Liver Transplantation for Incidental Cholangiocarcinoma or Combined Hepatocellular Carcinoma/Cholangiocarcinoma—Own Experiences and Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
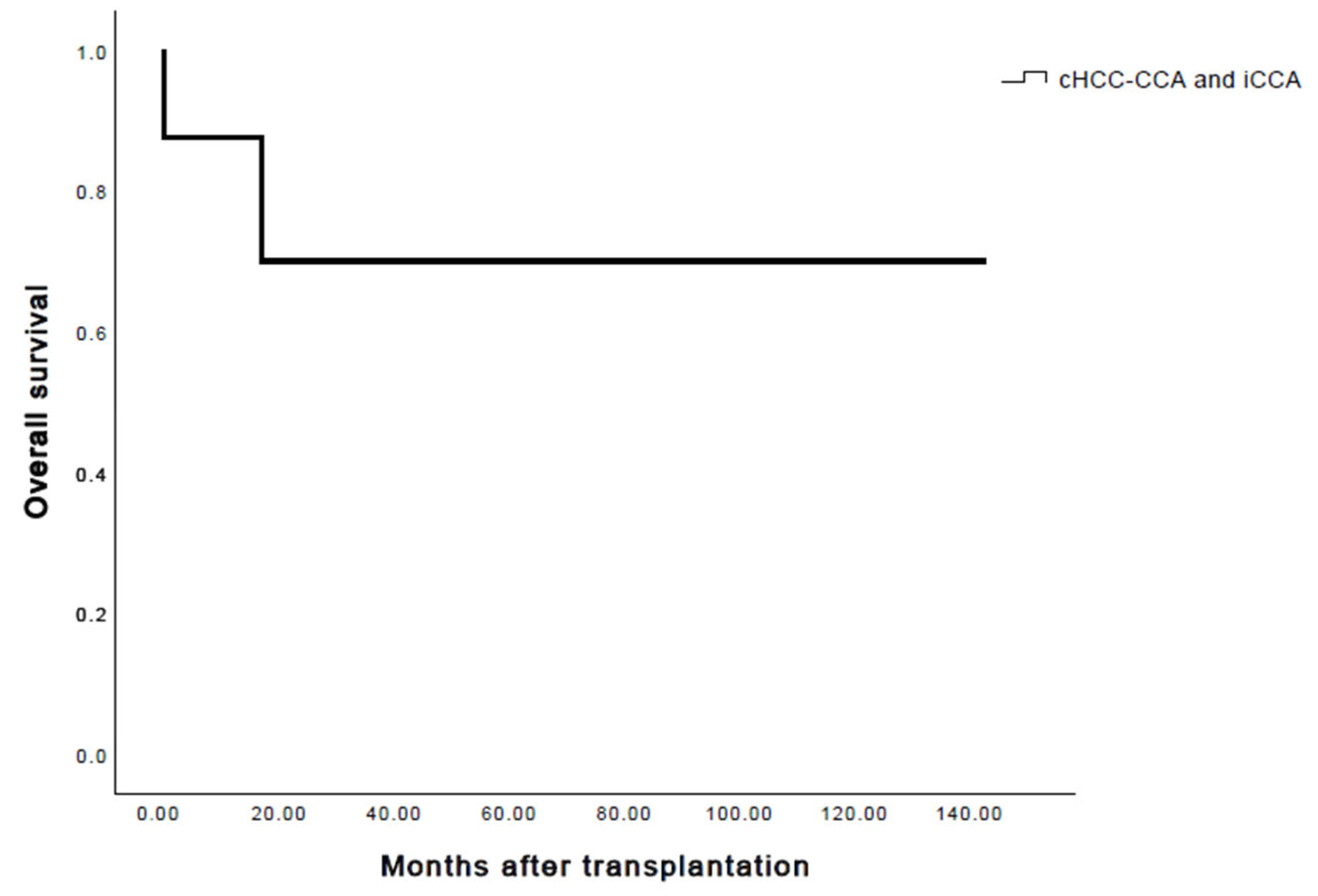
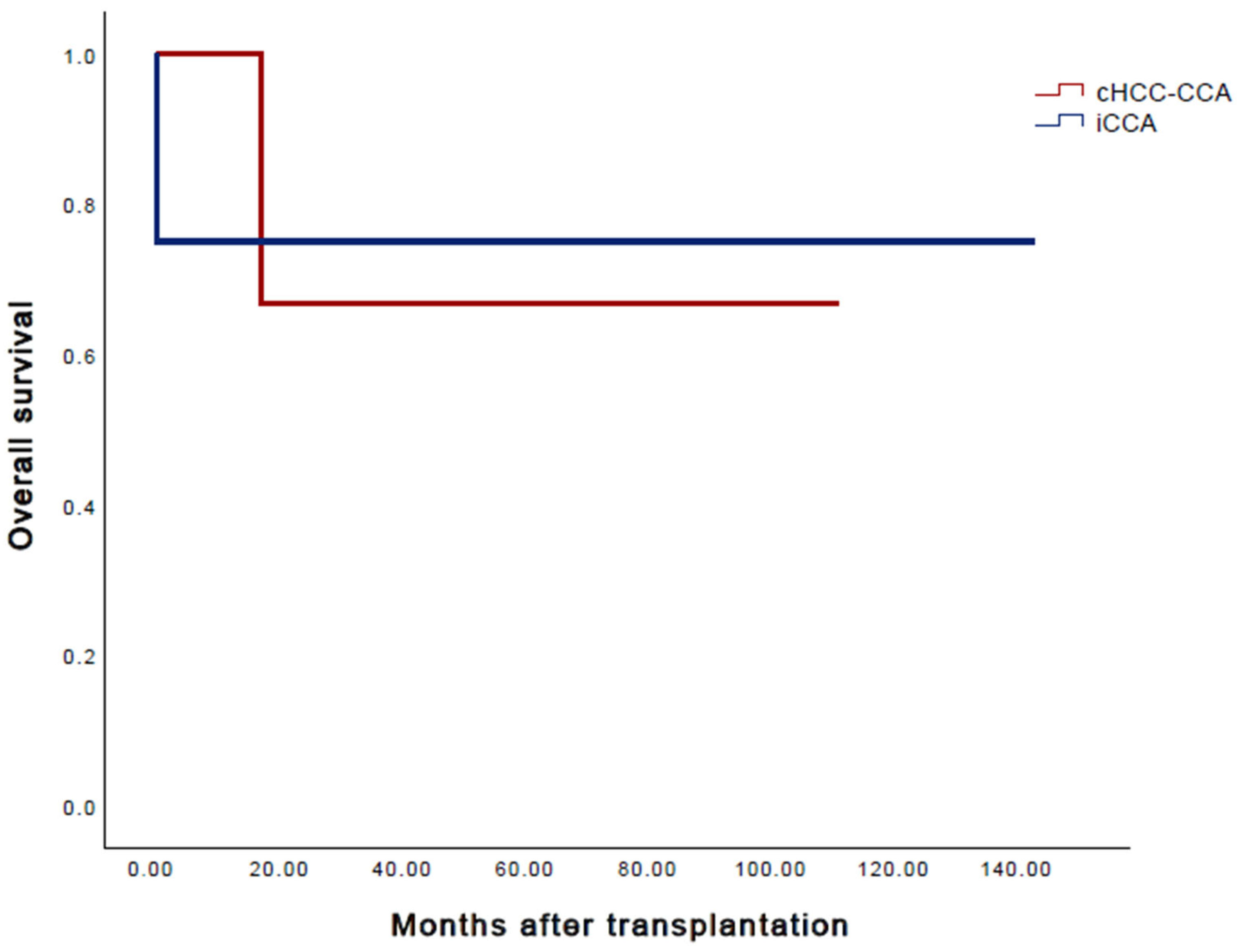
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voesch, S.; Bitzer, M.; Blödt, S.; Follmann, M.; Freudenberger, P.; Langer, T.; Lorenz, P.; Jansen, P.L.; Steubesand, N.; Galle, P.; et al. S3-Leitlinie: Diagnostik und Therapie des hepatozellulären Karzinoms und biliärer Karzinome—Version 2.0—Juni 2021, AWMF-Registernummer: 032-053OL. Z. Gastroenterol. 2022, 60, e131–e185. [Google Scholar] [CrossRef] [PubMed]
- Florio, A.A.; Ferlay, J.; Znaor, A.; Ruggieri, D.; Alvarez, C.S.; Laversanne, M.; Bray, F.; McGlynn, K.A.; Petrick, J.L. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer 2020, 126, 2666–2678. [Google Scholar] [CrossRef] [PubMed]
- Komuta, M. Intrahepatic cholangiocarcinoma: Tumour heterogeneity and its clinical relevance. Clin. Mol. Hepatol. 2022, 28, 396–407. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Weber, S.; Tickoo, S.K.; Koea, J.B.; Obiekwe, S.; Fong, Y.; DeMatteo, R.P.; Blumgart, L.H.; Klimstra, D. Combined hepatocellular and cholangiocarcinoma: Demographic, clinical, and prognostic factors. Cancer 2002, 94, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Goodman, Z.D.; Ishak, K.G.; Langloss, J.M.; Sesterhenn, I.A.; Rabin, L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer 1985, 55, 124–135. [Google Scholar] [CrossRef]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Stavraka, C.; Rush, H.; Ross, P. Combined hepatocellular cholangiocarcinoma (cHCC-CC): An update of genetics, molecular biology, and therapeutic interventions. J. Hepatocell Carcinoma 2019, 6, 11–21. [Google Scholar] [CrossRef]
- Wells, H.G. Primary carcinoma of the liver. Am. J. Med. Sci. 1903, 126, 403. [Google Scholar] [CrossRef]
