Simple Summary
Ocular malignancies encompass a broad range of disorders that affect the eyelids, orbit, and eye and have significant impacts for national healthcare systems. Due to its exposure to various stressors, the eye is an anatomical site susceptible to cellular toxicity and tissue damage, which can result in significant vision loss. In this context, similar to other tissue types, p53 plays a crucial role in maintaining ocular homeostasis. However, few in vitro experimentation and clinical trials of p53 pathway modulators have been conducted. The aim of this review is to discuss the potential of pharmacological p53 activators as a novel targeted therapy for managing ocular tumors.
Abstract
The pivotal role of p53 in the regulation of a vast array of cellular functions has been the subject of extensive research. The biological activity of p53 is not strictly limited to cell cycle arrest but also includes the regulation of homeostasis, DNA repair, apoptosis, and senescence. Thus, mutations in the p53 gene with loss of function represent one of the major mechanisms for cancer development. As expected, due to its key role, p53 is expressed throughout the human body including the eye. Specifically, altered p53 signaling pathways have been implicated in the development of conjunctival and corneal tumors, retinoblastoma, uveal melanoma, and intraocular melanoma. As non-selective cancer chemotherapies as well as ionizing radiation can be associated with either poor efficacy or dose-limiting toxicities in the eye, reconstitution of the p53 signaling pathway currently represents an attractive target for cancer therapy. The present review discusses the role of p53 in the pathogenesis of these ocular tumors and outlines the various pharmacological activators of p53 that are currently under investigation for the treatment of ocular malignancies.
1. Introduction
The eye is an anatomical site exposed to multiple stressors, such as microorganisms (viruses, bacteria, and protozoa), environmental factors (e.g., UV radiation, which leads to oxidative photodegradation), and degenerative disorders, which can cause moderate-to-severe vision loss and blindness.
Ocular diseases encompass various pathologies, including ocular malignancies and other complex eye diseases. The pathogenesis of various ocular tumors remains largely unknown, which limits the development of diagnostic and treatment approaches for these diseases that are relevant for health, society, and the national health system. Ocular malignancies may originate from any tissues of the eye, involving the conjunctiva, cornea, sclera, uvea, and/or retina with melanocytic, fibrous, epithelial, and other form of lesions [1]. In the United States and Northern Europe, the incidence of retinoblastoma is estimated to be approximately one in 16,000–18,000 live births [2,3]. On the other hand, melanoma is the most common malignant primary intraocular tumor in adults [4]. Resection and radiation therapy are the first-line treatment options for ocular melanoma, with enucleation being last resort [5].
Common eye disorders and diseases hold the potential to worsen the overall quality of life of patients and are commonly found in elderly people. These eye disorders include age-related macular degeneration (AMD), glaucoma, diabetic retinopathy, cataracts, and dry eye. These disorders are rising with an increase of blindness and vision loss patients from 12.44 million in 1990 to 22.56 million in 2019 [6].
In addition to diseases that mainly originate from tissues of the eye, paraneoplastic ocular syndrome may occur. In paraneoplastic ocular syndrome, molecular mimicry drives the autoantibodies response against normal eye tissue, leading to ophthalmic symptoms including, but not limited to, cancer-associated retinopathy, melanoma-associated retinopathy, cancer-associated cone dysfunction, paraneoplastic vitelliform maculopathy, and paraneoplastic optic neuritis [7].
In this context, the expression of p53 has been demonstrated throughout the human body, including the eye. The transcription factor p53 directly regulates the expression of target genes for maintaining tissue homeostasis during various physiological conditions such as development and differentiation [8,9,10]. Therefore, a mutation in the gene encoding p53 or the inactivation of the pathway under its governance may contribute to malignant transformation. In this regard, p53 preserves tissues by restricting genome alteration which may lead to aberrant mitoses [11]. Since the discovery that p53 plays a pivotal role in the induction of apoptotic cell death of DNA-damaged cells, the activation of p53 has become an attractive therapeutic strategy for various malignancies.
After the identification of compounds that modulate the p53 pathways, which arrest cell growth and induce apoptosis, multiple inhibitors of the p53–MDM2 interaction have been suggested as potential treatments for solid tumors, hematological cancers, and ocular diseases [12,13]. Alongside these inhibitors, recent research has identified traditional Chinese herbal medicine as a promising candidate for cancer therapy with fewer side effects than chemotherapy. Chinese herbal medicine may be considered as a complementary or alternative option in the field of oncology [14]. Natural products possess nutraceutical potential in a diverse range of diseases and are readily available with cost-effective pricing and fewer harmful side-effects [15,16,17,18]. Among these agents, baicalein, a flavonoid extracted from the dried root of Scutellaria baicalensis, has demonstrated significant efficacy in the treatment and prevention of many types of cancers. Baicalein exerts its actions by inhibiting numerous complex cascades and increasing the expression of the tumor suppression proteins p38 and p53, leading to cell cycle arrest and apoptosis [19].
The p53 network coordinates gene expression among cells and tissues, ensuring a central role in maintain organismal homeostasis [20].
Normal cells conserve pathways for maintaining genomic integrity, thus recognizing, and repairing damaged DNA. In response to certain types of DNA damage, the WRN gene, which encodes a DNA helicase, may activate p53 and potentiate p53-mediated apoptosis [21]. Indeed, as recently shown from Hao et al., the loss of WRN triggers DNA damage leading to the activation of p53/PUMA and subsequent cell death [22]. On the other hand, in progeria Werner’s syndrome, the ablation of the WNR gene may lead to the development of myeloid malignancies as result of competitive fitness by inactivating p53 [23]. Patients with Werner’s syndrome are also characterized by a high predisposition to various cancer types and as well as ocular cataracts [23,24]. The increased incidence of ocular cataracts in patients with Werner’s syndrome may be explained by the role of p53 in preventing cataracts and the existing relationship between WNR and p53. Indeed, single nucleotide polymorphisms (SNPs) of WRN might interfere with the binding of p53 to WNR, reducing the apoptotic function of p53 [25]. Furthermore, as shown by Reichel et al., p53 ablation increases the frequency of persistent hyperplastic primary vitreous and cataracts in a mouse model [26].
Therefore, given its role in tissue homeostasis, p53 is also widely expressed in the whole eye of an adult, where it plays a relevant role in maintaining ocular stability.
This review aims to address the involvement of p53 in ocular stability and highlight how the putative use of MDM2-p53 binding inhibitors, currently under clinical trial for other diseases, may improve ocular therapy, where the treatment methods might be the primary factor influencing the recurrence of the disease [27,28,29,30].
2. The Role of p53 in Tissue and Ocular Homeostasis
The p53 gene was first identified in 1979 as a 53 kDa protein associating with the middle T (mt) antigen of the SV40 virus [31]. Initially, it was believed to be an oncogene, due to its overexpression in SV40 transformed cells. Subsequent studies have shown it to be a humoral target in SV40-induced cancer model mice, and it was found to be expressed in 9% of patients with breast cancer [32,33]. In 1983, this protein was later named tumor suppression protein p53 [34], and finally further evidence from murine model leukemia and human leukemia cell line, wherein the gene encoding murine p53 protein was inactivated or deleted, suggested that p53 might be involved in tumor suppression [35,36].
Many pathways contribute to the p53 activation, including intracellular and extracellular stresses such as heat shock, UV light, inflammatory cytokines, oxidative stress, hypoxia, and mitogenic oncogenes, as well as physiologically cellular metabolic pathways observed during stem cell self-renewal and homeostasis. In response to these conditions, p53 is activated thereby promoting cell cycle arrest, DNA repair, senescence, and activation of the apoptotic pathway [37,38].
Under physiological conditions, the regulation of p53 depends on its post-translational protein turnover, which is mainly regulated by the murine double minus 2 (MDM2). MDM2 is primarily located at the nuclear level, by its intrinsic nuclear localization signal (NLS), and exerts the p53 nuclear export sequence to the cytoplasm, where proteasomal degradation can occur [39,40]. MDM2 acts as an E3 ligase that binds to the NH2 terminal domain of p53, targeting its ubiquitination and degradation by the 26S proteasome [41]. The E3 ubiquitin ligase activity of MDM2 relies on its interaction with the murine double minute X (MDMX also known as MDM4), forming an MDM2/MDMX complex with stable E3 ligase activity [42]. In mice models, targeted deletion of the MDM2 gene results in embryonic lethality due to p53-mediated apoptosis [43]. Therefore, MDM2 becomes a critical factor that transduces intrinsic and extrinsic signals to regulate the p53 effects in response to the perturbation of homeostasis [44].
It is well-known that p53 plays a role as a growth inhibitory factor and is incompatible with cancer cell proliferation. Indeed, p53 resides in the middle of the growth signals where growth-promoting conditions engage Akt which then mediates the phosphorylation of MDMX with the consequent stabilization of MDM2 [45]. This also demonstrates the pro-survival oncogenic activity of Akt and the cross talk between p53 and mTOR pathways [46,47]. These latter observations are in line with the alteration of the mTOR/AKT/PI3K pathway seen during diabetic retinopathy [48], the most common complication of diabetes mellitus (DM), a chronic metabolic disease characterized by hyperglycemia [49,50]. These findings support a putative relationship between p53 and the mTOR/AKT/PI3K pathway in the pathogenesis of diabetic retinopathy [48,51,52,53].
Activation of p53 relies on its intracellular increase via several mechanisms including the downregulation of p53 degradation, migration to the mitochondria and nucleus, and post-translational modification [54,55,56,57], which in turn suppress the interaction of p53 and MDM2 [58]. Once activated and phosphorylated at Ser15, p53 oligomerizes and forms tetramers that bind DNA to regulate transcription of target genes such as CDKN1A (p21, CIP1, WAF1), GADD45A13, pro-apoptotic genes such as NFRSF10B/TRAIL-R2, PUMA, and BAX, the pro-apoptotic Sept4/Apoptosis-related protein in the TGF-β signaling pathway (ARTS) gene, and interestingly also MDM2 [59,60,61,62,63,64,65,66]. Thus, the p53–MDM2 interaction is involved in a negative feedback loop that is important for p53 expression [67]. In addition, p21, which is a 21 kDa protein also known as WAF1, acts as a potent cyclin-dependent kinase 4 (CdK4)/cyclin D1 complex inhibitor essential for inducing cell cycle arrest mediating the downregulation of G1/S genes [68] and halting the cell cycle in the S and G2 phases in response to DNA damage [69]. Alternatively, activation of CdK4/Cyclin D1 leads to phosphorylation of the retinoblastoma protein (pRb) and cell cycle progression from G1 to S [70]. Many genes are under the p53-p21-pRb signaling pathway whereby DNA replication and repair processes are most prominently associated and in which p21 plays a crucial role in regulating pRb phosphorylation [71]. The resulting loss of function of the RB1 locus, following genetic mutation or deletion, leads to the activation of E2F family proteins, uncontrolled cell proliferation, and initiation of retinoblastoma [72,73].
