, J.P.; Coleman, M.; Wierda, W.G.; et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2013, 369, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Furman, R.R.; Liu, T.M.; Ozer, H.G.; Zapatka, M.; Ruppert, A.S.; Xue, L.; Li, D.H.; Steggerda, S.M.; Versele, M.; et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 2014, 370, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.C.; Mato, A.R. Treatment of Chronic Lymphocytic Leukemia After Discontinuation of Bruton’s Tyrosine Kinase Inhibitors. Hematol. Oncol. Clin. N. Am. 2021, 35, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Nabhan, C.; Thompson, M.C.; Lamanna, N.; Brander, D.M.; Hill, B.; Howlett, C.; Skarbnik, A.; Cheson, B.D.; Zent, C.; et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: A real-world analysis. Haematologica 2018, 103, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, K.J.; Ruppert, A.S.; Lozanski, G.; Heerema, N.A.; Zhao, W.; Abruzzo, L.; Lozanski, A.; Davis, M.; Gordon, A.; Smith, L.L.; et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA Oncol. 2015, 1, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.E.M.; Montraveta, A.; Niemann, C.U.; Mora-Jensen, H.; Gulrajani, M.; Krantz, F.; Mantel, R.; Smith, L.L.; McClanahan, F.; Harrington, B.K.; et al. The Bruton Tyrosine Kinase (BTK) Inhibitor Acalabrutinib Demonstrates Potent On-Target Effects and Efficacy in Two Mouse Models of Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2017, 23, 2831–2841. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Guinn, D.; Lehman, A.; Blachly, J.S.; Lozanski, A.; Heerema, N.A.; Zhao, W.; Coleman, J.; Jones, D.; et al. BTK(C481S)-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2017, 35, 1437–1443. [Google Scholar] [CrossRef]
- Ahn, I.E.; Underbayev, C.; Albitar, A.; Herman, S.E.; Tian, X.; Maric, I.; Arthur, D.C.; Wake, L.; Pittaluga, S.; Yuan, C.M.; et al. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood 2017, 129, 1469–1479. [Google Scholar] [CrossRef]
- Scarfo, L.; Bonfiglio, S.; Sutton, L.; Ljungstrom, V.; Pandzic, T.; Cortese, D.; Gaidano, G.; Trentin, L.; Bonello, L.; Reda, G.; et al. BTK and PLCG2 Mutations in Patients with Chronic Lymphocytic Leukemia Relapsing on Ibrutinib: A European Research Initiative on CLL (ERIC) Study Based on Real-World Evidence. EHA Libr. 2020, S161. [Google Scholar]
- Woyach, J.; Huang, Y.; Rogers, K.; Bhat, S.A.; Grever, M.R.; Lozanski, A.; Doong, T.-J.; Blachly, J.S.; Lozanski, G.; Jones, D.; et al. Resistance to Acalabrutinib in CLL Is Mediated Primarily By BTK Mutations. Blood 2019, 134, 504. [Google Scholar] [CrossRef]
- Blombery, P.; Thompson, E.R.; Lew, T.E.; Tiong, I.S.; Bennett, R.; Cheah, C.Y.; Lewis, K.L.; Handunnetti, S.M.; Tang, C.P.S.; Roberts, A.; et al. Enrichment of BTK Leu528Trp mutations in patients with CLL on zanubrutinib: Potential for pirtobrutinib cross-resistance. Blood Adv. 2022, 6, 5589–5592. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Shah, N.N.; Jurczak, W.; Cheah, C.Y.; Pagel, J.M.; Woyach, J.A.; Fakhri, B.; Eyre, T.A.; Lamanna, N.; Patel, M.R.; et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 2021, 397, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.; Flinn, I.W.; Awan, F.T.; Eradata, H.; Brander, D.; Tees, M.; Parikh, S.A.; Phillips, T.; Wayne, W.; Reddy, N.M.; et al. Nemtabrutinib (MK-1026), A Non-Covalent Inhibitor of Wild-Type and C481S Mutated Bruton’s Tyrosine Kinase for B-cell Malignancies: Efficacy and Safety of the Phase 2 Dose-Expansion Bellwave-001 Study. EHA Libr. 2022, P682. [Google Scholar]
