Deep Learning for Medical Image-Based Cancer Diagnosis
Abstract
Simple Summary
Abstract
1. Introduction
- (i)
- The principle and application of radiological and histopathological images in cancer diagnosis are introduced in detail;
- (ii)
- This paper introduces 9 basic architectures of deep learning, 12 classical pretrained models, and 5 typical methods to overcome overfitting. In addition, advanced deep neural networks are introduced, such as vision transformers, transfer learning, ensemble learning, graph neural network, and explainable deep neural networks;
- (iii)
- The application of deep learning technology in medical imaging cancer diagnosis is deeply analyzed, including image classification, image reconstruction, image detection, image segmentation, image registration, and image fusion;
- (iv)
- The current challenges and future research hotspots are discussed and analyzed around data, labels, and models.
2. Common Imaging Techniques
2.1. Computed Tomography
2.2. Magnetic Resonance Imaging
| MRI | Description |
|---|---|
| Conception |
|
| Feature |
|
2.3. Ultrasound
| Ultrasound | Description |
|---|---|
| Conception |
|
| Feature |
|
2.4. X-ray
| X-ray | Description |
|---|---|
| Conception |
|
| Feature |
|
2.5. Positron Emission Tomography
| PET | Description |
|---|---|
| Conception |
|
| Feature |
|
2.6. Histopathology
3. Deep Learning
3.1. Basic Model
3.1.1. Convolutional Neural Network
3.1.2. Fully Convolutional Network
3.1.3. Autoencoder
| Author | Method | Year | Description | Feature |
|---|---|---|---|---|
| Bengio et al. [159] | SAE 1 | 2007 | Use layer-wise training to learn network parameters. | The pre-trained network fits the structure of the training data to a certain extent, which makes the initial value of the entire network in a suitable state, which is convenient for the supervised stage to speed up the iterative convergence. |
| Vincent et al. [160] | DAE 2 | 2008 | Add random noise perturbation to the input data. | Representation reconstructs high-level information from chaotic information, allowing high learning capacity while preventing learning a useless identity function in the encoder and decoder, improving algorithm robustness, and obtaining a more efficient representation of the input. |
| Vincent et al. [152] | SDAE 3 | 2010 | Multiple DAEs are stacked together to form a deep architecture. The input is corroded (noised) only during training; once the training is complete, there is no need to corrode. | It has strong feature extraction ability and good robustness. It is just a feature extractor and does not have a classification function. |
| Ng [151] | Sparse autoencoder | 2011 | A regular term controlling sparsity is added to the original loss function. | Features can be automatically learned from unlabeled data and better feature descriptions can be given than the original data. |
| Rifai et al. [154] | CAE 4 | 2011 | The autoencoder object function is constrained by the encoder’s Jacobian matrix norm so that the encoder can learn abstract features with anti-jamming. | It mainly mines the inherent characteristics of the training samples, which entails using the gradient information of the samples themselves. |
| Masci et al. [161] | Convolutional autoencoder | 2011 | Utilizes the unsupervised learning method of the traditional autoencoder, combining the convolution and pooling operations of the convolutional neural network. | Through the convolution operation, the convolutional autoencoder can well preserve the spatial information of the two-dimensional signal. |
| Kingma et al. [153] | VAE 5 | 2013 | Addresses the problem of non-regularized latent spaces in autoencoders and provides generative capabilities for the entire space. | It is probabilistic and the output is contingent; new instances that look like input data can be generated. |
| Srivastava et al. [162] | Dropout autoencoder | 2014 | Reduce the expressive power of the network and prevent overfitting by randomly disconnecting the network. | The degree of overfitting can be reduced and the training time is long. |
| Srivastava et al. [163] | LAE 6 | 2015 | Compressive representations of sequence data can be learned. | Representation helps improve classification accuracy, especially when there are few training examples. |
| Makhzani et al. [164] | AAE 7 | 2015 | An additional discriminator network is used to determine whether hidden variables of dimensionality reduction are sampled from prior distributions. | Minimize the reconstruction error of traditional autoencoders; match the aggregated posterior distribution of the latent variables of the autoencoder with an arbitrary prior distribution. |
| Xu et al. [155] | SSAE 8 | 2015 | Advanced feature representations of pixel intensity can be captured in an unsupervised manner. | Only advanced features are learned from pixel intensity to identify the distinguishing features of the kernel; efficient coding can be achieved. |
| Higgins et al. [165] | beta-VAE | 2017 | beta-VAE is a generalization of VAE that only changes the ratio between reconstruction loss and divergence loss. The scalar β denotes the influence factor of the divergence loss. | The potential channel capacity and independence constraints can be balanced with the reconstruction accuracy. Training is stable, makes few assumptions about the data, and relies on tuning a single hyperparameter. |
| Zhao et al. [166] | info-VAE | 2017 | The ELBO objective is modified to address issues where variational autoencoders cannot perform amortized inference or learn meaningful latent features. | Significantly improves the quality of variational posteriors and allows the efficient use of latent features. |
| Van Den Oord et al. [167] | vq-VAE 9 | 2017 | Combining VAEs with vector quantization for discrete latent representations. | Encoder networks output discrete rather than continuous codes; priors are learned rather than static. |
| Dupont [168] | Joint-VAE | 2018 | Augment the continuous latent distribution of a variational autoencoder using a relaxed discrete distribution and control the amount of information encoded in each latent unit. | Stable training and large sample diversity, modeling complex continuous and discrete generative factors. |
| Kim et al. [169] | factorVAE | 2018 | The algorithm motivates the distribution of the representation so that it becomes factorized and independent in the whole dimension. | It outperforms β-VAE in disentanglement and reconstruction. |
3.1.4. Deep Convolutional Extreme Learning Machine
3.1.5. Recurrent Neural Network
3.1.6. Long Short-Term Memory
3.1.7. Generative Adversarial Network
3.1.8. Deep Belief Network
3.1.9. Deep Boltzmann Machine
3.2. Classical Pretrained Model
3.2.1. LeNet-5
3.2.2. AlexNet
3.2.3. ZF-Net
3.2.4. VGGNet
3.2.5. GoogLeNet
3.2.6. ResNet
3.2.7. DenseNet
3.2.8. MobileNet
3.2.9. ShuffleNet
3.2.10. SqueezeNet
3.2.11. XceptionNet
3.2.12. U-net
3.3. Advanced Deep Neural Network
3.3.1. Transfer Learning
- (i)
- Instance-based transfer learning entails reusing part of the data in the source domain through the heavy weight method of the target domain learning;
- (ii)
- Feature-representation transfer learning aims to learn a good feature representation through the source domain, encode knowledge in the form of features, and transfer it from source domain to the target domain for improving the effect of target domain tasks. Feature-based transfer learning is based on the assumption that the target and source domains share some overlapping common features. In feature-based methods, a feature transformation strategy is usually adopted to transform each original feature into a new feature representation for knowledge transfer [279];
- (iii)
- Parameter-transfer learning means that the tasks of the target domain and source domain share the same model parameters or obey the same prior distribution. It is based on the assumption that individual models for related tasks should share a prior distribution of some parameters or hyperparameters. Generally, there are usually two specific ways to achieve this. One is to initialize a new model with the parameters of the source model and then fine-tune it. Secondly, the source model or some layers in the source model are solidified as feature extractors in the new model. Then an output layer is added for the target problem and learning on this basis can effectively utilize previous knowledge and reduce training costs [274];
- (iv)
- Relational-knowledge transfer learning involves knowledge transfer between related domains, which needs to assume that the source and target domains are similar and can share some logical relationship, and attempts to transfer the logical relationship among data from the source domain to the target domain.
3.3.2. Ensemble Learning
3.3.3. Graph Neural Network
3.3.4. Explainable Deep Neural Network
3.3.5. Vision Transformer
- (i)
- The image of H × W × C is changed into a sequence of N × (P2 × C), where P is the size of the image block. This sequence can be viewed as a series of flattened image patches. That is, the image is cut into small patches and then flattened. The sequence contains a total of N = H × W/P2 image patches and the dimension of each image patch is (P2 × C). After the above transformation, N can be regarded as the length of the sequence;
- (ii)
- Since the dimension of each image patch is (P2 × C) and the vector dimension we actually need is D, we also need to Embed the image patch. That is, each image patch will be linearly transformed and the dimension will be compressed to D.
3.4. Overfitting Prevention Technique
3.4.1. Batch Normalization
3.4.2. Dropout
3.4.3. Weight Initialization
3.4.4. Data Augmentation
4. Application of Deep Learning in Cancer Diagnoses
4.1. Image Classification
4.2. Image Detection
4.3. Image Segmentation
4.4. Image Registration
4.5. Image Reconstruction
4.6. Image Synthesis
5. Discussion
5.1. Data
5.1.1. Less Training Data
5.1.2. Class Imbalance
5.1.3. Image Fusion
5.2. Label
5.2.1. Insufficient Annotation Data
5.2.2. Noisy Labels
5.2.3. Supervised Paradigm
5.3. Model
5.3.1. Model Explainability
5.3.2. Model Robustness and Generalization
5.4. Radiomics
6. Conclusions
6.1. Limitations and Challenges
- (i)
- Datasets problems.
- (ii)
- The model lacks explainability.
- (iii)
- Poor generalization ability.
- (iv)
- Lack of high-performance models for multi-modal images.
6.2. Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2022, 10, 451. [Google Scholar]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.; Hindocha, S.; Lee, R.W. The Role of Artificial Intelligence in Early Cancer Diagnosis. Cancers 2022, 14, 1524. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Su, W.; Ao, J.; Wang, M.; Jiang, Q.; He, J.; Gao, H.; Lei, S.; Nie, J.; Yan, X.; et al. Instant diagnosis of gastroscopic biopsy via deep-learned single-shot femtosecond stimulated Raman histology. Nat. Commun. 2022, 13, 4050. [Google Scholar] [CrossRef]
- Attallah, O. Cervical cancer diagnosis based on multi-domain features using deep learning enhanced by handcrafted descriptors. Appl. Sci. 2023, 13, 1916. [Google Scholar] [CrossRef]
- Sargazi, S.; Laraib, U.; Er, S.; Rahdar, A.; Hassanisaadi, M.; Zafar, M.; Díez-Pascual, A.; Bilal, M. Application of Green Gold Nanoparticles in Cancer Therapy and Diagnosis. Nanomaterials 2022, 12, 1102. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, B. Analysis of the Clinical Characteristics of Tuberculosis Patients based on Multi-Constrained Computed Tomography (CT) Image Segmentation Algorithm. Pak. J. Med. Sci. 2021, 37, 1705–1709. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Liu, S.; Yue, X. Design Computer-Aided Diagnosis System Based on Chest CT Evaluation of Pulmonary Nodules. Comput. Math. Methods Med. 2022, 2022, 7729524. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.-C.; Yeh, C.-H.; Yen, T.-C.; Ng, S.-H.; Chang, J.T.-C.; Lin, C.-Y.; Yen-Ming, T.; Fan, K.-H.; Huang, B.-S.; Hsu, C.-L.; et al. Clinical utility of simultaneous whole-body 18F-FDG PET/MRI as a single-step imaging modality in the staging of primary nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zheng, W.; Zhang, L.; Tian, H. Segmentation of ultrasound images of thyroid nodule for assisting fine needle aspiration cytology. Health Inf. Sci. Syst. 2013, 1, 5. [Google Scholar] [CrossRef]
- Janiesch, C.; Zschech, P.; Heinrich, K. Machine learning and deep learning. Electron. Mark. 2021, 31, 685–695. [Google Scholar] [CrossRef]
- Kavitha, R.; Jothi, D.K.; Saravanan, K.; Swain, M.P.; Gonzáles, J.L.A.; Bhardwaj, R.J.; Adomako, E. Ant colony optimization-enabled CNN deep learning technique for accurate detection of cervical cancer. BioMed Res. Int. 2023, 2023, 1742891. [Google Scholar] [CrossRef]
- Castiglioni, I.; Rundo, L.; Codari, M.; Di Leo, G.; Salvatore, C.; Interlenghi, M.; Gallivanone, F.; Cozzi, A.; D’Amico, N.C.; Sardanelli, F. AI applications to medical images: From machine learning to deep learning. Phys. Medica 2021, 83, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Ker, J.; Wang, L.; Rao, J.; Lim, T. Deep Learning Applications in Medical Image Analysis. IEEE Access 2018, 6, 9375–9389. [Google Scholar] [CrossRef]
- Greenspan, H.; van Ginneken, B.; Summers, R.M. Guest Editorial Deep Learning in Medical Imaging: Overview and Future Promise of an Exciting New Technique. IEEE Trans. Med. Imaging 2016, 35, 1153–1159. [Google Scholar] [CrossRef]
- Ghanem, N.M.; Attallah, O.; Anwar, F.; Ismail, M.A. AUTO-BREAST: A fully automated pipeline for breast cancer diagnosis using AI technology. In Artificial Intelligence in Cancer Diagnosis and Prognosis, Volume 2: Breast and Bladder Cancer; IOP Publishing: Bristol, UK, 2022. [Google Scholar]
- Yildirim, K.; Bozdag, P.G.; Talo, M.; Yildirim, O.; Karabatak, M.; Acharya, U.R. Deep learning model for automated kidney stone detection using coronal CT images. Comput. Biol. Med. 2021, 135, 104569. [Google Scholar] [CrossRef] [PubMed]
- Attallah, O.; Aslan, M.F.; Sabanci, K. A framework for lung and colon cancer diagnosis via lightweight deep learning models and transformation methods. Diagnostics 2022, 12, 2926. [Google Scholar] [CrossRef]
- Ragab, D.A.; Attallah, O.; Sharkas, M.; Ren, J.; Marshall, S. A framework for breast cancer classification using multi-DCNNs. Comput. Biol. Med. 2021, 131, 104245. [Google Scholar] [CrossRef]
- Punn, N.S.; Agarwal, S. Modality specific U-Net variants for biomedical image segmentation: A survey. Artif. Intell. Rev. 2022, 55, 5845–5889. [Google Scholar] [CrossRef]
- Du, G.; Cao, X.; Liang, J.; Chen, X.; Zhan, Y. Medical image segmentation based on u-net: A review. J. Imaging Sci. Technol. 2020, 64, jist0710. [Google Scholar] [CrossRef]
- Kaur, C.; Garg, U. Artificial intelligence techniques for cancer detection in medical image processing: A review. Mater. Today Proc. 2021, 81, 806–809. [Google Scholar] [CrossRef]
- Rapidis, A.D. Orbitomaxillary mucormycosis (zygomycosis) and the surgical approach to treatment: Perspectives from a maxillofacial surgeon. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2010, 15 (Suppl. 5), 98–102. [Google Scholar] [CrossRef]
- Kim, W.; Park, M.S.; Lee, S.H.; Kim, S.H.; Jung, I.J.; Takahashi, T.; Misu, T.; Fujihara, K.; Kim, H.J. Characteristic brain magnetic resonance imaging abnormalities in central nervous system aquaporin-4 autoimmunity. Mult. Scler. 2010, 16, 1229–1236. [Google Scholar] [CrossRef]
- Brinkley, C.K.; Kolodny, N.H.; Kohler, S.J.; Sandeman, D.C.; Beltz, B.S. Magnetic resonance imaging at 9.4 T as a tool for studying neural anatomy in non-vertebrates. J. Neurosci. Methods 2005, 146, 124–132. [Google Scholar]
- Grüneboom, A.; Kling, L.; Christiansen, S.; Mill, L.; Maier, A.; Engelke, K.; Quick, H.; Schett, G.; Gunzer, M. Next-generation imaging of the skeletal system and its blood supply. Nat. Rev. Rheumatol. 2019, 15, 533–549. [Google Scholar] [CrossRef]
- Yang, Z.H.; Gao, J.B.; Yue, S.W.; Guo, H.; Yang, X.H. X-ray diagnosis of synchronous multiple primary carcinoma in the upper gastrointestinal tract. World J. Gastroenterol. 2011, 17, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Jin, M.; Hao, D.; Lv, Y.; Liu, Q.; Zhu, Y.; Ding, S.; Zhao, J.; Fei, B. Accurate vessel extraction via tensor completion of background layer in X-ray coronary angiograms. Pattern Recognit. 2019, 87, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Geleijns, J.; Wondergem, J. X-ray imaging and the skin: Radiation biology, patient dosimetry and observed effects. Radiat. Prot. Dosim. 2005, 114, 121–125. [Google Scholar] [CrossRef]
- Sebastian, T.B.; Tek, H.; Crisco, J.J.; Kimia, B.B. Segmentation of carpal bones from CT images using skeletally coupled deformable models. Med. Image Anal. 2003, 7, 21–45. [Google Scholar] [CrossRef]
- Furukawa, A.; Sakoda, M.; Yamasaki, M.; Kono, N.; Tanaka, T.; Nitta, N.; Kanasaki, S.; Imoto, K.; Takahashi, M.; Murata, K.; et al. Gastrointestinal tract perforation: CT diagnosis of presence, site, and cause. Abdom. Imaging 2005, 30, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Cademartiri, F.; Nieman, K.; Aad, V.D.L.; Raaijmakers, R.H.; Mollet, N.; Pattynama, P.M.T.; De Feyter, P.J.; Krestin, G.P. Intravenous contrast material administration at 16-detector row helical CT coronary angiography: Test bolus versus bolus-tracking technique. Radiology 2004, 233, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, X.; Sun, S.; Wu, D.; Bai, J.; Yin, Y.; Liu, X.; Zhang, H.; de Albuquerque, V.H.C. Learning physical properties in complex visual scenes: An intelligent machine for perceiving blood flow dynamics from static CT angiography imaging. Neural Netw. 2020, 123, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.; Rademacher, G.; Ekkernkamp, A.; Güthoff, C.; Mutze, S. Emergency ultrasound-based algorithms for diagnosing blunt abdominal trauma. Cochrane Database Syst. Rev. 2015, 2015, CD004446. [Google Scholar] [CrossRef]
- Chew, C.; Halliday, J.L.; Riley, M.M.; Penny, D.J. Population-based study of antenatal detection of congenital heart disease by ultrasound examination. Ultrasound Obstet. Gynecol. 2010, 29, 619–624. [Google Scholar] [CrossRef]
- Garne, E.; Stoll, C.; Clementi, M. Evaluation of prenatal diagnosis of congenital heart diseases by ultrasound: Experience from 20 European registries. Ultrasound Obstet. Gynecol. 2002, 17, 386–391. [Google Scholar] [CrossRef]
- Fledelius, H.C. Ultrasound in ophthalmology. Ultrasound Med. Biol. 1997, 23, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Abinader, R.W.; Steven, L. Benefits and Pitfalls of Ultrasound in Obstetrics and Gynecology. Obstet. Gynecol. Clin. N. Am. 2019, 46, 367–378. [Google Scholar] [CrossRef]
- Videbech, P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: A critical review. Acta Psychiatr. Scand. 2010, 101, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Taghanaki, S.A.; Duggan, N.; Ma, H.; Hou, X.; Celler, A.; Benard, F.; Hamarneh, G. Segmentation-free direct tumor volume and metabolic activity estimation from PET scans. Comput. Med. Imaging Graph. 2018, 63, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Schöder, H.; Gönen, M. Screening for Cancer with PET and PET/CT: Potential and Limitations. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2007, 48 (Suppl. 1), 4S–18S. [Google Scholar]
- Seeram, E. Computed tomography: Physical principles and recent technical advances. J. Med. Imaging Radiat. Sci. 2010, 41, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Gładyszewski, K.; Gro, K.; Bieberle, A.; Schubert, M.; Hild, M.; Górak, A.; Skiborowski, M. Evaluation of performance improvements through application of anisotropic foam packings in rotating packed beds. Chem. Eng. Sci. 2021, 230, 116176. [Google Scholar] [CrossRef]
- Sera, T. Computed Tomography, in Transparency in Biology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 167–187. [Google Scholar]
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019, 25, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; He, B.; Mu, W.; Liu, K.; Liu, L.; Zeng, H.; Liu, Y.; Jiang, L.; Zhou, P.; Huang, Z.; et al. Assessing PD-L1 expression in non-small cell lung cancer and predicting responses to immune checkpoint inhibitors using deep learning on computed tomography images. Theranostics 2021, 11, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Best, T.D.; Mercaldo, S.F.; Bryan, D.S.; Marquardt, J.P.; Wrobel, M.M.; Bridge, C.P.; Troschel, F.M.; Javidan, C.; Chung, J.H.; Muniappan, A. Multilevel body composition analysis on chest computed tomography predicts hospital length of stay and complications after lobectomy for lung cancer: A multicenter study. Ann. Surg. 2022, 275, e708–e715. [Google Scholar] [CrossRef]
- Vangelov, B.; Bauer, J.; Kotevski, D.; Smee, R.I. The use of alternate vertebral levels to L3 in computed tomography scans for skeletal muscle mass evaluation and sarcopenia assessment in patients with cancer: A systematic review. Br. J. Nutr. 2022, 127, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A. Principles of magnetic resonance imaging. Rev. Mex. De Física 2004, 50, 272–286. [Google Scholar]
- Vasireddi, A.K.; Leo, M.E.; Squires, J.H. Magnetic resonance imaging of pediatric liver tumors. Pediatr. Radiol. 2022, 52, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-Y.; Wang, S.-Y.; Lin, S.-M.; Chen, K.-Y.; Tseng, J.-H.; Ho, M.-C.; Lee, R.-C.; Liang, P.-C.; Liao, L.-Y.; Huang, K.-W.; et al. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med. Assoc. 2021, 120, 1051–1060. [Google Scholar] [CrossRef]
- Yang, J.; Yu, S.; Gao, L.; Zhou, Q.; Zhan, S.; Sun, F. Current global development of screening guidelines for hepatocellular carcinoma: A systematic review. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi 2020, 41, 1126–1137. [Google Scholar]
- Pedrosa, I.; Alsop, D.C.; Rofsky, N.M. Magnetic resonance imaging as a biomarker in renal cell carcinoma. Cancer 2009, 115, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Kwon, Y.S.; Labib, M.; Foran, D.J.; Singer, E.A. Magnetic resonance imaging as a biomarker for renal cell carcinoma. Dis. Markers 2015, 2015, 648495. [Google Scholar] [CrossRef] [PubMed]
- Schima, W.; Ba-Ssalamah, A.; Goetzinger, P.; Scharitzer, M.; Koelblinger, C. State-of-the-art magnetic resonance imaging of pancreatic cancer. Top. Magn. Reson. Imaging 2007, 18, 421–429. [Google Scholar] [CrossRef]
- Saisho, H.; Yamaguchi, T. Diagnostic imaging for pancreatic cancer: Computed tomography, magnetic resonance imaging, and positron emission tomography. Pancreas 2004, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Srivastava, S.; Pant, M. Brain tumor segmentation and classification from magnetic resonance images: Review of selected methods from 2014 to 2019. Pattern Recognit. Lett. 2020, 131, 244–260. [Google Scholar] [CrossRef]
- Tonarelli, L. Magnetic Resonance Imaging of Brain Tumor; CEwebsource.com: Diepoldsau, Switzerland, 2013. [Google Scholar]
- Hernández, M.L.; Osorio, S.; Florez, K.; Ospino, A.; Díaz, G.M. Abbreviated magnetic resonance imaging in breast cancer: A systematic review of literature. Eur. J. Radiol. Open 2021, 8, 100307. [Google Scholar] [CrossRef]
- Park, J.W.; Jeong, W.G.; Lee, J.E.; Lee, H.-J.; Ki, S.Y.; Lee, B.C.; Kim, H.O.; Kim, S.K.; Heo, S.H.; Lim, H.S.; et al. Pictorial review of mediastinal masses with an emphasis on magnetic resonance imaging. Korean J. Radiol. 2021, 22, 139–154. [Google Scholar] [CrossRef]
- Bak, S.H.; Kim, C.; Kim, C.H.; Ohno, Y.; Lee, H.Y. Magnetic resonance imaging for lung cancer: A state-of-the-art review. Precis. Future Med. 2022, 6, 49–77. [Google Scholar] [CrossRef]
- Xia, L. Auxiliary Diagnosis of Lung Cancer with Magnetic Resonance Imaging Data under Deep Learning. Comput. Math. Methods Med. 2022, 2022, 1994082. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Magnetic resonance imaging for detection of parametrial invasion in cervical cancer: An updated systematic review and meta-analysis of the literature between 2012 and 2016. Eur. Radiol. 2018, 28, 530–541. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, S.; Chen, X.; Wang, Y.; Dong, L.; Liu, Z.; Tian, J.; Wang, M. Radiomics analysis of magnetic resonance imaging improves diagnostic performance of lymph node metastasis in patients with cervical cancer. Radiother. Oncol. 2019, 138, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, S.; Zhang, S.; Wang, M.; Ding, Y.; Fang, J.; Qian, W.; Liu, Z.; Sun, K.; Jin, Y.; et al. Development of a deep learning model to identify lymph node metastasis on magnetic resonance imaging in patients with cervical cancer. JAMA Netw. Open 2020, 3, e2011625. [Google Scholar] [CrossRef]
- Panebianco, V.; Barchetti, F.; de Haas, R.J.; Pearson, R.A.; Kennish, S.J.; Giannarini, G.; Catto, J.W. Improving staging in bladder cancer: The increasing role of multiparametric magnetic resonance imaging. Eur. Urol. Focus 2016, 2, 113–121. [Google Scholar] [CrossRef]
- Green, D.A.; Durand, M.; Gumpeni, N.; Rink, M.; Cha, E.K.; Karakiewicz, P.I.; Scherr, D.S.; Shariat, S.F. Role of magnetic resonance imaging in bladder cancer: Current status and emerging techniques. BJU Int. 2012, 110, 1463–1470. [Google Scholar] [CrossRef]
- Zhao, Y.; Simpson, B.S.; Morka, N.; Freeman, A.; Kirkham, A.; Kelly, D.; Whitaker, H.C.; Emberton, M.; Norris, J.M. Comparison of multiparametric magnetic resonance imaging with prostate-specific membrane antigen positron-emission tomography imaging in primary prostate cancer diagnosis: A systematic review and meta-analysis. Cancers 2022, 14, 3497. [Google Scholar] [CrossRef]
- Emmett, L.; Buteau, J.; Papa, N.; Moon, D.; Thompson, J.; Roberts, M.J.; Rasiah, K.; Pattison, D.A.; Yaxley, J.; Thomas, P.; et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): A prospective multicentre study. Eur. Urol. 2021, 80, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.; Radcliffe, A.; Newcombe, R.; Dallimore, N.; Bourne, M.; Williams, G. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br. J. Surg. 2003, 90, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Akasu, T.; Iinuma, G.; Takawa, M.; Yamamoto, S.; Muramatsu, Y.; Moriyama, N. Accuracy of high-resolution magnetic resonance imaging in preoperative staging of rectal cancer. Ann. Surg. Oncol. 2009, 16, 2787–2794. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, J.; Zhang, Z.; Li, S.; Zhang, X.; Zhou, Y.; Zhang, X.; Lu, Y. Evaluation of rectal cancer circumferential resection margin using faster region-based convolutional neural network in high-resolution magnetic resonance images. Dis. Colon Rectum 2020, 63, 143–151. [Google Scholar] [CrossRef]
- Lu, W.; Jing, H.; Ju-Mei, Z.; Shao-Lin, N.; Fang, C.; Xiao-Ping, Y.; Qiang, L.; Biao, Z.; Su-Yu, Z.; Ying, H. Intravoxel incoherent motion diffusion-weighted imaging for discriminating the pathological response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Sci. Rep. 2017, 7, 8496. [Google Scholar] [CrossRef]
- Lu, B.; Yang, X.; Xiao, X.; Chen, Y.; Yan, X.; Yu, S. Intravoxel incoherent motion diffusion-weighted imaging of primary rectal carcinoma: Correlation with histopathology. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- de Lussanet, Q.G.; Backes, W.H.; Griffioen, A.W.; Padhani, A.R.; Baeten, C.I.; van Baardwijk, A.; Lambin, P.; Beets, G.L.; van Engelshoven, J.M.; Beets-Tan, R.G. Dynamic contrast-enhanced magnetic resonance imaging of radiation therapy-induced microcirculation changes in rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Ciolina, M.; Caruso, D.; De Santis, D.; Zerunian, M.; Rengo, M.; Alfieri, N.; Musio, D.; De Felice, F.; Ciardi, A.; Tombolini, V.; et al. Dynamic contrast-enhanced magnetic resonance imaging in locally advanced rectal cancer: Role of perfusion parameters in the assessment of response to treatment. La Radiol. Medica 2019, 124, 331–338. [Google Scholar] [CrossRef]
- Wen, Z.; Chen, Y.; Yang, X.; Lu, B.; Liu, Y.; Shen, B.; Yu, S. Application of magnetic resonance diffusion kurtosis imaging for distinguishing histopathologic subtypes and grades of rectal carcinoma. Cancer Imaging 2019, 19, 8. [Google Scholar] [CrossRef]
- Hu, F.; Tang, W.; Sun, Y.; Wan, D.; Cai, S.; Zhang, Z.; Grimm, R.; Yan, X.; Fu, C.; Tong, T.; et al. The value of diffusion kurtosis imaging in assessing pathological complete response to neoadjuvant chemoradiation therapy in rectal cancer: A comparison with conventional diffusion-weighted imaging. Oncotarget 2017, 8, 75597–75606. [Google Scholar] [CrossRef]
