Elderly and Patients with Large Breast Volume Have an Increased Risk of Seroma Formation after Mastectomy—Results of the SerMa Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
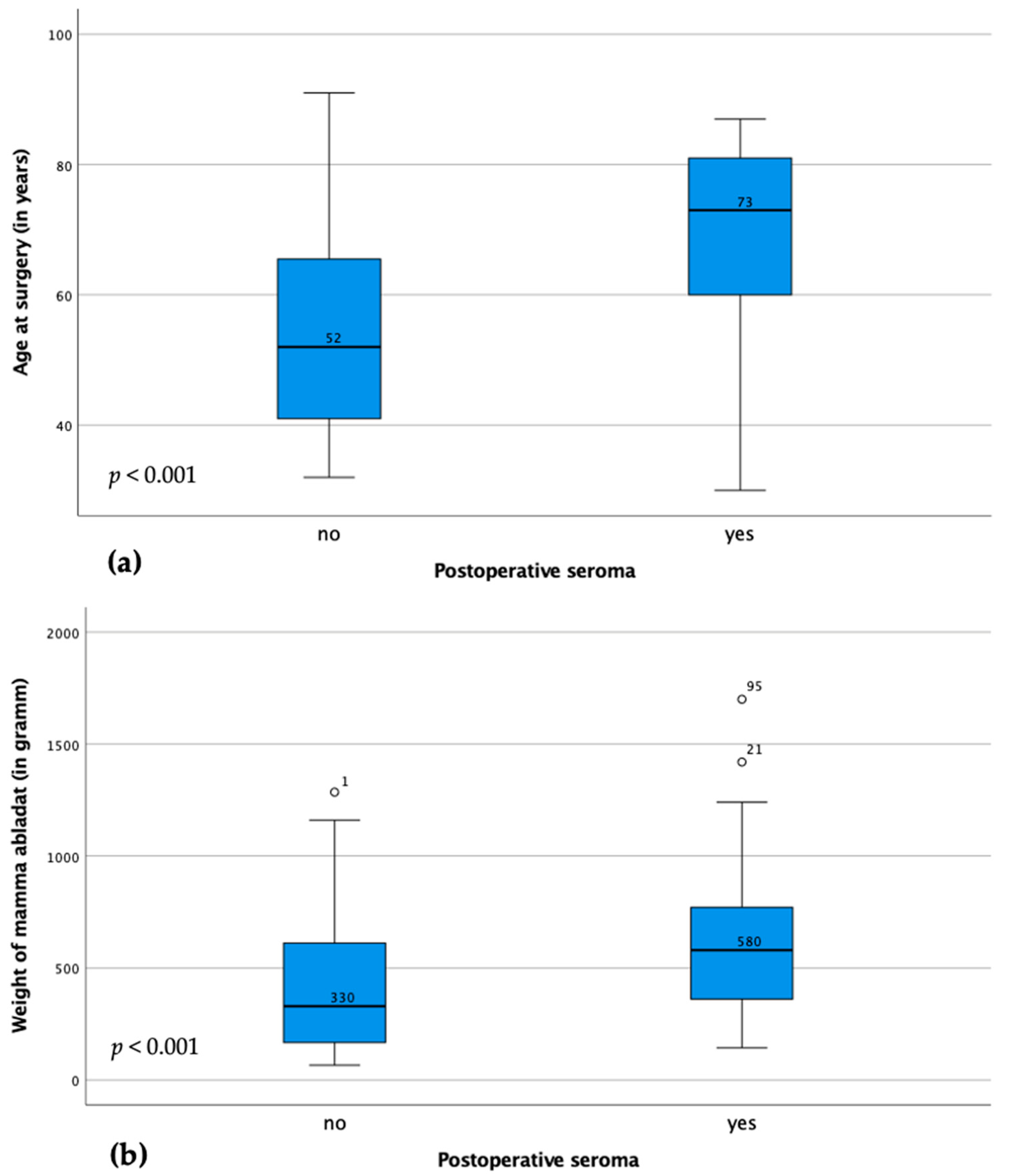
3. Results
3.1. Cohort Description
| Median (Min.–Max.) | |
|---|---|
| Age at surgery (in years) | 63 (30–91) |
| Body-Mass-Index (BMI) | 25.9 (18.9–56.6) |
| n (number) | |
| Histopathological type | |
| NST | 68 |
| Invasive lobular | 16 |
| Mucinous | 3 |
| Papillary | 3 |
| Other | 1 |
| CIS | 9 |
| Hormone receptor status | |
| HR+ | 84 |
| ER−/PR− | 16 |
| HER-2 | |
| Positive (IHC +++ or FISH pos.) | 10 |
| Negative (IHC 0) | 67 |
| Low (IHC + or ++, FISH neg.) | 14 |
| Not determined * | 9 |
| Ki67 | |
| <20% | 45 |
| ≥20% | 46 |
| Not determined * | 9 |
| Tumor size | |
| ypT0 | 16 |
| pTis | 8 |
| pT1 | 30 |
| pT2 | 24 |
| pT3 | 17 |
| pT4 | 5 |
| Axillary nodal status | |
| pN0 | 64 |
| pN1 | 20 |
| pN2 | 10 |
| pN3 | 2 |
| pNx | 4 |
| Grading | |
| G1 | 13 |
| G2 | 52 |
| G3 | 33 |
| No information available ** | 2 |
3.2. Correlation between Clinicopathological Parameters and Seroma Formation
3.3. Adjustment for Confounders by Binary Logistic Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALND | Axillary lymph node dissection |
| BMI | Body-Mass-Index |
| CI | Confidence interval |
| CIS | Carcinoma in situ |
| ER | Estrogen receptor |
| FISH | Fluorescence in situ hybridisation |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Hormone receptor |
| IHC | Immunohistochemistry |
| NST | Non special type |
| OR | Odds ratio |
| PR | Progesterone receptor |
| SerMa (study) | Seroma formations of the mammary gland in breast cancer patients after mastectomy (study) |
| SLND | Sentinel lymph node biopsy |
| TAD | Targeted axillary lymph node dissection |
| Th | T helper cell |
References
- Velotti, N.; Limite, G.; Vitiello, A.; Berardi, G.; Musella, M. Flap fixation in preventing seroma formation after mastectomy: An updated meta-analysis. Updates Surg. 2021, 73, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Montalto, E.; Mangraviti, S.; Costa, G.; Carrega, P.; Morandi, B.; Pezzino, G.; Bonaccorsi, I.; Cancellieri, A.; Mingari, M.C.; Mesiti, M.; et al. Seroma fluid subsequent to axillary lymph node dissection for breast cancer derives from an accumulation of afferent lymph. Immunol. Lett. 2010, 131, 67–72. Available online: https://www.ncbi.nlm.nih.gov/pubmed/20298720 (accessed on 7 July 2023). [CrossRef] [PubMed]
- Kuroi, K.; Shimozuma, K.; Taguchi, T.; Imai, H.; Yamashiro, H.; Ohsumi, S.; Saito, S. Pathophysiology of seroma in breast cancer. Breast Cancer 2005, 12, 288–293. [Google Scholar] [CrossRef]
