Trends in Cancer Incidence in Different Antiretroviral Treatment-Eras amongst People with HIV
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
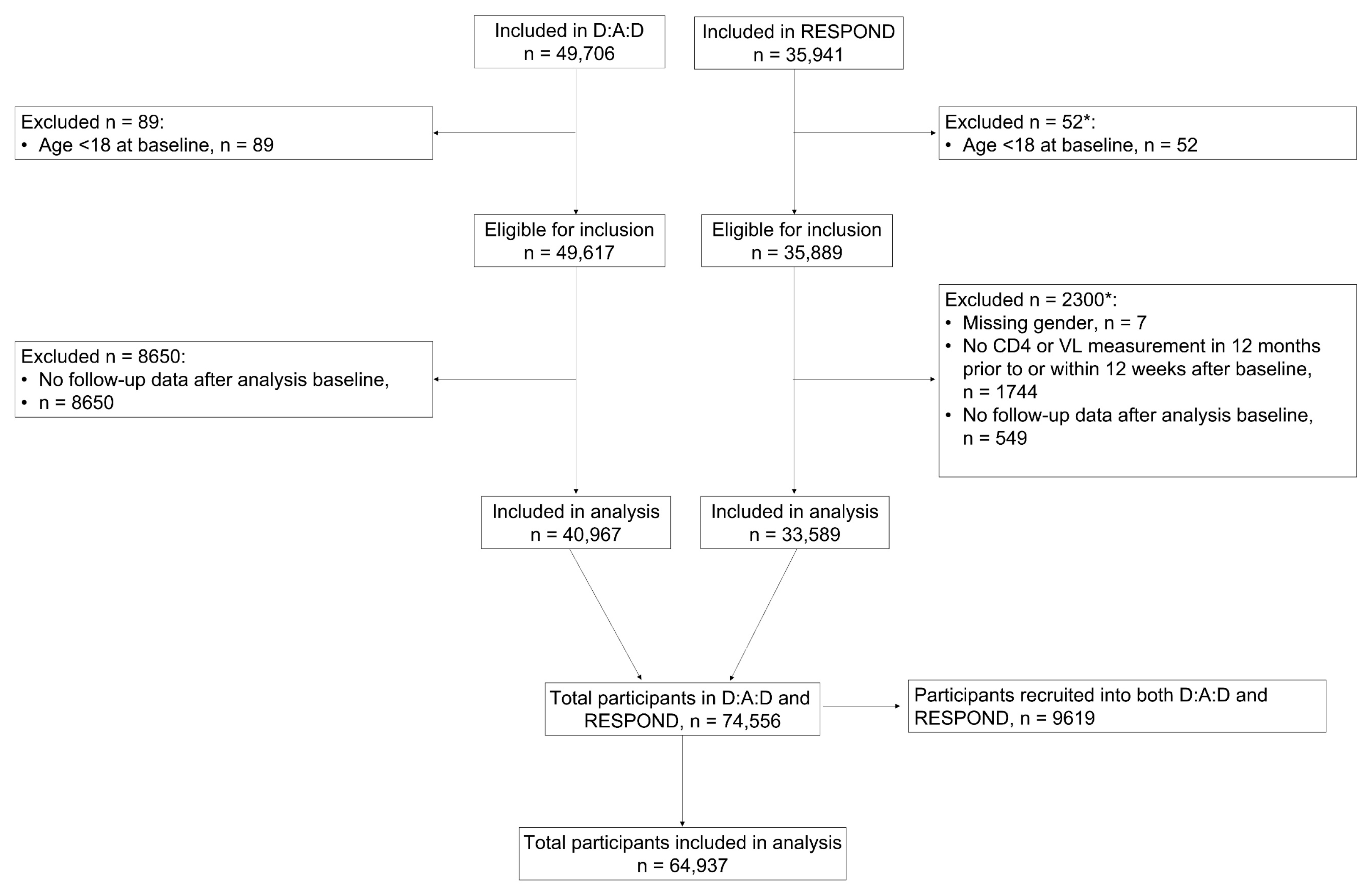
2.2. Participants
2.3. Outcome Definitions
2.4. Statistical Methods
2.4.1. Subgroup Analysis
2.4.2. Missing Data
3. Results
3.1. Baseline Characteristics
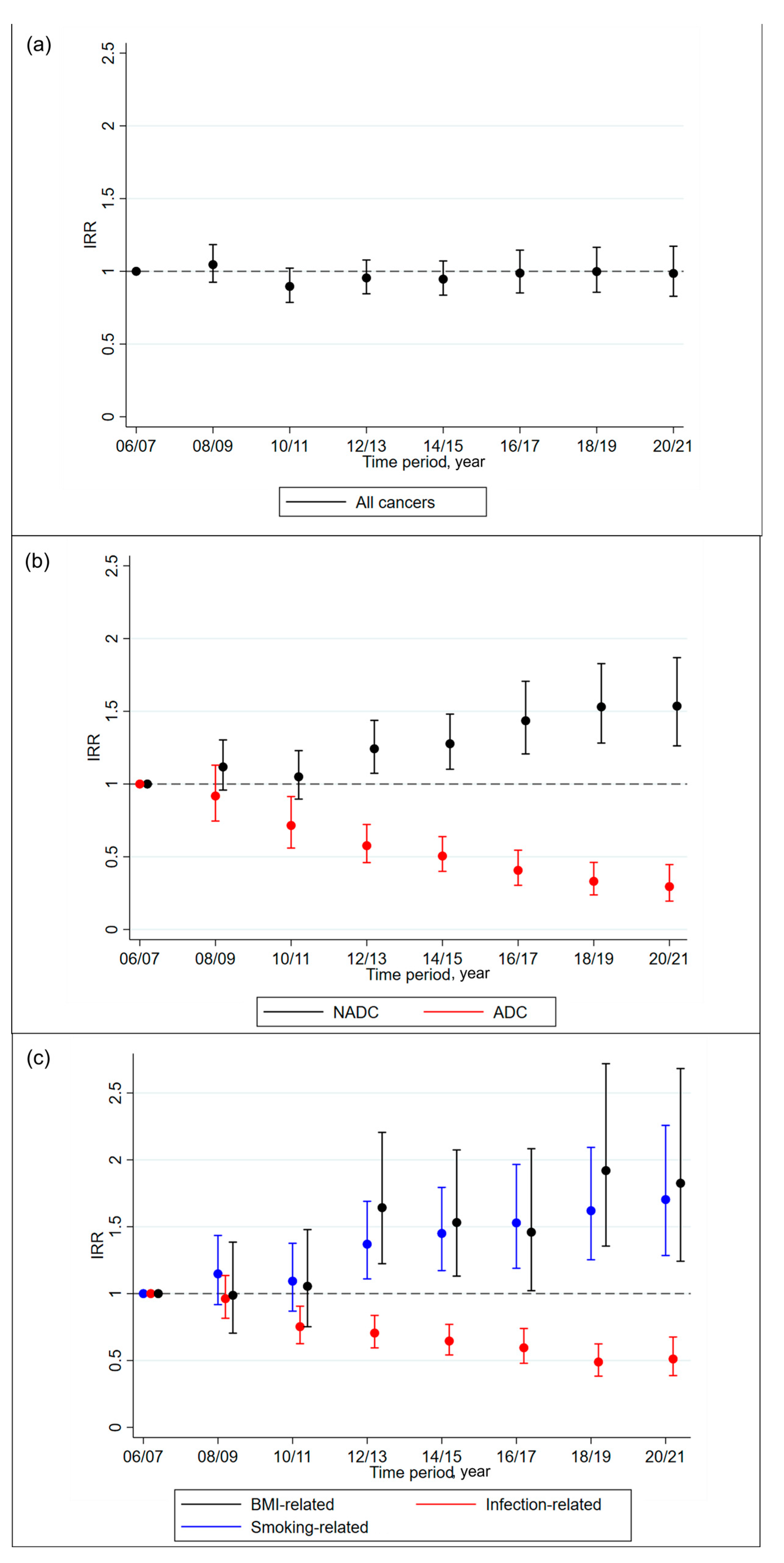
3.2. Cancer Trends
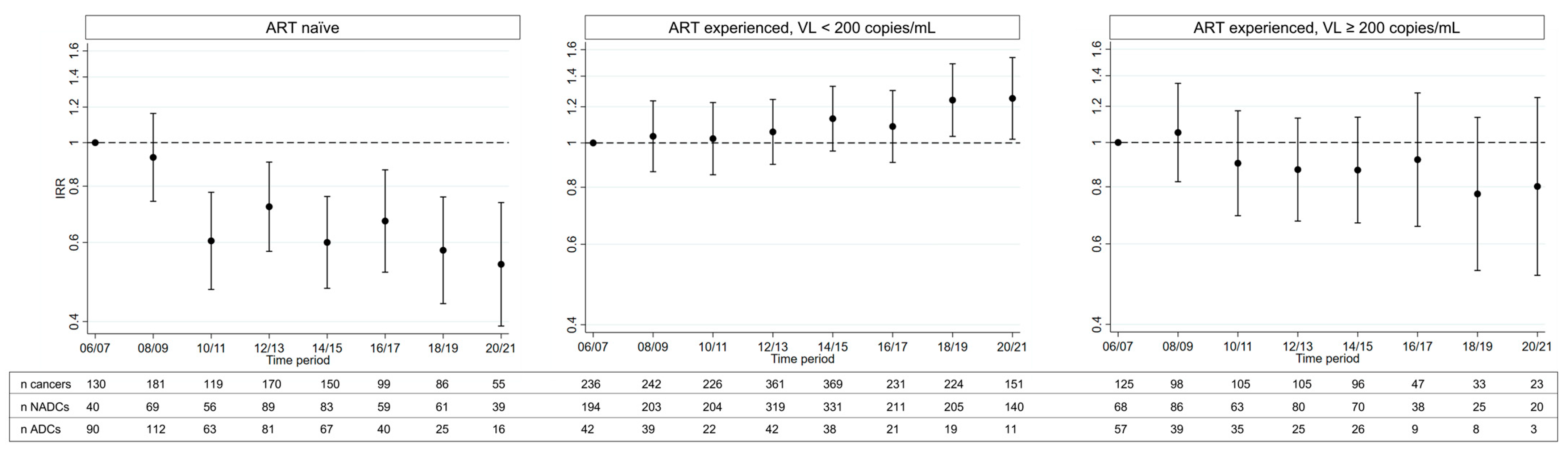
3.3. Subgroup Analyses
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- The Antiretroviral Therapy Cohort Collaboration. Causes of Death in HIV-1–Infected Patients Treated with Antiretroviral Therapy, 1996–2006: Collaborative Analysis of 13 HIV Cohort Studies. Clin. Infect. Dis. 2010, 50, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Ruppik, M.; Rickenbach, M.; Spoerri, A.; Furrer, H.; Battegay, M.; Cavassini, M.; Calmy, A.; Bernasconi, E.; Schmid, P.; et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013, 14, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Ryom, L.; Weber, R.; Morlat, P.; Pradier, C.; Reiss, P.; Kowalska, J.D.; de Wit, S.; Law, M.; el Sadr, W.; et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet 2014, 384, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–28. [Google Scholar] [CrossRef]
- Hulvat, M.C. Cancer Incidence and Trends. Surg. Clin. N. Am. 2020, 100, 469–481. [Google Scholar] [CrossRef]
- Engels, E.A.; Biggar, R.J.; Hall, H.I.; Cross, H.; Crutchfield, A.; Finch, J.L.; Grigg, R.; Hylton, T.; Pawlish, K.S.; McNeel, T.S.; et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int. J. Cancer 2008, 123, 187–194. [Google Scholar] [CrossRef]
- Hernández-Ramírez, R.U.; Shiels, M.S.; Dubrow, R.; Engels, E.A. Cancer risk in HIV-infected people in the USA from 1996 to 2012: A population-based, registry-linkage study. Lancet HIV 2017, 4, e495–e504. [Google Scholar] [CrossRef]
- Silverberg, M.J.; Lau, B.; Achenbach, C.J.; Jing, Y.; Althoff, K.N.; D’Souza, G.; Engels, E.A.; Hessol, N.A.; Brooks, J.T.; Burchell, A.N.; et al. Cumulative incidence of cancer among persons with HIV in North America: A cohort study. Ann. Intern. Med. 2015, 163, 507–518. [Google Scholar] [CrossRef]