Along with the nuclear migration and the regulation of the cell cycle, p53 may also promote the intrinsic apoptotic pathway, also known as mitochondrial apoptosis [63,74]. The intrinsic apoptotic pathway is driven by the Bcl-2 family of proteins, the activation of which depends on the p53 activity. This family includes many anti-apoptotic proteins (BCL-2, BCL-XL, BCL-W, MCL-1, and BFL-1/A1) and pro-apoptotic proteins (pore-formers BAX, BAK, and BOK as well as BH3-only BAD, BID, BIK, BIM, BMF, HRK, NOXA, and PUMA) [75]. The balance of these proteins determines the fate of the cell. The protein p53 exerts its apoptotic function through both direct and indirect protein–protein interactions. Once it has migrated to the nucleus, p53 may act as transcriptional activator, upregulating the expression of PUMA. PUMA acts as key a mediator of p53-driven apoptosis in two ways [76]: it directly mediates the inhibition of anti-apoptotic BCL-2, the inhibitor of cell death, thereby removing the direct the effect on other BCL-2 family protein, as well as by engaging and activating the pro-apoptotic BAX and BAK proteins [77,78]. The homo-oligomerization of BAX and BAK results in concomitant mitochondrial outer membrane permeabilization (MOMP) and the release of pro-apoptotic cytochrome c located in the mitochondrial membrane gap. Alternatively, p53 may mediate the mitochondrial release of cytochrome c through direct interaction in the cytosol with BAX [79].
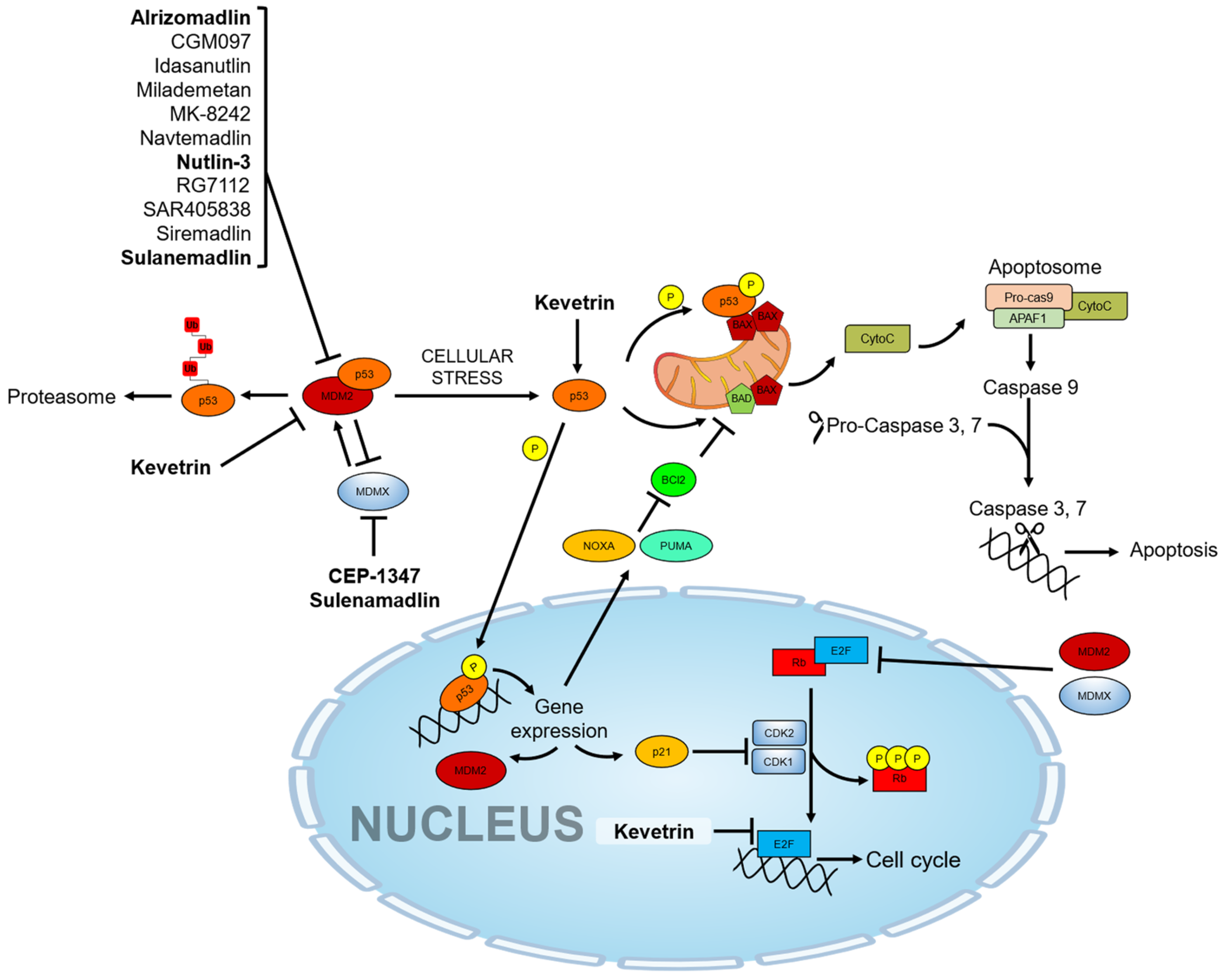
Cytochrome c can then activate a cascade of sequential activation of caspases by the formation of a complex so-called “apoptosome”, which contains cytochrome c, Apaf-1, and pro-caspase-9 [80]. Once caspase-9 is activated and associated with the apoptosome as a holoenzyme [81], it cleaves and activates downstream effector caspases as caspase-3 and caspase-7 [82]. Effector caspases are ultimately responsible for executing apoptosis by DNA fragmentation, subsequent cell shrinkage, and membrane blebbing (Figure 1).
Figure 1.
p53 molecular pathway and pharmacological activators. The drugs under investigation in ocular pathologies are highlighted in bold.
Hypoxic stress also induces the accumulation of the p53 protein, which mediates the mitochondrial apoptotic pathway [83]. Conversely, most solid tumors contain regions with inadequate oxygen supply where the hypoxia-inducible factor (HIF), an oxygen-sensitive transcription factor, promotes tissue neovascularization aiding tumor cells to survive in the hypoxic environment, thereby contributing tumor growth. HIF-1α induces the protein phosphatase-1 nuclear-targeting subunit (PNUTS), which increases the proteasomal-dependent degradation of MDM2, indirectly rescuing p53 from the MDM2-mediated ubiquitination and leading it to p53-activation and mediated apoptosis [84]. In this cancer model, further evidence suggests that MDM2 also regulates angiogenesis by increasing the expression levels of transcription factors such as HIF-1α and vascular endothelial growth factor (VEGF), thereby promoting tissue neovascularization [85]. Hypoxia is a hallmark factor in the development of solid tumors as well as in the development of retinal diseases, including diabetic retinopathy (DR), age-related macular degeneration (AMD), and degeneration of retinal ganglion cells (RGCs) [48,86,87]. Among these, during retinal ischemia, hypoxic conditions invoke both p53 gene and protein expression, which in turn induce cell death [88,89]. This highlights that the pharmacological targeting of p53-related pathways may provide additional therapeutic benefits also to non-cancer ocular disease.
p53 is expressed at high levels during normal embryogenesis and development, regulating cell cycle and apoptosis [90], in the central nervous system (CNS) as well as other anatomical compartments such as olfactory bulb and eye [91,92,93,94,95,96]. Studies in an animal model showed a marked role of p53 during early embryonic ocular development, highlighting ocular abnormalities of hyaloid vasculature, vitreal opacities, retinal folding, and nerve fiber hypoplasia in mice defective for p53, according to their genetic background [97]. It is noteworthy that p53 exhibits widespread expression in various regions of the brain and the entire eye of adult mice. The expression is particularly high in the retina and optic nerve, while also accounting for a substantial portion, up to 70%, of the overall promoter activity expression in the cornea [98,99,100]. These observations are consistent with the high cytoplasmic expression of p53 in both the corneal and conjunctival epithelium of a typical murine eye, as well as the lack of its inhibitory modulator [98,101].
In this view, it is noteworthy that p53 is widely expressed in the normal eye, and it may play a role during ocular malignancies. The eye is an anatomic site exposed to multiple stressors such as microorganisms (viruses, bacteria, and protozoa), environments (UV radiation leads to oxidative photodegradation), and degenerative disorders all of which led to moderate-to-severe vision impairment and blindness [102,103,104]. Chronic inflammation resulting from the infectious disease may lead to the initiation of cancer, hampering growth regulators such as tumor suppressor p53 and affecting pathways of DNA repair with an accumulation of DNA damage [105]. Among these microorganisms, viruses have the capability to control physiological functions and pathways of host cells regulating growth arrest and apoptosis. Several studies have shown that human papillomavirus (HPV) is implicated in the development of pterygia and other related neoplasia of the ocular adnexa by the expression of viral oncoprotein that suppresses p53 activity [106,107,108]. However, Dushku et al. reported that HPV is not required as a cofactor in the development of pterygia and limbal tumors [109].
Intense light exposure can cause photochemical injury to the retina, ultimately leading to damage and apoptosis of retinal pigmented epithelial (RPE) cells, photoreceptors, and the entire neural retina [110,111,112]. The role of p53 in light-induced ocular degeneration may be mixed. As shown in the p53-/- mice model exposed to the intense blue light, apoptosis of photoreceptors may be both p53-independent [113,114], with the ocular degeneration mainly driven by the accumulation of the lipofuscin in response of the RPE cells to the light, and p53-dependent, with the upregulation of p53 and related genes leading to RPE cell death [115,116,117]. On the other hand, blue light clearly induces apoptosis of retinal Müller Cells [118].
Further, the eye is constantly subjected to oxidative stress due to its exposure to light, its high metabolic activity, and the oxygen-rich environment. Among those, UV radiation is the major source of reactive oxygen species, inducing a redox imbalance affecting various structures of the eye including the cornea, sclera, lens, and retina [119]. Under normal physiological conditions p53-induced processes cooperate to lower the ROS by promoting glutathione-dependent ROS scavenging and stimulating the expression of genes that reduce oxidative stress [120,121,122]. Consequently, oxidative stress also triggers oxidative DNA damage and cellular senescence, upregulating the p16INk4a/Rb and p53/p21Cip1 pathway and finally leading to cell cycle arrest [123]. In this light, oxidative stress and p53 become key factors in the development of eye-related diseases.
Therefore, p53 is widely expressed in the healthy human eye and plays an important role in the various function of eye tissues. Based on the relationship existing between p53/MDM2 in the ocular tissues, it appears that a therapeutic intervention with drugs that disrupt p53 inhibition by MDM2 and MDMX becomes interesting in the field of ocular diseases and merits pursuit.