- Jordan, K.W.; Nordenstam, J.; Lauwers, G.Y.; Rothenberger, D.A.; Alavi, K.; Garwood, M.; Cheng, L.L. Metabolomic characterization of human rectal adenocarcinoma with intact tissue magnetic resonance spectroscopy. Dis. Colon Rectum 2009, 52, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Xie, P.; Yu, L.; Chen, H.; Zheng, J.; Meng, X.; Wan, X. A new magnetic resonance imaging tumour response grading scheme for locally advanced rectal cancer. Br. J. Cancer 2022, 127, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Clough, T.J.; Jiang, L.; Wong, K.-L.; Long, N.J. Ligand design strategies to increase stability of gadolinium-based magnetic resonance imaging contrast agents. Nat. Commun. 2019, 10, 1420. [Google Scholar] [CrossRef]
- Bottrill, M.; Kwok, L.; Long, N.J. Lanthanides in magnetic resonance imaging. Chem. Soc. Rev. 2006, 35, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Fitch, A.A.; Rudisill, S.S.; Harada, G.K.; An, H.S. Magnetic Resonance Imaging Techniques for the Evaluation of the Subaxial Cervical Spine. In Atlas of Spinal Imaging; Elsevier: Amsterdam, The Netherlands, 2022; pp. 75–105. [Google Scholar]
- Lei, Y.; Shu, H.-K.; Tian, S.; Jeong, J.J.; Liu, T.; Shim, H.; Mao, H.; Wang, T.; Jani, A.B.; Curran, W.J.; et al. Magnetic resonance imaging-based pseudo computed tomography using anatomic signature and joint dictionary learning. J. Med. Imaging 2018, 5, 034001. [Google Scholar]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Diagnostic performance of magnetic resonance imaging for the detection of bone metastasis in prostate cancer: A systematic review and meta-analysis. Eur. Urol. 2018, 73, 81–91. [Google Scholar] [CrossRef]
- Fritz, B.; Müller, D.A.; Sutter, R.; Wurnig, M.C.; Wagner, M.W.; Pfirrmann, C.W.; Fischer, M.A. Magnetic resonance imaging–based grading of cartilaginous bone tumors: Added value of quantitative texture analysis. Investig. Radiol. 2018, 53, 663–672. [Google Scholar] [CrossRef]
- Hetland, M.; Østergaard, M.; Stengaard-Pedersen, K.; Junker, P.; Ejbjerg, B.; Jacobsen, S.; Ellingsen, T.; Lindegaard, H.; Pødenphant, J.; Vestergaard, A. Anti-cyclic citrullinated peptide antibodies, 28-joint Disease Activity Score, and magnetic resonance imaging bone oedema at baseline predict 11 years’ functional and radiographic outcome in early rheumatoid arthritis. Scand. J. Rheumatol. 2019, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, M.; Boesen, M. Imaging in rheumatoid arthritis: The role of magnetic resonance imaging and computed tomography. La Radiol. Medica 2019, 124, 1128–1141. [Google Scholar] [CrossRef]
- Kijowski, R.; Gold, G.E. Routine 3D magnetic resonance imaging of joints. J. Magn. Reson. Imaging 2011, 33, 758–771. [Google Scholar] [CrossRef]
- Johnstone, E.; Wyatt, J.J.; Henry, A.M.; Short, S.C.; Sebag-Montefiore, D.; Murray, L.; Kelly, C.G.; McCallum, H.M.; Speight, R. Systematic review of synthetic computed tomography generation methodologies for use in magnetic resonance imaging–only radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Klenk, C.; Gawande, R.; Uslu, L.; Khurana, A.; Qiu, D.; Quon, A.; Donig, J.; Rosenberg, J.; Luna-Fineman, S.; Moseley, M.; et al. Ionising radiation-free whole-body MRI versus 18F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: A prospective, non-randomised, single-centre study. Lancet Oncol. 2014, 15, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, M.; Sapra, A. Magnetic resonance imaging contraindications. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Mohan, J.; Krishnaveni, V.; Guo, Y. A survey on the magnetic resonance image denoising methods. Biomed. Signal Process. Control 2014, 9, 56–69. [Google Scholar] [CrossRef]
- Wells, P.N. Ultrasonic imaging of the human body. Rep. Prog. Phys. 1999, 62, 671. [Google Scholar] [CrossRef]
- Rajamanickam, K. Role of Ultrasonography in Cancer Theranostic Applications. Arch. Intern. Med. Res. 2020, 3, 32–43. [Google Scholar] [CrossRef]
- Nayak, G.; Bolla, V.; Balivada, S.K.; Prabhudev, P. Technological Evolution of Ultrasound Devices: A Review. Int. J. Health Technol. Innov. 2022, 1, 24–32. [Google Scholar]
- Bogani, G.; Chiappa, V.; Lopez, S.; Salvatore, C.; Interlenghi, M.; D’Oria, O.; Giannini, A.; Maggiore, U.L.R.; Chiarello, G.; Palladino, S.; et al. Radiomics and Molecular Classification in Endometrial Cancer (The ROME Study): A Step Forward to a Simplified Precision Medicine. Healthcare 2022, 10, 2464. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, P.R.; Anderson, T.; Sharp, M.; Meagher, S.; McGillivray, T.; McDicken, W.N. Ultrasound B-mode 360/spl deg/tomography in mice. In Proceedings of the IEEE Ultrasonics Symposium, Montreal, QC, Canada, 23–27 August 2004. [Google Scholar]
- Fite, B.Z.; Wang, J.; Ghanouni, P.; Ferrara, K.W. A review of imaging methods to assess ultrasound-mediated ablation. BME Front. 2022, 2022, 9758652. [Google Scholar] [CrossRef]
- Jain, A.; Tiwari, A.; Verma, A.; Jain, S.K. Ultrasound-based triggered drug delivery to tumors. Drug Deliv. Transl. Res. 2018, 8, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Y.; Zhang, T.; Shung, K.K.; Zhu, B. Recent advancements in ultrasound transducer: From material strategies to biomedical applications. BME Front. 2022, 2022, 9764501. [Google Scholar] [CrossRef]
- Shalaby, T.; Gawish, A.; Hamad, H. A Promising Platform of Magnetic Nanofluid and Ultrasonic Treatment for Cancer Hyperthermia Therapy: In Vitro and in Vivo Study. Ultrasound Med. Biol. 2021, 47, 651–665. [Google Scholar] [CrossRef]
- Leighton, T.G. What is ultrasound? Prog. Biophys. Mol. Biol. 2007, 93, 3–83. [Google Scholar] [CrossRef] [PubMed]
- Carovac, A.; Smajlovic, F.; Junuzovic, D. Application of ultrasound in medicine. Acta Inform. Medica 2011, 19, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, O.; Biswas, A.; Bhattacharya, B.B. Bone-cancer assessment and destruction pattern analysis in long-bone X-ray image. J. Digit. Imaging 2019, 32, 300–313. [Google Scholar] [CrossRef]
- Gaál, G.; Maga, B.; Lukács, A. Attention u-net based adversarial architectures for chest x-ray lung segmentation. arXiv 2020, arXiv:2003.10304. [Google Scholar]
- Bradley, S.H.; Abraham, S.; Callister, M.E.; Grice, A.; Hamilton, W.T.; Lopez, R.R.; Shinkins, B.; Neal, R.D. Sensitivity of chest X-ray for detecting lung cancer in people presenting with symptoms: A systematic review. Br. J. Gen. Pract. 2019, 69, e827–e835. [Google Scholar] [CrossRef]
- Foley, R.W.; Nassour, V.; Oliver, H.C.; Hall, T.; Masani, V.; Robinson, G.; Rodrigues, J.C.; Hudson, B.J. Chest X-ray in suspected lung cancer is harmful. Eur. Radiol. 2021, 31, 6269–6274. [Google Scholar] [CrossRef] [PubMed]
- Gang, P.; Zhen, W.; Zeng, W.; Gordienko, Y.; Kochura, Y.; Alienin, O.; Rokovyi, O.; Stirenko, S. Dimensionality reduction in deep learning for chest X-ray analysis of lung cancer. In Proceedings of the 2018 Tenth International Conference on Advanced Computational Intelligence (ICACI), Xiamen, China, 29–31 March 2018. [Google Scholar]
- Gordienko, Y.; Gang, P.; Hui, J.; Zeng, W.; Kochura, Y.; Alienin, O.; Rokovyi, O.; Stirenko, S. Deep learning with lung segmentation and bone shadow exclusion techniques for chest X-ray analysis of lung cancer. In Proceedings of the International Conference on Computer Science, Engineering and Education Applications, Kiev, Ukraine, 18–20 January 2018; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Lu, L.; Sun, M.; Lu, Q.; Wu, T.; Huang, B. High energy X-ray radiation sensitive scintillating materials for medical imaging, cancer diagnosis and therapy. Nano Energy 2021, 79, 105437. [Google Scholar] [CrossRef]
- Zeng, F.; Zhu, Z. Design of X-ray energy detector. Energy Rep. 2022, 8, 456–460. [Google Scholar] [CrossRef]
- Preshlock, S.; Tredwell, M.; Gouverneur, V. 18F-Labeling of arenes and heteroarenes for applications in positron emission tomography. Chem. Rev. 2016, 116, 719–766. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, S.S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2002, 2, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, D.; Weder, W.; Hany, T.F.; Kamel, E.M.; Korom, S.; Seifert, B.; von Schulthess, G.K.; Steinert, H.C. Staging of non–small-cell lung cancer with integrated positron-emission tomography and computed tomography. N. Engl. J. Med. 2003, 348, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Anttinen, M.; Ettala, O.; Malaspina, S.; Jambor, I.; Sandell, M.; Kajander, S.; Rinta-Kiikka, I.; Schildt, J.; Saukko, E.; Rautio, P.; et al. A Prospective Comparison of 18F-prostate-specific Membrane Antigen-1007 Positron Emission Tomography Computed Tomography, Whole-body 1.5 T Magnetic Resonance Imaging with Diffusion-weighted Imaging, and Single-photon Emission Computed Tomography/Computed Tomography with Traditional Imaging in Primary Distant Metastasis Staging of Prostate Cancer (PROSTAGE). Eur. Urol. Oncol. 2021, 4, 635–644. [Google Scholar] [PubMed]
- Garg, P.K.; Singh, S.K.; Prakash, G.; Jakhetiya, A.; Pandey, D. Role of positron emission tomography-computed tomography in non-small cell lung cancer. World J. Methodol. 2016, 6, 105–111. [Google Scholar] [CrossRef]
- Czernin, J.; Phelps, M.E. Positron emission tomography scanning: Current and future applications. Annu. Rev. Med. 2002, 53, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Phelps, M.E. Positron emission tomography provides molecular imaging of biological processes. Proc. Natl. Acad. Sci. USA 2000, 97, 9226–9233. [Google Scholar] [CrossRef]
- Cherry, S.R. Fundamentals of positron emission tomography and applications in preclinical drug development. J. Clin. Pharmacol. 2001, 41, 482–491. [Google Scholar] [CrossRef]
- Van der Laak, J.; Litjens, G.; Ciompi, F. Deep learning in histopathology: The path to the clinic. Nat. Med. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Arevalo, J.; Cruz-Roa, A.; González, O.F.A. Histopathology image representation for automatic analysis: A state-of-the-art review. Rev. Med. 2014, 22, 79–91. [Google Scholar] [CrossRef]
- Gurcan, M.N.; Boucheron, L.E.; Can, A.; Madabhushi, A.; Rajpoot, N.M.; Yener, B. Histopathological image analysis: A review. IEEE Rev. Biomed. Eng. 2009, 2, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Dabeer, S.; Khan, M.M.; Islam, S. Cancer diagnosis in histopathological image: CNN based approach. Inform. Med. Unlocked 2019, 16, 100231. [Google Scholar] [CrossRef]
- Das, A.; Nair, M.S.; Peter, S.D. Computer-aided histopathological image analysis techniques for automated nuclear atypia scoring of breast cancer: A review. J. Digit. Imaging 2020, 33, 1091–1121. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Liu, X.; Zeng, N.; Liu, Y.; Alsaadi, F.E. A survey of deep neural network architectures and their applications. Neurocomputing 2017, 234, 11–26. [Google Scholar] [CrossRef]
- Raiko, T.; Valpola, H.; Lecun, Y. Deep Learning Made Easier by Linear Transformations in Perceptrons. In Proceedings of the Fifteenth International Conference on Artificial Intelligence and Statistics, La Palma, Canary Islands, 21–23 April 2012; Neil, D.L., Mark, G., Eds.; PMLR, Proceedings of Machine Learning Research: Cambridge, MA, USA, 2012; pp. 924–932. [Google Scholar]
- Lecun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef]
- Shelhamer, E.; Long, J.; Darrell, T. Fully Convolutional Networks for Semantic Segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning Representations by Back Propagating Errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Pang, S.; Yang, X. Deep convolutional extreme learning machine and its application in handwritten digit classification. Comput. Intell. Neurosci. 2016, 2016, 3049632. [Google Scholar] [CrossRef] [PubMed]
- Elman, J.L. Finding Structure in Time. Cogn. Sci. 1990, 14, 179–211. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I.J.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative Adversarial Networks. arXiv 2014, arXiv:1406.2661. [Google Scholar] [CrossRef]
- Hinton, G.E.; Osindero, S.; Teh, Y.-W. A fast learning algorithm for deep belief nets. Neural Comput. 2006, 18, 1527–1554. [Google Scholar] [CrossRef]
- Salakhutdinov, R.; Hinton, G.E. Deep Boltzmann Machines. J. Mach. Learn. Res. 2009, 5, 1967–2006. [Google Scholar]
- Tajbakhsh, N.; Shin, J.Y.; Gurudu, S.R.; Hurst, R.T.; Kendall, C.B.; Gotway, M.B.; Liang, J. Convolutional Neural Networks for Medical Image Analysis: Full Training or Fine Tuning? IEEE Trans. Med. Imaging 2016, 35, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.M.; Majid, M.; Qayyum, A.; Awais, M.; Alnowami, M.; Khan, M.K. Medical Image Analysis using Convolutional Neural Networks: A Review. J. Med. Syst. 2018, 42, 226. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, W.; Lin, Z.; Zhu, W.; Zhou, J.; Wong, J.; Ding, Z. Brain tumor grading based on Neural Networks and Convolutional Neural Networks. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015. [Google Scholar]
- Abiyev, R.H.; Ma’aitah, M.K.S. Deep Convolutional Neural Networks for Chest Diseases Detection. J. Healthc. Eng. 2018, 2018, 4168538. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Into Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, J.; Dong, W.; Yu, J.; Xie, C.; Li, R.; Chen, T.; Chen, H. A crop pests image classification algorithm based on deep convolutional neural network. TELKOMNIKA (Telecommun. Comput. Electron. Control) 2017, 15, 1239–1246. [Google Scholar] [CrossRef]
- Ben-Cohen, A.; Diamant, I.; Klang, E.; Amitai, M.; Greenspan, H. Fully Convolutional Network for Liver Segmentation and Lesions Detection. In Deep Learning and Data Labeling for Medical Applications; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Li, S.; Zhao, X.; Zhou, G. Automatic pixel-level multiple damage detection of concrete structure using fully convolutional network. Comput.-Aided Civ. Infrastruct. Eng. 2019, 34, 616–634. [Google Scholar] [CrossRef]
- Bi, L.; Feng, D.; Kim, J. Dual-Path Adversarial Learning for Fully Convolutional Network (FCN)-Based Medical Image Segmentation. Vis. Comput. 2018, 34, 1043–1052. [Google Scholar] [CrossRef]
- Huang, L.; Xia, W.; Zhang, B.; Qiu, B.; Gao, X. MSFCN-multiple supervised fully convolutional networks for the osteosarcoma segmentation of CT images. Comput. Methods Programs Biomed. 2017, 143, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Jiang, H.; Zhao, H.; Wang, F. A novel deep autoencoder feature learning method for rotating machinery fault diagnosis. Mech. Syst. Signal Process. 2017, 95, 187–204. [Google Scholar] [CrossRef]
- Yousuff, M.; Babu, R. Deep autoencoder based hybrid dimensionality reduction approach for classification of SERS for melanoma cancer diagnostics. J. Intell. Fuzzy Syst. 2022; preprint. [Google Scholar] [CrossRef]
- Suk, H.-I.; Lee, S.-W.; Shen, D. Latent feature representation with stacked auto-encoder for AD/MCI diagnosis. Brain Struct. Funct. 2015, 220, 841–859. [Google Scholar] [CrossRef]
- Ng, A. Sparse autoencoder. CS294A Lect. Notes 2011, 72, 1–19. [Google Scholar]
- Vincent, P.; Larochelle, H.; Lajoie, I.; Bengio, Y.; Manzagol, P.-A.; Bottou, L. Stacked denoising autoencoders: Learning useful representations in a deep network with a local denoising criterion. J. Mach. Learn. Res. 2010, 11, 3371–3408. [Google Scholar]
- Kingma, D.P.; Welling, M. Auto-encoding variational bayes. arXiv 2013, arXiv:1312.6114. [Google Scholar]
- Rifai, S.; Vincent, P.; Muller, X.; Glorot, X.; Bengio, Y. Contractive auto-encoders: Explicit invariance during feature extraction. In Proceedings of the 28th International Conference on International Conference on Machine Learning, Bellevue WA, USA, 28 June–2 July 2011; Omnipress2600 Anderson: St. Madison, WI, USA, 2011. [Google Scholar]
- Xu, J.; Xiang, L.; Liu, Q.; Gilmore, H.; Wu, J.; Tang, J.; Madabhushi, A. Stacked sparse autoencoder (SSAE) for nuclei detection on breast cancer histopathology images. IEEE Trans. Med. Imaging 2015, 35, 119–130. [Google Scholar] [CrossRef]
- Abraham, B.; Nair, M.S. Computer-aided diagnosis of clinically significant prostate cancer from MRI images using sparse autoencoder and random forest classifier. Biocybern. Biomed. Eng. 2018, 38, 733–744. [Google Scholar] [CrossRef]
- Huang, G.; Wang, H.; Zhang, L. Sparse-Coding-Based Autoencoder and Its Application for Cancer Survivability Prediction. Math. Probl. Eng. 2022, 2022, 8544122. [Google Scholar] [CrossRef]
- Munir, M.A.; Aslam, M.A.; Shafique, M.; Ahmed, R.; Mehmood, Z. Deep stacked sparse autoencoders-a breast cancer classifier. Mehran Univ. Res. J. Eng. Technol. 2022, 41, 41–52. [Google Scholar] [CrossRef]
- Bengio, Y.; Lamblin, P.; Popovici, D.; Larochelle, H. Greedy layer-wise training of deep networks. In Advances in Neural Information Processing Systems 19 (NIPS 2006); The MIT Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Vincent, P.; Larochelle, H.; Bengio, Y.; Manzagol, P.-A. Extracting and composing robust features with denoising autoencoders. In Proceedings of the 25th International Conference on Machine Learning, Helsinki, Finland, 5–9 July 2008; Association for Computing Machinery: New York, NY, USA, 2008. [Google Scholar]
- Masci, J.; Meier, U.; Cireşan, D.; Schmidhuber, J. Stacked convolutional auto-encoders for hierarchical feature extraction. In Artificial Neural Networks and Machine Learning–ICANN 2011, Proceedings of the 21st International Conference on Artificial Neural Networks, Espoo, Finland, 14–17 June 2011, Proceedings, Part I 21; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Srivastava, N.; Hinton, G.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R. Dropout: A simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 2014, 15, 1929–1958. [Google Scholar]
- Srivastava, N.; Mansimov, E.; Salakhudinov, R. Unsupervised learning of video representations using lstms. In Proceedings of the International Conference on Machine Learning, Lille, France, 6–11 July 2015; PMLR: Cambridge, MA, USA, 2015. [Google Scholar]
- Makhzani, A.; Shlens, J.; Jaitly, N.; Goodfellow, I.; Frey, B. Adversarial autoencoders. arXiv 2015, arXiv:1511.05644. [Google Scholar]
- Higgins, I.; Matthey, L.; Pal, A.; Burgess, C.; Glorot, X.; Botvinick, M.; Mohamed, S.; Lerchner, A. Beta-vae: Learning basic visual concepts with a constrained variational framework. In Proceedings of the International Conference on Learning Representations, Toulon, France, 24–26 April 2017. [Google Scholar]
- Zhao, S.; Song, J.; Ermon, S. Infovae: Information maximizing variational autoencoders. arXiv 2017, arXiv:1706.02262. [Google Scholar]
- Van Den Oord, A.; Vinyals, O. Neural discrete representation learning. In Advances in Neural Information Processing Systems 30; The MIT Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Dupont, E. Learning disentangled joint continuous and discrete representations. In Advances in Neural Information Processing Systems 31; The MIT Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Kim, H.; Mnih, A. Disentangling by factorising. In Proceedings of the International Conference on Machine Learning, Stockholm, Sweden, 10–15 July 2018; PMLR: Cambridge, MA, USA, 2018. [Google Scholar]
- Huang, G.-B.; Zhu, Q.-Y.; Siew, C.-K. Extreme learning machine: Theory and applications. Neurocomputing 2006, 70, 489–501. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Hao, Y. A method combining CNN and ELM for feature extraction and classification of SAR image. J. Sens. 2019, 2019, 6134610. [Google Scholar] [CrossRef]
- Huang, G.-B.; Chen, L.; Siew, C.K. Universal approximation using incremental constructive feedforward networks with random hidden nodes. IEEE Trans. Neural Netw. 2006, 17, 879–892. [Google Scholar] [CrossRef]
- Niu, X.-X.; Suen, C.Y. A novel hybrid CNN–SVM classifier for recognizing handwritten digits. Pattern Recognit. 2012, 45, 1318–1325. [Google Scholar] [CrossRef]
- Chen, L.; Pan, X.; Zhang, Y.-H.; Liu, M.; Huang, T.; Cai, Y.-D. Classification of widely and rarely expressed genes with recurrent neural network. Comput. Struct. Biotechnol. J. 2019, 17, 49–60. [Google Scholar] [CrossRef]
- Aher, C.N.; Jena, A.K. Rider-chicken optimization dependent recurrent neural network for cancer detection and classification using gene expression data. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2021, 9, 174–191. [Google Scholar] [CrossRef]
- Navaneethakrishnan, M.; Vairamuthu, S.; Parthasarathy, G.; Cristin, R. Atom search-Jaya-based deep recurrent neural network for liver cancer detection. IET Image Process. 2021, 15, 337–349. [Google Scholar] [CrossRef]
- Yan, R.; Ren, F.; Wang, Z.; Wang, L.; Zhang, T.; Liu, Y.; Rao, X.; Zheng, C.; Zhang, F. Breast cancer histopathological image classification using a hybrid deep neural network. Methods 2020, 173, 52–60. [Google Scholar] [CrossRef]
- Moitra, D.; Mandal, R.K. Prediction of Non-small Cell Lung Cancer Histology by a Deep Ensemble of Convolutional and Bidirectional Recurrent Neural Network. J. Digit. Imaging 2020, 33, 895–902. [Google Scholar] [CrossRef]
- Selvanambi, R.; Natarajan, J.; Karuppiah, M.; Islam, S.; Hassan, M.M.; Fortino, G. Lung cancer prediction using higher-order recurrent neural network based on glowworm swarm optimization. Neural Comput. Appl. 2020, 32, 4373–4386. [Google Scholar] [CrossRef]
- Yang, Y.; Fasching, P.A.; Tresp, V. Predictive Modeling of Therapy Decisions in Metastatic Breast Cancer with Recurrent Neural Network Encoder and Multinomial Hierarchical Regression Decoder. In Proceedings of the 2017 IEEE International Conference on Healthcare Informatics (ICHI), Park City, UT, USA, 23–26 August 2017. [Google Scholar]
- Moitra, D.; Mandal, R.K. Automated AJCC staging of non-small cell lung cancer (NSCLC) using deep convolutional neural network (CNN) and recurrent neural network (RNN). Health Inf. Sci. Syst. 2019, 7, 14. [Google Scholar] [CrossRef]
- Pan, Q.; Zhang, Y.; Chen, D.; Xu, G. Character-Based Convolutional Grid Neural Network for Breast Cancer Classification. In Proceedings of the 2017 International Conference on Green Informatics (ICGI), Fuzhou, China, 15–17 August 2017. [Google Scholar]
- Liu, S.; Li, T.; Ding, H.; Tang, B.; Wang, X.; Chen, Q.; Yan, J.; Zhou, Y. A hybrid method of recurrent neural network and graph neural network for next-period prescription prediction. Int. J. Mach. Learn. Cybern. 2020, 11, 2849–2856. [Google Scholar] [CrossRef]
- Nurtiyasari, D.; Rosadi, D.; Abdurakhman. The application of Wavelet Recurrent Neural Network for lung cancer classification. In Proceedings of the 2017 3rd International Conference on Science and Technology-Computer (ICST), Yogyakarta, Indonesia, 11–12 July 2017. [Google Scholar]
- Tng, S.S.; Le, N.Q.K.; Yeh, H.-Y.; Chua, M.C.H. Improved prediction model of protein lysine Crotonylation sites using bidirectional recurrent neural networks. J. Proteome Res. 2021, 21, 265–273. [Google Scholar] [CrossRef]
- Azizi, S.; Bayat, S.; Yan, P.; Tahmasebi, A.; Kwak, J.T.; Xu, S.; Turkbey, B.; Choyke, P.; Pinto, P.; Wood, B.; et al. Deep recurrent neural networks for prostate cancer detection: Analysis of temporal enhanced ultrasound. IEEE Trans. Med. Imaging 2018, 37, 2695–2703. [Google Scholar] [CrossRef] [PubMed]
- SivaSai, J.G.; Srinivasu, P.N.; Sindhuri, M.N.; Rohitha, K.; Deepika, S. An Automated segmentation of brain MR image through fuzzy recurrent neural network. In Bio-Inspired Neurocomputing; Springer: Berlin/Heidelberg, Germany, 2021; pp. 163–179. [Google Scholar]
- Bengio, Y.; Simard, P.; Frasconi, P. Learning long-term dependencies with gradient descent is difficult. IEEE Trans. Neural Netw. 1994, 5, 157–166. [Google Scholar] [CrossRef]
- Gers, F.A.; Schmidhuber, J.; Cummins, F. Learning to forget: Continual prediction with LSTM. Neural Comput. 2000, 12, 2451–2471. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Tang, Y.; Xu, K.; Huo, Y.; Bao, S.; Antic, S.L.; Epstein, E.S.; Deppen, S.; Paulson, A.B.; Sandler, K.L.; et al. Time-distanced gates in long short-term memory networks. Med. Image Anal. 2020, 65, 101785. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, H.-Y.; Shi, P.; Sun, R.; Wang, X.; Luo, Z.; Zeng, F.; Lebowitz, M.S.; Lin, W.-Y.; Lu, J.-J.; et al. Long short-term memory model—A deep learning approach for medical data with irregularity in cancer predication with tumor markers. Comput. Biol. Med. 2022, 144, 105362. [Google Scholar] [CrossRef]
- Elsheikh, A.; Yacout, S.; Ouali, M.S. Bidirectional handshaking LSTM for remaining useful life prediction. Neurocomputing 2019, 323, 148–156. [Google Scholar] [CrossRef]
- Koo, K.C.; Lee, K.S.; Kim, S.; Min, C.; Min, G.R.; Lee, Y.H.; Han, W.K.; Rha, K.H.; Hong, S.J.; Yang, S.C.; et al. Long short-term memory artificial neural network model for prediction of prostate cancer survival outcomes according to initial treatment strategy: Development of an online decision-making support system. World J. Urol. 2020, 38, 2469–2476. [Google Scholar] [CrossRef]
- Creswell, A.; White, T.; Dumoulin, V.; Arulkumaran, K.; Sengupta, B.; Bharath, A.A. Generative Adversarial Networks: An Overview. IEEE Signal Process. Mag. 2017, 35, 53–65. [Google Scholar] [CrossRef]
- Gonzalez-Abril, L.; Angulo, C.; Ortega, J.-A.; Lopez-Guerra, J.-L. Generative Adversarial Networks for Anonymized Healthcare of Lung Cancer Patients. Electronics 2021, 10, 2220. [Google Scholar] [CrossRef]
- Hua, Y.; Guo, J.; Zhao, H. Deep belief networks and deep learning. In Proceedings of the 2015 International Conference on Intelligent Computing and Internet of Things, Harbin, China, 17–18 January 2015. [Google Scholar]
- Xing, Y.; Yue, J.; Chen, C.; Xiang, Y.; Chen, Y.; Shi, M. A deep belief network combined with modified grey wolf optimization algorithm for PM2.5 concentration prediction. Appl. Sci. 2019, 9, 3765. [Google Scholar] [CrossRef]
- Hinton, G.E. Deep belief networks. Scholarpedia 2009, 4, 5947. [Google Scholar] [CrossRef]
- Mohamed, A.-R.; Dahl, G.E.; Hinton, G. Acoustic modeling using deep belief networks. IEEE Trans. Audio Speech Lang. Process. 2011, 20, 14–22. [Google Scholar] [CrossRef]
- Zhang, C.; Lim, P.; Qin, A.K.; Tan, K.C. Multiobjective Deep Belief Networks Ensemble for Remaining Useful Life Estimation in Prognostics. IEEE Trans. Neural Netw. Learn. Syst. 2017, 28, 2306–2318. [Google Scholar] [CrossRef]
- Novitasari, D.C.R.; Foeady, A.Z.; Thohir, M.; Arifin, A.Z.; Niam, K.; Asyhar, A.H. Automatic Approach for Cervical Cancer Detection Based on Deep Belief Network (DBN) Using Colposcopy Data. In Proceedings of the 2020 International Conference on Artificial Intelligence in Information and Communication (ICAIIC), Fukuoka, Japan, 19–21 February 2020. [Google Scholar]
- Ronoud, S.; Asadi, S. An evolutionary deep belief network extreme learning-based for breast cancer diagnosis. Soft Comput. 2019, 23, 13139–13159. [Google Scholar] [CrossRef]
- Eslami, S.; Heess, N.; Williams, C.K.; Winn, J. The shape boltzmann machine: A strong model of object shape. Int. J. Comput. Vis. 2014, 107, 155–176. [Google Scholar] [CrossRef]
- Wu, J.; Mazur, T.R.; Ruan, S.; Lian, C.; Daniel, N.; Lashmett, H.; Ochoa, L.; Zoberi, I.; Anastasio, M.A.; Gach, H.M.; et al. A deep Boltzmann machine-driven level set method for heart motion tracking using cine MRI images. Med. Image Anal. 2018, 47, 68–80. [Google Scholar] [CrossRef]
- Syafiandini, A.F.; Wasito, I.; Yazid, S.; Fitriawan, A.; Amien, M. Multimodal Deep Boltzmann Machines for feature selection on gene expression data. In Proceedings of the 2016 International Conference on Advanced Computer Science and Information Systems (ICACSIS), Malang, Indonesia, 15–16 October 2016. [Google Scholar]
- Hess, M.; Lenz, S.; Binder, H. A deep learning approach for uncovering lung cancer immunome patterns. bioRxiv 2018, 291047. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Hong, Q.-Q.; Teku, R.; Wang, S.-H.; Zhang, Y.-D. Transfer learning for medical images analyses: A survey. Neurocomputing 2022, 489, 230–254. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G. ImageNet Classification with Deep Convolutional Neural Networks. In Advances in Neural Information Processing Systems 25; The MIT Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Zeiler, M.D.; Fergus, R. Visualizing and understanding convolutional networks. In Proceedings of the European Conference on Computer Vision, Zurich, Switzerland, 6–12 September 2014; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015, Proceedings of the 18th International Conference, Munich, Germany, 5–9 October 2015, Proceedings, Part III 18; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Howard, A.G.; Zhu, M.; Chen, B.; Kalenichenko, D.; Wang, W.; Weyand, T.; Andreetto, M.; Adam, H. Mobilenets: Efficient convolutional neural networks for mobile vision applications. arXiv 2017, arXiv:1704.04861. [Google Scholar]
- Iandola, F.N.; Han, S.; Moskewicz, M.W.; Ashraf, K.; Dally, W.J.; Keutzer, K. SqueezeNet: AlexNet-level accuracy with 50x fewer parameters and <0.5 MB model size. arXiv 2016, arXiv:1602.07360. [Google Scholar]
- Redmon, J. Darknet: Open Source Neural Networks in C. 2013–2016. 2016. Available online: http://pjreddie.com/darknet/ (accessed on 20 April 2023).