- Bedimo, R.; Chen, R.Y.; Accortt, N.A.; Raper, J.L.; Linn, C.; Allison, J.J.; Dubay, J.; Saag, M.S.; Hoesley, C.J. Trends in AIDS-Defining and Non–AIDS-Defining Malignancies among HIV-Infected Patients: 1989–2002. CID 2004, 39, 1380–1384. [Google Scholar] [CrossRef]
- Appleby, P.; Beral, V.; Newton, R.; Reeves, G.; Carpenter, L. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J. Natl. Cancer Inst. 2000, 92, 1823–1830. [Google Scholar]
- Franceschi, S.; the Swiss HIV Cohort Study; Lise, M.; Clifford, G.M.; Rickenbach, M.; Levi, F.; Maspoli, M.; Bouchardy, C.; Dehler, S.; Jundt, G.; et al. Changing patterns of cancer incidence in the early- and late-HAART periods: The Swiss HIV Cohort Study. Br. J. Cancer 2010, 103, 416–422. [Google Scholar] [CrossRef]
- Lundgren, J.D.; Borges, A.H.; Neaton, J.D. Serious Non-AIDS Conditions in HIV: Benefit of Early ART. Curr. HIV/AIDS Rep. 2018, 15, 162–171. [Google Scholar] [CrossRef]
- Dubrow, R.; Silverberg, M.J.; Park, L.S.; Crothers, K.; Justice, A.C. HIV infection, aging, and immune function: Implications for cancer risk and prevention. Curr. Opin. Oncol. 2012, 24, 506–516. [Google Scholar] [CrossRef]
- Borges, Á.H.; Silverberg, M.J.; Wentworth, D.; Grulich, A.E.; Fätkenheuer, G.; Mitsuyasu, R.; Tambussi, G.; Sabin, C.A.; Neaton, J.D.; Lundgren, J.D.; et al. Predicting risk of cancer during HIV infection: The role of inflammatory and coagulation biomarkers. Aids 2013, 27, 1433–1441. [Google Scholar] [CrossRef]
- Worm, S.W.; Bower, M.; Reiss, P.; Bonnet, F.; Law, M.; Fätkenheuer, G.; Monforte, A.D.; Abrams, D.I.; Grulich, A.; Fontas, E.; et al. Non-AIDS defining cancers in the D:A: D Study-time trends and predictors of survival: A cohort study. BMC Infect. Dis. 2013, 1, 471. [Google Scholar]
- Wada, N.; Jacobson, L.P.; Cohen, M.; French, A.; Phair, J.; Muñoz, A. Cause-Specific Life Expectancies After 35 Years of Age for Human Immunodeficiency Syndrome-Infected and Human Immunodeficiency Syndrome-Negative Individuals Followed Simultaneously in Long-term Cohort Studies, 1984–2008. Am. J. Epidemiol. 2013, 177, 116–125. [Google Scholar] [CrossRef]