- Lin, C.W.; Wu, T.C.; Lin, H.Y.; Hung, C.M.; Hsieh, P.M.; Yeh, J.H.; Hsiao, P.; Huang, Y.L.; Li, Y.C.; Wang, Y.C.; et al. Clinical features and outcomes of combined hepatocellular carcinoma and cholangiocarcinoma versus hepatocellular carcinoma versus cholangiocarcinoma after surgical resection: A propensity score matching analysis. BMC Gastroenterol. 2021, 21, 20. [Google Scholar] [CrossRef]
- Beaufrère, A.; Calderaro, J.; Paradis, V. Combined hepatocellular-cholangiocarcinoma: An update. J. Hepatol. 2021, 74, 1212–1224. [Google Scholar] [CrossRef]
- Sapisochin, G.; Facciuto, M.; Rubbia-Brandt, L.; Marti, J.; Mehta, N.; Yao, F.Y.; Vibert, E.; Cherqui, D.; Grant, D.R.; Hernandez-Alejandro, R.; et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016, 64, 1178–1188. [Google Scholar] [CrossRef]
- Sapisochin, G.; de Lope, C.R.; Gastaca, M.; de Urbina, J.O.; López-Andujar, R.; Palacios, F.; Ramos, E.; Fabregat, J.; Castroagudín, J.F.; Varo, E.; et al. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: A Spanish matched cohort multicenter study. Ann. Surg. 2014, 259, 944–952. [Google Scholar] [CrossRef]
- Jaradat, D.; Bagias, G.; Lorf, T.; Tokat, Y.; Obed, A.; Oezcelik, A. Liver transplantation for combined hepatocellular-cholangiocarcinoma: Outcomes and prognostic factors for mortality. A multicenter analysis. Clin. Transplant. 2021, 35, e14094. [Google Scholar] [CrossRef]
- Bahra, M.; Yahyazadeh, A. Surgical Strategies for Combined Hepatocellular-Cholangiocarcinoma (cHCC-CC). Cancers 2023, 15, 774. [Google Scholar] [CrossRef]
- Park, Y.H.; Hwang, S.; Ahn, C.S.; Kim, K.H.; Moon, D.B.; Ha, T.Y.; Song, G.W.; Jung, D.H.; Park, G.C.; Namgoong, J.M.; et al. Long-term outcome of liver transplantation for combined hepatocellular carcinoma and cholangiocarcinoma. Transplant. Proc. 2013, 45, 3038–3040. [Google Scholar] [CrossRef]