- Huang, G.; Liu, Z.; Van Der Maaten, L.; Weinberger, K.Q. Densely connected convolutional networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the inception architecture for computer vision. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Szegedy, C.; Ioffe, S.; Vanhoucke, V.; Alemi, A. Inception-v4, inception-resnet and the impact of residual connections on learning. In Proceedings of the AAAI Conference on Artificial Intelligence, San Francisco, CA, USA, 4–9 February 2017. [Google Scholar]
- Zhang, X.; Zhou, X.; Lin, M.; Sun, J. Shufflenet: An extremely efficient convolutional neural network for mobile devices. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018. [Google Scholar]
- Zoph, B.; Vasudevan, V.; Shlens, J.; Le, Q.V. Learning transferable architectures for scalable image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018. [Google Scholar]
- Tan, M.; Le, Q. Efficientnet: Rethinking model scaling for convolutional neural networks. In Proceedings of the International Conference on Machine Learning, Long Beach, CA, USA, 9–15 June 2019. [Google Scholar]
- Tian, Y. Artificial Intelligence Image Recognition Method Based on Convolutional Neural Network Algorithm. IEEE Access 2020, 8, 125731–125744. [Google Scholar] [CrossRef]
- Sun, Y.; Ou, Z.; Chen, J.; Qi, X.; Guo, Y.; Cai, S.; Yan, X. Evaluating Performance, Power and Energy of Deep Neural Networks on CPUs and GPUs. In Theoretical Computer Science. NCTCS 2021. Communications in Computer and Information Science; Springer: Singapore, 2021. [Google Scholar]
- Samir, S.; Emary, E.; El-Sayed, K.; Onsi, H. Optimization of a Pre-Trained AlexNet Model for Detecting and Localizing Image Forgeries. Information 2020, 11, 275. [Google Scholar] [CrossRef]
- Li, M.; Tang, H.; Chan, M.D.; Zhou, X.; Qian, X. DC-AL GAN: Pseudoprogression and true tumor progression of glioblastoma multiform image classification based on DCGAN and AlexNet. Med. Phys. 2020, 47, 1139–1150. [Google Scholar] [CrossRef]
- Suryawati, E.; Sustika, R.; Yuwana, R.S.; Subekti, A.; Pardede, H.F. Deep structured convolutional neural network for tomato diseases detection. In Proceedings of the 2018 International Conference on Advanced Computer Science and Information Systems (ICACSIS), Yogyakarta, Indonesia, 27–28 October 2018. [Google Scholar]
- Lv, X.; Zhang, X.; Jiang, Y.; Zhang, J. Pedestrian Detection Using Regional Proposal Network with Feature Fusion. In Proceedings of the 2018 Eighth International Conference on Image Processing Theory, Tools and Applications (IPTA), Xi’an, China, 7–10 November 2018. [Google Scholar]
- Zou, Z.; Wang, N.; Zhao, P.; Zhao, X. Feature recognition and detection for ancient architecture based on machine vision. In Smart Structures and NDE for Industry 4.0; SPIE: Bellingham, WA, USA, 2018. [Google Scholar]
- Yu, S.; Liu, J.; Shu, H.; Cheng, Z. Handwritten Digit Recognition using Deep Learning Networks. In Proceedings of the 2022 IEEE Conference on Telecommunications, Optics and Computer Science (TOCS), Dalian, China, 11–12 December 2022. [Google Scholar]
- Cai, L.; Gao, J.; Zhao, D. A review of the application of deep learning in medical image classification and segmentation. Ann. Transl. Med. 2020, 8, 713. [Google Scholar] [CrossRef]
- Li, G.; Shen, X.; Li, J.; Wang, J. Diagonal-kernel convolutional neural networks for image classification. Digit. Signal Process. 2021, 108, 102898. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Wang, X.; Wang, W.; Li, J. Development of convolutional neural network and its application in image classification: A survey. Opt. Eng. 2019, 58, 040901. [Google Scholar] [CrossRef]
- Li, H.; Zhuang, S.; Li, D.-A.; Zhao, J.; Ma, Y. Benign and malignant classification of mammogram images based on deep learning. Biomed. Signal Process. Control 2019, 51, 347–354. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Luo, J.-H.; Wei, X.-S.; Wu, J. In defense of fully connected layers in visual representation transfer. In Proceedings of the Pacific Rim Conference on Multimedia, Harbin, China, 28–29 September 2017; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Attallah, O.; Zaghlool, S. AI-based pipeline for classifying pediatric medulloblastoma using histopathological and textural images. Life 2022, 12, 232. [Google Scholar] [CrossRef]
- Yao, X.; Wang, X.; Wang, S.-H.; Zhang, Y.-D. A comprehensive survey on convolutional neural network in medical image analysis. Multimed. Tools Appl. 2020, 81, 41361–41405. [Google Scholar] [CrossRef]
- Yu, X.; Yu, Z.; Ramalingam, S. Learning strict identity mappings in deep residual networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018. [Google Scholar]
- Zagoruyko, S.; Komodakis, N. Wide residual networks. arXiv 2016, arXiv:1605.07146. [Google Scholar]
- Pleiss, G.; Chen, D.; Huang, G.; Li, T.; Van Der Maaten, L.; Weinberger, K.Q. Memory-efficient implementation of densenets. arXiv 2017, arXiv:1707.06990. [Google Scholar]
- Huang, G.; Liu, Z.; Pleiss, G.; Maaten, L.V.D.; Weinberger, K.Q. Convolutional Networks with Dense Connectivity. IEEE Trans. Pattern Anal. Mach. Intell. 2022, 44, 8704–8716. [Google Scholar] [CrossRef] [PubMed]
- Sae-Lim, W.; Wettayaprasit, W.; Aiyarak, P. Convolutional neural networks using MobileNet for skin lesion classification. In Proceedings of the 2019 16th International Joint Conference on Computer Science and Software Engineering (JCSSE), Chonburi, Thailand, 10–12 July 2019. [Google Scholar]
- Tseng, F.-H.; Yeh, K.-H.; Kao, F.-Y.; Chen, C.-Y. MiniNet: Dense squeeze with depthwise separable convolutions for image classification in resource-constrained autonomous systems. ISA Trans. 2022, 132, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, J.; Duan, Y.; Fu, B.; Kang, J.-R.; Shi, Y. MobileNet based apple leaf diseases identification. Mob. Netw. Appl. 2022, 27, 172–180. [Google Scholar] [CrossRef]
- Dhouibi, M.; Salem, A.K.B.; Saidi, A.; Saoud, S.B. Accelerating deep neural networks implementation: A survey. IET Comput. Digit. Tech. 2021, 15, 79–96. [Google Scholar] [CrossRef]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L.-C. Mobilenetv2: Inverted residuals and linear bottlenecks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018. [Google Scholar]
- Howard, A.; Sandler, M.; Chu, G.; Chen, L.-C.; Chen, B.; Tan, M.; Wang, W.; Zhu, Y.; Pang, R.; Vasudevan, V. Searching for mobilenetv3. In Proceedings of the IEEE/CVF International Conference on Computer Vision, Seoul, Republic of Korea, 27 October–2 November 2019. [Google Scholar]
- Attallah, O.; Anwar, F.; Ghanem, N.M.; Ismail, M.A. Histo-CADx: Duo cascaded fusion stages for breast cancer diagnosis from histopathological images. PeerJ Comput. Sci. 2021, 7, e493. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, X.; Zheng, H.-T.; Sun, J. Shufflenet v2: Practical guidelines for efficient cnn architecture design. In Proceedings of the European Conference on Computer Vision (ECCV), Munich, Germany, 8–14 September 2018. [Google Scholar]
- Obayya, M.; Maashi, M.S.; Nemri, N.; Mohsen, H.; Motwakel, A.; Osman, A.E.; Alneil, A.A.; Alsaid, M.I. Hyperparameter optimizer with deep learning-based decision-support systems for histopathological breast cancer diagnosis. Cancers 2023, 15, 885. [Google Scholar] [CrossRef]
- Chollet, F. Xception: Deep learning with depthwise separable convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- Zhou, T.; Hou, S.; Lu, H.; Zhao, Y.; Dang, P.; Dong, Y. Exploring and analyzing the improvement mechanism of U-Net and its application in medical image segmentation. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi J. Biomed. Eng. Shengwu Yixue Gongchengxue Zazhi 2022, 39, 806–825. [Google Scholar]
- Jégou, S.; Drozdzal, M.; Vazquez, D.; Romero, A.; Bengio, Y. The one hundred layers tiramisu: Fully convolutional densenets for semantic segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition Workshops, Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- AlGhamdi, M.; Abdel-Mottaleb, M.; Collado-Mesa, F. DU-Net: Convolutional Network for the Detection of Arterial Calcifications in Mammograms. IEEE Trans. Med. Imaging 2020, 39, 3240–3249. [Google Scholar] [CrossRef]
- Khanna, A.; Londhe, N.D.; Gupta, S.; Semwal, A. A deep Residual U-Net convolutional neural network for automated lung segmentation in computed tomography images. Biocybern. Biomed. Eng. 2020, 40, 1314–1327. [Google Scholar] [CrossRef]
- Lu, L.; Jian, L.; Luo, J.; Xiao, B. Pancreatic Segmentation via Ringed Residual U-Net. IEEE Access 2019, 7, 172871–172878. [Google Scholar] [CrossRef]
- Lee, S.; Negishi, M.; Urakubo, H.; Kasai, H.; Ishii, S. Mu-net: Multi-scale U-net for two-photon microscopy image denoising and restoration. Neural Netw. 2020, 125, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, W.; Qin, S.; Wang, L. MDFA-Net: Multiscale dual-path feature aggregation network for cardiac segmentation on multi-sequence cardiac MR. Knowl.-Based Syst. 2021, 215, 106776. [Google Scholar] [CrossRef]
- Coupé, P.; Mansencal, B.; Clément, M.; Giraud, R.; de Senneville, B.D.; Ta, V.-T.; Lepetit, V.; Manjon, J.V. AssemblyNet: A large ensemble of CNNs for 3D whole brain MRI segmentation. NeuroImage 2020, 219, 117026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, C.; Coleman, S.; Kerr, D. DENSE-INception U-net for medical image segmentation. Comput. Methods Programs Biomed. 2020, 192, 105395. [Google Scholar] [CrossRef]
- Xu, G.; Cao, H.; Udupa, J.K.; Tong, Y.; Torigian, D.A. DiSegNet: A deep dilated convolutional encoder-decoder architecture for lymph node segmentation on PET/CT images. Comput. Med. Imaging Graph. 2021, 88, 101851. [Google Scholar] [CrossRef]
- Li, J.; Yu, Z.L.; Gu, Z.; Liu, H.; Li, Y. Dilated-inception net: Multi-scale feature aggregation for cardiac right ventricle segmentation. IEEE Trans. Biomed. Eng. 2019, 66, 3499–3508. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, Y.; Chen, W.; Luo, X.; He, Y.; Gao, Y.; Li, F. ANU-Net: Attention-based Nested U-Net to exploit full resolution features for medical image segmentation. Comput. Graph. 2020, 90, 11–20. [Google Scholar] [CrossRef]
- Guo, C.; Szemenyei, M.; Yi, Y.; Wang, W.; Chen, B.; Fan, C. SA-UNet: Spatial attention U-Net for retinal vessel segmentation. In Proceedings of the 2020 25th International Conference on Pattern Recognition (ICPR), Milan, Italy, 10–15 January 2021. [Google Scholar]
- Li, Y.; Yang, J.; Ni, J.; Elazab, A.; Wu, J. TA-Net: Triple attention network for medical image segmentation. Comput. Biol. Med. 2021, 137, 104836. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, S.; Chen, G.; Wang, W.; Lei, B.; Wen, Z. FAT-Net: Feature adaptive transformers for automated skin lesion segmentation. Med. Image Anal. 2022, 76, 102327. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, Ö.; Abdulkadir, A.; Lienkamp, S.S.; Brox, T.; Ronneberger, O. 3D U-Net: Learning dense volumetric segmentation from sparse annotation. In Medical Image Computing and Computer-Assisted Intervention–MICCAI 2016, Proceedings of the 19th International Conference, Athens, Greece, 17–21 October 2016, Proceedings, Part II 19; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Oktay, O.; Schlemper, J.; Folgoc, L.L.; Lee, M.; Heinrich, M.; Misawa, K.; Mori, K.; McDonagh, S.; Hammerla, N.Y.; Kainz, B. Attention u-net: Learning where to look for the pancreas. arXiv 2018, arXiv:1804.03999. [Google Scholar]
- Alom, M.Z.; Hasan, M.; Yakopcic, C.; Taha, T.M.; Asari, V.K. Recurrent residual convolutional neural network based on u-net (r2u-net) for medical image segmentation. arXiv 2018, arXiv:1802.06955. [Google Scholar]
- Zhou, Z.; Siddiquee, M.M.R.; Tajbakhsh, N.; Liang, J. Unet++: A nested u-net architecture for medical image segmentation. In Deep Learning in Medical Image Analysis and Multimodal Learning for Clinical Decision Support, Proceedings of the 4th International Workshop, DLMIA 2018, and 8th International Workshop, ML-CDS 2018, Held in Conjunction with MICCAI 2018, Granada, Spain, 20 September 2018, Proceedings 4; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ibtehaz, N.; Rahman, M.S. MultiResUNet: Rethinking the U-Net architecture for multimodal biomedical image segmentation. Neural Netw. 2020, 121, 74–87. [Google Scholar] [CrossRef]
- Yeung, M.; Sala, E.; Schönlieb, C.-B.; Rundo, L. Focus U-Net: A novel dual attention-gated CNN for polyp segmentation during colonoscopy. Comput. Biol. Med. 2021, 137, 104815. [Google Scholar] [CrossRef]
- Beeche, C.; Singh, J.P.; Leader, J.K.; Gezer, N.S.; Oruwari, A.P.; Dansingani, K.K.; Chhablani, J.; Pu, J. Super U-Net: A modularized generalizable architecture. Pattern Recognit. 2022, 128, 108669. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.; Wang, S.-H.; Zhang, Y.-D. A review of deep learning on medical image analysis. Mob. Netw. Appl. 2021, 26, 351–380. [Google Scholar] [CrossRef]
- Khan, S.; Islam, N.; Jan, Z.; Din, I.U.; Rodrigues, J.J.C. A novel deep learning based framework for the detection and classification of breast cancer using transfer learning. Pattern Recognit. Lett. 2019, 125, 1–6. [Google Scholar] [CrossRef]
- Zhen, X.; Chen, J.; Zhong, Z.; Hrycushko, B.; Zhou, L.; Jiang, S.; Albuquerque, K.; Gu, X. Deep convolutional neural network with transfer learning for rectum toxicity prediction in cervical cancer radiotherapy: A feasibility study. Phys. Med. Biol. 2017, 62, 8246–8263. [Google Scholar] [CrossRef]
- Weiss, K.; Khoshgoftaar, T.M.; Wang, D. A survey of transfer learning. J. Big Data 2016, 3, 9. [Google Scholar] [CrossRef]
- Pan, S.J.; Yang, Q. A survey on transfer learning. IEEE Trans. Knowl. Data Eng. 2010, 22, 1345–1359. [Google Scholar] [CrossRef]
- Zhuang, F.; Qi, Z.; Duan, K.; Xi, D.; Zhu, Y.; Zhu, H.; Xiong, H.; He, Q. A comprehensive survey on transfer learning. Proc. IEEE 2020, 109, 43–76. [Google Scholar] [CrossRef]
- Sagi, O.; Rokach, L. Ensemble learning: A survey. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2018, 8, e1249. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Zhou, Z.-H. Ensemble Learning; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Tsai, C.-F.; Sung, Y.-T. Ensemble feature selection in high dimension, low sample size datasets: Parallel and serial combination approaches. Knowl.-Based Syst. 2020, 203, 106097. [Google Scholar] [CrossRef]
- Breiman, L. Bagging predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hastie, T.; Rosset, S.; Zhu, J.; Zou, H. Multi-class adaboost. Stat. Its Interface 2009, 2, 349–360. [Google Scholar] [CrossRef]
- Friedman, J.H. Stochastic gradient boosting. Comput. Stat. Data Anal. 2002, 38, 367–378. [Google Scholar] [CrossRef]
- Müller, D.; Soto-Rey, I.; Kramer, F. An analysis on ensemble learning optimized medical image classification with deep convolutional neural networks. IEEE Access 2022, 10, 66467–66480. [Google Scholar] [CrossRef]
- Brunese, L.; Mercaldo, F.; Reginelli, A.; Santone, A. An ensemble learning approach for brain cancer detection exploiting radiomic features. Comput. Methods Programs Biomed. 2020, 185, 105134. [Google Scholar] [CrossRef] [PubMed]
- Abdar, M.; Makarenkov, V. CWV-BANN-SVM ensemble learning classifier for an accurate diagnosis of breast cancer. Measurement 2019, 146, 557–570. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Geng, N.; Wang, Y.; Yin, Y.; Jin, Y. Stacking-based ensemble learning of decision trees for interpretable prostate cancer detection. Appl. Soft Comput. 2019, 77, 188–204. [Google Scholar] [CrossRef]
- Scarselli, F.; Gori, M.; Tsoi, A.C.; Hagenbuchner, M.; Monfardini, G. The graph neural network model. IEEE Trans. Neural Netw. 2008, 20, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, X.; Qi, X.; Li, C.-G.; Xiao, R. Learning graph normalization for graph neural networks. Neurocomputing 2022, 493, 613–625. [Google Scholar] [CrossRef]
- Xue, J.; Jiang, N.; Liang, S.; Pang, Q.; Yabe, T.; Ukkusuri, S.V.; Ma, J. Quantifying the spatial homogeneity of urban road networks via graph neural networks. Nat. Mach. Intell. 2022, 4, 246–257. [Google Scholar] [CrossRef]
- Gao, J.; Lyu, T.; Xiong, F.; Wang, J.; Ke, W.; Li, Z. Predicting the survival of cancer patients with multimodal graph neural network. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021, 19, 699–709. [Google Scholar] [CrossRef]
- Schulte-Sasse, R.; Budach, S.; Hnisz, D.; Marsico, A. Integration of multiomics data with graph convolutional networks to identify new cancer genes and their associated molecular mechanisms. Nat. Mach. Intell. 2021, 3, 513–526. [Google Scholar] [CrossRef]
- Baul, S.; Ahmed, K.T.; Filipek, J.; Zhang, W. omicsGAT: Graph Attention Network for Cancer Subtype Analyses. Int. J. Mol. Sci. 2022, 23, 10220. [Google Scholar] [CrossRef]
- Miller, T. Explanation in Artificial Intelligence: Insights from the Social Sciences. Artif. Intell. 2017, 267, 1–38. [Google Scholar] [CrossRef]
- Gulum, M.A.; Trombley, C.M.; Kantardzic, M. A review of explainable deep learning cancer detection models in medical imaging. Appl. Sci. 2021, 11, 4573. [Google Scholar] [CrossRef]
- Angelov, P.; Soares, E. Towards explainable deep neural networks (xDNN). Neural Netw. 2020, 130, 185–194. [Google Scholar] [CrossRef]
- Marmolejo-Saucedo, J.A.; Kose, U. Numerical grad-CAM based explainable convolutional neural network for brain tumor diagnosis. Mob. Netw. Appl. 2022, 1–10. [Google Scholar] [CrossRef]
- Windisch, P.; Weber, P.; Fürweger, C.; Ehret, F.; Kufeld, M.; Zwahlen, D.; Muacevic, A. Implementation of model explainability for a basic brain tumor detection using convolutional neural networks on MRI slices. Neuroradiology 2020, 62, 1515–1518. [Google Scholar] [CrossRef]
- Dosovitskiy, A.; Beyer, L.; Kolesnikov, A.; Weissenborn, D.; Zhai, X.; Unterthiner, T.; Dehghani, M.; Minderer, M.; Heigold, G.; Gelly, S. An image is worth 16x16 words: Transformers for image recognition at scale. arXiv 2020, arXiv:2010.11929. [Google Scholar]
- Lin, S.; Wang, Y.; Zhang, L.; Chu, Y.; Liu, Y.; Fang, Y.; Jiang, M.; Wang, Q.; Zhao, B.; Xiong, Y. MDF-SA-DDI: Predicting drug–drug interaction events based on multi-source drug fusion, multi-source feature fusion and transformer self-attention mechanism. Brief. Bioinform. 2022, 23, bbab421. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention is all you need. In Advances in Neural Information Processing Systems 30; The MIT Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Zeng, Y.; Fu, J.; Chao, H. Learning joint spatial-temporal transformations for video inpainting. In Computer Vision–ECCV 2020, Proceedings of the 16th European Conference, Glasgow, UK, 23–28 August 2020, Proceedings, Part XVI 16; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Fu, Y.; Xu, T.; Wu, X.; Kittler, J. Ppt fusion: Pyramid patch transformerfor a case study in image fusion. arXiv 2021, arXiv:2107.13967. [Google Scholar]
- Hoang, G.M.; Kim, U.-H.; Kim, J.G. Vision transformers for the prediction of mild cognitive impairment to Alzheimer’s disease progression using mid-sagittal sMRI. Front. Aging Neurosci. 2023, 15, 1102869. [Google Scholar] [CrossRef]
- Aladhadh, S.; Alsanea, M.; Aloraini, M.; Khan, T.; Habib, S.; Islam, M. An effective skin cancer classification mechanism via medical vision transformer. Sensors 2022, 22, 4008. [Google Scholar] [CrossRef]
- Ikromjanov, K.; Bhattacharjee, S.; Hwang, Y.-B.; Sumon, R.I.; Kim, H.-C.; Choi, H.-K. Whole slide image analysis and detection of prostate cancer using vision transformers. In Proceedings of the 2022 International Conference on Artificial Intelligence in Information and Communication (ICAIIC), Jeju Island, Republic of Korea, 21–24 February 2022. [Google Scholar]