- Seaberg, E.C.; Wiley, D.; Martínez-maza, O.; Chmiel, J.S.; Kingsley, L.; Tang, Y.; Margolick, J.B.; Jacobson, L.P.; Multicenter AIDS Cohort Study (MACS). Cancer incidence in the Multicenter AIDS Cohort Study before and during the HAART era: 1984–2007. Cancer 2010, 116, 5507–5516. [Google Scholar] [CrossRef]
- Park, L.S.; Tate, J.P.; Sigel, K.; Rimland, D.; Crothers, K.; Gibert, C.; Rodriguez-Barradas, M.C.; Goetz, M.B.; Bedimo, R.J.; Brown, S.T.; et al. Time trends in cancer incidence in persons living with HIV/AIDS in the antiretroviral therapy era: 1997–2012. AIDS 2016, 30, 1795–1806. [Google Scholar] [CrossRef]
- Hleyhel, M. Writing committee of the cancer risk group of the French hospital database on HIV (FHDH-ANRS CO4). Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: Results from a French cohort Mira Hleyhel a, b Writing committee of the Cancer Risk Group of the. AIDS 2014, 28, 2109–2118. [Google Scholar] [PubMed]
- Albini, L.; Calabresi, A.; Gotti, D.; Ferraresi, A.; Festa, A.; Donato, F.; Magoni, M.; Castelli, F.; Quiros-Roldan, E. Burden of Non-AIDS-Defining and Non-Virus-Related Cancers Among HIV-Infected Patients in the Combined Antiretroviral Therapy Era. AIDS Res. Hum. Retrovir. 2013, 29, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, M.J.; Abrams, D.I. AIDS-defining and non-AIDS-defining malignancies: Cancer occurrence in the antiretroviral therapy era. Curr. Opin. Oncol. 2007, 19, 446–451. [Google Scholar] [CrossRef] [PubMed]
- The RESPOND Study Group. The interrelationship of smoking, CD4+ cell count, viral load and cancer in persons living with HIV. AIDS 2021, 35, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Chammartin, F.; Lodi, S.; Logan, R.; Ryom, L.; Mocroft, A.; Kirk, O.; Monforte, A.; Reiss, P.; Phillips, A.; El-Sadr, W.; et al. Risk for Non-AIDS-Defining and AIDS-Defining Cancer of Early Versus Delayed Initiation of Antiretroviral Therapy: A Multinational Prospective Cohort Study. Ann. Intern. Med. 2021, 174, 768–776. [Google Scholar] [CrossRef]
- European AIDS Clinical Society (EACS). EACS Guidelines, Version 11.1; EACS: Europe, 2022.