3. Pharmacological Activators of the p53 Pathway
Several strategies can be used to target MDM2/MDMX for cancer therapy [124]. The currently available p53 cancer therapy relies on the interaction of p53 with its negative regulator MDM2, which can, in turn, be inhibited by the MDM2–p53 binding inhibitors including Nutlins. Nutlins (Nutlin-1, -2, and -3) are the first synthetic molecules developed by Hoffmann-La Roche in Nutley, based on 1,2,4,5-tetrasubstituted 4,5-cis-imidazolines, which interact with the p53-binding pocket of MDM2 and activate the p53 pathway, leading to cell proliferation arrest and/or apoptosis [125,126,127]. Nutlins are selective non-genotoxic inhibitors that do not induce the phosphorylation of p53 on Ser15 [65]. Among these, Nutlin-3 is a synthetic small molecule cis-imidazoline analog that mimics highly conserved hydrophobic amino acid residues including Phe19, Trp23, Leu22, and Leu26 within the hydrophobic pocket of MDM2 [126]. Nutlins have been shown to induce cell cycle arrest and cell death in a variety of solid tumors as well as in several types of hematological malignancies, viral infections, and cancer models with wild-type p53 including osteosarcoma, prostate cancer, Kaposi’s sarcoma-associated herpesvirus lymphomas, and neuroblastoma [128,129,130,131,132,133,134].
Since the discovery of Nutlins in 2004, several p53–MDM2 interaction inhibitors have been investigated for use in patients with various solid tumors and hematological cancers, as reported in Table 1.

Table 1.
p53–MDM2 interaction inhibitors under clinical trials.
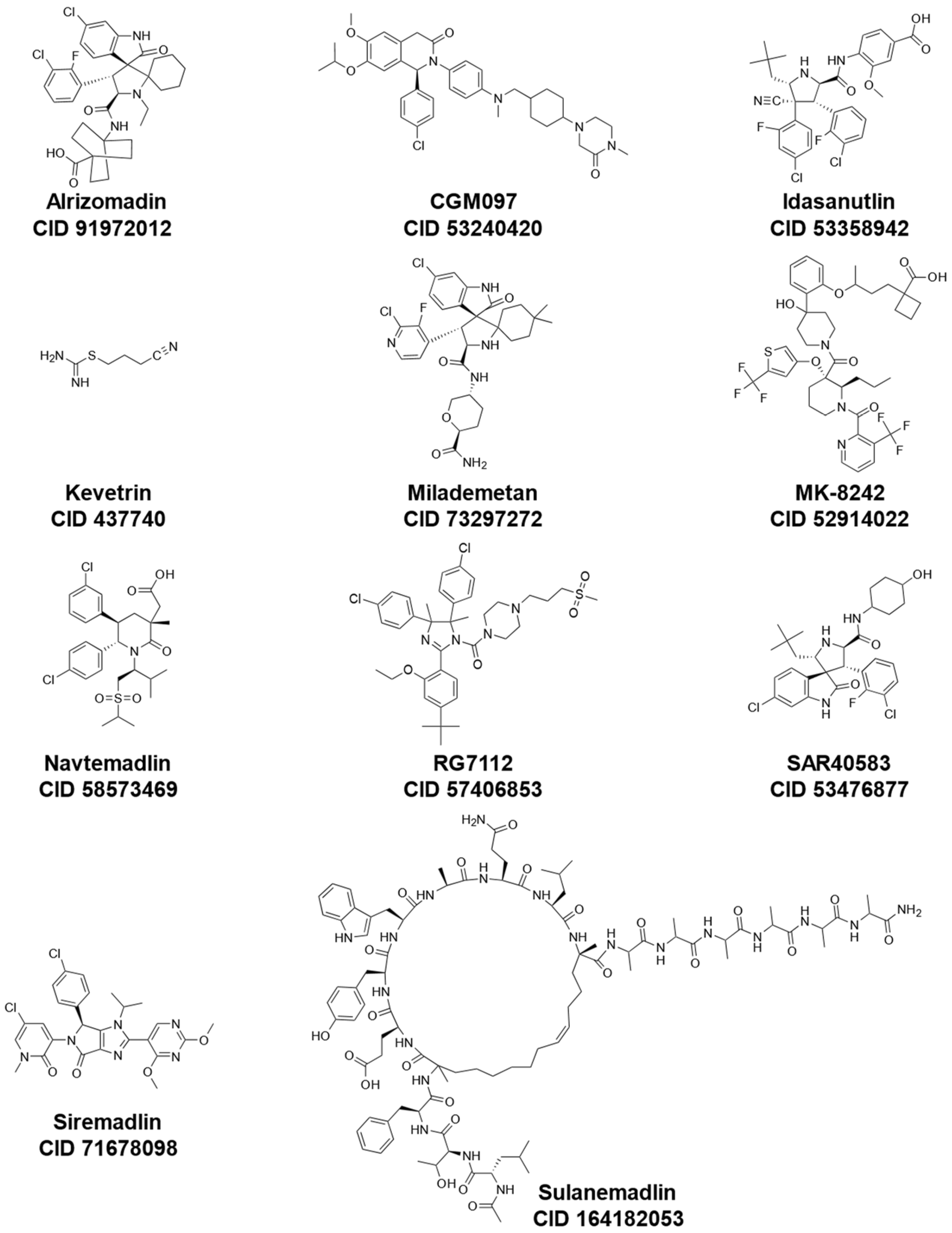
These inhibitors include Idasanutlin (also known as RG7388 or RO5503781) [135,136], RG7112 (also known as RO5045337) [137], Alrizomadlin (also known as APG-115) [138], SAR405838 (also known as MI-77301) [139], MK-8242 [140], Kevetrin [141], ALRN-6924 [142], Siremadlin (also known as HDM201) [143], Milademetan [144], CGM097 (also known as NVP-CGM097) [145], and AMG-232 (also known as KRT-232) [146]. In addition to small molecule inhibitors of MDM2, other compounds such as CEP-1347, originally developed for the treatment of other diseases (i.e., Parkinson’s Disease), have shown effectiveness in the activation of the p53 pathway [147]. An overview of mechanisms of actions, synonyms, molecular formulas, and chemical structures of these various drugs under investigation is shown in Table 2 and Figure 2.

Table 2.
p53–MDM2 interaction inhibitors.
Figure 2.
Chemical structures of representative p53-MDM2 interaction inhibitors cited in Table 2 (all obtained from PubChem; CID, CompoundID).
4. Role of p53 Therapy in Pterygium
Although previously classified as a chronic degenerative condition, pterygium is now considered an uncontrolled cellular proliferation secondary to an abnormal expression of p53 protein within the conjunctival epithelium [148]. Histopathologically, pterygium is characterized by a focal fibrovascular proliferation of the conjunctival tissue with alterations in limbal stem cells, squamous metaplasia of conjunctival epithelium, dissolution of Bowman’s membrane, and excess proliferation of the stroma and extracellular matrix. Primary pterygium is locally invasive exhibiting tumorigenicity ranging from mild dysplasia to carcinoma in situ.
While the pathogenesis of pterygia remains poorly understood, the interaction between UV exposure and p53 mutations may likely play a role in its development and recurrence. Extensive data have demonstrated a dose-related relationship between chronic ultraviolet radiation and pterygium formation [149]. Similar to those seen in other skin cancer, UV irradiation may induce DNA damage in the p53 tumor suppressor gene including C to T transitions and CC to TT tandem mutations [150,151].
In a study by Spandidos et al., 60% of pterygia exhibited several DNA mutations including loss of heterozygosity and microsatellite instability, which result in alterations of the DNA repair pathways [152]. Through immunohistochemical analysis, mutant p53 protein has been found to be highly expressed in the epithelium overlying the pterygium [153,154,155,156]. Variability in p53 immunopositivity may reflect differences in race or environmental exposure [157]. The abnormal expression of p53 likely represents a failure in the regulation and control of the cell cycle caused by ultraviolet radiation, a well-known risk factor for pterygium formation [158]. Coupled expression of p53 and Bcl-2 is thought to result in a disruption of the transcriptional fidelity of p53 [159].
Surgical intervention is indicated in cases associated with high astigmatism, recurrent inflammation, or visual loss secondary to the involvement of the visual axis. However, following surgical excision, pterygium is associated with high recurrence rates ranging from 25% to up to 70% [156]. Thus, similar to the treatment of neoplastic diseases, the management of pterygium requires a multimodal approach including wide excision, antimetabolite chemotherapy, and irradiation. In order to overcome surgical infection, the intraoperative use of mitomycin C is commonly used. However, it is associated with several complications including necrotizing scleritis, scleral calcification, ulceration, damage to the corneal epithelium and endothelium, corneal edema, iritis, hypotony by injury of the ciliary body, glaucoma, and cataracts [160].
Activation of p53 by small-molecule antagonists of MDM2 can potentially induce apoptosis and regression in pterygium. Specifically, Nutlin has been proposed as a potential pharmacological treatment for pterygium. Indeed, experiments based on primary cell cultures, established from surgically excised specimens of primary pterygium, have demonstrated that treatment with Nutlin results in a 39-fold reduction in cell proliferation with dose-dependent inhibition of cell migration [161]. Additionally, no significant changes in cell viability and migration were observed in normal conjunctival cells treated with Nutlin. Further studies are required to evaluate the use of Nutlin and other MDM2 antagonists for the non-surgical treatment of pterygium.
5. Role of p53 Therapy in Conjunctival Melanoma
Conjunctival malignant melanoma is a rare, potentially life-threatening ocular malignancy that arises from the basal cells of the conjunctival epithelium [162]. Recognized risk factors for malignant conjunctival tumors include white race, older age, and exposure to ultraviolet light. Malignant transformation of primary acquired melanosis and, less commonly, conjunctival nevi has also been reported [163]. The presence of significant atypia associated with primary acquired melanosis is a significant prognostic indicator for malignant transformation.
Around 75% of conjunctival melanomas are believed to arise from primary acquired melanosis while approximately 5–10% arise from melanocytic nevi with atypia. In the remaining 5–10%, conjunctival melanoma arises de novo [164].
The typical presentation of conjunctival melanoma is a focal nodular melanotic epibulbar mass with multiple prominent feeder vessels extending to and from the lesion most commonly found at the limbus within the interpalpebral fissure. Some melanomas may be hypomelanotic or even amelanotic. Conjunctival melanomas have a strong propensity to metastasize to the preauricular or anterior cervical lymph nodes. Even with wide surgical excision and adjuvant chemotherapy, the overall response to treatment is poor with high rates of local recurrence and metastasis [165]. Adjuvant therapy may include topical mitomycin, plaque-brachytherapy, or proton-beam therapy. In advanced cases with lymphatic and hematogenous spread, enucleation and even orbital exenteration may be required [166]. Hence, novel therapeutic targets need to be explored to improve prognosis.