- Watt-Boolsen, S.; Nielsen, V.B.; Jensen, J.; Bak, S. Postmastectomy seroma. A study of the nature and origin of seroma after mastectomy. Dan. Med. Bull. 1989, 36, 487–489. Available online: https://www.ncbi.nlm.nih.gov/pubmed/2509147 (accessed on 7 July 2023). [PubMed]
- Agrawal, A.; Ayantunde, A.A.; Cheung, K.L. Concepts of seroma formation and prevention in breast cancer surgery. ANZ J. Surg. 2006, 76, 1088–1095. [Google Scholar] [CrossRef]
- Ebner, F.; Friedl, T.W.P.; de Gregorio, A.; Lato, K.; Bekes, I.; Janni, W.; de Gregorio, N. Seroma in breast surgery: All the surgeons fault? Arch. Gynecol. Obstet. 2018, 298, 951–959. [Google Scholar] [CrossRef]
- De Rooij, L.; Bosmans, J.; van Kuijk, S.M.J.; Vissers, Y.L.J.; Beets, G.L.; van Bastelaar, J. A systematic review of seroma formation following drain-free mastectomy. Eur. J. Surg. Oncol. 2021, 47, 757–763. [Google Scholar] [CrossRef]
- Tansawet, A.; Nakchuai, P.; Techapongsatorn, S.; Sukhvibul, P.; Lolak, S. Prediction of seroma after total mastectomy using an artificial neural network algorithm. Breast Dis. 2022, 41, 21–26. [Google Scholar] [CrossRef]
- Pochert, N.; Schneider, M.; Ansorge, N.; Strieder, A.; Sagasser, J.; Reiger, M.; Traidl-Hoffmann, C.; Neumann, A.; Jeschke, U.; Dannecker, C.; et al. Seroma after simple mastectomy in breast cancer-the role of cd4+ t helper cells and the evidence as a possible specific immune process. Int. J. Mol. Sci. 2022, 23, 4848. [Google Scholar] [CrossRef]
- Pochert, N.; Schneider, M.; Köpke, M.B.; Wild, M.; Mattmer, A.; Sagasser, J.; Golas, M.M.; Banys-Paluchowski, M.; Metz, A.; Hinske, C.; et al. Th2/th17 cell associated cytokines found in seroma fluids after breast cancer surgery. Arch. Gynecol. Obstet. 2023. [Google Scholar] [CrossRef]
- Basu, A.; Ramamoorthi, G.; Albert, G.; Gallen, C.; Beyer, A.; Snyder, C.; Koski, G.; Disis, M.L.; Czerniecki, B.J.; Kodumudi, K. Differentiation and regulation of t(h) cells: A balancing act for cancer immunotherapy. Front. Immunol. 2021, 12, 669474. [Google Scholar] [CrossRef] [PubMed]
- van Bemmel, A.J.; van de Velde, C.J.; Schmitz, R.F.; Liefers, G.J. Prevention of seroma formation after axillary dissection in breast cancer: A systematic review. Eur. J. Surg. Oncol. 2011, 37, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lal, B.; Misra, M.C. Post-mastectomy seroma: A new look into the aetiology of an old problem. J. R. Coll. Surg. Edinb. 1995, 40, 292–294. [Google Scholar]
- Pogson, C.J.; Adwani, A.; Ebbs, S.R. Seroma following breast cancer surgery. Eur. J. Surg. Oncol. 2003, 29, 711–717. [Google Scholar] [CrossRef]
- Akinci, M.; Cetin, B.; Aslan, S.; Kulacoglu, H. Factors affecting seroma formation after mastectomy with full axillary dissection. Acta Chir. Belg. 2009, 109, 481–483. Available online: https://www.ncbi.nlm.nih.gov/pubmed/19803259 (accessed on 7 July 2023). [CrossRef]