- Chao, C.; Leyden, W.A.; Xu, L.; Horberg, M.A.; Klein, D.; Towner, W.J.; Quesenberry, C.P.J.; Abrams, D.I.; Silverberg, M.J. Exposure to antiretroviral therapy and risk of cancer in HIV-infected persons. AIDS 2012, 26, 2223–2231. [Google Scholar] [CrossRef]
- Mbang, P.A.; Kowalkowski, M.A.; Amirian, E.S.; Giordano, T.P.; Richardson, P.A.; Hartman, C.M.; Chiao, E.Y. Association between Time on Protease Inhibitors and the Incidence of Squamous Cell Carcinoma of the Anus among U.S. Male Veterans. PLoS ONE 2015, 10, e0142966. [Google Scholar] [CrossRef]
- Bruyand, M.; Ryom, L.; Shepherd, L.; Fatkenheuer, G.; Grulich, A.; Reiss, P.; de Wit, S.; d´Arminio Monforte, A.; Furrer, H.; Pradier, C.; et al. Cancer risk and use of protease inhibitor or nonnucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy: The D:A:D study. J. Acquir. Immune Defic. Syndr. 2015, 68, 568–577. [Google Scholar] [CrossRef]
- van der Zee, R.P.; Wit, F.W.N.M.; Richel, O.; van der Valk, M.; Reiss, P.; de Vries, H.J.C.; Prins, J.M. Effect of the introduction of screening for cancer precursor lesions on anal cancer incidence over time in people living with HIV: A nationwide cohort study. Lancet HIV 2023, 10, e97–e106. [Google Scholar] [CrossRef]
- Greenberg, L.; Ryom, L.; Neesgaard, B.; Miró, J.M.; Rasmussen, L.D.; Zangerle, R.; Grabmeier-Pfistershammer, K.; Günthard, H.F.; Kusejko, K.; Smith, C.; et al. Integrase Strand Transfer Inhibitor Use and Cancer Incidence in a Large Cohort Setting. Open Forum. Infect. Dis. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- The RESPOND Study Group. How to RESPOND to modern challenges for people living with HIV: A profile for a new cohort consortium. Microorganisms 2020, 8, 1–17. [Google Scholar]
- Friis-Møller, N.; Weber, R.; Reiss, P.; Thiébaut, R.; Kirk, O.; Monforte, A.D.A.; Pradier, C.; Morfeldt, L.; Mateu, S.; Law, M.; et al. Cardiovascular disease risk factors in HIV patients—Association with antiretroviral therapy. Results from the DAD study. Aids 2003, 17, 1179–1193. [Google Scholar] [CrossRef]
- D:A:D Manual of Operations (MOOP) for Clinical Events v1.4. 2013. Available online: https://chip.dk/Research/Studies/DAD/Study-Documents (accessed on 21 September 2022).