- Tambaro, F.P.; De Novellis, D.; Wierda, W.G. The Role of BTK Inhibition in the Treatment of Chronic Lymphocytic Leukemia: A Clinical View. J. Exp. Pharmacol. 2021, 13, 923–935. [Google Scholar] [CrossRef]
- Lewis, K.L.; Cheah, C.Y. Non-Covalent BTK Inhibitors-The New BTKids on the Block for B-Cell Malignancies. J. Pers. Med. 2021, 11, 764. [Google Scholar] [CrossRef]
- Gu, D.; Tang, H.; Wu, J.; Li, J.; Miao, Y. Targeting Bruton tyrosine kinase using non-covalent inhibitors in B cell malignancies. J. Hematol. Oncol. 2021, 14, 40. [Google Scholar] [CrossRef]
- Zain, R.; Vihinen, M. Structure-Function Relationships of Covalent and Non-Covalent BTK Inhibitors. Front. Immunol. 2021, 12, 694853. [Google Scholar] [CrossRef]
- Liang, C.; Tian, D.; Ren, X.; Ding, S.; Jia, M.; Xin, M.; Thareja, S. The development of Bruton’s tyrosine kinase (BTK) inhibitors from 2012 to 2017: A mini-review. Eur. J. Med. Chem. 2018, 151, 315–326. [Google Scholar] [CrossRef]
- Cuozzo, J.W.; Centrella, P.A.; Gikunju, D.; Habeshian, S.; Hupp, C.D.; Keefe, A.D.; Sigel, E.A.; Soutter, H.H.; Thomson, H.A.; Zhang, Y.; et al. Discovery of a Potent BTK Inhibitor with a Novel Binding Mode by Using Parallel Selections with a DNA-Encoded Chemical Library. Chembiochem 2017, 18, 864–871. [Google Scholar] [CrossRef]
- Lu, D.; Jiang, J.; Liang, Z.; Sun, M.; Luo, C.; Shen, B.; Hu, G. Molecular dynamic simulation to explore the molecular basis of Btk-PH domain interaction with Ins(1,3,4,5)P4. Sci. World J. 2013, 2013, 580456. [Google Scholar] [CrossRef] [PubMed]
- Aslan, B.; Hubner, S.E.; Fox, J.A.; Taverna, P.; Wierda, W.G.; Kornblau, S.M.; Gandhi, V. Vecabrutinib inhibits B-cell receptor signal transduction in chronic lymphocytic leukemia cell types with wild-type or mutant Bruton tyrosine kinase. Haematologica 2022, 107, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.N.; Pinilla-Ibarz, J.; Gladstone, D.E.; Patel, K.; Sharman, J.P.; Wierda, W.G.; Choi, M.Y.; O’Brien, S.M.; Shadman, M.; Davids, M.S.; et al. Phase Ib dose-escalation study of the selective, non-covalent, reversible Bruton’s tyrosine kinase inhibitor vecabrutinib in B-cell malignancies. Haematologica 2022, 107, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Ondrisova, L.; Mraz, M. Genetic and Non-Genetic Mechanisms of Resistance to BCR Signaling Inhibitors in B Cell Malignancies. Front. Oncol. 2020, 10, 591577. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Yang, J.; Zhou, K. The resistance mechanisms and treatment strategies of BTK inhibitors in B-cell lymphoma. Hematol. Oncol. 2021, 39, 605–615. [Google Scholar] [CrossRef]
- Ran, F.; Liu, Y.; Wang, C.; Xu, Z.; Zhang, Y.; Liu, Y.; Zhao, G.; Ling, Y. Review of the development of BTK inhibitors in overcoming the clinical limitations of ibrutinib. Eur. J. Med. Chem. 2022, 229, 114009. [Google Scholar] [CrossRef]
- Byrd, J.C.; Smith, S.; Wagner-Johnston, N.; Sharman, J.; Chen, A.I.; Advani, R.; Augustson, B.; Marlton, P.; Renee Commerford, S.; Okrah, K.; et al. First-in-human phase 1 study of the BTK inhibitor GDC-0853 in relapsed or refractory B-cell NHL and CLL. Oncotarget 2018, 9, 13023–13035. [Google Scholar] [CrossRef]
- Oh, J.; Cohen, S.; Isenberg, D.; Maurer, M.; Galanter, J.; Chu, T.; Teterina, A.; Goodyear, A.; Mandel, C.; Lee, C.; et al. The Safety of Fenebrutinib in a Large Population of Patients With Diverse Autoimmune Indications Supports Investigation in Multiple Sclerosis (MS) (4564). Neurology 2021, 96, 4564. [Google Scholar]