- Hara, T.; Eguchi, S.; Yoshizumi, T.; Akamatsu, N.; Kaido, T.; Hamada, T.; Takamura, H.; Shimamura, T.; Umeda, Y.; Shinoda, M.; et al. Incidental intrahepatic cholangiocarcinoma in patients undergoing liver transplantation: A multi-center study in Japan. J. Hepatobiliary Pancreat. Sci. 2021, 28, 346–352. [Google Scholar] [CrossRef]
- Sapisochin, G.; Rodríguez de Lope, C.; Gastaca, M.; Ortiz de Urbina, J.; Suarez, M.A.; Santoyo, J.; Castroagudín, J.F.; Varo, E.; López-Andujar, R.; Palacios, F.; et al. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: Should liver transplantation be reconsidered in these patients? Am. J. Transplant. 2014, 14, 660–667. [Google Scholar] [CrossRef]
- McMillan, R.R.; Javle, M.; Kodali, S.; Saharia, A.; Mobley, C.; Heyne, K.; Hobeika, M.J.; Lunsford, K.E.; Victor, D.W., 3rd; Shetty, A.; et al. Survival following liver transplantation for locally advanced, unresectable intrahepatic cholangiocarcinoma. Am. J. Transplant. 2022, 22, 823–832. [Google Scholar] [CrossRef]
- Itoh, S.; Ikegami, T.; Yoshizumi, T.; Wang, H.; Takeishi, K.; Harimoto, N.; Yamashita, Y.; Kawanaka, H.; Aishima, S.; Shirabe, K.; et al. Long-term outcome of living-donor liver transplantation for combined hepatocellular-cholangiocarcinoma. Anticancer Res. 2015, 35, 2475–2476. [Google Scholar]
- Facciuto, M.E.; Singh, M.K.; Lubezky, N.; Selim, M.A.; Robinson, D.; Kim-Schluger, L.; Florman, S.; Ward, S.C.; Thung, S.N.; Fiel, M.; et al. Tumors with intrahepatic bile duct differentiation in cirrhosis: Implications on outcomes after liver transplantation. Transplantation 2015, 99, 151–157. [Google Scholar] [CrossRef]
- Lee, D.D.; Croome, K.P.; Musto, K.R.; Melendez, J.; Tranesh, G.; Nakhleh, R.; Taner, C.B.; Nguyen, J.H.; Patel, T.; Harnois, D.M. Liver transplantation for intrahepatic cholangiocarcinoma. Liver Transpl. 2018, 24, 634–644. [Google Scholar] [CrossRef]