- Zeid, M.A.-E.; El-Bahnasy, K.; Abo-Youssef, S. Multiclass colorectal cancer histology images classification using vision transformers. In Proceedings of the 2021 Tenth International Conference on Intelligent Computing and Information Systems (ICICIS), Cairo, Egypt, 5–7 December 2021. [Google Scholar]
- Gheflati, B.; Rivaz, H. Vision transformers for classification of breast ultrasound images. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, Scotland, UK, 11–15 July 2022. [Google Scholar]
- Zhou, X.; Tang, C.; Huang, P.; Tian, S.; Mercaldo, F.; Santone, A. ASI-DBNet: An Adaptive Sparse Interactive ResNet-Vision Transformer Dual-Branch Network for the Grading of Brain Cancer Histopathological Images. Interdiscip. Sci. Comput. Life Sci. 2022, 15, 15–31. [Google Scholar] [CrossRef]
- Chen, H.; Li, C.; Wang, G.; Li, X.; Rahaman, M.; Sun, H.; Hu, W.; Li, Y.; Liu, W.; Sun, C. Gashis-transformer: A multi-scale visual transformer approach for gastric histopathology image classification. arXiv 2021, arXiv:2104.14528. [Google Scholar]
- Zhang, T.; Feng, Y.; Feng, Y.; Zhao, Y.; Lei, Y.; Ying, N.; Yan, Z.; He, Y.; Zhang, G. Shuffle Instances-based Vision Transformer for Pancreatic Cancer ROSE Image Classification. arXiv 2022, arXiv:2208.06833. [Google Scholar]
- Ioffe, S.; Szegedy, C. Batch normalization: Accelerating deep network training by reducing internal covariate shift. In Proceedings of the International Conference on Machine Learning, Lille, France, 6–11 July 2015. [Google Scholar]
- Bjorck, N.; Gomes, C.P.; Selman, B.; Weinberger, K.Q. Understanding batch normalization. In Advances in Neural Information Processing Systems 31; The MIT Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Hinton, G.E.; Srivastava, N.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R.R. Improving neural networks by preventing co-adaptation of feature detectors. Comput. Sci. 2012, 3, 212–223. [Google Scholar]
- Wan, L.; Zeiler, M.; Zhang, S.; Le Cun, Y.; Fergus, R. Regularization of neural networks using dropconnect. In Proceedings of the International Conference on Machine Learning, Atlanta, GA, USA, 16–21 June 2013. [Google Scholar]
- Ba, J.; Frey, B. Adaptive dropout for training deep neural networks. In Advances in Neural Information Processing Systems 26; The MIT Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Wang, S.; Manning, C. Fast dropout training. In Proceedings of the International Conference on Machine Learning, Atlanta, GA, USA, 16–21 June 2013; PMLR: Cambridge, MA, USA, 2013. [Google Scholar]
- Kingma, D.P.; Salimans, T.; Welling, M. Variational dropout and the local reparameterization trick. In Advances in Neural Information Processing Systems 28; The MIT Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Go, J.; Baek, B.; Lee, C. Analyzing weight distribution of feedforward neural networks and efficient weight initialization. In Structural, Syntactic, and Statistical Pattern Recognition, Proceedings of the Joint IAPR International Workshops, SSPR 2004 and SPR 2004, Lisbon, Portugal, 18–20 August 2004. Proceedings; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Glorot, X.; Bengio, Y. Understanding the difficulty of training deep feedforward neural networks. In Proceedings of the Thirteenth International Conference on Artificial Intelligence and Statistics, Chia Laguna Resort, Sardinia, Italy, 13–15 May 2010. [Google Scholar]
- Saxe, A.M.; McClelland, J.L.; Ganguli, S. Exact solutions to the nonlinear dynamics of learning in deep linear neural networks. arXiv 2013, arXiv:1312.6120. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Delving deep into rectifiers: Surpassing human-level performance on imagenet classification. In Proceedings of the IEEE International Conference on Computer Vision, Santiago, Chile, 7–13 December 2015. [Google Scholar]
- Krähenbühl, P.; Doersch, C.; Donahue, J.; Darrell, T. Data-dependent initializations of convolutional neural networks. arXiv 2015, arXiv:1511.06856. [Google Scholar]
- Mishkin, D.; Matas, J. All you need is a good init. arXiv 2015, arXiv:1511.06422. [Google Scholar]
- Gray, S.; Radford, A.; Kingma, D.P. Gpu kernels for block-sparse weights. arXiv 2017, arXiv:1711.09224. [Google Scholar]
- Zhang, H.; Dauphin, Y.N.; Ma, T. Fixup initialization: Residual learning without normalization. arXiv 2019, arXiv:1901.09321. [Google Scholar]
- Zhao, J.; Schäfer, F.; Anandkumar, A. ZerO initialization: Initializing neural networks with only zeros and ones. arXiv 2021, arXiv:2110.12661. [Google Scholar]
- DuMont Schütte, A.; Hetzel, J.; Gatidis, S.; Hepp, T.; Dietz, B.; Bauer, S.; Schwab, P. Overcoming barriers to data sharing with medical image generation: A comprehensive evaluation. NPJ Digit. Med. 2021, 4, 141. [Google Scholar] [CrossRef]
- He, Y.; Li, T.; Ge, R.; Yang, J.; Kong, Y.; Zhu, J.; Shu, H.; Yang, G.; Li, S. Few-Shot Learning for Deformable Medical Image Registration With Perception-Correspondence Decoupling and Reverse Teaching. IEEE J. Biomed. Health Inform. 2022, 26, 1177–1187. [Google Scholar] [CrossRef]
- Shorten, C.; Khoshgoftaar, T.M. A survey on image data augmentation for deep learning. J. Big Data 2019, 6, 60. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, W.; Zhang, M.; Guo, S.; Zhao, J.; Shen, F. Image data augmentation for deep learning: A survey. arXiv 2022, arXiv:2204.08610. [Google Scholar]
- Chlap, P.; Min, H.; Vandenberg, N.; Dowling, J.; Holloway, L.; Haworth, A. A review of medical image data augmentation techniques for deep learning applications. J. Med. Imaging Radiat. Oncol. 2021, 65, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, N.E.; Loey, M.; Mirjalili, S. A comprehensive survey of recent trends in deep learning for digital images augmentation. Artif. Intell. Rev. 2022, 55, 2351–2377. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, P.; Zhao, L.; Zhang, Z. Review of data augmentation for image in deep learning. Image Graph. 2021, 26, 487–502. [Google Scholar]
- Tian, Y.; Zhang, Y. A comprehensive survey on regularization strategies in machine learning. Inf. Fusion 2022, 80, 146–166. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Peng, Y.; Bai, L. Learning more robust features with adversarial training. arXiv 2018, arXiv:1804.07757. [Google Scholar]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Commun. ACM 2017, 60, 84–90. [Google Scholar] [CrossRef]
- Haddad, R.A.; Akansu, A.N. A class of fast Gaussian binomial filters for speech and image processing. IEEE Trans. Signal Process. 1991, 39, 723–727. [Google Scholar] [CrossRef]
- Canny, J. A computational approach to edge detection. IEEE Trans. Pattern Anal. Mach. Intell. 1986, 6, 679–698. [Google Scholar] [CrossRef]
- Kang, G.; Dong, X.; Zheng, L.; Yang, Y. Patchshuffle regularization. arXiv 2017, arXiv:1707.07103. [Google Scholar]
- Zhong, Z.; Zheng, L.; Kang, G.; Li, S.; Yang, Y. Random erasing data augmentation. In Proceedings of the AAAI Conference on Artificial Intelligence, Palo Alto, CA, USA, 23–25 March 2020. [Google Scholar]
- DeVries, T.; Taylor, G.W. Improved regularization of convolutional neural networks with cutout. arXiv 2017, arXiv:1708.04552. [Google Scholar]
- Kumar Singh, K.; Lee, Y.J. Hide-and-seek: Forcing a network to be meticulous for weakly-supervised object and action localization. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017. [Google Scholar]
- Chen, P.; Liu, S.; Zhao, H.; Jia, J. Gridmask data augmentation. arXiv 2020, arXiv:2001.04086. [Google Scholar]
- Li, P.; Li, X.; Long, X. Fencemask: A data augmentation approach for pre-extracted image features. arXiv 2020, arXiv:2006.07877. [Google Scholar]
- Zhang, H.; Cisse, M.; Dauphin, Y.N.; Lopez-Paz, D. mixup: Beyond empirical risk minimization. arXiv 2017, arXiv:1710.09412. [Google Scholar]
- Inoue, H. Data augmentation by pairing samples for images classification. arXiv 2018, arXiv:1801.02929. [Google Scholar]
- Tokozume, Y.; Ushiku, Y.; Harada, T. Between-class learning for image classification. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018. [Google Scholar]
- Yun, S.; Han, D.; Oh, S.J.; Chun, S.; Choe, J.; Yoo, Y. Cutmix: Regularization strategy to train strong classifiers with localizable features. In Proceedings of the IEEE/CVF International Conference on Computer Vision, Seoul, Republic of Korea, 27 October–2 November 2019. [Google Scholar]
- Hendrycks, D.; Mu, N.; Cubuk, E.D.; Zoph, B.; Gilmer, J.; Lakshminarayanan, B. Augmix: A simple data processing method to improve robustness and uncertainty. arXiv 2019, arXiv:1912.02781. [Google Scholar]
- Verma, V.; Lamb, A.; Beckham, C.; Najafi, A.; Mitliagkas, I.; Lopez-Paz, D.; Bengio, Y. Manifold mixup: Better representations by interpolating hidden states. In Proceedings of the International Conference on Machine Learning, Long Beach, CA, USA, 9–15 June 2019. [Google Scholar]
- Harris, E.; Marcu, A.; Painter, M.; Niranjan, M.; Prügel-Bennett, A.; Hare, J. Fmix: Enhancing mixed sample data augmentation. arXiv 2020, arXiv:2002.12047. [Google Scholar]
- Lee, J.-H.; Zaheer, M.Z.; Astrid, M.; Lee, S.-I. Smoothmix: A simple yet effective data augmentation to train robust classifiers. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops, Virtual, 14–19 June 2020. [Google Scholar]
- Cheng, Z.; Ren, X.; Juefei-Xu, F.; Xue, W.; Guo, Q.; Ma, L.; Zhao, J. Deepmix: Online auto data augmentation for robust visual object tracking. In Proceedings of the 2021 IEEE International Conference on Multimedia and Expo (ICME), Shenzhen, China, 5–9 July 2021. [Google Scholar]
- Choi, J.; Lee, C.; Lee, D.; Jung, H. SalfMix: A Novel Single Image-Based Data Augmentation Technique Using a Saliency Map. Sensors 2021, 21, 8444. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Barea, F.J.; Strazzera, F.; Jerez, J.M.; Urda, D.; Franco, L. Forward noise adjustment scheme for data augmentation. In Proceedings of the 2018 IEEE Symposium Series on Computational Intelligence (SSCI), Bangalore, India, 18–21 November 2018. [Google Scholar]
- Xie, L.; Wang, J.; Wei, Z.; Wang, M.; Tian, Q. Disturblabel: Regularizing cnn on the loss layer. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- DeVries, T.; Taylor, G.W. Dataset augmentation in feature space. arXiv 2017, arXiv:1702.05538. [Google Scholar]
- Chu, P.; Bian, X.; Liu, S.; Ling, H. Feature space augmentation for long-tailed data. In Computer Vision–ECCV 2020, Proceedings of the 16th European Conference, Glasgow, UK, 23–28 August 2020, Proceedings, Part XXIX 16; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Ma, C.-Y.; Huang, J.-B.; Kira, Z. Featmatch: Feature-based augmentation for semi-supervised learning. In Computer Vision–ECCV 2020, Proceedings of the 16th European Conference, Glasgow, UK, 23–28 August 2020, Proceedings, Part XVIII 16; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Goodfellow, I.J.; Shlens, J.; Szegedy, C. Explaining and harnessing adversarial examples. arXiv 2014, arXiv:1412.6572. [Google Scholar]
- Madry, A.; Makelov, A.; Schmidt, L.; Tsipras, D.; Vladu, A. Towards deep learning models resistant to adversarial attacks. arXiv 2017, arXiv:1706.06083. [Google Scholar]
- Wong, E.; Rice, L.; Kolter, J.Z. Fast is better than free: Revisiting adversarial training. arXiv 2020, arXiv:2001.03994. [Google Scholar]
- Andriushchenko, M.; Flammarion, N. Understanding and improving fast adversarial training. Adv. Neural Inf. Process. Syst. 2020, 33, 16048–16059. [Google Scholar]
- Du, C.; Huo, C.; Zhang, L.; Chen, B.; Yuan, Y. Fast C&W: A fast adversarial attack algorithm to fool SAR target recognition with deep convolutional neural networks. IEEE Geosci. Remote Sens. Lett. 2021, 19, 1–5. [Google Scholar]
- Mirza, M.; Osindero, S. Conditional generative adversarial nets. arXiv 2014, arXiv:1411.1784. [Google Scholar]
- Radford, A.; Metz, L.; Chintala, S. Unsupervised representation learning with deep convolutional generative adversarial networks. arXiv 2015, arXiv:1511.06434. [Google Scholar]
- Denton, E.L.; Chintala, S.; Fergus, R. Deep generative image models using a laplacian pyramid of adversarial networks. In Advances in Neural Information Processing Systems 28; The MIT Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Chen, X.; Duan, Y.; Houthooft, R.; Schulman, J.; Sutskever, I.; Abbeel, P. Infogan: Interpretable representation learning by information maximizing generative adversarial nets. In Advances in Neural Information Processing Systems 29; The MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Zhao, J.; Mathieu, M.; LeCun, Y. Energy-based generative adversarial network. arXiv 2016, arXiv:1609.03126. [Google Scholar]
- Arjovsky, M.; Chintala, S.; Bottou, L. Wasserstein generative adversarial networks. In Proceedings of the International Conference on Machine Learning, Sydney, Australia, 6–11 August 2017. [Google Scholar]
- Berthelot, D.; Schumm, T.; Metz, L. Began: Boundary equilibrium generative adversarial networks. arXiv 2017, arXiv:1703.10717. [Google Scholar]
- Karras, T.; Aila, T.; Laine, S.; Lehtinen, J. Progressive growing of gans for improved quality, stability, and variation. arXiv 2017, arXiv:1710.10196. [Google Scholar]
- Brock, A.; Donahue, J.; Simonyan, K. Large scale GAN training for high fidelity natural image synthesis. arXiv 2018, arXiv:1809.11096. [Google Scholar]
- Karras, T.; Laine, S.; Aila, T. A style-based generator architecture for generative adversarial networks. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Long Beach, CA, USA, 15–20 June 2019. [Google Scholar]
- Ma, D.; Tang, P.; Zhao, L. SiftingGAN: Generating and sifting labeled samples to improve the remote sensing image scene classification baseline in vitro. IEEE Geosci. Remote Sens. Lett. 2019, 16, 1046–1050. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Park, T.; Isola, P.; Efros, A.A. Unpaired image-to-image translation using cycle-consistent adversarial networks. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017. [Google Scholar]
- Isola, P.; Zhu, J.-Y.; Zhou, T.; Efros, A.A. Image-to-image translation with conditional adversarial networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- Perez, L.; Wang, J. The effectiveness of data augmentation in image classification using deep learning. arXiv 2017, arXiv:1712.04621. [Google Scholar]
- Lemley, J.; Bazrafkan, S.; Corcoran, P. Smart augmentation learning an optimal data augmentation strategy. IEEE Access 2017, 5, 5858–5869. [Google Scholar] [CrossRef]
- Cubuk, E.D.; Zoph, B.; Mane, D.; Vasudevan, V.; Le, Q.V. Autoaugment: Learning augmentation policies from data. arXiv 2018, arXiv:1805.09501. [Google Scholar]
- Lim, S.; Kim, I.; Kim, T.; Kim, C.; Kim, S. Fast autoaugment. In Advances in Neural Information Processing Systems 32; The MIT Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Hataya, R.; Zdenek, J.; Yoshizoe, K.; Nakayama, H. Faster autoaugment: Learning augmentation strategies using backpropagation. In Computer Vision–ECCV 2020, Proceedings of the 16th European Conference, Glasgow, UK, 23–28 August 2020, Proceedings, Part XXV 16; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Hamdi, A.; Aboeleneen, A.; Shaban, K. MARL: Multimodal attentional representation learning for disease prediction. In Computer Vision Systems, Proceedings of the 13th International Conference, ICVS 2021, Virtual Event, 22–24 September 2021, Proceedings; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Cubuk, E.D.; Zoph, B.; Shlens, J.; Le, Q.V. Randaugment: Practical automated data augmentation with a reduced search space. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops, Virtual, 14–19 June 2020. [Google Scholar]
- Lo, S.-C.B.; Chan, H.-P.; Lin, J.-S.; Li, H.; Freedman, M.T.; Mun, S.K. Artificial convolution neural network for medical image pattern recognition. Neural Netw. 1995, 8, 1201–1214. [Google Scholar] [CrossRef]
- Fu’adah, Y.N.; Pratiwi, N.K.C.; Pramudito, M.A.; Ibrahim, N. Convolutional Neural Network (CNN) for Automatic Skin Cancer Classification System. IOP Conf. Ser. Mater. Sci. Eng. 2020, 982, 012005. [Google Scholar] [CrossRef]
- Al-Antari, M.A.; Al-Masni, M.A.; Park, S.-U.; Park, J.; Metwally, M.K.; Kadah, Y.M.; Han, S.-M.; Kim, T.-S. An automatic computer-aided diagnosis system for breast cancer in digital mammograms via deep belief network. J. Med. Biol. Eng. 2018, 38, 443–456. [Google Scholar] [CrossRef]
- Anand, D.; Arulselvi, G.; Balaji, G.; Chandra, G.R. A Deep Convolutional Extreme Machine Learning Classification Method to Detect Bone Cancer from Histopathological Images. Int. J. Intell. Syst. Appl. Eng. 2022, 10, 39–47. [Google Scholar]
- Beevi, K.S.; Nair, M.S.; Bindu, G.R. A Multi-Classifier System for Automatic Mitosis Detection in Breast Histopathology Images Using Deep Belief Networks. IEEE J. Transl. Eng. Health Med. 2017, 5, 4300211. [Google Scholar] [CrossRef]
- Shahweli, Z.N. Deep belief network for predicting the predisposition to lung cancer in TP53 gene. Iraqi J. Sci. 2020, 61, 171–177. [Google Scholar] [CrossRef]
- Kumar, T.S.; Arun, C.; Ezhumalai, P. An approach for brain tumor detection using optimal feature selection and optimized deep belief network. Biomed. Signal Process. Control 2022, 73, 103440. [Google Scholar] [CrossRef]
- Abdel-Zaher, A.M.; Eldeib, A.M. Breast cancer classification using deep belief networks. Expert Syst. Appl. 2016, 46, 139–144. [Google Scholar] [CrossRef]
- Jeyaraj, P.R.; Nadar, E.R.S. Deep Boltzmann machine algorithm for accurate medical image analysis for classification of cancerous region. Cogn. Comput. Syst. 2019, 1, 85–90. [Google Scholar] [CrossRef]
- Nawaz, M.; Sewissy, A.A.; Soliman, T.H.A. Multi-class breast cancer classification using deep learning convolutional neural network. Int. J. Adv. Comput. Sci. Appl. 2018, 9, 316–332. [Google Scholar] [CrossRef]
- Jabeen, K.; Khan, M.A.; Alhaisoni, M.; Tariq, U.; Zhang, Y.-D.; Hamza, A.; Mickus, A.; Damaševičius, R. Breast cancer classification from ultrasound images using probability-based optimal deep learning feature fusion. Sensors 2022, 22, 807. [Google Scholar] [CrossRef]
- El-Ghany, S.A.; Azad, M.; Elmogy, M. Robustness Fine-Tuning Deep Learning Model for Cancers Diagnosis Based on Histopathology Image Analysis. Diagnostics 2023, 13, 699. [Google Scholar] [CrossRef]
- Kavitha, T.; Mathai, P.P.; Karthikeyan, C.; Ashok, M.; Kohar, R.; Avanija, J.; Neelakandan, S. Deep learning based capsule neural network model for breast cancer diagnosis using mammogram images. Interdiscip. Sci. Comput. Life Sci. 2022, 14, 113–129. [Google Scholar] [CrossRef]
- Spanhol, F.A.; Oliveira, L.S.; Petitjean, C.; Heutte, L. Breast Cancer Histopathological Image Classification using Convolutional Neural Networks. In Proceedings of the International Joint Conference on Neural Networks (IJCNN 2016), Vancouver, BC, Canada, 24–29 July 2016. [Google Scholar]
- Das, A.; Acharya, U.R.; Panda, S.S.; Sabut, S. Deep learning based liver cancer detection using watershed transform and Gaussian mixture model techniques. Cogn. Syst. Res. 2019, 54, 165–175. [Google Scholar] [CrossRef]
- Mohsen, H.; El-Dahshan, E.-S.A.; El-Horbaty, E.-S.M.; Salem, A.-B.M. Classification using deep learning neural networks for brain tumors. Future Comput. Inform. J. 2018, 3, 68–71. [Google Scholar] [CrossRef]
- Attallah, O. CerCan Net: Cervical Cancer Classification Model via Multi-layer Feature Ensembles of Lightweight CNNs and Transfer Learning. Expert Syst. Appl. 2023, 229, 120624. [Google Scholar] [CrossRef]