- Reiff, S.D.; Mantel, R.; Smith, L.L.; Greene, J.T.; Muhowski, E.M.; Fabian, C.A.; Goettl, V.M.; Tran, M.; Harrington, B.K.; Rogers, K.A.; et al. The BTK Inhibitor ARQ 531 Targets Ibrutinib-Resistant CLL and Richter Transformation. Cancer Discov. 2018, 8, 1300–1315. [Google Scholar] [CrossRef]
- Woyach, J.; Stephens, D.M.; Flinn, I.W.; Bhat, S.A.; Savage, R.E.; Chai, F.; Eathiraj, S.; Granlund, L.; Szuszkiewicz, L.A.; Schwartz, B.; et al. Final Results of Phase 1, Dose Escalation Study Evaluating ARQ 531 in Patients with Relapsed or Refractory B-Cell Lymphoid Malignancies. Blood 2019, 134, 4298. [Google Scholar] [CrossRef]
- Woyach, J.A.; Flinn, I.W.; Awan, F.T.; Eradat, H.; Brander, D.; Tees, M.; Parikh, S.A.; Phillips, T.J.; Ghori, R.; Reddy, N.M.; et al. Efficacy and Safety of Nemtabrutinib, a Wild-Type and C481S-Mutated Bruton Tyrosine Kinase Inhibitor for B-Cell Malignancies: Updated Analysis of the Open-Label Phase 1/2 Dose-Expansion Bellwave-001 Study. Blood 2022, 140, 7004–7006. [Google Scholar] [CrossRef]
- Mato, A.R.; Pagel, J.M.; Coombs, C.C.; Shah, N.N.; Lamanna, N.; Lech-Marańda, E.; Eyre, T.A.; Woyach, J.A.; Wierda, W.G.; Cheah, C.Y.; et al. LOXO-305, A Next Generation, Highly Selective, Non-Covalent BTK Inhibitor in Previously Treated CLL/SLL: Results from the Phase 1/2 BRUIN Study. Blood 2020, 136, 35–37. [Google Scholar] [CrossRef]
- Jensen, J.L.; Mato, A.R.; Pena, C.; Roeker, L.E.; Coombs, C.C. The potential of pirtobrutinib in multiple B-cell malignancies. Ther. Adv. Hematol. 2022, 13, 20406207221101697. [Google Scholar] [CrossRef]
- Mato, A.R.; Woyach, J.A.; Brown, J.R.; Ghia, P.; Patel, K.; Eyre, T.A.; Munir, T.; Lech-Marańda, E.; Lamanna, N.; Tam, C.S.; et al. Efficacy of Pirtobrutinib in Covalent BTK-Inhibitor Pre-Treated Relapsed/Refractory CLL/SLL: Additional Patients and Extended Follow-up from the Phase 1/2 BRUIN Study. Blood 2022, 140, 2316–2320. [Google Scholar] [CrossRef]
- Wang, M.L.; Shah, N.N.; Jurczak, W.; Zinzani, P.L.; Eyre, T.A.; Cheah, C.Y.; Ujjani, C.S.; Koh, Y.; Izutsu, K.; Gerson, J.N.; et al. Efficacy of Pirtobrutinib in Covalent BTK-Inhibitor Pre-Treated Relapsed/Refractory Mantle Cell Lymphoma: Additional Patients and Extended Follow-up from the Phase 1/2 BRUIN Study. Blood 2022, 140, 9368–9372. [Google Scholar] [CrossRef]
- Nakhoda, S.; Vistarop, A.; Wang, Y.L. Resistance to Bruton tyrosine kinase inhibition in chronic lymphocytic leukaemia and non-Hodgkin lymphoma. Br. J. Haematol. 2023, 200, 137–149. [Google Scholar] [CrossRef]
- Wang, E.; Mi, X.; Thompson, M.C.; Montoya, S.; Notti, R.Q.; Afaghani, J.; Durham, B.H.; Penson, A.; Witkowski, M.T.; Lu, S.X.; et al. Mechanisms of Resistance to Noncovalent Bruton’s Tyrosine Kinase Inhibitors. N. Engl. J. Med. 2022, 386, 735–743. [Google Scholar] [CrossRef]
- Qi, J.; Endres, S.; Yosifov, D.Y.; Tausch, E.; Dheenadayalan, R.P.; Gao, X.; Müller, A.; Schneider, C.; Mertens, D.; Gierschik, P.; et al. Acquired BTK mutations associated with resistance to non-covalent BTK inhibitors. Blood Adv. 2023. [Google Scholar] [CrossRef]
- Montoya, S.; Bourcier, J.; Thompson, M.C.; Noviski, M.; Tan, M.; Wang, E.; Mi, X.; Brathaban, N.; Barrientos Risso, C.; Tsai, D.; et al. Kinase Dead BTK Mutations Confer Resistance to Covalent and Noncovalent BTK Inhibitors but Are Susceptible to Clinical Stage BTK Degraders. Blood 2022, 140, 1811–1813. [Google Scholar] [CrossRef]