- Kim, P.; Littau, M.; Baker, T.B.; Abdelsattar, Z.; Tonelli, C.; Bunn, C.; Kulshrestha, S.; Luchette, F.A.; Baker, M.S. Intrahepatic cholangiocarcinoma: Is there a role for liver transplantation? Surgery 2022, 171, 741–746. [Google Scholar] [CrossRef] [PubMed]
- De Martin, E.; Rayar, M.; Golse, N.; Dupeux, M.; Gelli, M.; Gnemmi, V.; Allard, M.A.; Cherqui, D.; Sa Cunha, A.; Adam, R.; et al. Analysis of Liver Resection Versus Liver Transplantation on Outcome of Small Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma in the Setting of Cirrhosis. Liver Transpl. 2020, 26, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Vallin, M.; Sturm, N.; Lamblin, G.; Guillaud, O.; Hilleret, M.N.; Hervieu, V.; Joubert, J.; Abergel, A.; Leroy, V.; Boillot, O.; et al. Unrecognized intrahepatic cholangiocarcinoma: An analysis of 993 adult cirrhotic liver explants. Clin. Transplant. 2013, 27, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Antwi, S.O.; Habboush, Y.Y.; Chase, L.A.; Lee, D.D.; Patel, T. Response to Loco-Regional Therapy Predicts Outcomes After Liver Transplantation for Combined Hepatocellular-Cholangiocarcinoma. Ann. Hepatol. 2018, 17, 969–979. [Google Scholar] [CrossRef]
| Age (Years) | Height (cm) | Weight (kg) | BMI (kg/m2) | ICU Stay (Days) | Steatosis % | ASAT/ALAT Pre-Explant µmol/L*s | Sodium mmol/L | Bilirubin µmol/L | Viral Hepatitis | Sepsis | Meningitis | SARS-CoV-2-Infection | Malignant Tumor | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | 57 | 163 | 76 | 29 | 12 | no | 33/48 | 139 | 4.9 | negative | negative | negative | n.a. | post-explant-lung |
| II | 66 | 173 | 60 | 20 | 7 | no | 64/46 | 147 | 17.4 | negative | negative | negative | n.a. | negative |
| III | 48 | 190 | 90 | 25 | 3 | yes (61–70% microvesicular steatosis) | 102/84 | 131 | 3 | HBs Ab positive | negative | negative | negative | negative |
| IV | 62 | 180 | 85 | 26 | 3 | no | 109/25 | 128 | 13.7 | negative | negative | negative | n.a. | negative |