- Woo, K.J.; Paik, J.M.; Mun, G.H.; Pyon, J.K.; Bang, S.I. Risk factors for complications in immediate expander-implant breast reconstruction for non-obese patients: Impact of breast size on complications. Aesthetic. Plast. Surg. 2016, 40, 71–78. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26530484 (accessed on 7 July 2023). [CrossRef] [PubMed]
- Unger, J.; Rutkowski, R.; Kohlmann, T.; Paepke, S.; Zygmunt, M.; Ohlinger, R. Potential risk factors influencing the formation of postoperative seroma after breast surgery—A prospective study. Anticancer. Res. 2021, 41, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.; Gibson, T.; Li, Z.; Diehl, N.; Bagaria, S.; McLaughlin, S. Symptomatic axillary seroma after sentinel lymph node biopsy: Incidence and treatment. Ann. Surg. Oncol. 2016, 23, 3347–3353. [Google Scholar] [CrossRef] [PubMed]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-mediated inflammation in normal and diabetic wound healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef]
- Gallagher, K.A.; Joshi, A.; Carson, W.F.; Schaller, M.; Allen, R.; Mukerjee, S.; Kittan, N.; Feldman, E.L.; Henke, P.K.; Hogaboam, C.; et al. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes 2015, 64, 1420–1430. [Google Scholar] [CrossRef]
- Sadighi Akha, A.A. Aging and the immune system: An overview. J. Immunol. Methods 2018, 463, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Granzier, R.W.Y.; van Bastelaar, J.; van Kuijk, S.M.J.; Hintzen, K.F.H.; Heymans, C.; Theunissen, L.L.B.; van Haaren, E.R.M.; Janssen, A.; Beets, G.L.; Vissers, Y.L.J. Reducing seroma formation and its sequelae after mastectomy by closure of the dead space: The interim analysis of a multi-center, double-blind randomized controlled trial (sam trial). Breast 2019, 46, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Docimo, G.; Limongelli, P.; Conzo, G.; Gili, S.; Bosco, A.; Rizzuto, A.; Amoroso, V.; Marsico, S.; Leone, N.; Esposito, A.; et al. Axillary lymphadenectomy for breast cancer in elderly patients and fibrin glue. BMC Surg. 2013, 2 (Suppl. S13), S8. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.S.; Hutson, K.; Kalra, L.; Bonomi, R. The role of fibrin glue instillation under skin flaps in the prevention of seroma formation and related morbidities following breast and axillary surgery for breast cancer: A meta-analysis. J. Surg. Oncol. 2012, 106, 783–795. [Google Scholar] [CrossRef]
- DeLong, M.R.; Tandon, V.J.; Bertrand, A.A.; MacEachern, M.; Goldberg, M.; Salibian, A.; Pusic, A.L.; Festekjian, J.H.; Wilkins, E.G. Review of outcomes in prepectoral prosthetic breast reconstruction with and without surgical mesh assistance. Plast Reconstr. Surg. 2021, 147, 305–315. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33177453 (accessed on 7 July 2023). [CrossRef]