- Zhao, Z.-Q.; Zheng, P.; Xu, S.-T.; Wu, X. Object detection with deep learning: A review. IEEE Trans. Neural Netw. Learn. Syst. 2019, 30, 3212–3232. [Google Scholar] [CrossRef]
- Girshick, R.; Donahue, J.; Darrell, T.; Malik, J. Rich feature hierarchies for accurate object detection and semantic segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Columbus, OH, USA, 23–28 June 2014. [Google Scholar]
- Girshick, R. Fast R-CNN. In Proceedings of the IEEE International Conference on Computer Vision, Santiago, Chile, 7–13 December 2015. [Google Scholar]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster R-CNN: Towards real-time object detection with region proposal networks. In Advances in Neural Information Processing Systems 28; The MIT Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Welikala, R.A.; Remagnino, P.; Lim, J.H.; Chan, C.S.; Rajendran, S.; Kallarakkal, T.G.; Zain, R.B.; Jayasinghe, R.D.; Rimal, J.; Kerr, A.R.; et al. Automated detection and classification of oral lesions using deep learning for early detection of oral cancer. IEEE Access 2020, 8, 132677–132693. [Google Scholar] [CrossRef]
- He, K.; Gkioxari, G.; Dollár, P.; Girshick, R. Mask R-CNN. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017. [Google Scholar]
- Cao, G.; Song, W.; Zhao, Z. Gastric Cancer Diagnosis with Mask R-CNN. In Proceedings of the 2019 11th International Conference on Intelligent Human-Machine Systems and Cybernetics (IHMSC), Hangzhou, China, 24–25 August 2019. [Google Scholar]
- Zhang, Y.; Chan, S.; Park, V.Y.; Chang, K.-T.; Mehta, S.; Kim, M.J.; Combs, F.J.; Chang, P.; Chow, D.; Parajuli, R.; et al. Automatic Detection and Segmentation of Breast Cancer on MRI Using Mask R-CNN Trained on Non–Fat-Sat Images and Tested on Fat-Sat Images. Acad. Radiol. 2022, 29, S135–S144. [Google Scholar] [CrossRef]
- Redmon, J.; Divvala, S.; Girshick, R.; Farhadi, A. You only look once: Unified, real-time object detection. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Liu, W.; Anguelov, D.; Erhan, D.; Szegedy, C.; Reed, S.; Fu, C.-Y.; Berg, A.C. Ssd: Single shot multibox detector. In Computer Vision–ECCV 2016, Proceedings of the 14th European Conference, Amsterdam, The Netherlands, 11–14 October 2016, Proceedings, Part I 14; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Gao, R.; Huo, Y.; Bao, S.; Tang, Y.; Antic, S.L.; Epstein, E.S.; Balar, A.B.; Deppen, S.; Paulson, A.B.; Sandler, K.L.; et al. Distanced LSTM: Time-Distanced Gates in Long Short-Term Memory Models for Lung Cancer Detection. In Machine Learning in Medical Imaging; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Zhou, J.; Luo, L.Y.; Dou, Q.; Chen, H.; Chen, C.; Li, G.-J.; Jiang, Z.-F.; Heng, P.-A. Weakly supervised 3D deep learning for breast cancer classification and localization of the lesions in MR images. J. Magn. Reson. Imaging 2019, 50, 1144–1151. [Google Scholar] [CrossRef]
- Asuntha, A.; Srinivasan, A. Deep learning for lung Cancer detection and classification. Multimed. Tools Appl. 2020, 79, 7731–7762. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, N.; Phang, J.; Park, J.; Liu, K.; Tyagi, S.; Heacock, L.; Kim, S.G.; Moy, L.; Cho, K.; et al. An interpretable classifier for high-resolution breast cancer screening images utilizing weakly supervised localization. Med. Image Anal. 2021, 68, 101908. [Google Scholar] [CrossRef]
- Ranjbarzadeh, R.; Kasgari, A.B.; Ghoushchi, S.J.; Anari, S.; Naseri, M.; Bendechache, M. Brain tumor segmentation based on deep learning and an attention mechanism using MRI multi-modalities brain images. Sci. Rep. 2021, 11, 10930. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Song, T.; Wang, G.; Chen, J.; Chen, Y.; Li, K.; Metaxas, D.N.; Zhang, S. SCPM-Net: An anchor-free 3D lung nodule detection network using sphere representation and center points matching. Med. Image Anal. 2022, 75, 102287. [Google Scholar] [CrossRef]
- Chatterjee, S.; Biswas, S.; Majee, A.; Sen, S.; Oliva, D.; Sarkar, R. Breast cancer detection from thermal images using a Grunwald-Letnikov-aided Dragonfly algorithm-based deep feature selection method. Comput. Biol. Med. 2022, 141, 105027. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Tang, K.; Zhao, Y.; Lu, Z.; Feng, Q. DDTNet: A dense dual-task network for tumor-infiltrating lymphocyte detection and segmentation in histopathological images of breast cancer. Med. Image Anal. 2022, 78, 102415. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Damaševičius, R.; Maskeliūnas, R. TTCNN: A breast cancer detection and classification towards computer-aided diagnosis using digital mammography in early stages. Appl. Sci. 2022, 12, 3273. [Google Scholar] [CrossRef]
- Ari, A.; Hanbay, D. Deep learning based brain tumor classification and detection system. Turk. J. Electr. Eng. Comput. Sci. 2018, 26, 2275–2286. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Maitra, M. MRI-based brain tumour image detection using CNN based deep learning method. Neurosci. Inform. 2022, 2, 100060. [Google Scholar] [CrossRef]
- Azad, R.; Aghdam, E.K.; Rauland, A.; Jia, Y.; Avval, A.H.; Bozorgpour, A.; Karimijafarbigloo, S.; Cohen, J.P.; Adeli, E.; Merhof, D. Medical image segmentation review: The success of u-net. arXiv 2022, arXiv:2211.14830. [Google Scholar]
- Asgari Taghanaki, S.; Abhishek, K.; Cohen, J.P.; Cohen-Adad, J.; Hamarneh, G. Deep semantic segmentation of natural and medical images: A review. Artif. Intell. Rev. 2021, 54, 137–178. [Google Scholar] [CrossRef]
- Shi, J.; Malik, J. Normalized cuts and image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2000, 22, 888–905. [Google Scholar]
- Boykov, Y.; Funka-Lea, G. Graph cuts and efficient nd image segmentation. Int. J. Comput. Vis. 2006, 70, 109–131. [Google Scholar] [CrossRef]
- Rother, C.; Kolmogorov, V.; Blake, A. “GrabCut” interactive foreground extraction using iterated graph cuts. ACM Trans. Graph. (TOG) 2004, 23, 309–314. [Google Scholar] [CrossRef]
- Poudel, R.P.; Lamata, P.; Montana, G. Recurrent fully convolutional neural networks for multi-slice MRI cardiac segmentation. In Reconstruction, Segmentation, and Analysis of Medical Images, Proceedings of the First International Workshops, RAMBO 2016 and HVSMR 2016, Held in Conjunction with MICCAI 2016, Athens, Greece, 17 October 2016, Revised Selected Papers 1; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Wang, Y.; Zheng, B.; Gao, D.; Wang, J. Fully convolutional neural networks for prostate cancer detection using multi-parametric magnetic resonance images: An initial investigation. In Proceedings of the 2018 24th International Conference on Pattern Recognition (ICPR), Beijing, China, 20–24 August 2018. [Google Scholar]
- Dong, X.; Zhou, Y.; Wang, L.; Peng, J.; Lou, Y.; Fan, Y. Liver Cancer Detection Using Hybridized Fully Convolutional Neural Network Based on Deep Learning Framework. IEEE Access 2020, 8, 129889–129898. [Google Scholar] [CrossRef]
- Shukla, P.K.; Zakariah, M.; Hatamleh, W.A.; Tarazi, H.; Tiwari, B. AI-DRIVEN novel approach for liver cancer screening and prediction using cascaded fully convolutional neural network. J. Healthc. Eng. 2022, 2022, 4277436. [Google Scholar] [CrossRef] [PubMed]
- Michael, E.; Ma, H.; Li, H.; Kulwa, F.; Li, J. Breast cancer segmentation methods: Current status and future potentials. BioMed Res. Int. 2021, 2021, 9962109. [Google Scholar] [CrossRef]
- Ayalew, Y.A.; Fante, K.A.; Mohammed, M.A. Modified U-Net for liver cancer segmentation from computed tomography images with a new class balancing method. BMC Biomed. Eng. 2021, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xiang, X.; Tran, T.D.; Hager, G.D.; Xie, X. Adversarial deep structured nets for mass segmentation from mammograms. In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018. [Google Scholar]
- Al-Antari, M.A.; Al-Masni, M.A.; Choi, M.-T.; Han, S.-M.; Kim, T.-S. A fully integrated computer-aided diagnosis system for digital X-ray mammograms via deep learning detection, segmentation, and classification. Int. J. Med. Inform. 2018, 117, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, D.; Nailon, W.H.; Davies, M.E.; Laurenson, D. Improved breast mass segmentation in mammograms with conditional residual u-net. In Image Analysis for Moving Organ, Breast, and Thoracic Images, Proceedings of the Third International Workshop, RAMBO 2018, Fourth International Workshop, BIA 2018, and First International Workshop, TIA 2018, Held in Conjunction with MICCAI 2018, Granada, Spain, 16 and 20 September 2018, Proceedings 3; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Shen, T.; Gou, C.; Wang, J.; Wang, F.-Y. Simultaneous segmentation and classification of mass region from mammograms using a mixed-supervision guided deep model. IEEE Signal Process. Lett. 2019, 27, 196–200. [Google Scholar] [CrossRef]
- Li, S.; Dong, M.; Du, G.; Mu, X. Attention dense-u-net for automatic breast mass segmentation in digital mammogram. IEEE Access 2019, 7, 59037–59047. [Google Scholar] [CrossRef]
- Hossain, M.S. Microc alcification segmentation using modified u-net segmentation network from mammogram images. J. King Saud Univ.-Comput. Inf. Sci. 2019, 34, 86–94. [Google Scholar] [CrossRef]
- Sun, H.; Li, C.; Liu, B.; Liu, Z.; Wang, M.; Zheng, H.; Feng, D.D.; Wang, S. AUNet: Attention-guided dense-upsampling networks for breast mass segmentation in whole mammograms. Phys. Med. Biol. 2020, 65, 055005. [Google Scholar] [CrossRef]
- Min, H.; Wilson, D.; Huang, Y.; Liu, S.; Crozier, S.; Bradley, A.P.; Chandra, S.S. Fully automatic computer-aided mass detection and segmentation via pseudo-color mammograms and mask r-cnn. In Proceedings of the 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI), Iowa City, IA, USA, 3–7 April 2020. [Google Scholar]
- Al-Antari, M.A.; Al-Masni, M.A.; Kim, T.-S. Deep learning computer-aided diagnosis for breast lesion in digital mammogram. Deep. Learn. Med. Image Anal. Chall. Appl. 2020, 1213, 59–72. [Google Scholar]
- Abdelhafiz, D.; Bi, J.; Ammar, R.; Yang, C.; Nabavi, S. Convolutional neural network for automated mass segmentation in mammography. BMC Bioinform. 2020, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, N.R.; Vidhyapriya, R.; Elango, N.; Ramesh, N. Deeply supervised u-net for mass segmentation in digital mammograms. Int. J. Imaging Syst. Technol. 2020, 31, 59–71. [Google Scholar]
- Saffari, N.; Rashwan, H.A.; Abdel-Nasser, M.; Singh, V.K.; Arenas, M.; Mangina, E.; Herrera, B.; Puig, D. Fully automated breast density segmentation and classification using deep learning. Diagnostics 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Rashwan, H.A.; Romani, S.; Akram, F.; Pandey, N.; Sarker, M.M.K.; Saleh, A.; Arenas, M.; Arquez, M.; Puig, D.; et al. Breast tumor segmentation and shape classification in mammograms using generative adversarial and convolutional neural network. Expert Syst. Appl. 2020, 139, 112855. [Google Scholar] [CrossRef]
- Ahmed, L.; Iqbal, M.M.; Aldabbas, H.; Khalid, S.; Saleem, Y.; Saeed, S. Images data practices for semantic segmentation of breast cancer using deep neural network. J. Ambient. Intell. Humaniz. Comput. 2020, 1–17. [Google Scholar] [CrossRef]
- Bhatti, H.M.A.; Li, J.; Siddeeq, S.; Rehman, A.; Manzoor, A. Multi-detection and segmentation of breast lesions based on mask rcnn-fpn. In Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Republic of Korea, 16–19 December 2020. [Google Scholar]
- Zeiser, F.A.; da Costa, C.A.; Zonta, T.; Marques, N.M.; Roehe, A.V.; Moreno, M.; da, R.R.R. Segmentation of masses on mammograms using data augmentation and deep learning. J. Digit. Imaging 2020, 33, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzidis, L.; Koutla, P.; Costaridou, L.; Pratikakis, I. Integrating segmentation information into CNN for breast cancer diagnosis of mammographic masses. Comput. Methods Programs Biomed. 2021, 200, 105913. [Google Scholar] [CrossRef]
- Salama, W.M.; Aly, M.H. Deep learning in mammography images segmentation and classification: Automated CNN approach. Alex. Eng. J. 2021, 60, 4701–4709. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Song, G.; Li, Z.; Zhang, Y.; Fan, Y. A deep learning model integrating FCNNs and CRFs for brain tumor segmentation. Med. Image Anal. 2018, 43, 98–111. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hu, W.; Zhang, K.; Yuan, S.; Han, X.; Su, C.; Zhao, J.; Wang, G.; Wang, G.; Zhang, L. Image segmentation algorithm of lung cancer based on neural network model. Expert Syst. 2022, 39, e12822. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, Y.; Lan, J.; Wang, T.; Han, Z.; Huang, Y.; Zhang, H.; Wang, J.; Cheng, M.; Jiang, H.; et al. A multi-task deep learning framework for perineural invasion recognition in gastric cancer whole slide images. Biomed. Signal Process. Control 2023, 79, 104261. [Google Scholar] [CrossRef]
- Alpert, N.; Bradshaw, J.; Kennedy, D.; Correia, J. The principal axes transformation—A method for image registration. J. Nucl. Med. 1990, 31, 1717–1722. [Google Scholar] [PubMed]
- Wodzinski, M.; Ciepiela, I.; Kuszewski, T.; Kedzierawski, P.; Skalski, A. Semi-supervised deep learning-based image registration method with volume penalty for real-time breast tumor bed localization. Sensors 2021, 21, 4085. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hong, J.; Reyngold, M.; Crane, C.; Cuaron, J.; Hajj, C.; Mann, J.; Zinovoy, M.; Greer, H.; Yorke, E. Deep-learning-based image registration and automatic segmentation of organs-at-risk in cone-beam CT scans from high-dose radiation treatment of pancreatic cancer. Med. Phys. 2021, 48, 3084–3095. [Google Scholar] [CrossRef]
- Wei, W.; Haishan, X.; Alpers, J.; Rak, M.; Hansen, C. A deep learning approach for 2D ultrasound and 3D CT/MR image registration in liver tumor ablation. Comput. Methods Programs Biomed. 2021, 206, 106117. [Google Scholar] [CrossRef]
- Salehi, M.; Sadr, A.V.; Mahdavi, S.R.; Arabi, H.; Shiri, I.; Reiazi, R. Deep Learning-based Non-rigid Image Registration for High-dose Rate Brachytherapy in Inter-fraction Cervical Cancer. J. Digit. Imaging 2022, 36, 574–587. [Google Scholar] [CrossRef]
- Xie, H.; Lei, Y.; Fu, Y.; Wang, T.; Roper, J.; Bradley, J.D.; Patel, P.; Liu, T.; Yang, X. Deformable Image Registration using Unsupervised Deep Learning for CBCT-guided Abdominal Radiotherapy. arXiv 2022, arXiv:2208.13686. [Google Scholar] [CrossRef]
- Xie, X.; Song, Y.; Ye, F.; Yan, H.; Wang, S.; Zhao, X.; Dai, J. Improving deformable image registration with point metric and masking technique for postoperative breast cancer radiotherapy. Quant. Imaging Med. Surg. 2021, 11, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lei, Y.; Wang, T.; Higgins, K.; Bradley, J.D.; Curran, W.J.; Liu, T.; Yang, X. LungRegNet: An unsupervised deformable image registration method for 4D-CT lung. Med. Phys. 2020, 47, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, D.H.; Park, S.H.; Kim, J.; Lee, J.-G.; Ye, J.C. CycleMorph: Cycle consistent unsupervised deformable image registration. Med. Image Anal. 2021, 71, 102036. [Google Scholar] [CrossRef]
- Fu, Y.; Lei, Y.; Wang, T.; Curran, W.J.; Liu, T.; Yang, X. Deep learning in medical image registration: A review. Phys. Med. Biol. 2020, 65, 20TR01. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.; Gach, H.M.; Li, H.; Yang, D. GroupRegNet: A groupwise one-shot deep learning-based 4D image registration method. Phys. Med. Biol. 2021, 66, 045030. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Fu, Y.; Tian, Z.; Wang, T.; Dai, X.; Roper, J.; Yu, D.S.; McDonald, M.; Bradley, J.D.; Liu, T.; et al. Deformable CT image registration via a dual feasible neural network. Med. Phys. 2022, 49, 7545–7554. [Google Scholar] [CrossRef]
- Kim, B.; Lee, C.-M.; Jang, J.K.; Kim, J.; Lim, S.-B.; Kim, A.Y. Deep learning-based imaging reconstruction for MRI after neoadjuvant chemoradiotherapy for rectal cancer: Effects on image quality and assessment of treatment response. Abdom. Radiol. 2023, 48, 201–210. [Google Scholar] [CrossRef]
- Cheng, A.; Kim, Y.; Anas, E.M.; Rahmim, A.; Boctor, E.M.; Seifabadi, R.; Wood, B.J. Deep learning image reconstruction method for limited-angle ultrasound tomography in prostate cancer. In Medical Imaging 2019: Ultrasonic Imaging and Tomography; SPIE: Bellingham, WA, USA, 2019. [Google Scholar]
- Noda, Y.; Kawai, N.; Nagata, S.; Nakamura, F.; Mori, T.; Miyoshi, T.; Suzuki, R.; Kitahara, F.; Kato, H.; Hyodo, F.; et al. Deep learning image reconstruction algorithm for pancreatic protocol dual-energy computed tomography: Image quality and quantification of iodine concentration. Eur. Radiol. 2022, 32, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Kuanar, S.; Athitsos, V.; Mahapatra, D.; Rao, K.R.; Akhtar, Z.; Dasgupta, D. Low Dose Abdominal CT Image Reconstruction: An Unsupervised Learning Based Approach. In Proceedings of the 2019 IEEE International Conference on Image Processing (ICIP), Taipei, Taiwan, 22–25 September 2019. [Google Scholar]
- Gassenmaier, S.; Afat, S.; Nickel, M.D.; Mostapha, M.; Herrmann, J.; Almansour, H.; Nikolaou, K.; Othman, A.E. Accelerated T2-Weighted TSE Imaging of the Prostate Using Deep Learning Image Reconstruction: A Prospective Comparison with Standard T2-Weighted TSE Imaging. Cancers 2021, 13, 3593. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Gu, H.; Zhu, H.; Chang, K.; Hoebel, K.V.; Patel, J.B.; Kalpathy-Cramer, J.; Carp, S.A. FDU-Net: Deep Learning-Based Three-Dimensional Diffuse Optical Image Reconstruction. IEEE Trans. Med. Imaging 2023, 1. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, W.; Li, Z.; Jia, K.; Jiang, S.; Dehghani, H.; Pogue, B.W.; Paulsen, K.D. Deep-learning based image reconstruction for MRI-guided near-infrared spectral tomography. Optica 2022, 9, 264–267. [Google Scholar] [CrossRef]
- Wei, R.; Chen, J.; Liang, B.; Chen, X.; Men, K.; Dai, J. Real-time 3D MRI reconstruction from cine-MRI using unsupervised network in MRI-guided radiotherapy for liver cancer. Med. Phys. 2022, 50, 3584–3596. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Ohira, S.; Teraoka, Y.; Matsumi, A.; Imai, Y.; Akino, Y.; Miyazaki, M.; Nakamura, S.; Konishi, K.; Tanigawa, N.; et al. Pseudo low-energy monochromatic imaging of head and neck cancers: Deep learning image reconstruction with dual-energy CT. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1271–1279. [Google Scholar] [CrossRef]
- Wang, T.; Lei, Y.; Fu, Y.; Wynne, J.F.; Curran, W.J.; Liu, T.; Yang, X. A review on medical imaging synthesis using deep learning and its clinical applications. J. Appl. Clin. Med. Phys. 2021, 22, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Wang, Z.; Wang, Z.J.; Ward, R.K.; Wang, X. Deep learning for pixel-level image fusion: Recent advances and future prospects. Inf. Fusion 2018, 42, 158–173. [Google Scholar] [CrossRef]
- Sahiner, B.; Pezeshk, A.; Hadjiiski, L.M.; Wang, X.; Drukker, K.; Cha, K.H.; Summers, R.M.; Giger, M.L. Deep learning in medical imaging and radiation therapy. Med. Phys. 2019, 46, e1–e36. [Google Scholar] [CrossRef]
- Kanayama, T.; Kurose, Y.; Tanaka, K.; Aida, K.; Satoh, S.I.; Kitsuregawa, M.; Harada, T. Gastric cancer detection from endoscopic images using synthesis by GAN. In Medical Image Computing and Computer Assisted Intervention–MICCAI 2019, Proceedings of the 22nd International Conference, Shenzhen, China, 13–17 October 2019, Proceedings, Part V 22; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Yu, B.; Zhou, L.; Wang, L.; Fripp, J.; Bourgeat, P. 3D cGAN based cross-modality MR image synthesis for brain tumor segmentation. In Proceedings of the 2018 IEEE 15th international symposium on biomedical imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018. [Google Scholar]