| V | 72 | 170 | 90 | 31 | 4 | no | 29/23 | 132 | 14 | negative | negative | negative | n.a. | negative |
| VI | 57 | 180 | 90 | 28 | 3 | no | 28/24 | 135 | 10.3 | negative | negative | negative | n.a. | negative |
| VII | 76 | 175 | 80 | 26 | 6 | no | 129/53 | 141 | 4 | negative | negative | negative | negative | negative |
| Age (Years) | Height (cm) | Weight (kg) | BMI (kg/m2) | Blood Type | Relation to Recipient | Right or Left Lobe of the Liver | Mass of the Graft (cm3) | |
|---|---|---|---|---|---|---|---|---|
| I | 51 | 164 | 68 | 25 | 0 | mother | right | 1009 |
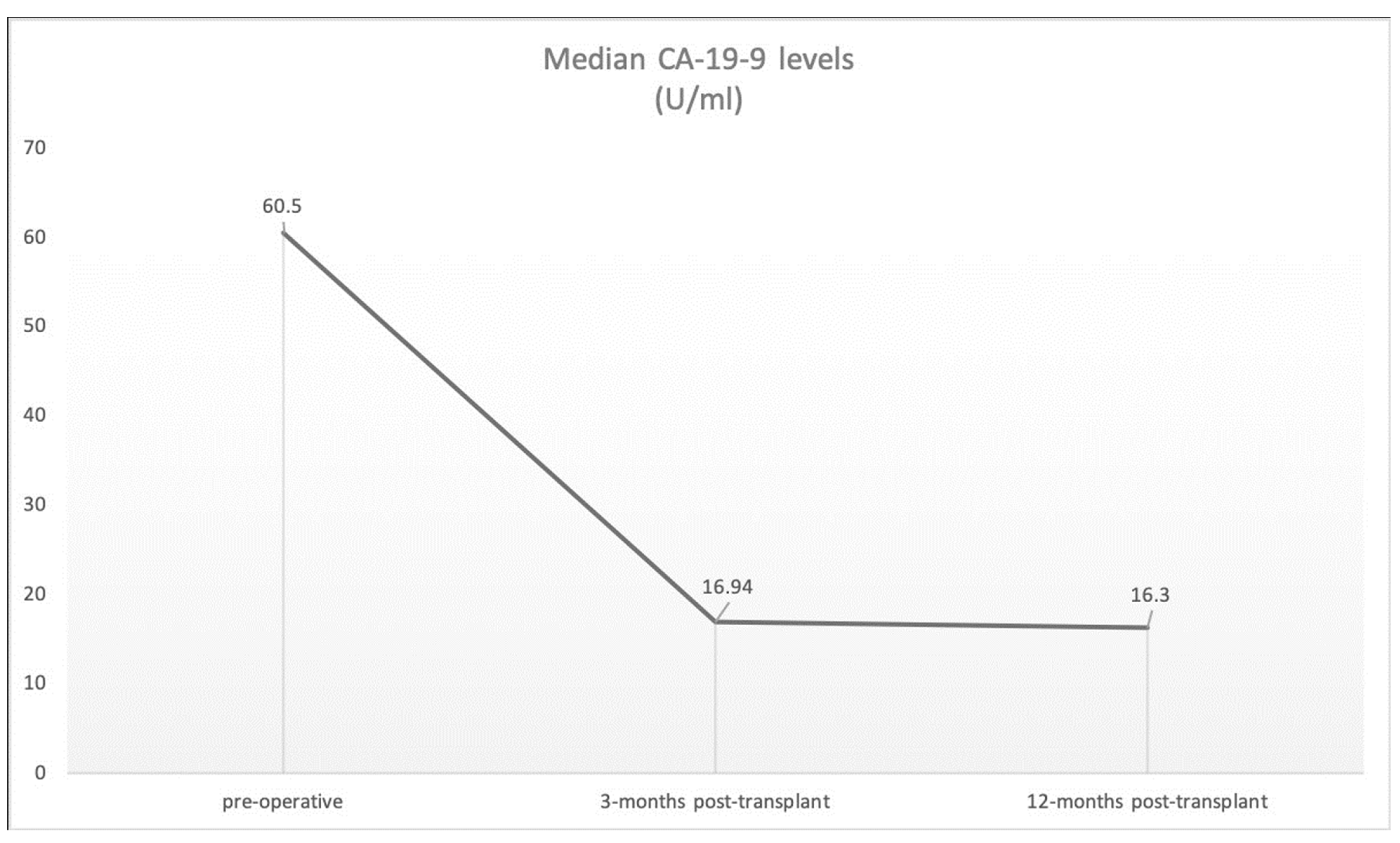
| Diagnosis | Localization | Child–Pugh SCORE | Blood Type | Meld Score | Largest Tumor Diameter (cm) | Number of Lesions | Tumor Classification | CA19-9 Pre- Transplant (U/mL) | CA19-9 3 and 12 Months Post- Transplant (U/mL) | AFP Pre- Transplant (ng/mL) | AFP 3 and 12 Months Post- Transplant (ng/mL) | CEA Pre- Transplant (ng/mL) | CEA 3 and 12 Months Post- Transplant (ng/mL) | Clavien–Dindo Complication Classification (30 days/90 days) | Rejection/Transplant Dysfunction | Dialysis Post-Transplant | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | cHCC-CCA | segment IV | A | 0 | 10 | 6 | 1 | not further classified | 42.9 | 27.8/13.4 | 468.9 | 2.4/3.9 | <1 | 0.9/0.9 | IIIb/0 | no | no |
| II | cHCC-CCA | segment II/III | B | A | 12 | 3.5 | 1 | pT1a, pN0 (0/4), L0, V0, Pn0, R0 G3 | 188 | 23.5/16.8 | 29.4 | 1.3/2.7 | 4.4 | 1.2/n.a. | IVa/IIIa | no | yes |
| III | cHCC-CCA | segment V/VI | A | A | 12 | 3.3 | 1 | ypT2, pN0 (0/2), L0, V0, Pn0, R0 | 24.4 | 22/21 | 842 | 3.2/2.9 | 2.1 | 0.9/1.5 | II/0 | mild rejection | no |
| IV | cHCC-CCA | segment VI, VII, VIII, IV, V | A | 0 | 12 | 4 | 3 | HCC: ypT2, pNx, L0, V0, Pn0, R0 iCCA: ypT1a, L0, V0, Pn0, R0 | 32.7 | 8.9/8.9 | 2.7 | 2.7/3.4 | 5 | 2.2/2.3 | 0/0 | no | no |