- Naeem, A.; Utro, F.; Wang, Q.; Cha, J.; Vihinen, M.; Martindale, S.P.; Zhou, Y.; Ren, Y.; Tyekucheva, S.P.D.; Kim, A.S.; et al. Pirtobrutinib Targets BTK C481S in Ibrutinib-Resistant CLL but Second-Site BTK Mutations Lead to Resistance. Blood Adv. 2022, 7, 1929–1943. [Google Scholar] [CrossRef]
- Dhami, K.; Chakraborty, A.; Gururaja, T.L.; Cheung, L.W.K.; Sun, C.; DeAnda, F.; Huang, X. Kinase-deficient BTK mutants confer ibrutinib resistance through activation of the kinase HCK. Sci. Signal. 2022, 15, eabg5216. [Google Scholar] [CrossRef] [PubMed]
- Scheffold, A.; Jebaraj, B.M.C.; Tausch, E.; Bloehdorn, J.; Ghia, P.; Yahiaoui, A.; Dolnik, A.; Blätte, T.J.; Bullinger, L.; Dheenadayalan, R.P.; et al. IGF1R as druggable target mediating PI3K-δ inhibitor resistance in a murine model of chronic lymphocytic leukemia. Blood 2019, 134, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Kersy, O.; Salmon-Divon, M.; Gomes da Silva, M.; Moita, A.F.; Cabecadas, J.; Klener, P., Jr.; Trněný, M.; Basood, M.; Shpilberg, O.; Hershkovitz-Rokah, O. Inhibition of MAPK-ERK Signaling Pathway Overcomes Microrna-Mediated Ibrutinib Resistance in Mantle Cell Lymphoma. Blood 2022, 140, 3109–3110. [Google Scholar] [CrossRef]
- Polcik, L.; Dannewitz Prosseda, S.; Pozzo, F.; Zucchetto, A.; Gattei, V.; Hartmann, T.N. Integrin Signaling Shaping BTK-Inhibitor Resistance. Cells 2022, 11, 2235. [Google Scholar] [CrossRef]
- Smith, C.I.E.; Burger, J.A. Resistance Mutations to BTK Inhibitors Originate From the NF-κB but Not From the PI3K-RAS-MAPK Arm of the B Cell Receptor Signaling Pathway. Front. Immunol. 2021, 12, 689472. [Google Scholar] [CrossRef]
- Mato, A.R.; Davids, M.S.; Sharman, J.; Roeker, L.E.; Kay, N.; Kater, A.P.; Rogers, K.; Thompson, M.C.; Rhodes, J.; Goy, A.; et al. Recognizing Unmet Need in the Era of Targeted Therapy for CLL/SLL: “What’s Past Is Prologue” (Shakespeare). Clin. Cancer Res. 2022, 28, 603–608. [Google Scholar] [CrossRef]
- Lew, T.E.; Lin, V.S.; Cliff, E.R.; Blombery, P.; Thompson, E.R.; Handunnetti, S.M.; Westerman, D.A.; Kuss, B.J.; Tam, C.S.; Huang, D.C.S.; et al. Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition. Blood Adv. 2021, 5, 4054–4058. [Google Scholar] [CrossRef]
- Eyre, T.A.; Hess, L.M.; Sugihara, T.; He, D.; Khanal, M.; Pagel, J.M.; Walgren, R.A.; Abada, P.B.; Konig, H.; Roeker, L.E.; et al. Clinical outcomes among patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) who received treatment with a covalent BTK and BCL2 inhibitor in the United States: A real-world database study. Leuk. Lymphoma 2023, 64, 1005–1016. [Google Scholar] [CrossRef]
- Aronson, J.H.; Skånland, S.S.; Roeker, L.E.; Thompson, M.C.; Mato, A.R. Approach to a patient with “double refractory” chronic lymphocytic leukemia: “Double, double toil and trouble” (Shakespeare). Am. J. Hematol. 2022, 97 (Suppl. 2), S19–S25. [Google Scholar] [CrossRef]
- Flinn, I.W.; Hillmen, P.; Montillo, M.; Nagy, Z.; Illés, Á.; Etienne, G.; Delgado, J.; Kuss, B.J.; Tam, C.S.; Gasztonyi, Z.; et al. The phase 3 DUO trial: Duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 2018, 132, 2446–2455. [Google Scholar] [CrossRef]