- Saha, M.; Guo, X.; Sharma, A. TilGAN: GAN for Facilitating Tumor-Infiltrating Lymphocyte Pathology Image Synthesis With Improved Image Classification. IEEE Access 2021, 9, 79829–79840. [Google Scholar] [CrossRef]
- Abhishek, K.; Hamarneh, G. Mask2Lesion: Mask-constrained adversarial skin lesion image synthesis. In Simulation and Synthesis in Medical Imaging, Proceedings of the 4th International Workshop, SASHIMI 2019, Held in Conjunction with MICCAI 2019, Shenzhen, China, 13 October 2019, Proceedings; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Qin, Z.; Liu, Z.; Zhu, P.; Xue, Y. A GAN-based image synthesis method for skin lesion classification. Comput. Methods Programs Biomed. 2020, 195, 105568. [Google Scholar] [CrossRef] [PubMed]
- Baydoun, A.; Xu, K.; Heo, J.U.; Yang, H.; Zhou, F.; Bethell, L.A.; Fredman, E.T.; Ellis, R.J.; Podder, T.K.; Traughber, M.S.; et al. Synthetic CT generation of the pelvis in patients with cervical cancer: A single input approach using generative adversarial network. IEEE Access 2021, 9, 17208–17221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, S.-G.; Gong, X.-C.; Yuan, X.-X.; Lin, J.-F.; Chen, Q.; Li, J.-G. Generating synthesized computed tomography from CBCT using a conditional generative adversarial network for head and neck cancer patients. Technol. Cancer Res. Treat. 2022, 21, 15330338221085358. [Google Scholar] [CrossRef]
- Sun, B.; Jia, S.; Jiang, X.; Jia, F. Double U-Net CycleGAN for 3D MR to CT image synthesis. Int. J. Comput. Assist. Radiol. Surg. 2023, 18, 149–156. [Google Scholar] [CrossRef]
- Chen, S.; Qin, A.; Zhou, D.; Yan, D. U-net-generated synthetic CT images for magnetic resonance imaging-only prostate intensity-modulated radiation therapy treatment planning. Med. Phys. 2018, 45, 5659–5665. [Google Scholar] [CrossRef]
- Bahrami, A.; Karimian, A.; Fatemizadeh, E.; Arabi, H.; Zaidi, H. A new deep convolutional neural network design with efficient learning capability: Application to CT image synthesis from MRI. Med. Phys. 2020, 47, 5158–5171. [Google Scholar] [CrossRef]
- Arita, Y.; Takahara, T.; Yoshida, S.; Kwee, T.C.; Yajima, S.; Ishii, C.; Ishii, R.; Okuda, S.; Jinzaki, M.; Fujii, Y. Quantitative assessment of bone metastasis in prostate cancer using synthetic magnetic resonance imaging. Investig. Radiol. 2019, 54, 638–644. [Google Scholar] [CrossRef]
- Pang, Y.; Chen, X.; Huang, Y.; Yap, P.-T.; Lian, J. Weakly Supervised MR-TRUS Image Synthesis for Brachytherapy of Prostate Cancer. In Medical Image Computing and Computer Assisted Intervention–MICCAI 2022, Proceedings of the 25th International Conference, Singapore, 18–22 September 2022, Proceedings, Part VI; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Razzak, M.I.; Naz, S.; Zaib, A. Deep learning for medical image processing: Overview, challenges and the future. Classif. BioApps 2018, 26, 323–350. [Google Scholar]
- Skandarani, Y.; Jodoin, P.-M.; Lalande, A. Gans for medical image synthesis: An empirical study. J. Imaging 2023, 9, 69. [Google Scholar] [CrossRef]
- De Angeli, K.; Gao, S.; Danciu, I.; Durbin, E.B.; Wu, X.-C.; Stroup, A.; Doherty, J.; Schwartz, S.; Wiggins, C.; Damesyn, M.; et al. Class imbalance in out-of-distribution datasets: Improving the robustness of the TextCNN for the classification of rare cancer types. J. Biomed. Inform. 2022, 125, 103957. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.M.; Shaukat, K.; Khan, W.A.; Hameed, I.A.; Almuqren, L.A.; Raza, M.A.; Aslam, M.; Luo, S. An Efficient Deep Learning-Based Skin Cancer Classifier for an Imbalanced Dataset. Diagnostics 2022, 12, 2115. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, J.; Lin, Z. Cancer diagnosis using generative adversarial networks based on deep learning from imbalanced data. Comput. Biol. Med. 2021, 135, 104540. [Google Scholar] [CrossRef]
- Yuan, X.; Xie, L.; Abouelenien, M. A regularized ensemble framework of deep learning for cancer detection from multi-class, imbalanced training data. Pattern Recognit. 2018, 77, 160–172. [Google Scholar] [CrossRef]
- Saini, M.; Susan, S. VGGIN-Net: Deep Transfer Network for Imbalanced Breast Cancer Dataset. IEEE/ACM Trans. Comput. Biol. Bioinform. 2023, 20, 752–762. [Google Scholar] [CrossRef] [PubMed]
- European Society of Radiology (ESR). Abdominal applications of ultrasound fusion imaging technique: Liver, kidney, and pancreas. Insights Into Imaging 2019, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Blum, R.S.; Liu, Z. Multi-Sensor Image Fusion and Its Applications; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Saleh, M.A.; Ali, A.A.; Ahmed, K.; Sarhan, A.M. A Brief Analysis of Multimodal Medical Image Fusion Techniques. Electronics 2022, 12, 97. [Google Scholar] [CrossRef]
- Kavita, P.; Alli, D.R.; Rao, A.B. Study of Image Fusion Optimization Techniques for Medical Applications. Int. J. Cogn. Comput. Eng. 2022, 3, 136–143. [Google Scholar] [CrossRef]
- Yao, D.; Wen, J.; Chen, A.; Fang, M.; Wei, X.; Pan, Z. Trimodal Fusion Network Combined Global-Local Feature Extraction Strategy and Spatial-Frequency Fusion Strategy. In Proceedings of the International Conference on Machine Learning for Cyber Security, Guangzhou, China, 2–4 December 2022; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Jin, C.; Guo, Z.; Lin, Y.; Luo, L.; Chen, H. Label-efficient deep learning in medical image analysis: Challenges and future directions. arXiv 2023, arXiv:2303.12484. [Google Scholar]
- Xu, Z.; Qi, C.; Xu, G. Semi-supervised attention-guided cyclegan for data augmentation on medical images. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019. [Google Scholar]
- Madani, A.; Moradi, M.; Karargyris, A.; Syeda-Mahmood, T. Semi-supervised learning with generative adversarial networks for chest X-ray classification with ability of data domain adaptation. In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018. [Google Scholar]
- Shurrab, S.; Duwairi, R. Self-supervised learning methods and applications in medical imaging analysis: A survey. PeerJ Comput. Sci. 2022, 8, e1045. [Google Scholar] [CrossRef]
- Srinidhi, C.L.; Kim, S.W.; Chen, F.-D.; Martel, A.L. Self-supervised driven consistency training for annotation efficient histopathology image analysis. Med. Image Anal. 2022, 75, 102256. [Google Scholar] [CrossRef]
- Tan, L.; Li, H.; Yu, J.; Zhou, H.; Wang, Z.; Niu, Z.; Li, J.; Li, Z. Colorectal cancer lymph node metastasis prediction with weakly supervised transformer-based multi-instance learning. Med. Biol. Eng. Comput. 2023, 61, 1565–1580. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhao, L.; Yuan, L.; Wen, X. Deep Multi-Instance Learning with Adaptive Recurrent Pooling for Medical Image Classification. In Proceedings of the 2022 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Las Vegas, NV, USA, 6–8 December 2022. [Google Scholar]
- Chen, X.; Wang, X.; Zhang, K.; Fung, K.-M.; Thai, T.C.; Moore, K.; Mannel, R.S.; Liu, H.; Zheng, B.; Qiu, Y. Recent advances and clinical applications of deep learning in medical image analysis. Med. Image Anal. 2022, 79, 102444. [Google Scholar] [CrossRef]
- Azizi, S.; Culp, L.; Freyberg, J.; Mustafa, B.; Baur, S.; Kornblith, S.; Chen, T.; MacWilliams, P.; Mahdavi, S.S.; Wulczyn, E. Robust and efficient medical imaging with self-supervision. arXiv 2022, arXiv:2205.09723. [Google Scholar]
- Wolf, D.; Regnery, S.; Tarnawski, R.; Bobek-Billewicz, B.; Polańska, J.; Götz, M. Weakly Supervised Learning with Positive and Unlabeled Data for Automatic Brain Tumor Segmentation. Appl. Sci. 2022, 12, 10763. [Google Scholar] [CrossRef]
- Karimi, D.; Dou, H.; Warfield, S.K.; Gholipour, A. Deep learning with noisy labels: Exploring techniques and remedies in medical image analysis. Med. Image Anal. 2020, 65, 101759. [Google Scholar] [CrossRef]
- Song, H.; Kim, M.; Park, D.; Shin, Y.; Lee, J.G. Learning From Noisy Labels With Deep Neural Networks: A Survey. IEEE Trans. Neural Netw. Learn. Syst. 2022; ahead of print. [Google Scholar]
- Qu, L.; Liu, S.; Liu, X.; Wang, M.; Song, Z. Towards label-efficient automatic diagnosis and analysis: A comprehensive survey of advanced deep learning-based weakly-supervised, semi-supervised and self-supervised techniques in histopathological image analysis. Phys. Med. Biol. 2022, 67, 20TR01. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bentley, P.; Mori, K.; Misawa, K.; Fujiwara, M.; Rueckert, D. Self-supervised learning for medical image analysis using image context restoration. Med. Image Anal. 2019, 58, 101539. [Google Scholar] [CrossRef]
- Zhou, Z.-H. A brief introduction to weakly supervised learning. Natl. Sci. Rev. 2018, 5, 44–53. [Google Scholar] [CrossRef]
- Alloghani, M.; Al-Jumeily, D.; Mustafina, J.; Hussain, A.; Aljaaf, A.J. A systematic review on supervised and unsupervised machine learning algorithms for data science. In Supervised and Unsupervised Learning for Data Science; Springer: Cham, Switzerland, 2020; pp. 3–21. [Google Scholar]
- Van Engelen, J.E.; Hoos, H.H. A survey on semi-supervised learning. Mach. Learn. 2020, 109, 373–440. [Google Scholar] [CrossRef]
- Ciga, O.; Xu, T.; Martel, A.L. Self supervised contrastive learning for digital histopathology. Mach. Learn. Appl. 2022, 7, 100198. [Google Scholar] [CrossRef]
- Mahapatra, D.; Poellinger, A.; Shao, L.; Reyes, M. Interpretability-Driven Sample Selection Using Self Supervised Learning for Disease Classification and Segmentation. IEEE Trans. Med. Imaging 2021, 40, 2548–2562. [Google Scholar] [CrossRef]
- Singh, A.; Sengupta, S.; Lakshminarayanan, V. Explainable deep learning models in medical image analysis. J. Imaging 2020, 6, 52. [Google Scholar] [CrossRef]
- Singh, R.K.; Gorantla, R.; Allada, S.G.; Pratap, N. Skinet: A deep learning solution for skin lesion diagnosis with uncertainty estimation and explainability. arXiv 2020, arXiv:2012.15049. [Google Scholar]
- Malafaia, M.; Silva, F.; Neves, I.; Pereira, T.; Oliveira, H.P. Robustness Analysis of Deep Learning-Based Lung Cancer Classification Using Explainable Methods. IEEE Access 2022, 10, 112731–112741. [Google Scholar] [CrossRef]
- Moustakidis, S.; Ntakolia, C.; Diamantis, D.E.; Papandrianos, N.; Papageorgiou, E.I. Application and post-hoc explainability of deep convolutional neural networks for bone cancer metastasis classification in prostate patients. In Artificial Intelligence in Cancer Diagnosis and Prognosis, Volume 3: Brain and Prostate Cancer; IOP Publishing: Bristol, UK, 2022. [Google Scholar]
- Van der Velden, B.H.; Kuijf, H.J.; Gilhuijs, K.G.; Viergever, M.A. Explainable artificial intelligence (XAI) in deep learning-based medical image analysis. Med. Image Anal. 2022, 79, 102470. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.; Hagiwara, Y.; Sudarshan, V. Precision medical imaging in big data: Radiomics. Chin. J. Bases Clin. General. Surg. 2016, 23, 752–755. [Google Scholar]
- Vallières, M.; Freeman, C.R.; Skamene, S.R.; El Naqa, I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys. Med. Biol. 2015, 60, 5471–5496. [Google Scholar] [CrossRef]
- Lo Gullo, R.; Daimiel, I.; Morris, E.A.; Pinker, K. Combining molecular and imaging metrics in cancer: Radiogenomics. Insights Imaging 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, V.; Kontopantelis, E.; Cuccu, I.; Sgamba, L.; D′Augè, T.G.; Pernazza, A.; Della Rocca, C.; Manganaro, L.; Catalano, C.; Perniola, G.; et al. Magnetic resonance imaging-radiomics in endometrial cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2023, 33, 1070–1076. [Google Scholar] [CrossRef]
- Avanzo, M.; Stancanello, J.; Pirrone, G.; Sartor, G. Radiomics and deep learning in lung cancer. Strahlenther. Und Onkol. 2020, 196, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Sushentsev, N.; Rundo, L.; Abrego, L.; Li, Z.; Nazarenko, T.; Warren, A.Y.; Gnanapragasam, V.J.; Sala, E.; Zaikin, A.; Barrett, T.; et al. Time series radiomics for the prediction of prostate cancer progression in patients on active surveillance. Eur. Radiol. 2023, 33, 3792–3800. [Google Scholar] [CrossRef]
- Ge, G.; Zhang, J. Feature selection methods and predictive models in CT lung cancer radiomics. J. Appl. Clin. Med. Phys. 2023, 24, e13869. [Google Scholar] [CrossRef]
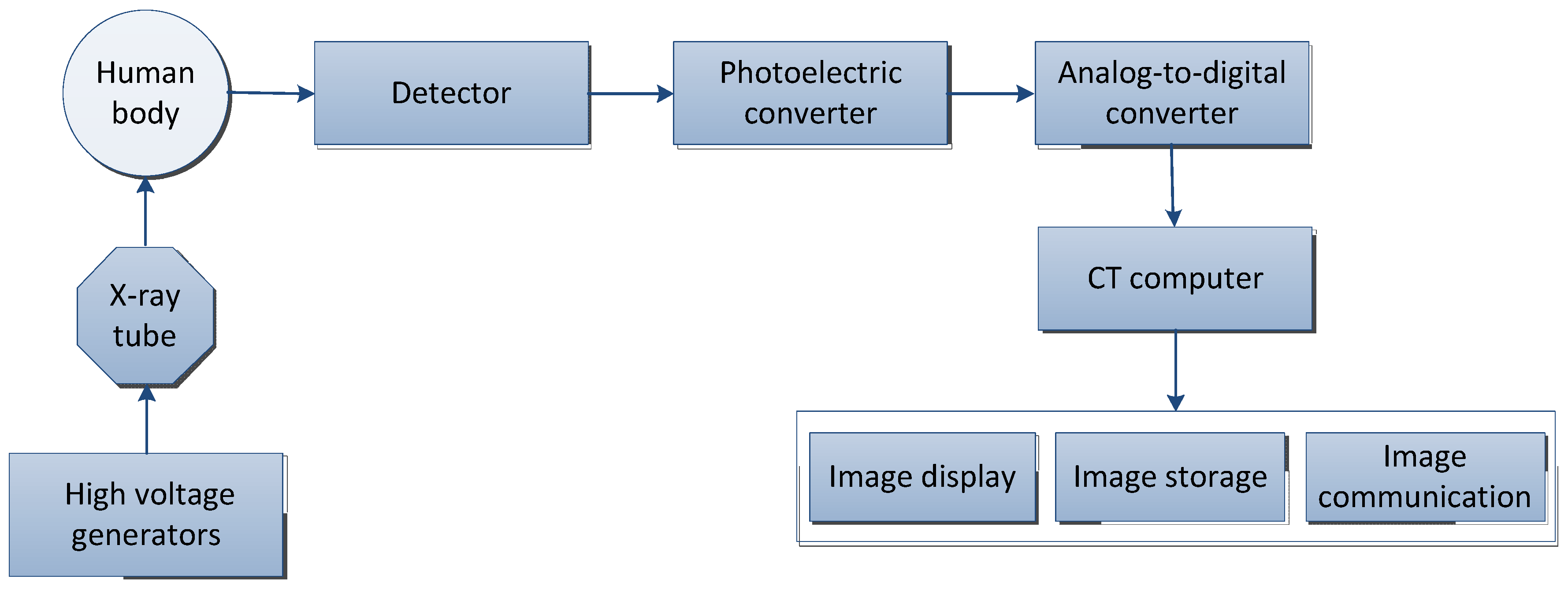
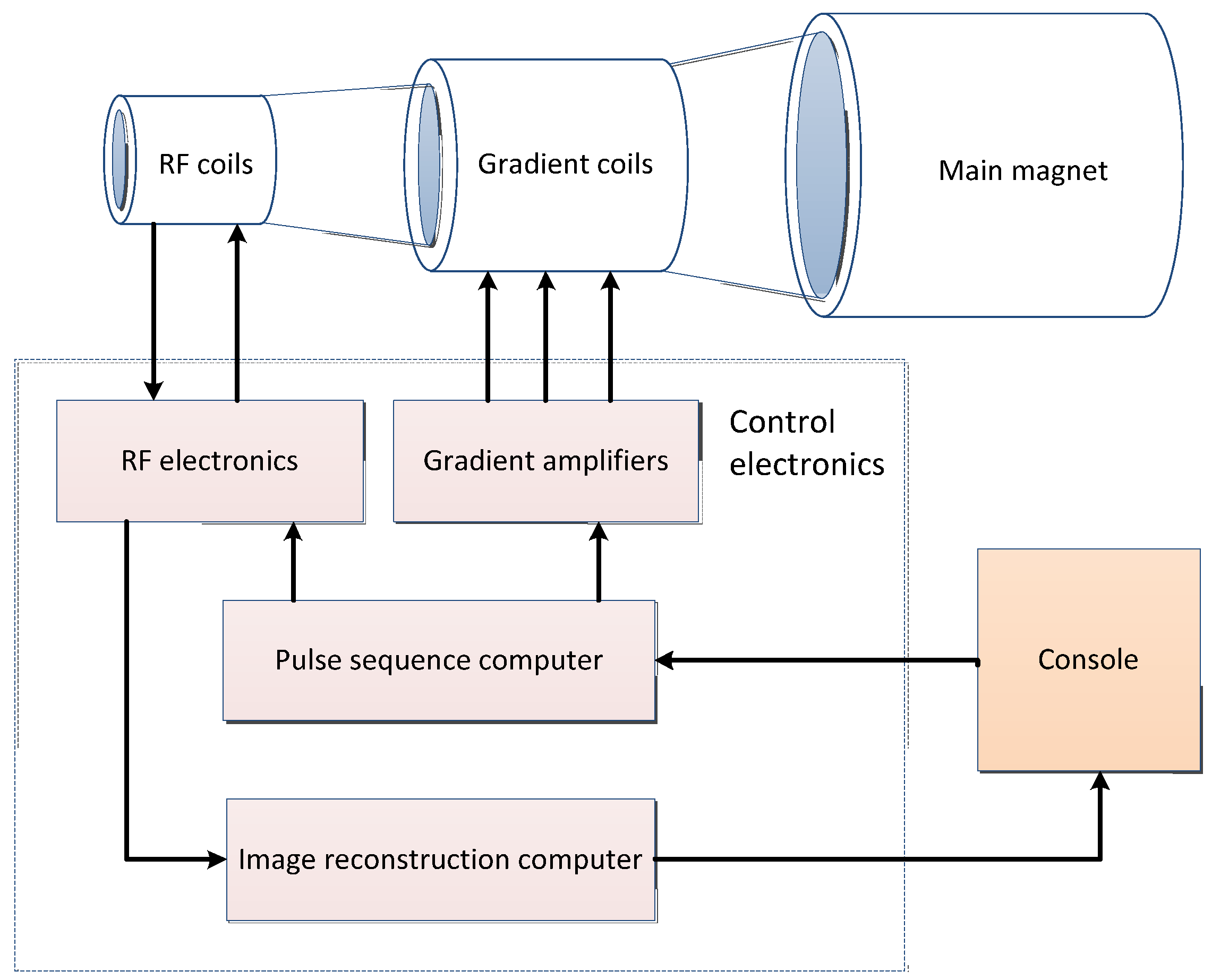

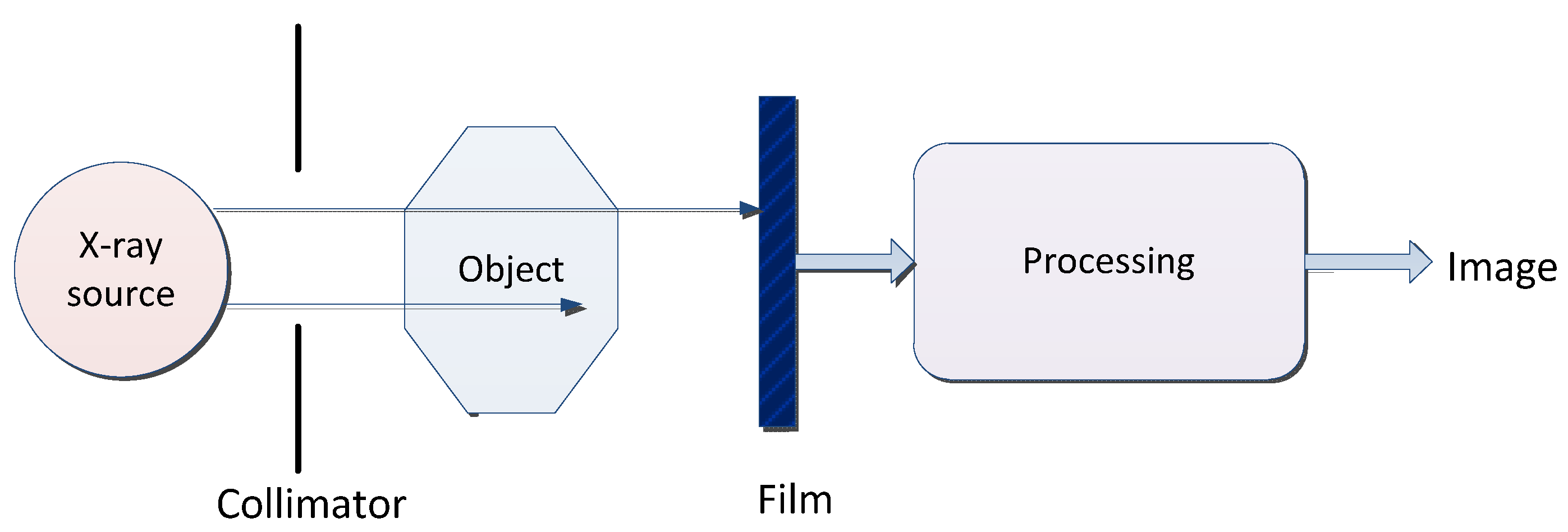
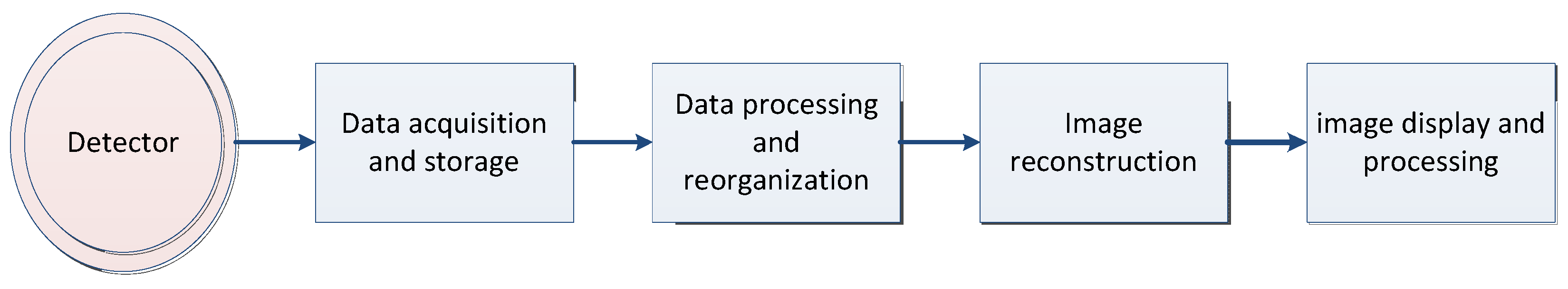
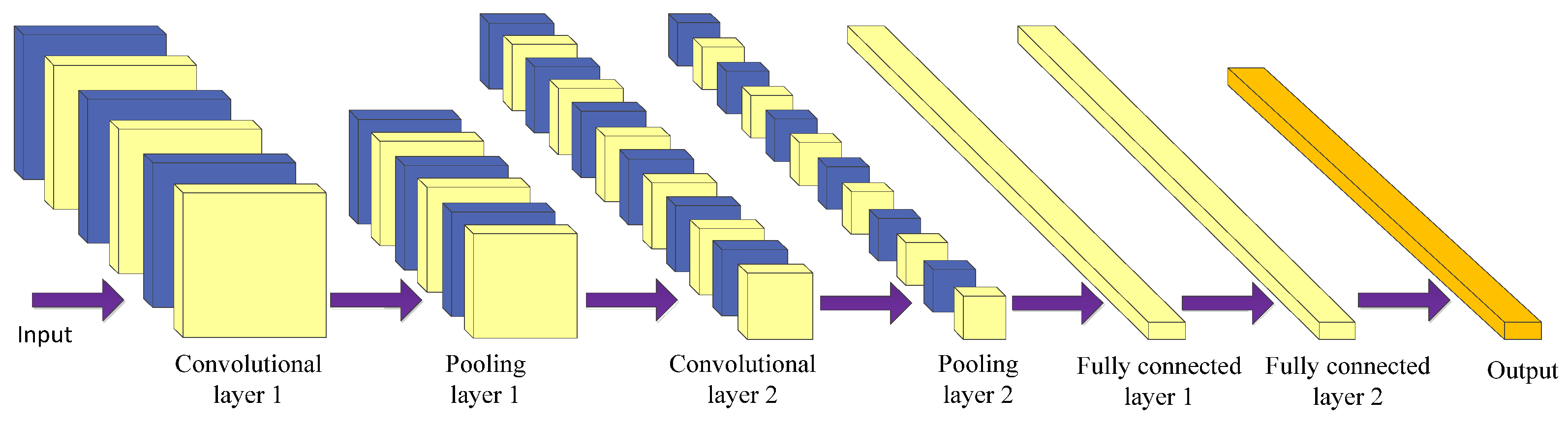
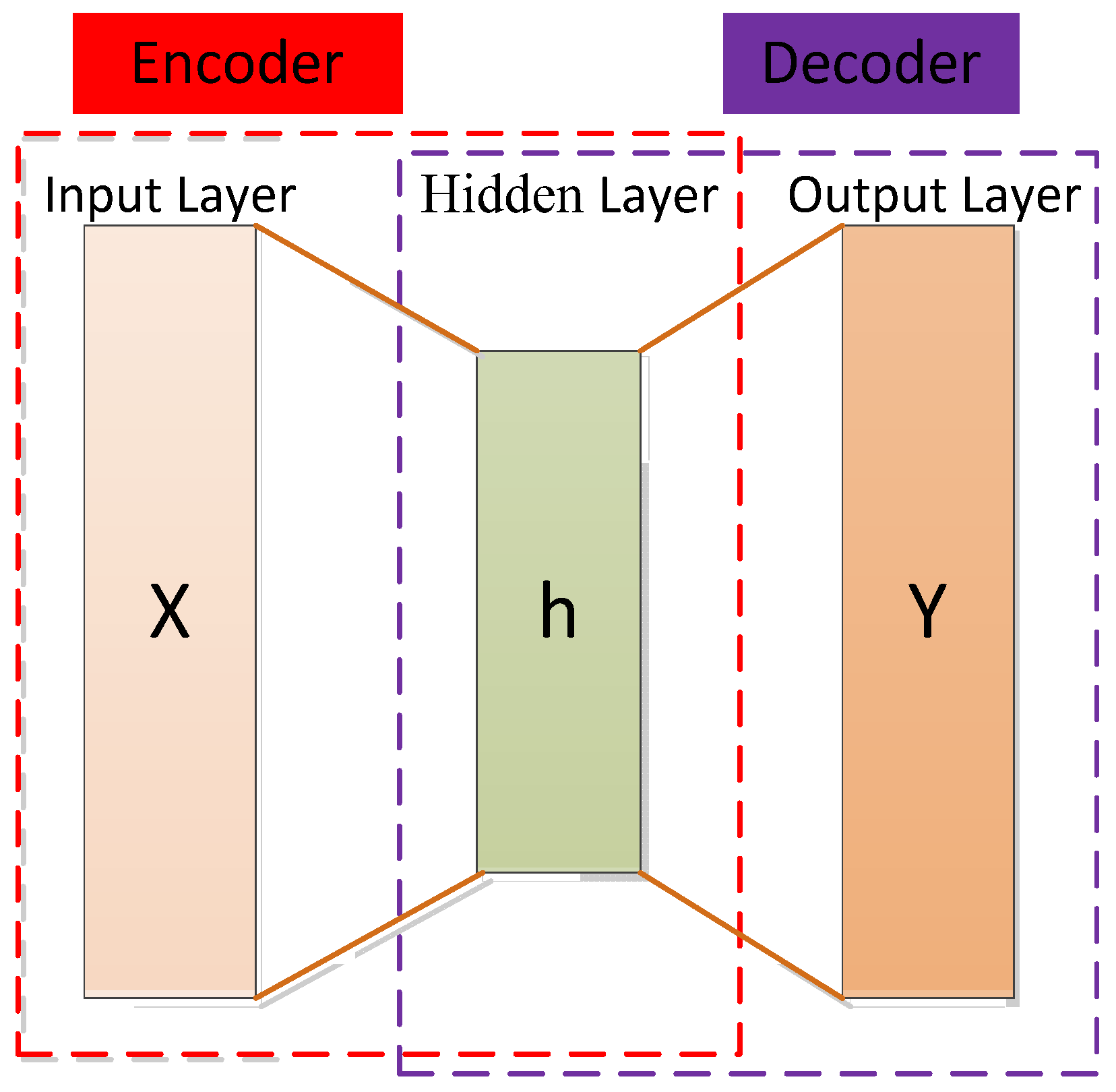
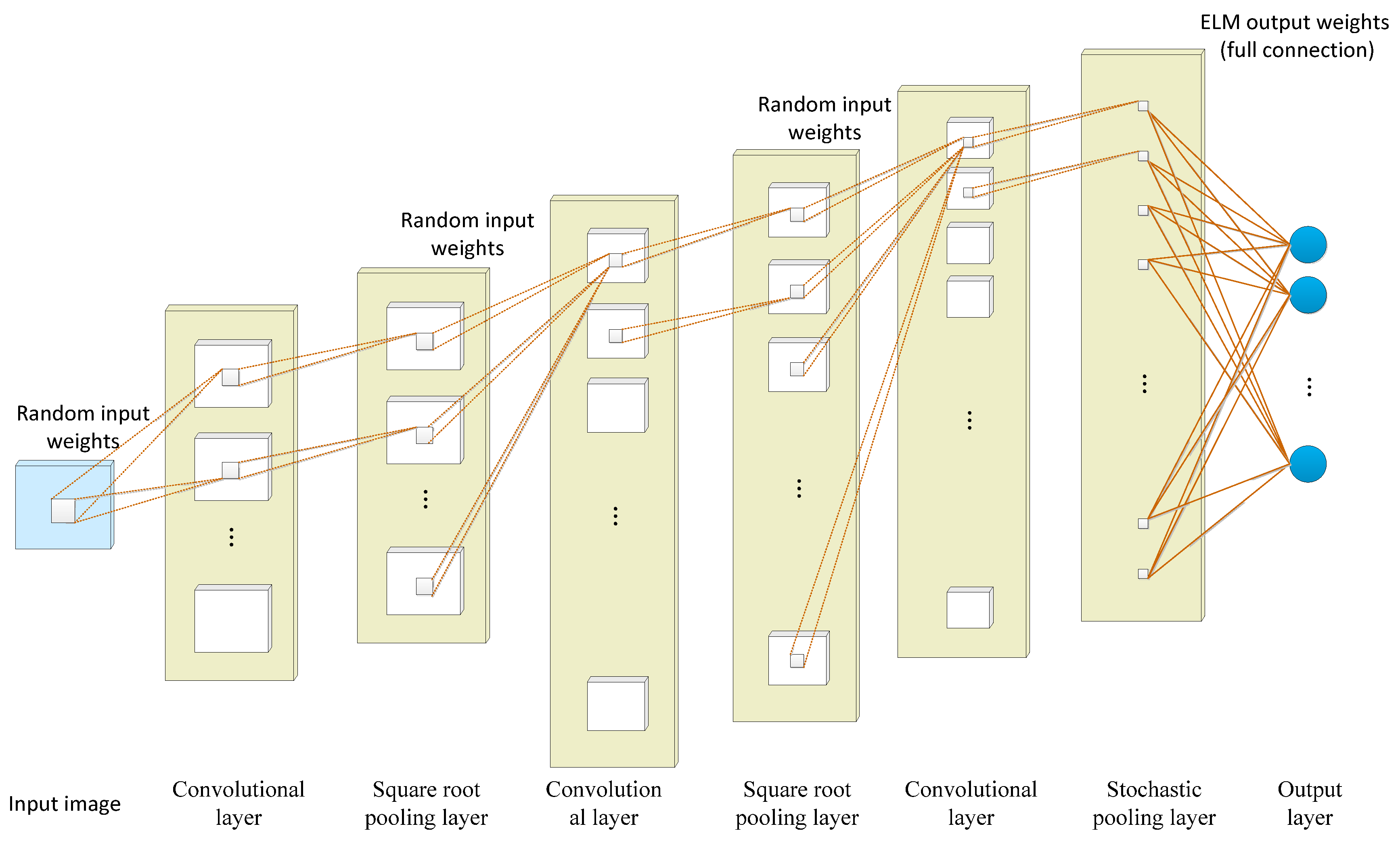
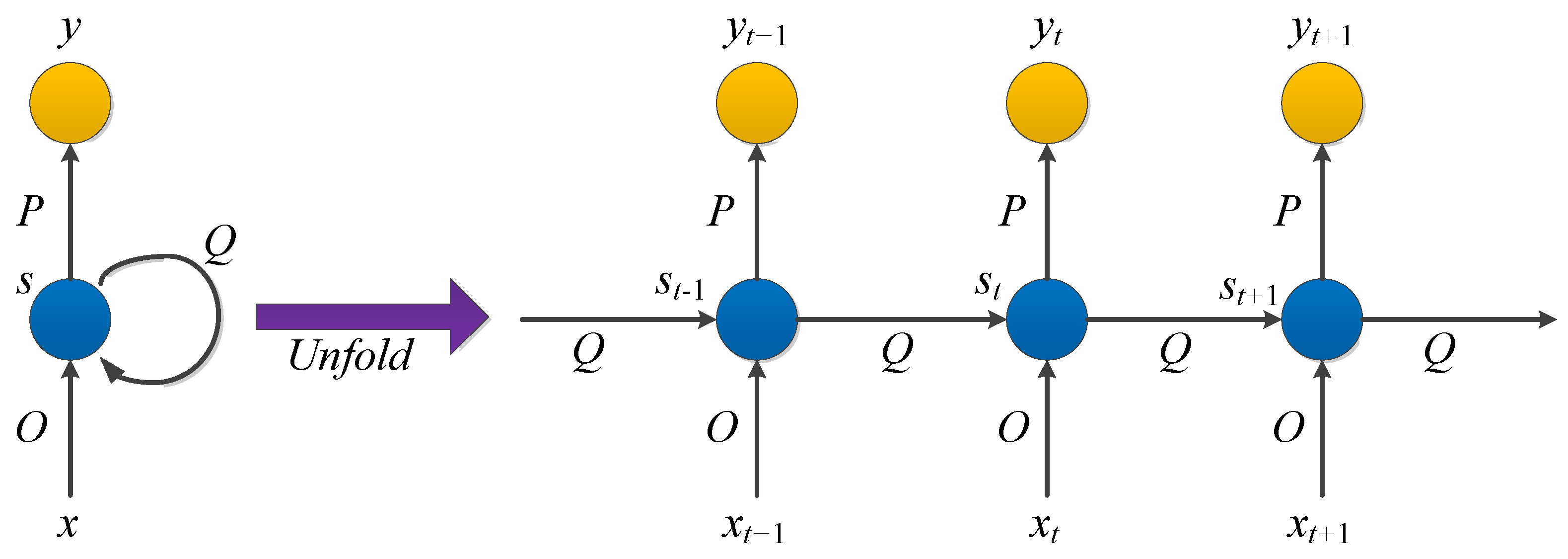
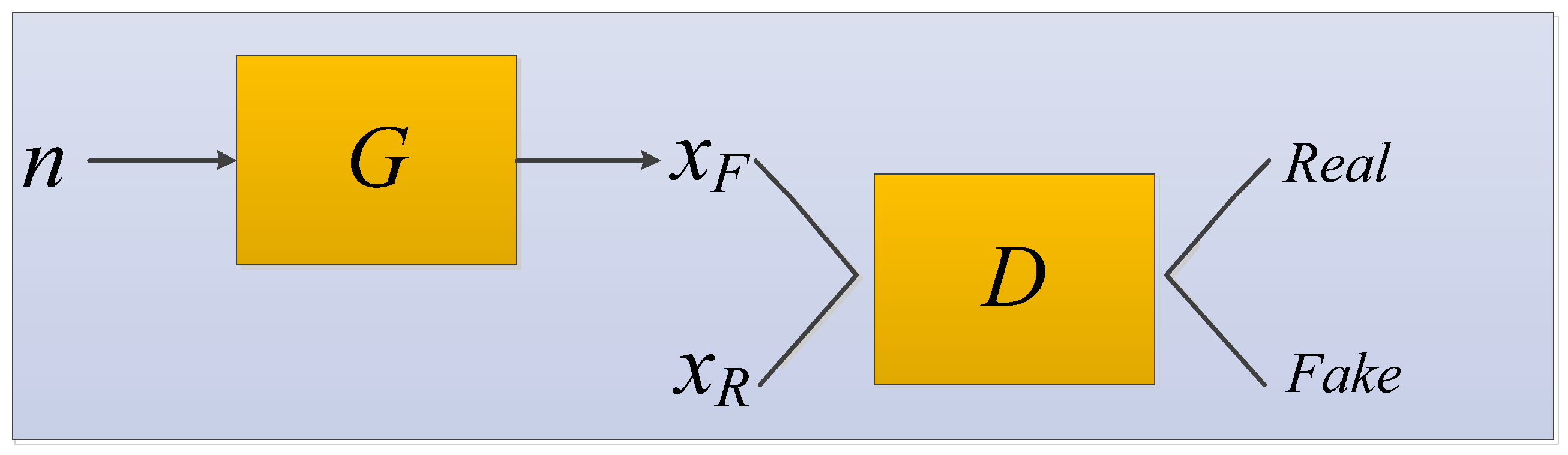
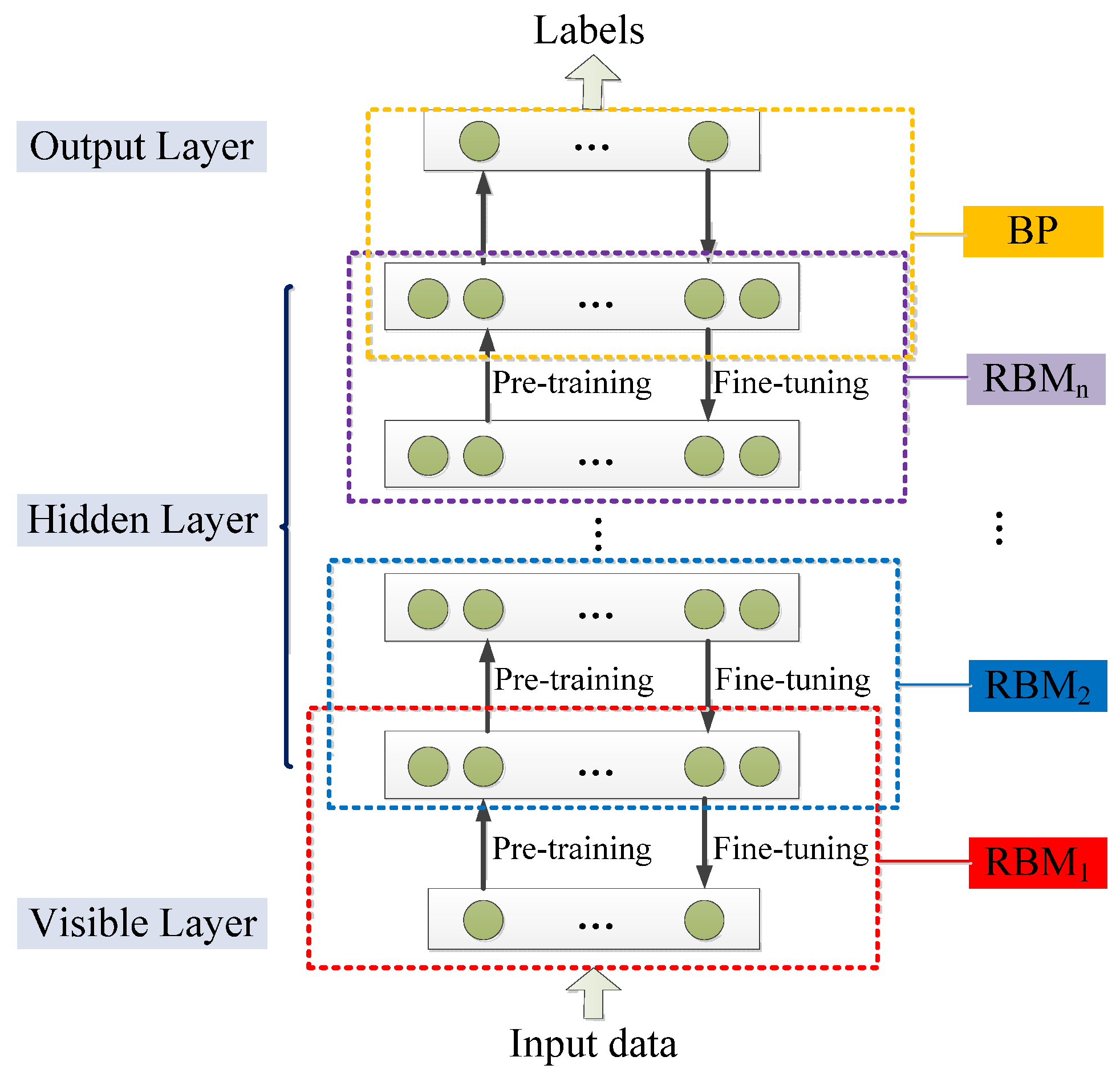
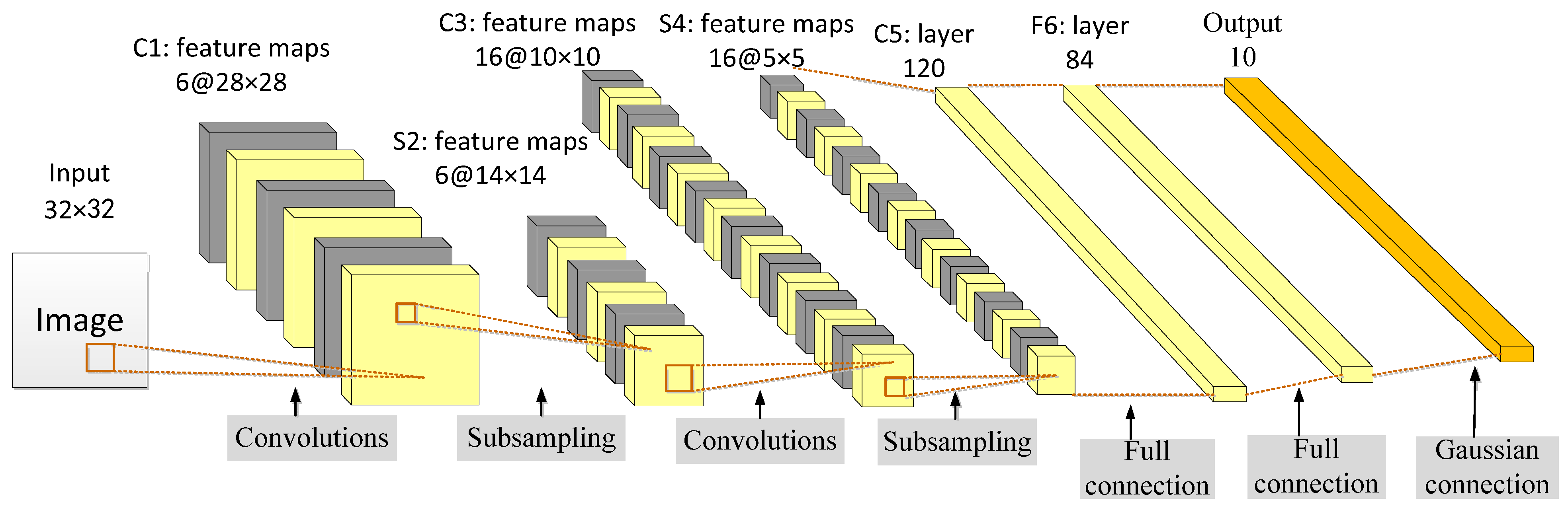

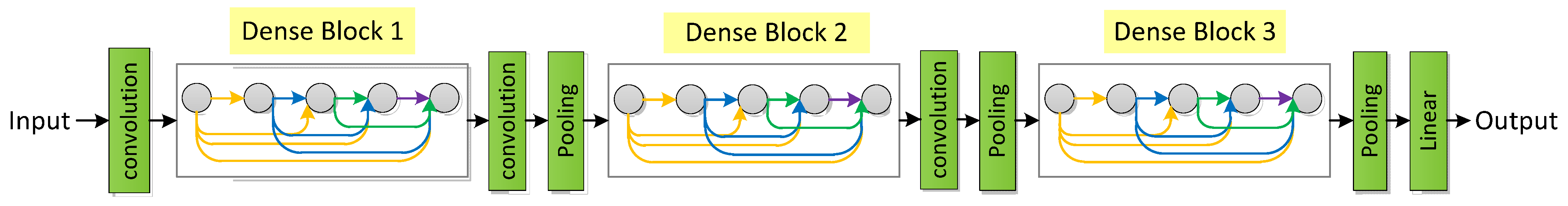
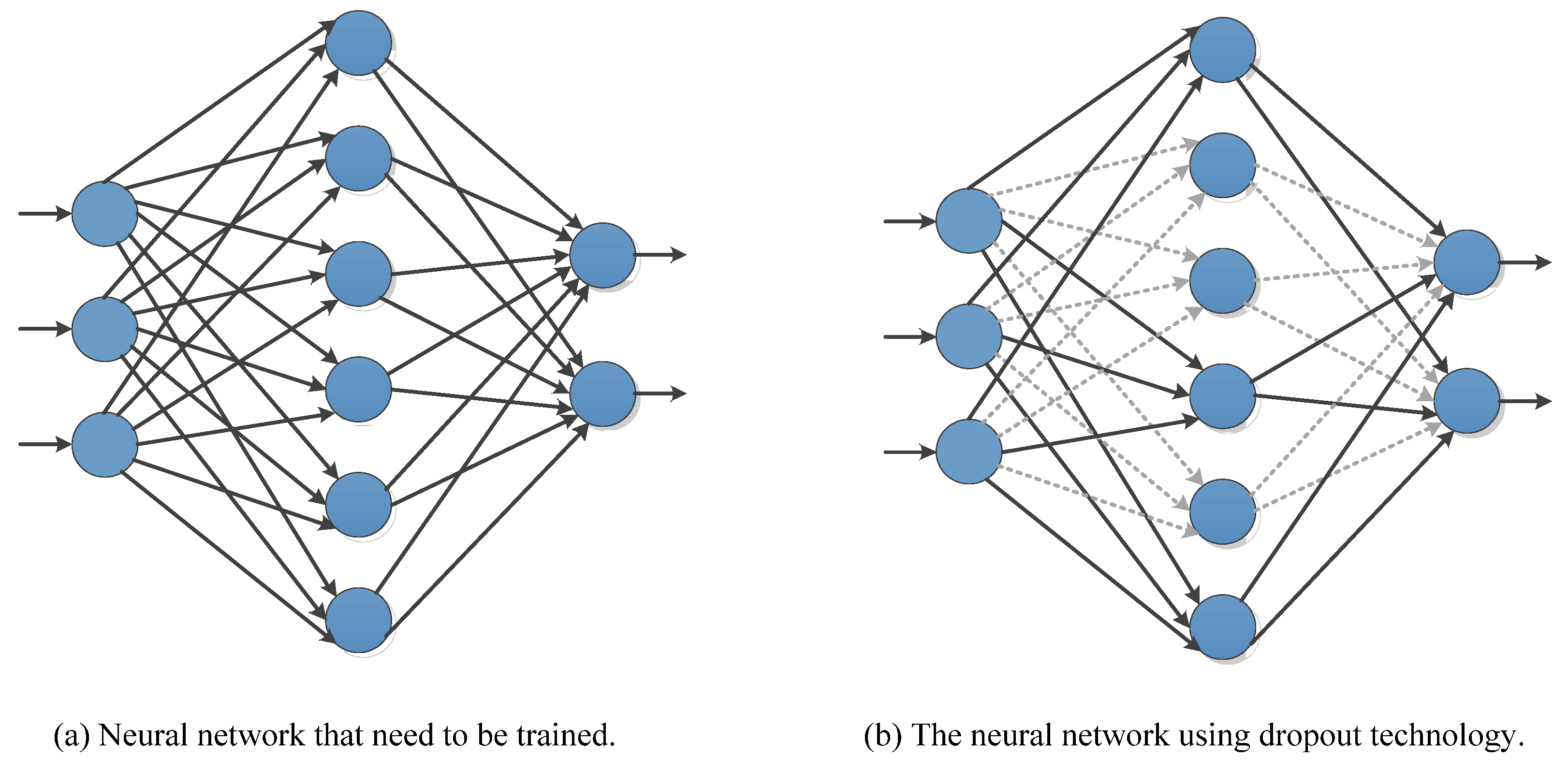
| Name | Medium | Imaging Method | Imaging Basis | Features | Advantages | Disadvantages | Radiation | Use Cases |
|---|---|---|---|---|---|---|---|---|
| MRI | Magnetic fields and radio waves | Mathematical reconstruction | A variety of parameters | Tomographic images, multi-parameter, multi-sequence imaging, rich grayscale information. | High soft-tissue resolution. | Long examination time, prone to motion artifacts, low spatial resolution, not suitable for patients with metal parts, high price. | No | soft tissue [23], nervous tissue [24], internal organs [25], etc. |
| X-ray | Ionizing radiation | Transmission projection | Density and thickness | Strong penetrability, wide dynamic range, suitable for image diagnosis with small grayscale differences. | Full picture, real-time, fine image, low cost. | Limited density resolution, overlapping images, poor identification of soft tissues. | Yes | Skeletal system [26], gastrointestinal tract [27], cardiovascular angiography and dynamic observation [28,29], etc. |
| CT | Ionizing radiation | Mathematical reconstruction | Absorption coefficient | Tomographic image, grayscale image, higher grayscale, can display the tissue density of the human body section. | Fast imaging speed, high-density resolution, no image overlap, further quantitative analysis. | Low spatial resolution, artifacts, and partial volume effects, reflecting only anatomical features. | Yes | Bones [30], lungs, internal organs [31], angiography [32,33], etc. |
| US | Sound waves | Mathematical reconstruction | Acoustic impedance interface | Suitable for moderate acoustic tissue measurements (soft tissue, muscle, etc.) and imaging of human anatomy and blood flow. | Safe and reliable, no radiation, low cost, can detect very subtle diseased tissue, real-time dynamic imaging. | Poor image contrast, limited field of view, difficult in displaying normal tissue and large lesions. | No | Abdominal organs [34], heart [35,36], ophthalmology [37], obstetrics and gynecology [38], etc. |
| PET | Radioactive tracer | Mathematical reconstruction | Using positron radionuclide labeling | Concentration image of positrons, showing biological metabolic activity. | High sensitivity, high specificity, whole-body imaging, accurate location can achieve the purpose of early diagnosis. | Low image clarity, poor specificity for inflammation, expensive, the examiner needs to have rich experience. | Yes | Brain blood flow [39], metabolic activity [40], cancer diagnosis [41], etc. |
| CT | Description |
|---|---|
| Conception |
|
| Feature |
|
| Histopathological Image | Description |
|---|---|
| Conception |
|
| Feature |
|
| Name | Brief Description | Basic Module |
|---|---|---|
| CNN [129] | Feed-forward neural network with convolutional computation and deep structure. | It consists of an input layer, an output layer, and multiple hidden layers. The hidden layers can be divided into convolutional layers, pooling layers, RELU, and fully connected layers. |
| FCN [130] | Pixel-level classification of images solves the problem of semantic-level image segmentation. | All layers in the model network are convolutional layers. |
| AE [131] | Efficient feature extraction and feature representation for high-dimensional data using unsupervised learning. | Encoder and decoder. |
| DC-ELM [132] | Combining the feature abstraction performance of convolutional neural networks and the fast training of extreme learning machines | It consists of an input layer, an output layer, and multiple alternating convolutional and pooling layers. |
| RNN [133] | A neural network with short-term memory is often used to process time-series data. | It consists of an input layer, a recurrently connected hidden layer, and an output layer. |
| LTSM [134] | A special kind of RNN capable of learning long dependencies. | A cell, an input gate, an output gate, and a forget gate. |
| GAN [135] | A deep generative model based on adversarial learning. | Generator and discriminator. |
| DBN [136] | The number of layers is increased by stacking multiple RBMs to increase expressive power. | Multi-layer RBM. |
| DBM [137] | A stack of multi-layer RBMs. The middle layer is bidirectionally connected to the adjacent layer. | Boltzmann distribution. |
| Name | Year | Brief Description |
|---|---|---|
| LeNet-5 [129] | 1998 | It was designed to solve the problem of handwritten digit recognition and is considered one of the pioneering works of CNN. |
| AlexNet [208] | 2012 | The first deep convolutional neural network structure on large-scale image datasets. |
| ZF-Net [209] | 2014 | Visualize and understand convolutional networks. |
| VGGNet [210] | 2014 | Deeper architecture and simpler form. |
| GoogLeNet [211] | 2014 | The introduced Inception combines feature information of different scales to obtain better feature representation. |
| U-net [212] | 2015 | Convolutional networks for biomedical image segmentation. |
| ResNet [213] | 2016 | The internal residual block uses a skip connection which alleviates the gradient disappearance problem caused by increasing the depth in the deep neural network. |
| MobileNet [214] | 2016 | Lightweight CNN for mobile vision problems. |
| SqueezeNet [215] | 2016 | Use the fire module to compress parameters. |
| DarkNet [216] | 2016 | An open source neural network framework written in C and CUDA. |
| DenseNet [217] | 2017 | Re-usage of feature maps. |
| XceptionNet [218] | 2017 | Better performed than Inception-v3. |
| Inception-ResNet [219] | 2017 | Residual connections were used to increase the training speed of Inception networks. |
| ShuffleNet [220] | 2018 | Lightweight CNN using pointwise group convolution and channel shuffle. |
| NasNet [221] | 2018 | An architectural building block is searched on a small dataset and then transferred to a larger dataset. |
| EfficientNet [222] | 2019 | A compound scaling method with extremely high parametric efficiency and speed. |
| Brief Description | Description | Typical Representative |
|---|---|---|
| Dense connection mechanism | There is a direct connection between any two layers. | Dense U-Net [253]; Denseblock U-Net [254] |
| Residual connection mechanism | The convolution layer of U-Net is replaced with a residual block. The skip connection uses a residual connection path. The encoder and decoder are replaced by a residual network. | Residual U-Net [255]; RRA-UNet [256] |
| Multi-scale mechanism | Images of multiple scales are input and the results are fused so that the final output combines the features from receptive fields of different sizes. | MU-Net [257]; MDFA-Net [258] |
| Ensemble mechanism | A group of neural networks processes the same input data in parallel and then combines their outputs to complete the segmentation. | AssemblyNet [259]; DIU-Net [260] |