| V | iCCA | segment V | B | AB | 22 | 5.5 | 1 | pT1, pN0 (0/3), PMx, L0, V0, Pn0, R0, G2 | 146 | 12.9/12.9 | 6.2 | 4.8/3.6 | 1.4 | 0.9/0.9 | IIIb/0 | no | no |
| VI | iCCA | segment VI | A | A | 8 | 4 | 1 | not further classified | 25 | - | 3.8 | - | 0.9 | - | V/- | transplant dysfunction | yes |
| VII | iCCA | segment IVb | B | AB | 9 | 1.7 | 1 | pT1a, pN0 (0/2), L0, V0, Pn0, R0, G1 | 12.7 | 12.8/8.9 | 9.7 | 3.9/2.7 | 4.3 | 2.4/2.5 | IIIa/0 | no | no |
| VIII | iCCA | multiple | B | A | 19 | 1.3 | multiple | ypT2, ypN0 (0/1), L0, V0, Pn0, R0, G2 | 12.3 | 10.7/32.2 | 11.6 | 5.3/2.8 | 2.5 | 3.1/3.6 | 0/IIIa | no | no |
| Serial Number | Study | Country/ Study Period | Total Number of iCCA/cHCC-CCA with LDLT | iCCC | cHCC-CCA | Neoadjuvant Therapy | Underlying Disease | Overall Survival 1, 3 and 5 Years | Recurrence-Free Survival 1, 3 and 5 Years |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Hara T. et al. | Japan 2001–2005 | 19 | 13 | 6 | TACE | Cirrhosis no further description | 79%/63%/46% | 79%/45%/45% |
| 2 | Serra V. et al. | Italy 2000–2015 | 1 | 1 | 0 | TACE, RFA | PSC | ||
| 3 | Itoh S. et al. | Japan 1999–2014 | 8 | 0 | 8 | n.a. | - | 87.5%/72.9%/72.9% | 85.7%/85.7%/85.7% |
| 4 | Park Y.-H. et al. | South Korea 1999–2009 | 14 | 0 | 15 | TACE | 14× HBV 1× NTLC | 66.7%/60%/60% | 60%/53.3%/53.3% |
| 5 | Fukuda A. et al. | Japan 2012 | 1 | 1 | 0 | no | Biliary atresia/ Kasai Op | died | died |
| 6 | Song S. et al. | South Korea 1995–2012 | 7 | 0 | 7 | n.a. | HBV | n.a./n.a./50% | n.a./n.a./37.5% |
| 7 | Nart D. et al. | Turkey 2012 | 2 | 2 | 0 | n.a. | 1× HBV 1× HCV | n.a. | n.a. |
| 8 | Chan A. et al. | China 2002–2003 | 2 | 0 | 3 | n.a. | 1× HBV 1× HCV | n.a. | 100%/100%/33.3% |
| 9 | Vilchez V. et al. | USA 1994–2013 | 6 | 0 | 6 | n.a. | - | 82%/47%/40% | n.a./93%/n.a. |
| 10 | Togashi J. et al. | Japan 1996–2015 | 3 | 1 | 2 | yes | - | 80%/n.a./78% | 5%/6%/6% |