Immunohistopathology studies have shown that p53 is rarely expressed in conjunctival melanomas [167]. Although no clinical studies have been performed, the tumor suppressor p53 represents a potential therapeutic target for conjunctival melanoma. In conjunctival melanoma cell lines, treatment with Nutlin-3, a p53/MDM2 inhibitor, resulted in a time and dose-dependent decrease in cell viability with an increase in both MDM2 and p53. Compared to mitomycin C, stabilization of p53 and downregulation of IGF-1R were more effectively induced by Nutlin-3 [168].
6. Role of p53 Therapy for Retinoblastoma
Retinoblastoma is the most common neoplasm affecting the eye in children under five years of age [169]. The typical presenting signs include leukocoria and strabismus. As the tumor advances, affected individuals may develop hyphema, neovascular glaucoma, vitreous hemorrhage, or exudative retinal detachment. Extraocular tumor extension may be associated with significant proptosis and orbital inflammation [170].
While retinoblastoma represents a potentially life-threatening condition, current treatment modalities have significantly improved the prognosis, with disease-free survival rates approaching up to 100% [171]. However, the visual prognosis of retinoblastoma remains poor. In advanced disease, enucleation remains the standard of care. Although various strategies for globe salvage have been developed as alternative interventions, the rate of successful ocular salvage rate remains around 50–70%. Moreover, current chemotherapies are associated with toxicity resulting in various side effects. With an improved understanding of the tumorigenesis of retinoblastoma, novel adjuvant agents have been developed to target specific components and pathways. In particular, treatment using molecular genetics may individualize therapy based on specific tumor characteristics [172].
In retinoblastoma, various genetic changes result in tumor growth, proliferation, and metastasis. Consistent with the Knudson “two-hit hypothesis”, the development of retinoblastoma requires two mutational events in both alleles of the RB1 tumor suppression gene located in the long arm of chromosome 13 (13q14) [173]. Germline mutations of the RB1 gene result in dysfunctional pRb, which is a 928 amino acid phosphoprotein responsible for the regulation of gene transcription. In normal cells, pRb regulates progression through the cell cycle by interacting with the cellular E2F transcription factor and thereby blocking the transition from G1 to S phase [174]. Like the p53 family, alterations in the pRb function can result from multiple mechanisms including mutations in the RB1 gene itself and altered function of the promoter sequence [175]. In a normally healthy cell, loss of RB1 activity activates the transcription of p14ARF which in turn inactivates MDM2, leading to p53-mediated apoptosis and exit from the cell cycle [176,177]. On the other hand, MDM2 is an E3 ubiquitin ligase that mediates the interaction of Rb with the C8 subunit of the 20S proteasome, resulting in the ubiquitin-proteasome-mediated degradation of the RB1 tumor suppression protein [178]. MDM2-dependent degradation of Rb also increases DNA methyltransferase DNMT3A activity which is associated with the silencing of tumor suppressor genes [179]. Nonetheless, MDMX may inhibit RB protein degradation via MDM2 although it may contribute to the pRb degradation in a MDM2-dependent manner [180]. The pharmacological inhibition of MDMX by CEP1347 in wild-type p53 retinoblastoma cell lines, which overexpresses MDMX, leads to an increased p53 expression and activation of the p53 pathway [147]. Notably, greater than 70% of pediatric retinoblastoma patients demonstrate MDMX overexpression [181]. In this context, it is important to remember that MDMX also acts as a negative regulator by binding and sequestering p53 [182]. Elevated levels of MDM2 and MDMX proteins have been observed in certain cancers such as melanoma, Ewing’s sarcoma, and colon carcinoma [44].
Therefore, in the light of the interplay between pRb/E2F, MDM2/MDMX, and p53, the pharmacological modulator of p53-MDM2 interaction may be an attractive therapeutic target for retinoblastoma. Currently Nutlin-3, Kevetrin, ALRN-6924, and CEP-1347 are under investigation for their potential use in treating retinoblastoma.
Much attention has been given to the recently developed Nutlin class of MDM2 antagonists (Nutlin-1, -2, -3, and -3a) because of their non-genotoxic nature and potency in activating p53 [126,183,184]. By blocking the interaction between p53 and MDM2, Nutlin-3 releases p53 from negative control resulting in p53 stabilization and accumulation only in cells expressing wild-type p53 protein, thereby activating p53-dependent cell cycle arrest and apoptosis [185]. Nutlin-3 has been shown to suppress the proliferation of retinoblastoma cells both in vitro and in vivo. Co-immunoprecipitation experiments have demonstrated that Nutlin-3 not only binds MDM2 but also MDMX, although with much lower affinity [186]. In contrast to the p53-deficient retinoblastoma cell line (SJMRBL-8), retinoblastoma cells (Weri1) with wild-type p53 and MDMX overexpression were sensitive to Nutlin-3 [187]. In preclinical retinoblastoma models, the combined subconjunctival injection of Nutlin-3 with a topoisomerase inhibitor, topotecan, induced a p53 response that is similar to that induced by 5 Gy of ionizing radiation. The combined topotecan and Nutlin-3 improve the therapeutic index via a synergistic antineoplastic activity resulting in an 82-fold reduction in tumor burden without causing systemic or ocular adverse effects associated with prolonged exposure to broad-spectrum chemotherapeutic drugs [188].
Recently, the United States Food and Drug Administration has granted a rare disease designation to Kevetrin (thioureidobutyronitrile or 3-cyanopropyl carbamimidothioate hydrochloride). Kevetrin induces cell cycle arrest and apoptosis by altering the E3 ligase of MDM2, activating of the p53 gene, and increasing expression of p53-associated tumor suppressor proteins such as p21. Kevetrin also has therapeutic potential in advanced solid tumors of the ovary, lung, and breast. In a phase 1 clinical trial, patients with advanced solid tumors treated with Kevetrin exhibited a greater than 10% increase in p21 expression 7 to 24 h after treatment (NCT01664000). Additionally, Kevetrin potentially targets the altered Rb-E2F tumor suppressor pathway by downregulating E2F1, thus becoming a useful candidate for the treatment of this pathology [141]. Currently, Kevetrin has secured orphan drug status for ovarian cancer, pancreatic cancer, and retinoblastoma.
Moreover, ALRN-6924, a stabilized, cell-permeating peptide that inhibits both MDM2 and MDMX, is under investigation (NCT03654716) for use in retinoblastoma. ALRN-6924 has shown antitumor activity in phase I clinical trial for patients with lymphoma and solid tumors [142,189]. The mechanism of action of ALRN-6924 involves the inhibition of the interaction between p53 and MDM2 and MDMX, thereby inducing cell-cycle arrest or apoptosis in TP53-wild-type (WT) tumors.
Initially developed for Parkinson’s disease, CEP-1347 is a pharmacological inhibitor of MDMX that has also been shown to suppress the expression of MDM4 in retinoblastoma cell lines [147,190].
While several of these therapeutic agents are still under investigation, the future of retinoblastoma treatment will likely include these forms of anti-cancer therapy that target specific molecular genetic changes and aspects of the tumor microenvironment.
7. Role of p53 in Uveal Melanoma
Uveal melanoma is the most common primary intraocular tumor in adults. Approximately 85 percent of all ocular melanomas arise from the melanocytes of the uveal tract of the eye including the iris, ciliary body, and choroid [191]. The most common site for uveal melanoma is the choroid [192]. Although the development of uveal melanoma is largely considered sporadic, several risk factors including fair skin, light eyes, propensity to sunburn, and cutaneous nevi may predispose individuals to uveal melanoma [193]. Common symptoms of uveal melanoma include blurring of vision, photophobia, floaters, and visual field defects.
Uveal melanoma is a potentially fatal metastatic cancer. In approximately 50% of patients, uveal melanoma of the choroid and ciliary body spreads through the bloodstream to the liver, lung, bone, and skin [194]. Historically, enucleation was the only treatment option for uveal melanoma. Most patients are currently treated conservatively by means of plaque brachytherapy using iodine 125 or ruthenium 106 as an applicator [195]. Alternatively, patients undergo surgical resection, proton beam radiation therapy, or stereotactic radiosurgery using a cyber knife, gamma knife, or linear accelerator [196]. However, uveal melanoma is highly radioresistant and therefore requires treatment with high doses of radiation [194]. Moreover, radiotherapy is associated with several adverse events including radiation retinopathy, secondary glaucoma, and phthisis bulbi [197,198]. Even after successful radiation therapy, over 50% of patients with uveal melanoma eventually develop metastatic disease [199]. Thus, there has been increased interest in finding alternative therapy which results in a high tumor control rate and an improved safety profile.
In uveal melanoma, UV radiation may indirectly cause DNA damage through cytosine to thymine (C > T) transitions [200]. p53 serves as the main mediator of radiation-induced DNA damage [201], suggesting that uveal melanoma may be associated with functional defects that interfere with the p53 pathway [202], where gene mutations of TP53 are rare [203].
Defects within various downstream components of the p53 pathway, such as MDM2, could contribute to the relative radio resistance of uveal melanoma [204]. Analysis of the p53 pathway’s functionality has revealed defects within various downstream components, such as p21 and BAX [202]. Although this finding shows defects in the p53 pathway, Decaulin et al. have also demonstrated Bcl-2/XL/W and MDM2 co-inhibition as a promising target for treatment of uveal melanoma [205].
Hence, the restoration of p53 function by inhibiting its interaction with an MDM2 homolog represents a promising therapeutic strategy for this type of cancer. Consequently, the combination therapy with targeted agents and immunotherapy may further improve treatment response.
Recently, the United States Food and Drug Administration granted fast-track status to a phase 2 trial of the novel MDM2–p53 inhibitor alrizomadlin (APG-115), which demonstrated preliminary antitumor activity also in uveal melanoma [138]. Alrizomadlin is a selective, orally active, and potent spirooxindole-based small-molecule MDM2–p53 antagonist that destabilizes the MDM2–p53 complex and restores TP53 function [206]. By inhibiting the interaction between MDM2 and p53, alrizomadlin acts also as an immunomodulator and a regulator of a tumor’s immune escape mechanism, leading to enhanced T-cell mediated antitumor immunity. In various tumor models, alrizomadlin is a pharmacological p53 activator that has been found to promote an antitumor microenvironment, sensitize tumors that are resistant to PD-1 blockade, and enhance the efficacy of a PD-1 blockade independent of p53 status [207]. The combination of alrizomadlin and immune checkpoint PD-1 inhibitor enhances an antitumor response by reprogramming and downregulating of immunosuppressive M2 macrophages.