| Dilated mechanism | The dilated convolution is used in the encoder and decoder to increase the size of the small convolution kernel while keeping the parameter amount of the convolution unchanged. | DiSegNet [261]; DIN [262] |
| Attention mechanism | Attention modules are added to the encoder, decoder, and skip connections. | ANU-Net [263]; SA-UNet [264] |
| Transformer mechanism | The operation of the encoder, decoder, and jump connection is changed to transformer. | TA-Net [265]; FAT-Net [266] |
| Author | Variation | Year | Features |
|---|---|---|---|
| Ronneberger et al. [212] | U-Net | 2015 | It consists of a contracting path and an expanding path, which are used to obtain contextual information and precise localization, respectively. These two paths are symmetrical to each other. |
| Çiçek et al. [267] | 3D U-Net | 2016 | The core structure still contains a contracting path and a symmetric expanding path. Overall, 3D volume segmentation is supported and all 2D operations are replaced by corresponding 3D operations, resulting in 3D segmented images that can be segmented with minimal annotation examples. |
| Oktay et al. [268] | Attention U-Net | 2018 | It is a hybrid structure that uses attention gates to focus on specific objects of importance and each level in the expansion path has an attention gate through which corresponding features from the contraction path must pass. |
| Alom et al. [269] | R2U-Net 1 | 2018 | It builds on residual and loop techniques, using a simpler concatenation of functions from the encoder to decoder. |
| Zhou et al. [270] | U-Net++ | 2018 | It is essentially a deeply supervised encoder–decoder network, where the encoder and decoder subnetworks are connected by a series of nested, dense skip pathways, aiming to reduce the semantic gap between feature maps. |
| Khanna et al. [255] | Residual U-Net | 2020 | Residuals are integrated into the contraction path of U-Net networks, thus reducing the computational burden and avoiding network degradation problems. |
| Ibtehaz et al. [271] | MultiResUNet | 2020 | Res paths are proposed to reconcile two incompatible sets of features. The two feature mappings are more uniform. MultiRes blocks are proposed to augment U-Net’s multiresolution analysis capabilities. It is lightweight and requires less memory. |
| Li et al. [263] | ANU-Net 2 | 2020 | A redesigned dense skip connection and attention mechanism are introduced and a new hybrid loss function is designed. |
| Zhang et al. [260] | DIU-Net 3 | 2020 | The Inception-Res module and the densely connecting convolutional module are integrated into the U-net structure. |
| Yeung et al. [272] | Focus U-Net | 2021 | Efficient spatial and channel attention are combined into Focus Gate. Focus Gate uses an adjustable focal parameter to control the degree of background suppression. Short-range skip connections and deep supervision are added. Hybrid focal loss is used to deal with class-imbalanced image segmentation. |
| Beeche et al. [273] | Super U-Net | 2022 | In the classical U-Net architecture, a dynamic receiving field module and a fusion up-sampling module are integrated. Image segmentation performance can be improved significantly by dynamic receptive field and fusion up sampling. |
| Author | Datasets | IoU (%) | Dice (%) | Precision (%) | Recall (%) | DSC 1 (%) | JI 2 (%) | Body Part or Organ |
|---|---|---|---|---|---|---|---|---|
| Ronneberger et al. [212] | LiTS | 89.49 | 94.45 | 93.24 | 95.70 | - | - | Liver |
| CHAOS | 84.46 | 91.58 | 89.20 | 94.08 | - | - | Kidney | |
| CHAOS | 76.18 | 86.48 | 82.34 | 91.06 | - | - | Spleen | |
| CHAOS | 75.37 | 85.96 | 87.31 | 84.65 | - | - | Liver | |
| Çiçek et al. [267] | Private dataset | 86.30 | - | - | - | - | - | The Xenopus kidney |
| Oktay et al. [268] | LiTS | 93.39 | 96.58 | 96.79 | 96.37 | - | - | Liver |
| CHAOS | 85.77 | 92.34 | 90.97 | 93.76 | - | - | Kidney | |
| CHAOS | 84.13 | 91.38 | 91.54 | 91.22 | - | - | Spleen | |
| CHAOS | 76.00 | 86.37 | 91.11 | 82.09 | - | - | Liver | |
| Alom et al. [269] | LiTS | 90.69 | 95.11 | 93.80 | 96.48 | - | - | Liver |
| CHAOS | 85.54 | 92.21 | 91.92 | 92.50 | - | - | Kidney | |
| CHAOS | 81.50 | 89.77 | 93.60 | 86.24 | - | - | Spleen | |
| CHAOS | 77.80 | 87.50 | 92.11 | 83.39 | - | - | Liver | |
| Khanna et al. [255] | LUNA16 | - | - | - | 98.61 ± 0.14 | 98.63 ± 0.05 | 97.32± 0.10 | Lung |
| VESSEL12 | - | - | - | 99.61 ± 0.01 | 99.62 ± 0.003 | 99.24 ± 0.007 | Lung | |
| HUG-ILD | - | - | - | 98.73 ± 0.001 | 98.68 ± 0.04 | 97.39 + 0.06 | Lung | |
| Zhou et al. [270] | LiTS | 94.46 | 97.15 | 98.16 | 96.17 | - | - | Liver |
| CHAOS | 86.58 | 92.81 | 90.87 | 94.82 | - | - | Kidney | |
| CHAOS | 81.05 | 89.53 | 86.37 | 92.93 | - | - | Spleen | |
| CHAOS | 84.23 | 91.39 | 93.06 | 89.79 | - | - | Liver | |
| Yeung et al. [272] | Kvasir-SEG | 0.845 (mIoU) | - | 91.70 | 91.60 | 0.910(mDSC) | - | colorectum |
| CVC-ClinicDB | 0.893 (mIoU) | - | 93.00 | 95.60 | 0.941(mDSC) | - | colorectum | |
| Ibtehaz et al. [271] | ISIC-2018 | - | - | - | - | - | 80.2988 ± 0.3717 | Skin lesions |
| CVC-ClinicDB | - | - | - | - | - | 82.0574 ± 1.5953 | Colon | |
| FMID 3 | - | - | - | - | - | 91.6537 ± 0.9563 | U2OS cells; NIH3T3 cells | |
| BraTS17 | - | - | - | - | - | 78.1936 ± 0.7868 | Glioblastoma; lower grade glioma | |
| Li et al. [263] | LiTS | 97.48 | 98.15 | 98.15 | 99.31 | - | - | Liver |
| CHAOS | 90.10 | 94.79 | 94.00 | 95.60 | - | - | Kidney | |
| CHAOS | 89.23 | 94.31 | 95.19 | 93.44 | - | - | Spleen | |
| CHAOS | 87.89 | 93.55 | 94.23 | 92.88 | - | - | Liver | |
| Zhang et al. [260] | KDSB2017 4 | - | 98.57 | - | - | - | - | Lung |
| DRIVE + STARE + CHASH_DB1 | - | 95.82 | - | - | - | - | Blood vessel | |
| MICCAI BraTS 2017 | - | 98.92 | - | - | - | - | Brain tumor | |
| Beeche et al. [273] | DRIVE (n = 40) | - | - | - | - | 80.80 ± 0.021 | - | Retinal vessels |
| Kvasir-SEG (n = 1000) | - | - | - | - | 80.40 ± 0.239 | - | GI polyps | |
| CHASE DB1 (n = 28) | - | - | - | - | 75.20 ± 0.019 | - | Retinal vessels | |
| ISIC (n = 200) | - | - | - | - | 87.70 ± 0.135 | - | Skin lesions |
| Method | Description | Typical Method | Features |
|---|---|---|---|
| Instance | Samples from different source tasks are collected and reused for learning of the target task. | TrAdaBoost | The method is simple, easy to implement, unstable, and more empirical. |
| Feature-representation | By introducing the source data features to help complete the learning task of the target data feature domain, the features of the source domain and the target domain are transformed into the same space through feature transformation. | Self-taught learning, multi-task structure learning | Applicable to most methods, the effect is better, it is difficult to solve, and it is prone to overfitting. |
| Parameter | When some parameters are shared between the source task and the target task, or the prior distribution of model hyperparameters is shared, the model of the source domain is transferred to the target domain. | Learning to learn, Regularized multi-task learning | The similarity between the models can be fully utilized and the model parameters are not easy to converge. |
| Relational-knowledge | It facilitates learning tasks on target data by mining relational patterns relevant to the target data from the source domain. | Mapping | Compatible for data with dependency and identical distribution |
| Explanation Type | Characteristics |
|---|---|
| Local | Provide explanations for individual samples. |
| Global | Provide explanations for a set of samples or the entire model. |
| Data Modality Specific | Explanation methods that apply only to specific data types. |
| Data Modality Agnostic | Explanation methods are applicable to any data type. |
| Ad-Hoc | The model itself is designed to be inherently explainable. |
| Post-Hoc | Provide explanations after classification is performed. |
| Model Agnostic | Can explain any model and is not limited to a certain type. |
| Model Specific | Only available on certain models. |
| Attribution | Attempts to compute the most important neural network inputs relative to the network result. |
| Non-Attribution | Develop and validate an explainability method for a given specialized problem. |
| Method | Year | Goal | Description | Features |
|---|---|---|---|---|
| Xavier initialization [323] | 2010 | Solve the problem of gradient disappearance or gradient explosion that may be caused by random initialization. | The range of weight initialization is determined according to the number of input neurons in the previous layer and the number of output neurons in the next layer. | It reduces the probability of gradient vanishing/exploding problems. The influence of the activation function on the output data distribution is not considered and the ReLU activation function does not perform well. |
| Orthogonal Initialization [324] | 2013 | Orthogonalize the weight matrix. | It solves the problem of gradient disappearance and gradient explosion under the deep network and is often used in RNN. | It can effectively reduce the redundancy and overfitting in the neural network and improve the generalization ability and performance of the network. Computational complexity is high, so it may not be suitable for large neural networks. |
| He initialization [325] | 2015 | The input and output data have the same variance; suitable for neural networks using the ReLU activation function. | In the ReLU network, it is assumed that half of the neurons in each layer are activated and the other half is 0, just divide by 2 on the basis of Xavier to keep the variance constant. | Simple and effective, especially suitable for the case where the activation function is ReLU. Compared with the Xavier initialization, it can effectively improve the training speed and performance of the network. In some cases, it may cause the weight to be too small or too large, thus affecting the network performance. |
| Data-dependent Initialization [326] | 2015 | Focus on behavior on smaller training sets, handle structured initialization, and improve pretrained networks. | It relies on the initialization process of the data. All units in the network train at roughly the same rate by setting the network’s weights. | CNN representations for tasks with limited labeled data are significantly improved and representations learned by self-supervised and unsupervised methods are improved. Early training of CNNs on large-scale datasets is greatly accelerated. |
| LSUV [327] | 2015 | Produces thin and very deep neural networks. | The weights of each convolutional or inner product layer are pre-initialized with an orthogonal matrix. The variance in the output of each layer is normalized to be equal to 1 from the first layer to the last layer. | It has minimal computation and very low computational overhead. Due to variability in the data, it is often not possible to normalize the variance with the required precision. |
| Sparse initialization [328] | 2017 | Achieving sparsity. | The weights are all initialized to 0. Some parameters are chosen randomly with some random values. | The parameters occupy less memory. Redundancy and overfitting in neural networks can be reduced. The generalization ability and performance of the network are improved. Some elements in the weight matrix may be too large or too small, thereby affecting the performance of the network. |
| Fixup [329] | 2019 | For networks with residual branches. | Standard initialization is rescaled appropriately. | Deep residual networks can be reliably trained without normalization. |
| ZerO Initialization [330] | 2021 | Deterministic weight initialization. | It is based on the identity transformation and the Hadamard transformation and only initializes the weights of the network with 0 and 1 (up to a normalization factor). | Ultra-deep networks without batch normalization are trained. It has obvious characteristics of low-rank learning and solves the problem of training decay. The trained model is more reproducible. |
| Data Augmentation Category | Advantages | Disadvantages |
|---|---|---|
| Geometric transformations | It is simple and easy to implement, which can increase the spatial geometry information of the data set and improve the robustness of the model in different perspectives and positions. | The amount of added information is limited. The data is repeatedly memorized. Inappropriate operations may change the original semantic annotation of the image. |
| Color space | The method is simple and easy to implement. The color information of the dataset is added to improve the robustness of the model under different lighting conditions. | The amount of added information is limited and repeated memory of the data may change the important color information in the image. |
| Kernel filter | It can improve the robustness of the model to motion blur and highlight the details of objects. | It is implemented by filtering, which is repeated with the internal mechanism of CNN. |
| Image Erasing | It can increase the robustness of the model under occlusion conditions, enable the model to learn more descriptive features in the image, and pay attention to the global information of the entire image. | The semantic information of the original image may be tampered with. Images may not be recognized after important partial information is erased. |
| Mixing images | The pixel value information of multiple images is mixed. | Lack of interpretability |
| Noise injection | This method enhances the filtering ability of the model to noise interference and redundant information and improves the recognition ability of the model of different quality images. | Unable to add new valid information. The improvement effect on model accuracy is not obvious. |
| Feature space augmentation | The feature information of multiple images is fused. | Vector data is difficult to interpret. |
| Adversarial training [339] | It can improve the weak links in the learned decision boundary and improve the robustness of the model. | Extremely slow and inaccurate. More data, deeper and more complex models are required. |
| GAN-based Data Augmentation | Sampling from the fitted data distribution generates an unlimited number of samples. | A certain number of training samples are required to train the GAN model, which is difficult to train and requires extra model training costs. In most cases, the quality of the generated images is difficult to guarantee and the generated samples cannot be treated as real samples. |
| Neural style transfer | It can realize mutual conversion between images of the same content and different modalities and can help solve special problems in many fields. | Two datasets from different domains need to be constructed to train the style transfer model, which requires additional training overhead. |
| Meta learning data augmentations | Neural network is used to replace the definite data augmentation method to train the model to learn better augmentation strategies. | Introducing additional networks requires additional training overhead. |
| Reinforcement learning data augmentation | Combining existing data augmentation methods to search for the optimal strategy. | The policy search space is large, the training complexity is high, and the calculation overhead is large. |
| Data Augmentation Category | Method | Year | Describe | M/D |
|---|---|---|---|---|
| Geometric transformations | Flipping | - | Usually, the image flip operation is performed about the horizontal or vertical axis. | M |
| Rotating | - | Select an angle and rotate the image left or right to change the orientation of the image content. | M | |
| Zoom | - | The image is enlarged and reduced according to a certain ratio without changing the content in the image. | M | |
| shearing | - | Move part of the image in one direction and another part in the opposite direction. | M | |
| translating, | - | Shifting the image left, right, up, or down avoids positional bias in the data. | M | |
| skew | - | Perspective transforms. | M | |
| Cropping | - | It is divided into uniform cropping and random cropping. Uniform cropping crops images of different sizes to a set size. Random cropping is similar to translation, the difference is that translation retains the original image size and cropping reduces the size. | M | |
| Color space | Color jittering [213] | 2016 | Randomly change the brightness, contrast, and saturation of the image. | M |
| PCA jittering [340] | 2017 | Principal component analysis is carried out on the image to obtain the principal component and then the principal component is added to the original image by Gaussian disturbance with mean of 0 and variance of 0.1 to generate a new image. | M | |
| Kernel filter | Gaussian blur filter [341] | 1991 | The image is blurred using Gaussian blur. | M |
| edge filter [342] | 1986 | Get an image with edge sharpening, highlighting more details of the object. | M | |
| PatchShuffle [343] | 2017 | Rich local variations are created by generating images and feature maps with internally disordered patches. | M | |
| Image Erasing | Random erasing [344] | 2020 | During training, a rectangular region in the image is randomly selected and its pixels are erased with random values. | M |
| Cutout [345] | 2017 | Randomly mask input square regions during training. | M | |
| HaS [346] | 2017 | Patches are hidden randomly in training images. When the most discriminative parts are hidden, forcing the network to find other relevant parts. | M | |
| GridMask [347] | 2020 | Based on the deletion of regions in the input image, the deleted regions are a set of spatially uniformly distributed squares that can be controlled in terms of density and size. | M | |
| FenceMask [348] | 2020 | The “ simulation of object occlusion” strategy is employed to achieve a balance between the information preservation of input data and object occlusion. | M | |
| Mixing images | Mixup [349] | 2017 | The neural network is trained on convex combinations of pairs of examples and their labels. | M |
| SamplePairing [350] | 2018 | Another image randomly selected from the training data is superimposed on one image to synthesize a new sample, that is, the average value of the two images is taken for each pixel. | M | |
| Between-Class Learning [351] | 2018 | Two images belonging to different classes are mixed in a random ratio to generate between-class images. | M | |
| CutMix [352] | 2019 | Patches were cut and pasted among the training images, where the ground truth labels was also mixed in proportion to the area of the patches. | M | |
| AugMix [353] | 2019 | Using random and diverse augmentation, Jensen–Shannon divergence consistency loss, and mixing multiple augmented images | M | |
| Manifold Mixup [354] | 2019 | Using semantic interpolation as an additional training signal, neural networks with smoother decision boundaries at multiple representation levels are obtained. | M | |
| Fmix [355] | 2020 | A mixed sample data augmentation using a random binary mask obtained by applying thresholds to low-frequency images sampled from Fourier space. | M | |
| SmoothMix [356] | 2020 | Image blending is conducted based on soft edges and training labels are computed accordingly. | M | |
| Deepmix [357] | 2021 | Takes embeddings of historical samples as input and generates augmented embeddings online. | M | |
| SalfMix [358] | 2021 | A data augmentation method for generating saliency map-based self-mixing images. | M | |