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Roeker, L.E.; Jacobs, R.; Hill, B.T.; Lamanna, N.; Brander, D.; Shadman, M.; Ujjani, C.S.; Yazdy, M.S.; Perini, G.F.; et al. Assessment of the Efficacy of Therapies Following Venetoclax Discontinuation in CLL Reveals BTK Inhibition as an Effective Strategy. Clin. Cancer Res. 2020, 26, 3589–3596. [Google Scholar] [CrossRef]
- Roeker, L.E.; Mato, A.R.; Brown, J.R.; Coombs, C.C.; Shah, N.N.; Wierda, W.G.; Patel, M.R.; Lewis, K.L.; Balbas, M.; Zhao, J.; et al. Abstract CT138: Pirtobrutinib, a highly selective, non-covalent (reversible) BTK inhibitor in combination with venetoclax ± rituximab in relapsed/refractory chronic lymphocytic leukemia: Results from the BRUIN phase 1b study. Cancer Res. 2022, 82, CT138. [Google Scholar] [CrossRef]
- Mato, A.R.; Wierda, W.G.; Ai, W.Z.; Flinn, I.W.; Tees, M.; Patel, M.R.; Patel, K.; O’Brien, S.; Bond, D.A.; Roeker, L.E.; et al. NX-2127-001, a First-in-Human Trial of NX-2127, a Bruton’s Tyrosine Kinase-Targeted Protein Degrader, in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia and B-Cell Malignancies. Blood 2022, 140, 2329–2332. [Google Scholar] [CrossRef]
- Blachly, J.S.; Stephens, D.M.; Ye, J.C.; Lamanna, N.; Jain, N.; Niesman, M.; Zhang, K.; Woyach, J.A. Initial Results from a Phase 1/2 Dose Escalation and Expansion Study Evaluating MS-553, a Novel and Selective PKCβ Inhibitor, in Patients with CLL/SLL. Blood 2022, 140, 2324–2325. [Google Scholar] [CrossRef]
- Siddiqi, T.; Soumerai, J.D.; Dorritie, K.A.; Stephens, D.M.; Riedell, P.A.; Arnason, J.; Kipps, T.J.; Gillenwater, H.H.; Gong, L.; Yang, L.; et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood 2022, 139, 1794–1806. [Google Scholar] [CrossRef]
| Study | Study Description | Study Population | Primary Outcome |
|---|---|---|---|
| BRUIN-CLL-313 (NCT05023980) | Pirtobrutinib vs. Bendamustine + Rituximab | Previously untreated CLL/SLL | PFS |
| BRUIN-CLL-314 (NCT05254743) | Pirtobrutinib vs. ibrutinib | Previously untreated CLL/SLL | ORR |
| BRUIN-CLL-321 (NCT04666038) | Pirtobrutinib vs. investigator’s choice (idelalisib + rituximab vs. bendamustine + rituximab) | R/R CLL/SLL | PFS |
| BRUIN-CLL-322 (NCT04965493) | Pirtobrutinib + venetoclax + rituximab vs. venetoclax + rituximab | R/R CLL | PFS |
| MIRACLE (NCT05677919) | Pirtobrutinib + Venetoclax | Previously untreated CLL | Undetectable minimal residual disease (<1/104) after 15 cycles of treatment |
| Time-limited triplet combination of pirtobrutinib, venetoclax and obinutuzumab (NCT05536349) | Pirtobrutinib + Venetoclax + Obinutuzumab | Previously untreated CLL or Richter Transformation (RT) | Undetectable measurable residual disease rate of PVO in patients with CLL cohort; ORR for RT cohort |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya, S.; Thompson, M.C. Non-Covalent Bruton’s Tyrosine Kinase Inhibitors in the Treatment of Chronic Lymphocytic Leukemia. Cancers 2023, 15, 3648. https://doi.org/10.3390/cancers15143648
Montoya S, Thompson MC. Non-Covalent Bruton’s Tyrosine Kinase Inhibitors in the Treatment of Chronic Lymphocytic Leukemia. Cancers. 2023; 15(14):3648. https://doi.org/10.3390/cancers15143648
Chicago/Turabian StyleMontoya, Skye, and Meghan C. Thompson. 2023. "Non-Covalent Bruton’s Tyrosine Kinase Inhibitors in the Treatment of Chronic Lymphocytic Leukemia" Cancers 15, no. 14: 3648. https://doi.org/10.3390/cancers15143648
APA StyleMontoya, S., & Thompson, M. C. (2023). Non-Covalent Bruton’s Tyrosine Kinase Inhibitors in the Treatment of Chronic Lymphocytic Leukemia. Cancers, 15(14), 3648. https://doi.org/10.3390/cancers15143648