- RESPOND Manual of Operations (MOOP) for Clinical Events v1.6. 2019. Available online: https://chip.dk/Research/Studies/RESPOND/Study-documents (accessed on 21 September 2022).
- Ryom, L.; Lundgren, J.D.; El-Sadr, W.; Reiss, P.; Kirk, O.; Law, M.; Phillips, A.; Weber, R.; Fontas, E.; Monforte, A.D.A.; et al. Cardiovascular disease and use of contemporary protease inhibitors: The D:A:D international prospective multicohort study. Lancet HIV 2018, 5, e291–e300. [Google Scholar] [CrossRef] [PubMed]
- Breslow, N.; Day, N. Statistical Methods in Cancer Research Volume II-The Design and Analysis of Cohort Studies; International Agency for Reserach on Cancer: Lyon, France, 1987. [Google Scholar]
- Eayres, D. Association of Public Health Observatories (APHO). Technical Briefing 3: Commonly Used Public Health Statistics and Their Confidence Intervals. 2008. Available online: https://fingertips.phe.org.uk/documents/APHO%20Tech%20Briefing%203%20Common%20PH%20Stats%20and%20CIs.pdf (accessed on 1 June 2023).
- Dobson, A.J.; Kuulasmaa, K.; Eberle, E.; Schere, J. Confidence Intervals for Weighted Sums of Poisson Parameters. Stat. Med. 1991, 10, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, L.; Ryom, L.; Wandeler, G.; Grabmeier-Pfistershammer, K.; Öllinger, A.; Neesgaard, B.; Stephan, C.; Calmy, A.; Rauch, A.; Castagna, A.; et al. Uptake and discontinuation of integrase inhibitors (INSTIs) in a large cohort setting. J. Acquir. Immune Defic. Syndr. 2020, 83, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Friis-Møller, N.; Reiss, P.; Sabin, C.; Weber, R.; Monforte, A.D.; El-Sadr, W.; Thiebaut, R.; De Wit, S.; Kirk, O.; Fontas, E.E.; et al. Class of Antiretroviral Drugs and the Risk of Myocardial Infarction. N. Engl. J. Med. 2007, 356, 1723–1735. [Google Scholar]
- Powles, T.; Robinson, D.; Stebbing, J.; Shamash, J.; Nelson, M.; Gazzard, B.; Mandelia, S.; Møller, H.; Bower, M. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J. Clin. Oncol. 2009, 27, 884–890. [Google Scholar] [CrossRef]
- Spagnuolo, V.; Galli, L.; Salpietro, S.; Gianotti, N.; Guffanti, M.; Cossarini, F.; Bigoloni, A.; Cinque, P.; Bossolasco, S.; Travi, G.; et al. Ten-year survival among HIV-1-infected subjects with AIDS or non-AIDS-defining malignancies. Int. J. Cancer 2012, 130, 2990–2996. [Google Scholar] [CrossRef]
- Silverberg, M.J.; Chao, C.; A Leyden, W.; Xu, L.; Tang, B.; A Horberg, M.; Klein, D.; Quesenberry, C.P.J.; Towner, W.J.; I Abrams, D. HIV infection and the risk of cancers with and without a known infectious cause. AIDS 2010, 23, 2337–2345. [Google Scholar] [CrossRef]
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.A.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, R.K.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. JNCI J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef]
- ECIS—European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu (accessed on 10 May 2023).