| 11 | Chang C. et al. | Taiwan 2006–2014 | 11 | 0 | 11 | yes | - | 90%/61.7%/n.a. | 80%/46.7%/n.a. |
| 12 | Jonas S. et al. | Germany 1999–2004 | 2 | 2 | 0 | - | Liver fibrosis | n.a. | n.a. |
| 13 | Sotiropoulos G.C. et al. | Germany | 1 | 1 | 0 | - | Recurrence after resection | ||
| 14 | Takatsuki M et al. | Japan 1997 | 1 | 1 | 0 | - | Caroli | alive | no recurrence |
| 15 | Hafeeq Bhatti AB et al. | Pakistan 2012–2019 | 16 | 9 | 7 | yes | 63.6%/n.a./63.6% | n.a./46.7%/n.a. | |
| 16 | Schwenk L et al. | Germany 2010–2022 | 1 | 6 | 4 | yes | - | 87.5%/75%./75% | 85.7%/85.7%/85.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwenk, L.; Rohland, O.; Ali-Deeb, A.; Dondorf, F.; Settmacher, U.; Rauchfuß, F. Liver Transplantation for Incidental Cholangiocarcinoma or Combined Hepatocellular Carcinoma/Cholangiocarcinoma—Own Experiences and Review of the Literature. Cancers 2023, 15, 3609. https://doi.org/10.3390/cancers15143609
Schwenk L, Rohland O, Ali-Deeb A, Dondorf F, Settmacher U, Rauchfuß F. Liver Transplantation for Incidental Cholangiocarcinoma or Combined Hepatocellular Carcinoma/Cholangiocarcinoma—Own Experiences and Review of the Literature. Cancers. 2023; 15(14):3609. https://doi.org/10.3390/cancers15143609
Chicago/Turabian StyleSchwenk, Laura, Oliver Rohland, Aladdin Ali-Deeb, Felix Dondorf, Utz Settmacher, and Falk Rauchfuß. 2023. "Liver Transplantation for Incidental Cholangiocarcinoma or Combined Hepatocellular Carcinoma/Cholangiocarcinoma—Own Experiences and Review of the Literature" Cancers 15, no. 14: 3609. https://doi.org/10.3390/cancers15143609
APA StyleSchwenk, L., Rohland, O., Ali-Deeb, A., Dondorf, F., Settmacher, U., & Rauchfuß, F. (2023). Liver Transplantation for Incidental Cholangiocarcinoma or Combined Hepatocellular Carcinoma/Cholangiocarcinoma—Own Experiences and Review of the Literature. Cancers, 15(14), 3609. https://doi.org/10.3390/cancers15143609