An open-label, sequential assignment, phase 1/2 clinical trial (NCT03611868) has evaluated alrizomadlin combined with pembrolizumab in patients with immunotherapy-resistant advanced solid tumors including melanoma, non–small cell lung cancer, STK-11–mutated lung adenocarcinoma, liposarcoma, urothelial carcinoma, and malignant peripheral nerve sheath tumors. Patients received 150 mg of alrizomadlin orally once every other day for two weeks, with one week off, and 200 mg of pembrolizumab was administered intravenously over 30 min on Day 1 of a 21-day cycle. Based on the preliminary and interim results of this study, patients in the uveal melanoma cohort who received alrizomadlin achieved an overall response rate of 14.3% due to one partial responder and a disease control rate of 71.4% with four cases of stable disease [208].
Interestingly, the p53 apoptosis effector related to PMP-22 (PERP) protein acts as the transcriptional target of p53 and has been found to influence tumorigenesis in uveal melanoma. Primarily localized in the plasma membrane, PERP is a tetraspan protein that stabilizes p53 through modulation of the interaction between p53 and MDM2. While its precise mechanism and function are currently unknown, PERP has been shown to induce p53-dependent apoptosis without resulting in cell cycle arrest [209]. Increased expression of PERP results in phosphorylation of the serine residues of p53, thereby disrupting the p53/MDM2 interaction between and enhancing pro-apoptotic gene transcription [210]. Preclinical studies have shown that PERP is a critical molecular determinant of apoptosis in primary uveal melanoma, where its downregulation is associated with aggressive disease. Therefore, PERP also represents a potential target for exploitation in enhancing p53 activity [210,211].
Currently, there are no approved targeted therapies for the treatment of early-stage ocular melanoma. However, the evolution of our understanding of the tumorigenesis and molecular characteristics of uveal melanoma has opened the possibility for targeted therapies.
8. Conclusions
Ocular tumors are a range of eye disorders that can cause moderate-to-severe vision loss, contributing to undesirable health outcomes. Degenerative disorders and causative agents (such as environmental and microbial factors) can prompt eye tissue alterations through the loss of intracellular processes that regulate cell cycle, DNA repair, and senescence and activate apoptotic pathways. The findings reported in this review suggest that the p53 pathway may be modulated in ocular disease and could represent a promising therapeutic target for ocular tumors. Although biological effects using ex vivo models have been demonstrated, only a few clinical trials of MDM2–p53 binding inhibitors for the treatment of ocular diseases have been conducted. Considering the multitude of effects driven by p53 activity in eye physiology, in vitro experimentation and clinical trials of these molecules could be undertaken to exploit the effects of p53 pathway activators for ocular disease treatment and develop novel targeted therapies for the management of ocular tumors.
Author Contributions
F.C. and A.C.Y.: conceptualization. F.C., E.Z. and A.C.Y.: writing—original draft preparation. F.C., E.Z., L.C., A.C.Y., S.A., S.A.-S., G.Z. and M.B.: writing—review and editing. F.C., A.C.Y., M.B. and G.Z.: revision process and final editing. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript was supported by local funds from the University of Ferrara, grant numbers: 2022-PRN-PR.A-SP_001, 2021-FAR.L-CF_002 and 2022-FAR.L-CF_004.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neupane, R.; Gaudana, R.; Boddu, S.H.S. Imaging Techniques in the Diagnosis and Management of Ocular Tumors: Prospects and Challenges. AAPS J. 2018, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Broaddus, E.; Topham, A.; Singh, A.D. Incidence of retinoblastoma in the USA: 1975-2004. Br. J. Ophthalmol. 2009, 93, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Seregard, S.; Lundell, G.; Svedberg, H.; Kivela, T. Incidence of retinoblastoma from 1958 to 1998 in Northern Europe: Advantages of birth cohort analysis. Ophthalmology 2004, 111, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Roelofsen, C.D.M.; Wierenga, A.P.A.; van Duinen, S.; Verdijk, R.M.; Bleeker, J.; Marinkovic, M.; Luyten, G.P.M.; Jager, M.J. Five Decades of Enucleations for Uveal Melanoma in One Center: More Tumors with High Risk Factors, No Improvement in Survival over Time. Ocul. Oncol. Pathol. 2021, 7, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, H.; Zhang, T.; Yin, X.; Man, J.; He, Q.; Lu, M. Global, regional, and national burden of blindness and vision loss due to common eye diseases along with its attributable risk factors from 1990 to 2019: A systematic analysis from the global burden of disease study 2019. Aging 2021, 13, 19614–19642. [Google Scholar] [CrossRef]
- Sarkar, P.; Mehtani, A.; Gandhi, H.C.; Bhalla, J.S.; Tapariya, S. Paraneoplastic ocular syndrome: A pandora’s box of underlying malignancies. Eye 2022, 36, 1355–1367. [Google Scholar] [CrossRef]
- Aboudehen, K.; Hilliard, S.; Saifudeen, Z.; El-Dahr, S.S. Mechanisms of p53 activation and physiological relevance in the developing kidney. Am. J. Physiol. Renal Physiol. 2012, 302, F928–F940. [Google Scholar] [CrossRef][Green Version]
- Saifudeen, Z.; Dipp, S.; El-Dahr, S.S. A role for p53 in terminal epithelial cell differentiation. J. Clin. Investig. 2002, 109, 1021–1030. [Google Scholar] [CrossRef]
- Jacobs, W.B.; Kaplan, D.R.; Miller, F.D. The p53 family in nervous system development and disease. J. Neurochem. 2006, 97, 1571–1584. [Google Scholar] [CrossRef]
- Eischen, C.M. Genome Stability Requires p53. Cold Spring Harb. Perspect. Med. 2016, 6, a026096. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, H.; Ji, Y.; Zhou, Q.; Du, Z.; Tian, L.; Jiang, Y.; Yao, K.; Zhou, Z. Targeting p53-MDM2 interaction by small-molecule inhibitors: Learning from MDM2 inhibitors in clinical trials. J. Hematol. Oncol. 2022, 15, 91. [Google Scholar] [CrossRef]
- Jiang, H.; Luo, J.; Lei, H. The roles of mouse double minute 2 (MDM2) oncoprotein in ocular diseases: A review. Exp. Eye Res. 2022, 217, 108910. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Feriotto, G.; Tagliati, F.; Giriolo, R.; Casciano, F.; Tabolacci, C.; Beninati, S.; Khan, M.T.H.; Mischiati, C. Caffeic Acid Enhances the Anti-Leukemic Effect of Imatinib on Chronic Myeloid Leukemia Cells and Triggers Apoptosis in Cells Sensitive and Resistant to Imatinib. Int. J. Mol. Sci. 2021, 22, 1644. [Google Scholar] [CrossRef] [PubMed]
- Melloni, E.; Marchesi, E.; Preti, L.; Casciano, F.; Rimondi, E.; Romani, A.; Secchiero, P.; Navacchia, M.L.; Perrone, D. Synthesis and Biological Investigation of Bile Acid-Paclitaxel Hybrids. Molecules 2022, 27, 471. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Casciano, F.; Stevanin, C.; Maietti, A.; Tedeschi, P.; Secchiero, P.; Marchetti, N.; Voltan, R. Anticancer Activity of Aqueous Extracts from Asparagus officinalis L. Byproduct on Breast Cancer Cells. Molecules 2021, 26, 6369. [Google Scholar] [CrossRef] [PubMed]
- Morshed, A.; Paul, S.; Hossain, A.; Basak, T.; Hossain, M.S.; Hasan, M.M.; Hasibuzzaman, M.A.; Rahaman, T.I.; Mia, M.A.R.; Shing, P.; et al. Baicalein as Promising Anticancer Agent: A Comprehensive Analysis on Molecular Mechanisms and Therapeutic Perspectives. Cancers 2023, 15, 2128. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Campbell, H.; Drummond, C.J.; Li, K.; Murray, K.; Slatter, T.; Bourdon, J.C.; Braithwaite, A.W. Adaptive homeostasis and the p53 isoform network. EMBO Rep. 2021, 22, e53085. [Google Scholar] [CrossRef]
- Blander, G.; Zalle, N.; Leal, J.F.; Bar-Or, R.L.; Yu, C.E.; Oren, M. The Werner syndrome protein contributes to induction of p53 by DNA damage. FASEB J. 2000, 14, 2138–2140. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Tong, J.; Jha, A.; Risnik, D.; Lizardo, D.; Lu, X.; Goel, A.; Opresko, P.L.; Yu, J.; Zhang, L. Synthetical lethality of Werner helicase and mismatch repair deficiency is mediated by p53 and PUMA in colon cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2211775119. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Maezawa, Y.; Nishijima, D.; Iwamoto, E.; Takeda, J.; Kanamori, T.; Yamaga, M.; Mishina, T.; Takeda, Y.; Izumi, S.; et al. A high prevalence of myeloid malignancies in progeria with Werner syndrome is associated with p53 insufficiency. Exp. Hematol. 2022, 109, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, M.; Oshima, J.; von Kobbe, C.; Cheng, W.H.; Leistritz, D.F.; Bohr, V.A. The clinical characteristics of Werner syndrome: Molecular and biochemical diagnosis. Hum. Genet. 2008, 124, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lee, L.; Hanson, N.B.; Lenaerts, C.; Hoehn, H.; Poot, M.; Rubin, C.D.; Chen, D.F.; Yang, C.C.; Juch, H.; et al. The spectrum of WRN mutations in Werner syndrome patients. Hum. Mutat. 2006, 27, 558–567. [Google Scholar] [CrossRef]
- Reichel, M.B.; Ali, R.R.; D’Esposito, F.; Clarke, A.R.; Luthert, P.J.; Bhattacharya, S.S.; Hunt, D.M. High frequency of persistent hyperplastic primary vitreous and cataracts in p53-deficient mice. Cell Death Differ. 1998, 5, 156–162. [Google Scholar] [CrossRef]