- Andrades, P.; Prado, A. Composition of postabdominoplasty seroma. Aesthetic. Plast. Surg. 2007, 31, 514–518. [Google Scholar] [CrossRef]
| Cases with Postoperative Seroma | Cases without Postoperative Seroma | p-Value | |
|---|---|---|---|
| Use of a foreign body | p < 0.001 | ||
| No foreign body Implant (with Mesh) Expander | 43 7 2 | 24 21 3 | |
| Axillary intervention | p = 0.137 | ||
| None | 1 | 3 | |
| SLND | 31 | 22 | |
| ALND | 17 | 8 | |
| TAD | 1 | 7 | |
| SLN + ALND | 2 | 6 | |
| TAD + ALND | 2 | 0 | |
| Number of removed axillary lymph nodes | p = 0.792 | ||
| 0 | 0 | 2 | |
| 1 | 16 | 11 | |
| 2 | 9 | 11 | |
| 3 | 3 | 6 | |
| 4–10 | 7 | 4 | |
| >10 | 17 | 13 | |
| Number of positive axillary lymph nodes | p = 0.186 | ||
| 0 | 31 | 32 | |
| 1 | 5 | 5 | |
| 2 | 4 | 3 | |
| 3 | 2 | 2 | |
| 4 | 2 | 1 | |
| >4 | 8 | 3 | |
| Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|
| BMI | 0.987 | 0.874–1.113 | 0.828 |
| Age at surgery | 1.054 | 1.014–1.095 | 0.007 * |
| Grading | 0.318 | 0.122–0.831 | 0.019 * |
| Ablation weight | 1.002 | 1.000–1.005 | 0.109 |
| Use of foreign body | 1.025 | 0.627–1.678 | 0.920 |
| Tumor stage (pT) | 0.930 | 0.769–1.125 | 0.456 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köpke, M.B.; Wild, C.M.; Schneider, M.; Pochert, N.; Schneider, F.; Sagasser, J.; Kühn, T.; Untch, M.; Hinske, C.; Reiger, M.; et al. Elderly and Patients with Large Breast Volume Have an Increased Risk of Seroma Formation after Mastectomy—Results of the SerMa Pilot Study. Cancers 2023, 15, 3606. https://doi.org/10.3390/cancers15143606
Köpke MB, Wild CM, Schneider M, Pochert N, Schneider F, Sagasser J, Kühn T, Untch M, Hinske C, Reiger M, et al. Elderly and Patients with Large Breast Volume Have an Increased Risk of Seroma Formation after Mastectomy—Results of the SerMa Pilot Study. Cancers. 2023; 15(14):3606. https://doi.org/10.3390/cancers15143606
Chicago/Turabian StyleKöpke, Melitta Beatrice, Carl Mathis Wild, Mariella Schneider, Nicole Pochert, Felicitas Schneider, Jacqueline Sagasser, Thorsten Kühn, Michael Untch, Christian Hinske, Matthias Reiger, and et al. 2023. "Elderly and Patients with Large Breast Volume Have an Increased Risk of Seroma Formation after Mastectomy—Results of the SerMa Pilot Study" Cancers 15, no. 14: 3606. https://doi.org/10.3390/cancers15143606
APA StyleKöpke, M. B., Wild, C. M., Schneider, M., Pochert, N., Schneider, F., Sagasser, J., Kühn, T., Untch, M., Hinske, C., Reiger, M., Traidl-Hoffmann, C., Dannecker, C., Jeschke, U., & Ditsch, N. (2023). Elderly and Patients with Large Breast Volume Have an Increased Risk of Seroma Formation after Mastectomy—Results of the SerMa Pilot Study. Cancers, 15(14), 3606. https://doi.org/10.3390/cancers15143606