- The INSIGHT START Study Group; Lundgren, J.; Babiker, A.; Gordin, F.; Emery, S.; Grund, B. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N. Engl. J. Med. 2015, 373, 795–807. [Google Scholar]
- Koethe, J.R.; Jenkins, C.A.; Lau, B.; Shepherd, B.E.; Justice, A.C.; Tate, J.P.; Buchacz, K.; Napravnik, S.; Mayor, A.M.; Horberg, M.A.; et al. Rising Obesity Prevalence and Weight Gain in the United States and Canada. AIDS Res. Hum. Retrovir. 2016, 32, 50–58. [Google Scholar] [CrossRef]
- Bailin, S.S.; Gabriel, C.L.; Wanjalla, C.N.; Koethe, J.R. Obesity and weight gain in persons with HIV. Curr. HIV/AIDS Rep. 2020, 17, 138–150. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Bannister, W.P.; Mast, T.C.; Wit, D.; Gerstoft, J.; Wiese, L.; Milinkovic, A.; Hadziosmanovic, V.; Clarke, A.; Rasmussen, L.D.; Lacombe, K.; et al. Changes in body mass index and clinical outcomes after initiation of contemporary antiretroviral regimens ere. AIDS 2022, 36, 2107–2119. [Google Scholar] [CrossRef]
- Bansi-Matharu, L.; Phillips, A.; Oprea, C.; Grabmeier-Pfistershammer, K.; Günthard, H.F.; De Wit, S.; Guaraldi, G.; Vehreschild, J.J.; Wit, F.; Law, M.; et al. Contemporary antiretrovirals and body-mass index: A prospective study of the RESPOND cohort consortium. Lancet HIV 2021, 8, e711–e722. [Google Scholar] [CrossRef]
- Taramasso, L.; Bonfanti, P.; Ricci, E.; Orofino, G.; Squillace, N.; Menzaghi, B.; De Socio, G.V.; Madeddu, G.; Pellicanò, G.F.; Pagnucco, L.; et al. Factors associated with weight gain in people treated with dolutegravir. Open Forum. Infect. Dis. 2020, 7, ofaa195. [Google Scholar] [CrossRef]
- Mallon, P.W.; Brunet, L.; Hsu, R.K.; Fusco, J.S.; Mounzer, K.C.; Prajapati, G.; Beyer, A.P.; Wohlfeiler, M.B.; Fusco, G.P. Weight gain before and after switch from TDF to TAF in a U.S. cohort study. J. Int. AIDS Soc. 2021, 24, 25–30. [Google Scholar]
- Altekruse, S.F.; Shiels, M.S.; Modur, S.P.; Land, S.R.; Crothers, K.; Kitahata, M.M.; Thorne, J.E.; Mathews, W.C.; Fernández-Santos, D.M.; Mayor, A.M.; et al. Cancer burden attributable to cigarette smoking among HIV- infected people in North America. AIDS 2018, 32, 513–521. [Google Scholar] [CrossRef]
- Ther, R.; Giles, M.L.; Gartner, C.; Boyd, M.A. Smoking and HIV: What are the risks and what harm reduction strategies do we have at our disposal? AIDS Res. Ther. 2018, 15, 26. [Google Scholar]
- Frazier, E.L.; Sutton, M.Y.; Brooks, J.T.; Shouse, R.L.; Weiser, J. Trends in cigarette smoking among adults with HIV compared with the general adult population, United States-2009–2014. Prev. Med. 2018, 111, 231–234. [Google Scholar] [CrossRef]
- Mdodo, R.; Frazier, E.L.; Dube, S.R.; Mattson, C.L.; Sutton, M.Y.; Brooks, J.T.; Skarbinski, J. Cigarette Smoking Prevalence Among Adults with HIV Compared with the General Adult Population in the United States. Ann. Intern. Med. 2015, 162, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Helleberg, M.; May, M.T.; Ingle, S.M.; Dabis, F.; Reiss, P.; Fätkenheuer, G.; Costagliola, D.; D’arminio, A.; Cavassini, M.; Smith, C.; et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. Aids 2015, 29, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, L.; Ryom, L.; Law, M.; Petoumenos, K.; Hatleberg, C.I.; d’Arminio Monforte, A.; Sabin, C.; Bower, M.; Bonnet, F.; Reiss, P.; et al. Cessation of cigarette smoking and the impact on cancer incidence in human immunodeficiency virus-infected persons: The data collection on adverse events of anti-HIV drugs study. Clin. Infect. Dis. 2019, 68, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Smolle, E.; Pichler, M. Non-smoking-associated lung cancer: A distinct entity in terms of tumor biology, patient characteristics and impact of hereditary cancer predisposition. Cancers 2019, 11, 204. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Light alcohol drinking and cancer: A meta-analysis. Ann. Oncol. 2013, 24, 301–308. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA-J. Am. Med. Assoc. 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Turati, F.; Edefonti, V.; Bosetti, C.; Ferraroni, M.; Malvezzi, M.; Franceschi, S.; Talamini, R.; Montella, M.; Levi, F.; Maso, L.D.; et al. Family history of cancer and the risk of cancer: A network of case-control studies. Ann. Oncol. 2013, 24, 2651–2656. [Google Scholar] [CrossRef]
- Pagano, J.S. Epstein-Barr Virus: The First Human Tumor Virus and its Role in Cancer. Proc. Assoc. Am. Physicians 1999, 111, 573–580. [Google Scholar] [CrossRef]
- Ye, Y.; Burkholder, G.A.; Wiener, H.W.; Griffin, R.; Aslibekyan, S.; Fry, K.; Khan, A.; Shrestha, S. Comorbidities associated with HPV infection among people living with HIV-1 in the southeastern US: A retrospective clinical cohort study. BMC Infect. Dis. 2020, 20, 144. [Google Scholar] [CrossRef]
- Sigel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]

| Overall (n = 64,937) | Cancer during Follow-Up (n = 3763) | No Cancer during Follow-Up (n = 61,174) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |||
| Gender § | Male | 48,208 | (74.2) | 3001 | (79.8) | 45,207 | (73.9) | |
| Female | 16,674 | (25.7) | 761 | (20.2) | 15,913 | (26.0) | ||
| Transgender | 51 | (0.1) | 1 | (0.0) | 50 | (0.1) | ||
| Ethnicity | White | 36,711 | (56.5) | 2188 | (58.1) | 34,523 | (56.4) | |
| Black | 6279 | (9.7) | 173 | (4.6) | 6106 | (10.0) | ||
| Other | 2299 | (3.5) | 62 | (1.6) | 2237 | (3.7) | ||
| Unknown | 19,648 | (30.3) | 1340 | (35.6) | 18,308 | (29.9) | ||
| Body Mass Index (kg/m2) | <18.5 | 2636 | (4.1) | 209 | (5.6) | 2427 | (4.0) | |
| 18.5–<25 | 31,838 | (49.0) | 1954 | (51.9) | 29,884 | (48.9) | ||
| 25–<30 | 3323 | (5.1) | 165 | (4.4) | 3158 | (5.2) | ||
| 30+ | 12,402 | (19.1) | 770 | (20.5) | 11,632 | (19.0) | ||
| Unknown | 14,738 | (22.7) | 665 | (17.7) | 14,073 | (23.0) | ||
| Geographical Region a | Western Europe | 25,415 | (39.1) | 1626 | (43.2) | 23,789 | (38.9) | |
| Southern Europe | 11,630 | (18.8) | 684 | (18.2) | 10,946 | (17.9) | ||
| Northern Europe | 20,429 | (31.4) | 1286 | (34.1) | 19,143 | (31.3) | ||
| Eastern Europe | 6011 | (9.3) | 167 | (4.4) | 5844 | (9.6) | ||
| USA | 1452 | (2.2) | 0 | (0.0) | 1452 | (2.4) | ||
| Mode of HIV Acquisition | MSM | 29,410 | (45.3) | 1820 | (48.4) | 27,590 | (45.1) | |
| IDU | 8721 | (13.4) | 589 | (15.7) | 8132 | (13.3) | ||
| Heterosexual | 22,290 | (34.3) | 1103 | (29.3) | 21,187 | (34.6) | ||
| Other | 1380 | (2.1) | 83 | (2.2) | 1297 | (2.1) | ||
| Unknown | 3136 | (4.8) | 168 | (4.5) | 2968 | (4.9) | ||
| Smoking Status | Never | 16,340 | (25.2) | 809 | (21.5) | 15,531 | (25.4) | |
| Current | 23,836 | (36.7) | 1630 | (43.3) | 22,206 | (36.3) | ||
| Previous | 9874 | (15.2) | 697 | (18.5) | 9177 | (15.0) | ||
| Unknown | 14,887 | (22.9) | 627 | (16.7) | 14,260 | (23.3) | ||
| ART treatment history status | Naïve | 20,118 | (31.0) | 990 | (26.3) | 19,128 | (31.3) | |
| Experienced, VL < 200 cps/mL | 34,289 | (52.8) | 2040 | (54.2) | 32,249 | (52.7) | ||
| Experienced, VL ≥ 200 cps/mL | 9062 | (14.0) | 652 | (17.3) | 8410 | (13.7) | ||
| Experienced, unknown VL | 1468 | (2.3) | 81 | (2.2) | 1387 | (2.3) | ||
| Prior exposure to INSTIs | 1399 | (2.2) | 41 | (1.1) | 1358 | (2.2) | ||