- Damato, B. Does ocular treatment of uveal melanoma influence survival? Br. J. Cancer 2010, 103, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Li, A.S.; Shih, C.Y.; Rosen, L.; Steiner, A.; Milman, T.; Udell, I.J. Recurrence of Ocular Surface Squamous Neoplasia Treated With Excisional Biopsy and Cryotherapy. Am. J. Ophthalmol. 2015, 160, 213–219.e1. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; He, W. Clinical features and factors affecting prognosis and partial deterioration of ocular papilloma: A retrospective study of 298 cases. Graefes Arch. Clin. Exp. Ophthalmol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Garcia Tirado, A.; Boto de Los Bueis, A.; Rivas Jara, L. Ocular surface changes in recurrent pterygium cases post-operatively treated with 5-fluorouracil subconjunctival injections. Eur. J. Ophthalmol. 2019, 29, 9–14. [Google Scholar] [CrossRef]
- Linzer, D.I.; Maltzman, W.; Levine, A.J. The SV40 A gene product is required for the production of a 54,000 MW cellular tumor antigen. Virology 1979, 98, 308–318. [Google Scholar] [CrossRef]
- DeLeo, A.B.; Jay, G.; Appella, E.; Dubois, G.C.; Law, L.W.; Old, L.J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc. Natl. Acad. Sci. USA 1979, 76, 2420–2424. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.V.; Pim, D.C.; Bulbrook, R.D. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int. J. Cancer 1982, 30, 403–408. [Google Scholar] [CrossRef]
- Crawford, L. The 53,000-dalton cellular protein and its role in transformation. Int. Rev. Exp. Pathol. 1983, 25, 1–50. [Google Scholar] [PubMed]
- Wolf, D.; Rotter, V. Inactivation of p53 gene expression by an insertion of Moloney murine leukemia virus-like DNA sequences. Mol. Cell Biol. 1984, 4, 1402–1410. [Google Scholar]
- Wolf, D.; Rotter, V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc. Natl. Acad. Sci. USA 1985, 82, 790–794. [Google Scholar] [CrossRef]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.W.; Zaika, A.; Moll, U.M. Nuclear and cytoplasmic degradation of endogenous p53 and HDM2 occurs during down-regulation of the p53 response after multiple types of DNA damage. FASEB J. 2003, 17, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- do Patrocinio, A.B.; Rodrigues, V.; Guidi Magalhaes, L. P53: Stability from the Ubiquitin-Proteasome System and Specific 26S Proteasome Inhibitors. ACS Omega 2022, 7, 3836–3843. [Google Scholar] [CrossRef]
- Ringshausen, I.; O’Shea, C.C.; Finch, A.J.; Swigart, L.B.; Evan, G.I. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell 2006, 10, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yan, Z.; Liao, X.; Li, Y.; Yang, J.; Wang, Z.G.; Zuo, Y.; Kawai, H.; Shadfan, M.; Ganapathy, S.; et al. The p53 inhibitors MDM2/MDMX complex is required for control of p53 activity in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 12001–12006. [Google Scholar] [CrossRef] [PubMed]
- de Rozieres, S.; Maya, R.; Oren, M.; Lozano, G. The loss of mdm2 induces p53-mediated apoptosis. Oncogene 2000, 19, 1691–1697. [Google Scholar] [CrossRef]
- Wade, M.; Li, Y.C.; Wahl, G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer 2013, 13, 83–96. [Google Scholar] [CrossRef]
- Lopez-Pajares, V.; Kim, M.M.; Yuan, Z.M. Phosphorylation of MDMX mediated by Akt leads to stabilization and induces 14-3-3 binding. J. Biol. Chem. 2008, 283, 13707–13713. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Qu, R.; Liu, D.; Xiong, X.; Liang, T.; Zhao, Y. The Cross Talk Between p53 and mTOR Pathways in Response to Physiological and Genotoxic Stresses. Front. Cell Dev Biol. 2021, 9, 775507. [Google Scholar] [CrossRef]
- Pungsrinont, T.; Kallenbach, J.; Baniahmad, A. Role of PI3K-AKT-mTOR Pathway as a Pro-Survival Signaling and Resistance-Mediating Mechanism to Therapy of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 11088. [Google Scholar] [CrossRef]
- Casciano, F.; Zauli, E.; Rimondi, E.; Mura, M.; Previati, M.; Busin, M.; Zauli, G. The role of the mTOR pathway in diabetic retinopathy. Front. Med. 2022, 9, 973856. [Google Scholar] [CrossRef]
- Qin, X.; Zou, H. The role of lipopolysaccharides in diabetic retinopathy. BMC Ophthalmol. 2022, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Zauli, E.; Casciano, F.; Secchiero, P.; Zauli, G.; Fields, M.; Melloni, E. Palmitic Acid Induced a Long-Lasting Lipotoxic Insult in Human Retinal Pigment Epithelial Cells, which Is Partially Counteracted by TRAIL. Antioxidants 2022, 11, 2340. [Google Scholar] [CrossRef] [PubMed]
- Gurel, Z.; Zaro, B.W.; Pratt, M.R.; Sheibani, N. Identification of O-GlcNAc modification targets in mouse retinal pericytes: Implication of p53 in pathogenesis of diabetic retinopathy. PLoS ONE 2014, 9, e95561. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhao, H.; Chen, B. DJ-1/PARK7 inhibits high glucose-induced oxidative stress to prevent retinal pericyte apoptosis via the PI3K/AKT/mTOR signaling pathway. Exp. Eye Res. 2019, 189, 107830. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, H.; Zhang, Q. Procyanidin protects human retinal pigment epithelial cells from high glucose by inhibiting autophagy. Environ. Toxicol. 2022, 37, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Gu, W. p53 post-translational modification: Deregulated in tumorigenesis. Trends Mol. Med. 2010, 16, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tavana, O.; Gu, W. p53 modifications: Exquisite decorations of the powerful guardian. J. Mol. Cell. Biol. 2019, 11, 564–577. [Google Scholar] [CrossRef]
- Holley, A.K.; St Clair, D.K. Watching the watcher: Regulation of p53 by mitochondria. Future Oncol. 2009, 5, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chaiswing, L.; Velez, J.M.; Batinic-Haberle, I.; Colburn, N.H.; Oberley, T.D.; St Clair, D.K. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res. 2005, 65, 3745–3750. [Google Scholar] [CrossRef]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef]
- Fischer, M. Census and evaluation of p53 target genes. Oncogene 2017, 36, 3943–3956. [Google Scholar] [CrossRef]
- Secchiero, P.; Barbarotto, E.; Tiribelli, M.; Zerbinati, C.; di Iasio, M.G.; Gonelli, A.; Cavazzini, F.; Campioni, D.; Fanin, R.; Cuneo, A.; et al. Functional integrity of the p53-mediated apoptotic pathway induced by the nongenotoxic agent nutlin-3 in B-cell chronic lymphocytic leukemia (B-CLL). Blood 2006, 107, 4122–4129. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Choi, K.S.; Yoo, Y.H.; Kwon, T.K. Nutlin-3, a small-molecule MDM2 inhibitor, sensitizes Caki cells to TRAIL-induced apoptosis through p53-mediated PUMA upregulation and ROS-mediated DR5 upregulation. Anticancer Drugs 2013, 24, 260–269. [Google Scholar] [CrossRef]
- Valente, L.J.; Aubrey, B.J.; Herold, M.J.; Kelly, G.L.; Happo, L.; Scott, C.L.; Newbold, A.; Johnstone, R.W.; Huang, D.C.; Vassilev, L.T.; et al. Therapeutic Response to Non-genotoxic Activation of p53 by Nutlin3a Is Driven by PUMA-Mediated Apoptosis in Lymphoma Cells. Cell Rep. 2016, 14, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Chen, J.; Liao, J.; Huang, Y.; Gan, Y.; Larisch, S.; Zeng, S.X.; Lu, H.; Zhou, X. p53 induces ARTS to promote mitochondrial apoptosis. Cell Death Dis. 2021, 12, 204. [Google Scholar] [CrossRef]
- Li, L.; Song, M.; Zhou, J.; Sun, X.; Lei, Y. Ambient particulate matter exposure causes visual dysfunction and retinal neuronal degeneration. Ecotoxicol. Environ. Saf. 2022, 247, 114231. [Google Scholar] [CrossRef] [PubMed]
- Loughery, J.; Cox, M.; Smith, L.M.; Meek, D.W. Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive promoters. Nucleic Acids Res. 2014, 42, 7666–7680. [Google Scholar] [CrossRef] [PubMed]
- Aubry, A.; Pearson, J.D.; Huang, K.; Livne-Bar, I.; Ahmad, M.; Jagadeesan, M.; Khetan, V.; Ketela, T.; Brown, K.R.; Yu, T.; et al. Functional genomics identifies new synergistic therapies for retinoblastoma. Oncogene 2020, 39, 5338–5357. [Google Scholar] [CrossRef]
- Pant, V.; Xiong, S.; Jackson, J.G.; Post, S.M.; Abbas, H.A.; Quintas-Cardama, A.; Hamir, A.N.; Lozano, G. The p53-Mdm2 feedback loop protects against DNA damage by inhibiting p53 activity but is dispensable for p53 stability, development, and longevity. Genes Dev. 2013, 27, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, L.A.; Hamard, P.J.; Tonnessen, C.; Manfredi, J.J. E2F7, a novel target, is up-regulated by p53 and mediates DNA damage-dependent transcriptional repression. Genes Dev. 2012, 26, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, S.F.; de la Vega, M.B.; Calzetta, N.L.; Siri, S.O.; Gottifredi, V. CDK-Independent and PCNA-Dependent Functions of p21 in DNA Replication. Genes 2020, 11, 593. [Google Scholar] [CrossRef]
- Topacio, B.R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C.S.; Rubin, S.M.; Sage, J.; Koivomagi, M.; Skotheim, J.M. Cyclin D-Cdk4,6 Drives Cell-Cycle Progression via the Retinoblastoma Protein’s C-Terminal Helix. Mol. Cell 2019, 74, 758–770.e4. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Linn, P.; Kohno, S.; Sheng, J.; Kulathunga, N.; Yu, H.; Zhang, Z.; Voon, D.; Watanabe, Y.; Takahashi, C. Targeting RB1 Loss in Cancers. Cancers 2021, 13, 3737. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.D.; Dyer, M.A. Genetic and Epigenetic Discoveries in Human Retinoblastoma. Crit. Rev. Oncog. 2015, 20, 217–225. [Google Scholar] [CrossRef]
- Romani, A.; Zauli, E.; Zauli, G.; AlMesfer, S.; Al-Swailem, S.; Voltan, R. MDM2 inhibitors-mediated disruption of mitochondrial metabolism: A novel therapeutic strategy for retinoblastoma. Front. Oncol. 2022, 12, 1000677. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Wang, J.; Thomas, H.R.; Li, Z.; Yeo, N.C.F.; Scott, H.E.; Dang, N.; Hossain, M.I.; Andrabi, S.A.; Parant, J.M. Puma, noxa, p53, and p63 differentially mediate stress pathway induced apoptosis. Cell Death Dis. 2021, 12, 659. [Google Scholar] [CrossRef]