| Noise injection | forward noise adjustment scheme [359] | 2018 | Insert random values into an image to create a new image. | M |
| DisturbLabel [360] | 2016 | Randomly replace the labels of some samples during training and apply disturbance to the sample labels, which is equivalent to adding noise at the loss level. | M | |
| Feature space augmentation | Dataset Augmentation in Feature Space [361] | 2017 | Representation is first learned using encoder–decoder algorithm and then different transformations are applied to the representation, such as adding noise, interpolation, or extrapolation. | D |
| Feature Space Augmentation for Long-Tailed Data [362] | 2020 | Use features learned from the classes with sufficient samples to augment underrepresented classes in the feature space. | D | |
| SMOTE [363] | 2002 | Interpolate on the feature space to generate new samples. | D | |
| FeatMatch [364] | 2020 | A learned feature-based refinement and augmentation method that exploits information from within-class and across-class representations extracted through clustering to produce various complex sets of transformations. | D | |
| Adversarial training [339] | FGSM [365] | 2014 | This method believes that the attack is to add disturbance to increase the loss of the model and it should be best to generate attack samples along the gradient direction. It is a one-time attack. That is, adding a gradient to a graph only increases the gradient once. | D |
| PGD [366] | 2017 | As the strongest first-order adversary, it is an efficient solution to the internal maximization problem. | D | |
| FGSM+ random initialization [367] | 2020 | Eric Wong, Leslie Rice, and J. Zico Kolter. Fast is better than free: Revisiting adversarial training. In ICLR, 2020. Adversarial training using FGSM, combined with random initialization, is as effective as PGD-based training, but at a much lower cost. | D | |
| GradAlign [368] | 2020 | Catastrophic overfitting is prevented by explicitly maximizing the gradient alignment inside the perturbation set. | D | |
| Fast C and W [369] | 2021 | An accelerated SAR-TR AA algorithm. A network of trained deep encoder replaces the process of iteratively searching for the best perturbation of the input SAR image in the vanilla C and W algorithm. | D | |
| GAN-based Data Augmentation | GAN [194] | 2014 | sing GAN generative models to generate more data can be used as an oversampling technique to address class imbalance. | D |
| CGAN [370] | 2014 | Add some constraints to GAN to control the generation of images. | ||
| DCGAN [371] | 2015 | Combining CNN with GAN, deep convolutional adversarial pair learns representation hierarchies from object parts to scenes in the generator and discriminator. | D | |
| LapGAN [372] | 2015 | Images are generated in a coarse-to-fine fashion using a cascade of convolutional networks within the Laplacian Pyramid framework. | D | |
| InfoGAN [373] | 2016 | Interpretable representation learning in a completely unsupervised manner via information-maximizing GANs. | D | |
| EBGAN [374] | 2016 | An energy-based generative adversarial network model in which the discriminator is treated as a function of energy. | D | |
| WGAN [375] | 2017 | The loss function is derived by means of earth mover or Wasserstein distance. | D | |
| BEGAN [376] | 2017 | The generator and discriminator are balanced for training an autoencoder-based generative adversarial network. | D | |
| PGGAN [377] | 2017 | Generators and discriminators are progressively increasing. | D | |
| BigGAN [378] | 2018 | Large-scale GAN training for high-fidelity natural image synthesis based on GAN architecture. | D | |
| StyleGAN [379] | 2019 | A style-based GAN generator architecture controls the image synthesis process. | D | |
| SiftingGAN [380] | 2019 | The traditional GAN framework is extended to include an online output method for generating samples, a generative model screening method for model sifting, and a labeled sample discrimination method for sample sifting. | D | |
| Neural style transfer | CycleGAN [381] | 2017 | It only needs to build the respective sample sets of the two image style domains, and unpaired samples can be used for training, which greatly reduces the difficulty of building training sample sets and makes the style transfer between any image domains easier to realize. | D |
| Pix2Pix [382] | 2017 | Conditional adversarial networks as a general solution to image-to-image translation problems. | D | |
| Meta learning data augmentations | Neural augmentation [383] | 2017 | Before the classification network, an augmented network is introduced to input two randomly selected images of the same category, learn the common content information or style information of the two images through the neural network, and then obtain an “enhanced image”, which is input into the classification network together with the original image for classification model training. | D |
| Smart Augmentation [384] | 2017 | Reduce network losses by creating a network to learn how to generate enhanced data during the training of the target network. | D | |
| Reinforcement learning data augmentation | AutoAugment [385] | 2018 | The search space is designed and has a strategy consisting of many sub-strategies, the best data augmentation is found by an automatic search strategy. | D |
| Fast Autoaugment [386] | 2019 | The efficient search strategy based on density matching is used to find better data expansion, thus reducing the time of high order training. | D | |
| Faster AutoAugment [387] | 2020 | The differentiable policy search pipeline not only estimates the gradient for many conversion operations with discrete parameters but also provides an efficient mechanism for selecting operations. | D | |
| MARL [388] | 2021 | An automatic local patch augmentation method based on multi-agent collaboration and the first to use reinforcement learning to find a patch-level data augmentation strategy. | D | |
| RandAugment [389] | 2020 | Simplifies the search space, greatly reduces the computational cost of automatic augmentation, and allows the removal of a separate proxy task. | D |
| Reference | Year | Method | Dataset (s) | Imaging Modality | Type of Cancer | Evaluation Metric(s) |
|---|---|---|---|---|---|---|
| Kavitha et al. [402] | 2021 | BPNN | Mini-MIAS DDSM | Mammography | Breast Cancer | Accuracy: 98.50% Accuracy: 97.55% |
| Nawaz et al. [399] | 2018 | DenseNet | BreakHis | histopathological images | Breast Cancer | Accuracy: 95.4% |
| Spanhol et al. [403] | 2016 | CNN | BreaKHis | Histopathology | Breast cancer | Accuracy: between 98.87% and 99.34% for the binary classification; between 90.66% and 93.81% for the multi-class classification. |
| Fu’adah et al. [391] | 2020 | CNN | ISIC | - | skin cancer | Accuracy: 99% |
| Anand et al. [393] | 2022 | DC-ELM | private | histopathological images | Bone Cancer | Accuracy: 97.27% Sensitivity: 98.204% Specificity: 99.568% Precision: 87.832% |
| Beevi et al. [394] | 2017 | DBN | MITOS RCC | histopathology images | Breast cancer | F-score: 84.29% F-score: 75% |
| Shahweli [395] | 2020 | DBN | IARC TP53 | - | lung cancer | Accuracy: 96% |
| Abdel-Zaher et al. [397] | 2016 | DBN | WBCD | - | Breast cancer | Accuracy: 99.68% Sensitivity: 100% Specificity: 99.47% |
| Jabeen et al. [400] | 2022 | DarkNet-53 | BUSI | ultrasound images | Breast cancer | Accuracy: 99.1% |
| Das et al. [404] | 2019 | DNN | Private dataset | CT/3D | Liver | Accuracy: 99.38% Jaccard index: 98.18% |
| Mohsen et al. [405] | 2018 | DNN | Harvard Medical School website | MRI | Brain | Precision: 97% Rate: 96.9% Recall: 97% F-measure: 97% AUC: 98.4% |
| El-Ghany et al. [401] | 2023 | ResNet101 | LC25000 | histopathological images | Lung and colon cancer | Precision-99.84% Recall: 99.85% F1-score: 99.84% Specificity: 99.96% Accuracy: 99.94% |
| Attallah [406] | 2023 | CerCan·Net | SIPaKMeD and Mendeley | - | Cervical cancer | Accuracy: 97.7% (SIPaKMeD) Accuracy: 100% (Mendeley) |
| Reference | Year | Method | Imaging Modality | Type of Cancer | Datasets | Evaluation Metrics |
|---|---|---|---|---|---|---|
| Shen et al. [420] | 2021 | GMIC | Mammogram | Breast Cancer | NYUBCS + CBIS-DDSM | DSC 1 (Malignant): 0.325 ± 0.231 DSC (Benign): 0.240 ± 0.175 PxAP 2 (Malignant): 0.396 ± 0.275 PxAP (Benign): 0.283 ± 0.244 |
| Ranjbarzadeh et al. [421] | 2021 | C-ConvNet/C-CNN | MRI | Brain tumor | BRATS 2018 | Dice (mean): 0.9203 (Whole 3) 0.9113 (Enh 4), 0.8726 (Core 5) Sensitivity (mean): 0.9386 (Whole), 0.9217 (Enh), 0.9712 (Core) HAUSDORFF99 (mm): 1.427 (Whole), 1.669 (Enh), 2.408 (Core) |
| Ari et al. [426] | 2018 | ELM-LRF | MRI | Brain Cancer | Simulated datasets | Accuracy: 97.18% Sensitivity: 96.80% Specificity: 97.12% |
| Zhang et al. [414] | 2022 | Mask R-CNN | MRI | Breast Cancer | DCE-MRI | Accuracy (mean): 0.86 DSC: 0.82 |
| Asuntha et al. [419] | 2020 | FPSOCNN | CT | Lung cancer | WRT 6 | Average accuracy: 94.97% Average sensitivity: 96.68% Average specificity: 95.89% |
| LIDC | Average accuracy: 95.62% Average sensitivity: 97.93% Average specificity: 6.32% | |||||
| Zhou et al. [418] | 2019 | 3D CNN | MRI | Breast cancer | Private dataset | Accuracy: 83.7% Sensitivity: 90.8% Specificity: 69.3% Overall dice distance: 0.501 ± 0.274 |
| Welikala et al. [411] | 2020 | ResNet-101 + Faster R-CNN | MRI | Oral cancer | Private dataset | F1: 87.07% (for identification of images that contained lesions) F1: 78.30% (for the identification of images that required referral) |
| Zhang et al. [424] | 2022 | DDTNet | Histopathological image | Breast cancer | BCa-lym | F1: 0.885 Dice: 0.845 PQ 7: 0.731 Time: 0.0674 |
| Post-NAT-BRCA | F1: 0.892 Dice: 0.846 PQ: 0.782 Time: 0.0662 | |||||
| TCGA-lym | F1: 0.793 Dice: 0.788 PQ: 0.635 Time: 0.0647 | |||||
| Maqsood et al. [425] | 2022 | TTCNN | Mammogram | Breast cancer | DDSM + INbreast + MIAS | Accuracy: 97.49% |
| Chattopadhyay et al. [427] | 2022 | CNN | MRI | Brain cancer | BRATS | Accuracy: 99.74% |
| Luo et al. [422] | 2022 | SCPM-Net | 3D CT | Lung cancer | LUNA16 | Average sensitivity: 89.2% |
| Cao et al. [413] | 2019 | Mask R-CNN | Pathological images | Gastric Cancer | Private dataset | AP: 61.2 |
| Reference | Year | Method | Datasets | Imaging Modality | Type of Cancer | Evaluation Metrics |
|---|---|---|---|---|---|---|
| Zhu et al. [439] | 2018 | Adversarial FCN-CRF | Inbreast + DDSM-BCRP | Mammogram | Breast Cancer | Accuracy: 97.0% |
| Al-Antari et al. [440] | 2018 | Frcn | Inbreast | Mammogram | Breast Cancer | Dice: 92.69% MCC 1: 85.93% Accuracy: 92.97% JSC 2: 86.37% |
| Dong et al. [435] | 2020 | HFCNN | Private dataset | CT | Liver cancer | Dice: 92% |
| Shukla et al. [436] | 2022 | Cfcns | 3DIRCAD | CT | Liver cancer | Accuracy: 93.85% |
| Ayalew et al. [438] | 2021 | U-Net | 3Dircadb01 + LITS | CT | Liver cancer | Dice: 96% (liver segmentation) Dice: 74% (segmentation of tumors from the liver) Dice: 63% (segmentation of tumor from abdominal CT scan images) |
| Li et al. [441] | 2018 | CRU-Net | Inbreast DDSM-BCRP | Mammogram | Breast Cancer | Dice Index: 93.66% (Inbreast) Dice Index: 91.43% (DDSM-BCRP) |
| Shen et al. [442] | 2019 | Rescu-Net + MS-rescu-Net | Inbreast | Mammogram | Breast Cancer | Dice: 91.78% Jaccard index: 85.12% Accuracy: 94.16% |
| Li et al. [443] | 2019 | U-Net + AGS | DDSM | Mammogram | Breast Cancer | Accuracy: 78.38% Sensitivity: 77.89% F-score: 82.24% |
| Hossain [444] | 2019 | U-Net | DDSM | Mammogram | Breast Cancer | Dice: 97.80% F-score: 98.50% Jaccard index: 97.4% |
| Sun et al. [445] | 2020 | Aunet | INbreast CBIS-DDSM | Mammogram | Breast Cancer | Dice: 79.10% (INbreast) Dice: 81.80% (CBIS-DDSM) |
| Min et al. [446] | 2020 | Mask RCNN | Inbreast | Mammogram | Breast Cancer | Dice: 88.00% |
| Al-Antari et al. [447] | 2020 | FrCN | Inbreast | Mammogram | Breast Cancer | Dice: 92.69% Accuracy: 92.97% MCC: 85.93% JAC: 86.37% |
| Abdelhafiz et al. [448] | 2020 | Vanilla U-Net | Inbreast + DDSM | Mammogram | Breast Cancer | Accuracy (mean): 92.6% IoU 3: 90.90% |
| Rajalakshmi et al. [449] | 2020 | DS-U-Net | Inbreast CBIS-DDSM | Mammogram | Breast Cancer | Dice: 79% (Inbreast) Sensitivity: 81% (Inbreast) Dice: 82.7% (CBIS-DDSM) Sensitivity: 84.1% (CBIS-DDSM) |
| Saffari et al. [450] | 2020 | Cgan | Inbreast | Mammogram | Breast Cancer | Dice: 88.0% Jaccard index: 78.0% Accuracy: 98.0% Precision: 97.85% Sensitivity: 97.85% Specificity: 99.28% |
| Singh et al. [451] | 2020 | Cgan | DDSM | Mammogram | Breast Cancer | Dice: 94.0% IoU: 87.0% |
| Ahmed et al. [452] | 2020 | Mask RCNN Deep lab v3 | MIAS + CBIS-DDSM | Mammogram | Breast Cancer | Average precision: 80.0% (Mask RCNN) Average precision: 75.0% (Deep lab v3) |
| Bhatti et al. [453] | 2020 | Mask RCCN-FPN | DDSM | Mammogram | Breast Cancer | Accuracy: 91.0% Precision: 84.0% |
| Zeiser et al. [454] | 2020 | U-Net | DDSM | Mammogram | Breast Cancer | Dice Index: 79.39% Accuracy: 85.95% AUC 4: 86.40% Sensitivity: 92.32% Specificity: 80.47% |
| Tsochatzidis et al. [455] | 2021 | U-Net | DDSM-400 CBIS-DDSM | Mammogram | Breast Cancer | AUC: 89.8% (DDSM-400) AUC: 86.2% (CBIS-DDSM) |
| Salama et al. [456] | 2021 | U-Net | MIAS DDSM CBIS-DDSM | Mammogram | Breast Cancer | Dice: 98.87% (DDSM) AUC: 98.88% (DDSM) Sensitivity: 98.98% (DDSM) Precision: 98.79% (DDSM) F1 score: 97.99% (DDSM) |
| Zhao et al. [457] | 2018 | Fcnns | BRATS 2013 + BRATS 2015 + BRATS 2016 | MRI | Brain | Dice: 86% |
| He et al. [458] | 2022 | ANN | LIDC-IDRI | CT | Lung | Accuracy: 94.6% Sensitivity: 95.7% Specificity: 93.5% |
| Hu et al. [459] | 2023 | U-Net++ | GC-PNI | Pathological images | Gastric cancer | Sensitivity: 97.2% Specificity: 93.3% |
| Reference | Year | Method | Transformation | Supervision | ROI | Modality | Datasets | Evaluation Metrics |
|---|---|---|---|---|---|---|---|---|
| Fu et al. [467] | 2020 | LungRegNet | Deformable | Unsupervised | Lung images | 4D-CT | Private dataset + DIRLAB | TRE 1 (mean): 1.00 ± 0.53 mm Standard deviation: 1.59 ± 1.58 mm |
| Kim et al. [468] | 2021 | CycleMorph | Deformable | Unsupervised | Brain | MRI | OASIS-3 | CycleMorph, global: Dice: 0.750 (0.144) Time: 0.01 (GPU) CycleMorph, multi: Dice: 0.756 (0.141) Time: 2.18 (GPU) |
| Liver | 4D-CT | Private dataset | (1) Arterial → Portal: CycleMorph, global: TRE: 4.722 (3.294) Time: 0.06 (GPU) CycleMorph, multi: TRE: 4.720 (3.275) Time: 0.69 (GPU) (2) Delayed → Portal: CycleMorph, global: TRE: 3.902 (1.694) Time: 0.06 (GPU) CycleMorph, multi: TRE: 3.928 (1.696) Time: 0.69 (GPU) | |||||
| Xie et al. [466] | 2021 | IBs DIR 2 + PM 3 + MT 4 | Deformable | - | Breast cancer | CT | Private dataset | TRE (mm): 2.27 JD (mean) 5: 0.93 ± 0.18 |
| Wodzinski et al. [461] | 2021 | U-Net | Non-rigid | Semi-Supervised | Breast cancer | CT | Private dataset | TRE (mean): < 6.5 mm RVR 6 ≈ 0 Registration time < 1 s |
| Wei et al. [463] | 2021 | U-net + ResNet18 | Rigid | - | Liver | 2D US—3D CT/MR | Private dataset | Distance error: 4.7 ± 1.7 mm Angle errors: 11.6 ± 6.3° |
| Zhang et al. [470] | 2021 | GroupRegNet | Deformable | Unsupervised | Lung cancer | 4D-CT | DIR-Lab | Average RMSE: 1.48 mm (Landmark300) Average RMSE: 0.85 mm (LandmarkDense) |
| Salehi et al. [464] | 2022 | DIRNet | Non-rigid | - | Cervical Cancer | CT | Private dataset | Mean Dice indices: 0.89 ± 0.02 (cervix), 0.96 ± 0.01 (bladder), 0.93 ± 0.02 (rectum) ASSD 7: 1.61 ± 0.46 mm (cervix), 1.17 ± 0.15 mm (bladder), 1.06 ± 0.42 mm (rectum) Jaccard coefficient: 0.86 ± 0.04 (cervix), 0.93 ± 0.01 (bladder), 0.88 ± 0.04 (rectum) |
| Xie et al. [465] | 2022 | STN | Deformable | Unsupervised | Abdominal cancer | CBCT–CBCT | Private dataset | TRE: 1.91 ± 1.11 mm MAE: 33.42 ± 7.48 HU NCC 8: 0.94 ± 0.04 HU |
| Lei et al. [471] | 2022 | Dual-feasible framework | Deformable | - | Head-and-neck cancer | pCT-QA CT | Private dataset | MAE 9: 40.6 HU PSNR 10: 30.8 dB SSIM 11: 0.94 TRE: 2.0 mm |
| Reference | Year | Method | ROI | Modality | Datasets | Evaluation Metrics |
|---|---|---|---|---|---|---|
| Gassenmaier et al. [476] | 2021 | TSE | Prostate cancer | mpMRI | Private dataset | Acquisition time reduced by more than 60%. |
| Feng et al. [478] | 2022 | Z-Net | Breast cancer | MRI + NIRST | Simulation datasets | Number of parameter (M): 3.48 Training time (hours): 3.9 Average MSE 1: 0.05±0.04 Average PSNR 2: 43.3±3.8 dB Average SSIM 3: 0.99 |
| Wei et al. [479] | 2022 | - | Liver cancer | Cine-MRI | Private dataset | DSC (liver): >96.1% Standard deviation (liver) < 1.3% Localization error (blood vessel): <2.6 mm Standard deviation (blood vessel): <2.0 mm Time: ≈ 100 ms |
| Koike et al. [480] | 2022 | U-Net | head and neck cancer | SECT | Private dataset | Lowest MAE: 13.32 ± 2.20 HU Highest PSNR: 47.03 ± 2.33 dB SSIM: 0.9965 ± 0.0009 |
| Deng et al. [477] | 2023 | FDU-Net | Breast cancer | DOT | Simulated dataset | It has clear advantages over traditional DOT image reconstruction methods, providing over four orders of magnitude speedup in computation time. |
| Kim et al. [472] | 2023 | DLR | Rectal cancer | MRI | Private dataset | SNR (MRIDLR30): 9.44 ± 2.31 (p < 0.001) SNR (MRIDLR50): 11.83 ± 3.07 (p < 0.001) Sensitivity (MRIconv): 48.9% Specificity (MRIconv): 80.8% Sensitivity (MRIDLR30): 48.9% Specificity (MRIDLR30): 88.2%% Sensitivity (MRIDLR50): 38.8% Specificity (MRIDLR50): 86.7% |
| Reference | Year | Method | ROI | Modality | Datasets | Evaluation Metrics |
|---|---|---|---|---|---|---|
| Chen et al. [492] | 2018 | U-net | Prostate cancer | MR–CT | Private dataset | Computation time: 3.84–7.65 s MAE 1: 29.96 ± 4.87 HU Gamma pass rate (1%/1 mm) > 98.03% Gamma pass rate (2%/2 mm) > 99.36% |
| Abhishek et al. [487] | 2019 | Conditional GAN | Skin cancer | Dermoscopic images | ISIC | Achieve an improvement of 5.17% in the mean Dice score as compared to a model trained with only classical data augmentation techniques. |
| Kanayama et al. [484] | 2019 | GAN | Gastric cancer | Endoscopic images | Private dataset | The dataset bias was lessened. |
| Qin et al. [488] | 2020 | GAN | Skin cancer | Dermatoscopic images | ISIC | Accuracy: 95.2% Sensitivity: 83.2% Specificity: 74.3% Average precision: 96.6% Balanced multiclass accuracy: 83.1% |
| Bahrami et al. [493] | 2020 | eCNN | Prostate cancer | MRI-t0-CT | Private dataset | ME 2: 2.8 ± 10.3 HU MAE: 30.0 ± 10.4 HU |
| Saha et al. [486] | 2021 | TilGAN | TILs 3 | Pathological images | CGADR 8 | Accuracy: 97.83%, F1-score: 97.37% Area under the curve: 97% |
| Baydoun et al. [489] | 2021 | sU-cGAN | Cervical cancer | MR–CT | Private dataset | Mean MAPE 4: 72.83 ± 25.42 Mean RMSE 5: 115.74 ± 21.84 PSNR 6: 63.41 ± 1.67 SSIM 7: 0.839 ± 0.044 |
| Zhang et al. [490] | 2022 | Conditional GAN | Head and neck cancer | CBCT–sCT | Private dataset | MAE: 36.23 ± 20.24 HU RMSE: 104.60 ± 41.05 HU SSIM: 0.83 ± 0.08 PSNR: 25.34 ± 3.19 dB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Hu, Z.; Wang, S.; Zhang, Y. Deep Learning for Medical Image-Based Cancer Diagnosis. Cancers 2023, 15, 3608. https://doi.org/10.3390/cancers15143608
Jiang X, Hu Z, Wang S, Zhang Y. Deep Learning for Medical Image-Based Cancer Diagnosis. Cancers. 2023; 15(14):3608. https://doi.org/10.3390/cancers15143608
Chicago/Turabian StyleJiang, Xiaoyan, Zuojin Hu, Shuihua Wang, and Yudong Zhang. 2023. "Deep Learning for Medical Image-Based Cancer Diagnosis" Cancers 15, no. 14: 3608. https://doi.org/10.3390/cancers15143608
APA StyleJiang, X., Hu, Z., Wang, S., & Zhang, Y. (2023). Deep Learning for Medical Image-Based Cancer Diagnosis. Cancers, 15(14), 3608. https://doi.org/10.3390/cancers15143608