| Prior exposure to PIs | 30,693 | (47.3) | 2150 | (57.1) | 28,543 | (46.7) | ||
| Prior exposure to NNRTIs | 30,478 | (46.9) | 1919 | (51.0) | 28,559 | (46.7) | ||
| Prior exposure to NRTIs | 25,788 | (39.7) | 1900 | (50.5) | 23,888 | (39.1) | ||
| Prior AIDS | No | 51,896 | (79.9) | 2735 | (72.7) | 49,161 | (80.4) | |
| Yes | 13,041 | (20.1) | 1028 | (27.3) | 12,013 | (19.6) | ||
| Hepatitis C b | No | 42,872 | (66.0) | 2528 | (67.2) | 40,344 | (65.9) | |
| Yes | 12,449 | (19.2) | 806 | (21.4) | 11,643 | (19.0) | ||
| Unknown | 9616 | (14.8) | 429 | (11.4) | 9187 | (15.0) | ||
| Hepatitis B c | No | 52,794 | (81.3) | 3157 | (83.9) | 49,637 | (81.1) | |
| Yes | 2703 | (4.2) | 206 | (5.5) | 2497 | (4.1) | ||
| Unknown | 9440 | (14.5) | 400 | (10.6) | 9040 | (14.8) | ||
| Hypertension d | No | 51,164 | (78.8) | 2765 | (73.5) | 48,399 | (79.1) | |
| Yes | 9632 | (14.8) | 859 | (22.8) | 8773 | (14.3) | ||
| Unknown | 4141 | (6.4) | 139 | (3.7) | 4002 | (6.5) | ||
| Diabetes e | No | 63,054 | (97.1) | 3561 | (94.6) | 59,493 | (97.3) | |
| Yes | 1883 | (2.9) | 202 | (5.4) | 1681 | (2.7) | ||
| Prior Cancer | No | 61,053 | (94.0) | 3438 | (91.4) | 57,615 | (94.2) | |
| Yes | 3122 | (4.8) | 302 | (8.0) | 2820 | (4.6) | ||
| Unknown | 762 | (1.2) | 23 | (0.6) | 739 | (1.2) | ||
| Dyslipidaemia f | No | 25,705 | (39.6) | 1085 | (28.8) | 24,620 | (40.2) | |
| Yes | 39,232 | (60.4) | 2678 | (71.2) | 36,554 | (59.8) | ||
| Continuous variables | Median | (IQR) | Median | (IQR) | Median | (IQR) | ||
| Baseline date, month/year | 09/06 | (01/06, 01/12) | 01/06 | (01/06, 11/08) | 12/06 | (01/06, 01/12) | ||
| Age, years | 42 | (35, 49) | 48 | (41, 56) | 42 | (35, 49) | ||
| CD4 cell nadir, cells/mm3 g | 244 | (116, 400) | 194 | (77, 333) | 248 | (120, 401) | ||
| CD4 at baseline, cells/mm3 g | 470 | (312, 661) | 432 | (263, 635) | 472 | (315, 663) | ||
| VL at baseline, copies/mL | 50 | (49, 12400) | 50 | (49, 18986) | 50 | (46, 12100) | ||
| Total duration of previous ART for those who started ART, years | 6.5 | (2.5, 9.9) | 8.6 | (4.5, 11.8) | 6.4 | (2.5, 9.7) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greenberg, L.; Ryom, L.; Bakowska, E.; Wit, F.; Bucher, H.C.; Braun, D.L.; Phillips, A.; Sabin, C.; d’Arminio Monforte, A.; Zangerle, R.; et al. Trends in Cancer Incidence in Different Antiretroviral Treatment-Eras amongst People with HIV. Cancers 2023, 15, 3640. https://doi.org/10.3390/cancers15143640
Greenberg L, Ryom L, Bakowska E, Wit F, Bucher HC, Braun DL, Phillips A, Sabin C, d’Arminio Monforte A, Zangerle R, et al. Trends in Cancer Incidence in Different Antiretroviral Treatment-Eras amongst People with HIV. Cancers. 2023; 15(14):3640. https://doi.org/10.3390/cancers15143640
Chicago/Turabian StyleGreenberg, Lauren, Lene Ryom, Elzbieta Bakowska, Ferdinand Wit, Heiner C. Bucher, Dominique L. Braun, Andrew Phillips, Caroline Sabin, Antonella d’Arminio Monforte, Robert Zangerle, and et al. 2023. "Trends in Cancer Incidence in Different Antiretroviral Treatment-Eras amongst People with HIV" Cancers 15, no. 14: 3640. https://doi.org/10.3390/cancers15143640
APA StyleGreenberg, L., Ryom, L., Bakowska, E., Wit, F., Bucher, H. C., Braun, D. L., Phillips, A., Sabin, C., d’Arminio Monforte, A., Zangerle, R., Smith, C., De Wit, S., Bonnet, F., Pradier, C., Mussini, C., Muccini, C., Vehreschild, J. J., Hoy, J., Svedhem, V., ... on behalf of the RESPOND and D:A:D Study Groups. (2023). Trends in Cancer Incidence in Different Antiretroviral Treatment-Eras amongst People with HIV. Cancers, 15(14), 3640. https://doi.org/10.3390/cancers15143640