- Roufayel, R.; Younes, K.; Al-Sabi, A.; Murshid, N. BH3-Only Proteins Noxa and Puma Are Key Regulators of Induced Apoptosis. Life 2022, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Li, M. The role of P53 up-regulated modulator of apoptosis (PUMA) in ovarian development, cardiovascular and neurodegenerative diseases. Apoptosis 2021, 26, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, K.; Hertlein, V.; Jenner, A.; Dellmann, T.; Gojkovic, M.; Pena-Blanco, A.; Dadsena, S.; Wajngarten, N.; Danial, J.S.H.; Thevathasan, J.V.; et al. The interplay between BAX and BAK tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation. Mol. Cell 2022, 82, 933–949.e9. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H. Structural features of caspase-activating complexes. Int. J. Mol. Sci. 2012, 13, 4807–4818. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Lazebnik, Y. Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev. 1999, 13, 3179–3184. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [PubMed]
- Marchenko, N.D.; Moll, U.M. Mitochondrial death functions of p53. Mol. Cell. Oncol. 2014, 1, e955995. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Wang, J.; Zhang, T.; Xu, D.; Hu, W.; Feng, Z. The Interplay Between Tumor Suppressor p53 and Hypoxia Signaling Pathways in Cancer. Front. Cell Dev. Biol. 2021, 9, 648808. [Google Scholar] [CrossRef]
- Nieminen, A.L.; Qanungo, S.; Schneider, E.A.; Jiang, B.H.; Agani, F.H. Mdm2 and HIF-1alpha interaction in tumor cells during hypoxia. J. Cell. Physiol. 2005, 204, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Mammadzada, P.; Corredoira, P.M.; Andre, H. The role of hypoxia-inducible factors in neovascular age-related macular degeneration: A gene therapy perspective. Cell Mol. Life Sci. 2020, 77, 819–833. [Google Scholar] [CrossRef]
- Lee, D.; Kunimi, H.; Negishi, K.; Kurihara, T. Degeneration of retinal ganglion cells in hypoxic responses: Hypoxia-inducible factor inhibition, a new therapeutic insight. Neural Regen. Res. 2022, 17, 2230–2231. [Google Scholar] [PubMed]
- Rosenbaum, D.M.; Rosenbaum, P.S.; Gupta, H.; Singh, M.; Aggarwal, A.; Hall, D.H.; Roth, S.; Kessler, J.A. The role of the p53 protein in the selective vulnerability of the inner retina to transient ischemia. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2132–2139. [Google Scholar]
- Kaur, C.; Foulds, W.S.; Ling, E.A. Hypoxia-ischemia and retinal ganglion cell damage. Clin. Ophthalmol. 2008, 2, 879–889. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Raj, S.; DePamphilis, M.L. Developmental Acquisition of p53 Functions. Genes 2021, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, Y.; Xiong, S.; Williams-Villalobo, A.E. A Glance of p53 Functions in Brain Development, Neural Stem Cells, and Brain Cancer. Biology 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.D.; Pozniak, C.D.; Walsh, G.S. Neuronal life and death: An essential role for the p53 family. Cell Death Differ. 2000, 7, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Casciano, F.; Bianchi, N.; Borin, M.; Vellani, V.; Secchiero, P.; Bergamini, C.M.; Capsoni, S.; Pignatelli, A. Characterization by Gene Expression Analysis of Two Groups of Dopaminergic Cells Isolated from the Mouse Olfactory Bulb. Biology 2023, 12, 367. [Google Scholar] [CrossRef]
- Ogundele, O.M.; Sanya, O.J. Bax modulates neuronal survival while p53 is unaltered after Cytochrome C induced oxidative stress in the adult olfactory bulb in vivo. Ann. Neurosci. 2015, 22, 19–25. [Google Scholar] [CrossRef]
- Fatt, M.P.; Cancino, G.I.; Miller, F.D.; Kaplan, D.R. p63 and p73 coordinate p53 function to determine the balance between survival, cell death, and senescence in adult neural precursor cells. Cell Death Differ. 2014, 21, 1546–1559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vuong, L.; Brobst, D.E.; Ivanovic, I.; Sherry, D.M.; Al-Ubaidi, M.R. p53 selectively regulates developmental apoptosis of rod photoreceptors. PLoS ONE 2013, 8, e67381. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.; Brobst, D.E.; Saadi, A.; Ivanovic, I.; Al-Ubaidi, M.R. Pattern of expression of p53, its family members, and regulators during early ocular development and in the post-mitotic retina. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4821–4831. [Google Scholar] [CrossRef]
- Tendler, Y.; Panshin, A. Features of p53 protein distribution in the corneal epithelium and corneal tear film. Sci. Rep. 2020, 10, 10051. [Google Scholar] [CrossRef]
- Tendler, Y.; Weisinger, G.; Coleman, R.; Diamond, E.; Lischinsky, S.; Kerner, H.; Rotter, V.; Zinder, O. Tissue-specific p53 expression in the nervous system. Brain Res. Mol. Brain Res. 1999, 72, 40–46. [Google Scholar] [CrossRef]
- Shin, D.H.; Lee, H.Y.; Lee, H.W.; Kim, H.J.; Lee, E.; Cho, S.S.; Baik, S.H.; Lee, K.H. In situ localization of p53, bcl-2 and bax mRNAs in rat ocular tissue. Neuroreport 1999, 10, 2165–2167. [Google Scholar] [CrossRef]
- Pokroy, R.; Tendler, Y.; Pollack, A.; Zinder, O.; Weisinger, G. p53 expression in the normal murine eye. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1736–1741. [Google Scholar]
- Li, J.J.; Yi, S.; Wei, L. Ocular Microbiota and Intraocular Inflammation. Front. Immunol. 2020, 11, 609765. [Google Scholar] [CrossRef] [PubMed]
- Jaki Mekjavic, P.; Tipton, M.J.; Mekjavic, I.B. The eye in extreme environments. Exp. Physiol. 2021, 106, 52–64. [Google Scholar] [CrossRef]
- Blindness, G.B.D.; Vision Impairment, C.; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar]
- Zella, D.; Gallo, R.C. Viruses and Bacteria Associated with Cancer: An Overview. Viruses 2021, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Ramberg, I.; Heegaard, S. Human Papillomavirus Related Neoplasia of the Ocular Adnexa. Viruses 2021, 13, 1522. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.W.; Arruda, J.T.; Silva, R.E.; Moura, K.K. TP53 gene expression, codon 72 polymorphism and human papillomavirus DNA associated with pterygium. Genet. Mol. Res. 2008, 7, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Chang, C.C.; Chiang, C.C.; Yeh, K.T.; Chen, P.L.; Chang, C.H.; Chou, M.C.; Lee, H.; Cheng, Y.W. HPV infection and p53 inactivation in pterygium. Mol. Vis. 2009, 15, 1092–1097. [Google Scholar]
- Dushku, N.; Hatcher, S.L.; Albert, D.M.; Reid, T.W. p53 expression and relation to human papillomavirus infection in pingueculae, pterygia, and limbal tumors. Arch. Ophthalmol. 1999, 117, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Morgan, J.I.; Merigan, W.H.; Sliney, D.H.; Sparrow, J.R.; Williams, D.R. The susceptibility of the retina to photochemical damage from visible light. Prog. Retin. Eye Res. 2012, 31, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of blue light-induced eye hazard and protective measures: A review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef]
- Song, W.; Zhu, R.; Gao, W.; Xing, C.; Yang, L. Blue Light Induces RPE Cell Necroptosis, Which Can Be Inhibited by Minocycline. Front. Med. 2022, 9, 831463. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Hafezi, F.; Lansel, N.; Hegi, M.E.; Wenzel, A.; Grimm, C.; Niemeyer, G.; Reme, C.E. Light-induced cell death of retinal photoreceptors in the absence of p53. Investig. Ophthalmol. Vis. Sci. 1998, 39, 846–849. [Google Scholar]
- Lansel, N.; Hafezi, F.; Marti, A.; Hegi, M.; Reme, C.; Niemeyer, G. The mouse ERG before and after light damage is independent of p53. Doc. Ophthalmol. 1998, 96, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.L.; Deng, W.L.; Huang, N.; Wang, Y.Y.; Lei, X.L.; Xu, Z.Q.; Hu, D.N.; Cai, J.Q.; Lu, F.; Jin, Z.B. Upregulation of GADD45alpha in light-damaged retinal pigment epithelial cells. Cell Death Discov. 2016, 2, 16013. [Google Scholar] [CrossRef] [PubMed]
- Westlund, B.S.; Cai, B.; Zhou, J.; Sparrow, J.R. Involvement of c-Abl, p53 and the MAP kinase JNK in the cell death program initiated in A2E-laden ARPE-19 cells by exposure to blue light. Apoptosis 2009, 14, 31–41. [Google Scholar] [CrossRef]
- Lyu, Y.; Tschulakow, A.V.; Wang, K.; Brash, D.E.; Schraermeyer, U. Chemiexcitation and melanin in photoreceptor disc turnover and prevention of macular degeneration. Proc. Natl. Acad. Sci. USA 2023, 120, e2216935120. [Google Scholar] [CrossRef]
- Fietz, A.; Hurst, J.; Schnichels, S. Out of the Shadow: Blue Light Exposure Induces Apoptosis in Muller Cells. Int. J. Mol. Sci. 2022, 23, 14540. [Google Scholar] [CrossRef]
- Alvarez-Barrios, A.; Alvarez, L.; Garcia, M.; Artime, E.; Pereiro, R.; Gonzalez-Iglesias, H. Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins. Antioxidants 2021, 10, 89. [Google Scholar] [CrossRef]
- Sablina, A.A.; Budanov, A.V.; Ilyinskaya, G.V.; Agapova, L.S.; Kravchenko, J.E.; Chumakov, P.M. The antioxidant function of the p53 tumor suppressor. Nat. Med. 2005, 11, 1306–1313. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Wallace, D.M.; O’Brien, C.J.; Cotter, T.G. A novel antioxidant function for the tumor-suppressor gene p53 in the retinal ganglion cell. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4237–4244. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.J.; Gallego-Pinazo, R.; de Hoz, R.; Pinazo-Duran, M.D.; Rojas, B.; Ramirez, A.I.; Serrano, M.; Ramirez, J.M. "Super p53" mice display retinal astroglial changes. PLoS ONE 2013, 8, e65446. [Google Scholar] [CrossRef] [PubMed]
- Terao, R.; Ahmed, T.; Suzumura, A.; Terasaki, H. Oxidative Stress-Induced Cellular Senescence in Aging Retina and Age-Related Macular Degeneration. Antioxidants 2022, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Zhang, X.; Srivenugopal, K.S.; Wang, M.H.; Wang, W.; Zhang, R. Targeting MDM2-p53 interaction for cancer therapy: Are we there yet? Curr. Med. Chem. 2014, 21, 553–574. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Maki, C.G. Pharmacologic activation of p53 by small-molecule MDM2 antagonists. Curr. Pharm. Des. 2011, 17, 560–568. [Google Scholar] [CrossRef]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef]
- Neochoritis, C.; Estrada-Ortiz, N.; Khoury, K.; Dömling, A. p53–MDM2 and MDMX Antagonists. Annu. Rep. Med. Chem. 2014, 49, 167–187. [Google Scholar]
- Agnoletto, C.; Brunelli, L.; Melloni, E.; Pastorelli, R.; Casciano, F.; Rimondi, E.; Rigolin, G.M.; Cuneo, A.; Secchiero, P.; Zauli, G. The anti-leukemic activity of sodium dichloroacetate in p53mutated/null cells is mediated by a p53-independent ILF3/p21 pathway. Oncotarget 2015, 6, 2385–2396. [Google Scholar] [CrossRef][Green Version]
- Secchiero, P.; Bosco, R.; Celeghini, C.; Zauli, G. Recent advances in the therapeutic perspectives of Nutlin-3. Curr. Pharm. Des. 2011, 17, 569–577. [Google Scholar] [CrossRef]
- Zauli, G.; AlHilali, S.; Al-Swailem, S.; Secchiero, P.; Voltan, R. Therapeutic potential of the MDM2 inhibitor Nutlin-3 in counteracting SARS-CoV-2 infection of the eye through p53 activation. Front. Med. 2022, 9, 902713. [Google Scholar] [CrossRef]
- Milani, D.; Caruso, L.; Zauli, E.; Al Owaifeer, A.M.; Secchiero, P.; Zauli, G.; Gemmati, D.; Tisato, V. p53/NF-kB Balance in SARS-CoV-2 Infection: From OMICs, Genomics and Pharmacogenomics Insights to Tailored Therapeutic Perspectives (COVIDomics). Front. Pharmacol. 2022, 13, 871583. [Google Scholar] [CrossRef]
- Lodi, G.; Gentili, V.; Casciano, F.; Romani, A.; Zauli, G.; Secchiero, P.; Zauli, E.; Simioni, C.; Beltrami, S.; Fernandez, M.; et al. Cell cycle block by p53 activation reduces SARS-CoV-2 release in infected alveolar basal epithelial A549-hACE2 cells. Front. Pharmacol. 2022, 13, 1018761. [Google Scholar] [CrossRef]
- Tisato, V.; Voltan, R.; Gonelli, A.; Secchiero, P.; Zauli, G. MDM2/X inhibitors under clinical evaluation: Perspectives for the management of hematological malignancies and pediatric cancer. J. Hematol. Oncol. 2017, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, H.; Thuraisamy, A. MDM2/P53 Inhibitors as Sensitizing Agents for Cancer Chemotherapy. In Protein Kinase Inhibitors as Sensitizing Agents for Chemotherapy; Chen, Z.-S., Yang, D.-H., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 4, pp. 243–266. [Google Scholar]
- Papai, Z.; Chen, L.C.; Da Costa, D.; Blotner, S.; Vazvaei, F.; Gleave, M.; Jones, R.; Zhi, J. A single-center, open-label study investigating the excretion balance, pharmacokinetics, metabolism, and absolute bioavailability of a single oral dose of [(14)C]-labeled idasanutlin and an intravenous tracer dose of [(13)C]-labeled idasanutlin in a single cohort of patients with solid tumors. Cancer Chemother. Pharmacol. 2019, 84, 93–103. [Google Scholar] [PubMed]
- Zauli, G.; Tisato, V.; Secchiero, P. Rationale for Considering Oral Idasanutlin as a Therapeutic Option for COVID-19 Patients. Front. Pharmacol. 2020, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.; Wovkulich, P.; Pizzolato, G.; Lovey, A.; Ding, Q.; Jiang, N.; Liu, J.J.; Zhao, C.; Glenn, K.; Wen, Y.; et al. Discovery of RG7112: A Small-Molecule MDM2 Inhibitor in Clinical Development. ACS Med. Chem. Lett. 2013, 4, 466–469. [Google Scholar] [CrossRef]
- McKean, M.; Tolcher, A.W.; Reeves, J.A.; Chmielowski, B.; Shaheen, M.F.; Beck, J.T.; Orloff, M.M.; Somaiah, N.; Van Tine, B.A.; Drabick, J.J.; et al. Newly updated activity results of alrizomadlin (APG-115), a novel MDM2/p53 inhibitor, plus pembrolizumab: Phase 2 study in adults and children with various solid tumors. J. Clin. Oncol. 2022, 40, 9517. [Google Scholar] [CrossRef]
- Bill, K.L.; Garnett, J.; Meaux, I.; Ma, X.; Creighton, C.J.; Bolshakov, S.; Barriere, C.; Debussche, L.; Lazar, A.J.; Prudner, B.C.; et al. SAR405838: A Novel and Potent Inhibitor of the MDM2:p53 Axis for the Treatment of Dedifferentiated Liposarcoma. Clin. Cancer Res. 2016, 22, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.J.; Banerji, U.; Mahipal, A.; Somaiah, N.; Hirsch, H.; Fancourt, C.; Johnson-Levonas, A.O.; Lam, R.; Meister, A.K.; Russo, G.; et al. Phase I Trial of the Human Double Minute 2 Inhibitor MK-8242 in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2017, 35, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, R.; De Matteis, S.; Carloni, S.; Bruno, S.; Abbati, G.; Capelli, L.; Ghetti, M.; Bochicchio, M.T.; Liverani, C.; Mercatali, L.; et al. Kevetrin induces apoptosis in TP53 wild-type and mutant acute myeloid leukemia cells. Oncol. Rep. 2020, 44, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.N.; Patel, M.R.; Bauer, T.M.; Goel, S.; Falchook, G.S.; Shapiro, G.I.; Chung, K.Y.; Infante, J.R.; Conry, R.M.; Rabinowits, G.; et al. Phase 1 Trial of ALRN-6924, a Dual Inhibitor of MDMX and MDM2, in Patients with Solid Tumors and Lymphomas Bearing Wild-type TP53. Clin. Cancer Res. 2021, 27, 5236–5247. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; DeAngelo, D.J.; Chromik, J.; Chatterjee, M.; Bauer, S.; Lin, C.C.; Suarez, C.; de Vos, F.; Steeghs, N.; Cassier, P.A.; et al. Results from a First-in-Human Phase I Study of Siremadlin (HDM201) in Patients with Advanced Wild-Type TP53 Solid Tumors and Acute Leukemia. Clin. Cancer Res. 2022, 28, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Fujiwara, Y.; Nakano, K.; Shimizu, T.; Tomomatsu, J.; Koyama, T.; Ogura, M.; Tachibana, M.; Kakurai, Y.; Yamashita, T.; et al. Safety and pharmacokinetics of milademetan, a MDM2 inhibitor, in Japanese patients with solid tumors: A phase I study. Cancer Sci. 2021, 112, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Demetri, G.D.; Halilovic, E.; Dummer, R.; Meille, C.; Tan, D.S.W.; Guerreiro, N.; Jullion, A.; Ferretti, S.; Jeay, S.; et al. Pharmacokinetic-pharmacodynamic guided optimisation of dose and schedule of CGM097, an HDM2 inhibitor, in preclinical and clinical studies. Br. J. Cancer 2021, 125, 687–698. [Google Scholar] [CrossRef]
- Gluck, W.L.; Gounder, M.M.; Frank, R.; Eskens, F.; Blay, J.Y.; Cassier, P.A.; Soria, J.C.; Chawla, S.; de Weger, V.; Wagner, A.J.; et al. Phase 1 study of the MDM2 inhibitor AMG 232 in patients with advanced P53 wild-type solid tumors or multiple myeloma. Investig. New Drugs 2020, 38, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Togashi, K.; Okada, M.; Suzuki, S.; Sanomachi, T.; Seino, S.; Yamamoto, M.; Yamashita, H.; Kitanaka, C. Inhibition of Retinoblastoma Cell Growth by CEP1347 Through Activation of the P53 Pathway. Anticancer Res. 2020, 40, 4961–4968. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Xie, L.; He, X.; Bai, J. Progress in the pathogenesis of pterygium. Curr. Eye Res. 2013, 38, 1191–1197. [Google Scholar] [CrossRef]
- Threlfall, T.J.; English, D.R. Sun exposure and pterygium of the eye: A dose-response curve. Am. J. Ophthalmol. 1999, 128, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Reisman, D.; McFadden, J.W.; Lu, G. Loss of heterozygosity and p53 expression in Pterygium. Cancer Lett. 2004, 206, 77–83. [Google Scholar] [CrossRef]
- Tsai, Y.Y.; Cheng, Y.W.; Lee, H.; Tsai, F.J.; Tseng, S.H.; Chang, K.C. P53 gene mutation spectrum and the relationship between gene mutation and protein levels in pterygium. Mol. Vis. 2005, 11, 50–55. [Google Scholar]
- Spandidos, D.A.; Sourvinos, G.; Kiaris, H.; Tsamparlakis, J. Microsatellite instability and loss of heterozygosity in human pterygia. Br. J. Ophthalmol. 1997, 81, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.; Lim, A.S.; Goh, H.S.; Smith, D.R. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am. J. Ophthalmol. 1997, 123, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Onur, C.; Orhan, D.; Orhan, M.; Dizbay Sak, S.; Tulunay, O.; Irkec, M. Expression of p53 protein in pterygium. Eur. J. Ophthalmol. 1998, 8, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Dushku, N.; Reid, T.W. P53 expression in altered limbal basal cells of pingueculae, pterygia, and limbal tumors. Curr. Eye Res. 1997, 16, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Chowers, I.; Pe’er, J.; Zamir, E.; Livni, N.; Ilsar, M.; Frucht-Pery, J. Proliferative activity and p53 expression in primary and recurrent pterygia. Ophthalmology 2001, 108, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Chang, K.C.; Lin, C.L.; Lee, H.; Tsai, F.J.; Cheng, Y.W.; Tseng, S.H. p53 Expression in pterygium by immunohistochemical analysis: A series report of 127 cases and review of the literature. Cornea 2005, 24, 583–586. [Google Scholar] [CrossRef]
- Weinstein, O.; Rosenthal, G.; Zirkin, H.; Monos, T.; Lifshitz, T.; Argov, S. Overexpression of p53 tumor suppressor gene in pterygia. Eye 2002, 16, 619–621. [Google Scholar] [CrossRef]
- Tan, D.T.; Tang, W.Y.; Liu, Y.P.; Goh, H.S.; Smith, D.R. Apoptosis and apoptosis related gene expression in normal conjunctiva and pterygium. Br. J. Ophthalmol. 2000, 84, 212–216. [Google Scholar] [CrossRef][Green Version]
- Martins, T.G.; Costa, A.L.; Alves, M.R.; Chammas, R.; Schor, P. Mitomycin C in pterygium treatment. Int. J. Ophthalmol. 2016, 9, 465–468. [Google Scholar]
- Cao, D.; Chu, W.K.; Ng, T.K.; Yip, Y.W.Y.; Young, A.L.; Pang, C.P.; Jhanji, V. Cellular Proliferation and Migration of Human Pterygium Cells: Mitomycin Versus Small-Molecule Inhibitors. Cornea 2018, 37, 760–766. [Google Scholar] [CrossRef]
- Isager, P.; Engholm, G.; Overgaard, J.; Storm, H. Uveal and conjunctival malignant melanoma in denmark 1943-97: Observed and relative survival of patients followed through 2002. Ophthalmic Epidemiol. 2006, 13, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Markowitz, J.S.; Belinsky, I.; Schwartzstein, H.; George, N.S.; Lally, S.E.; Mashayekhi, A.; Shields, J.A. Conjunctival melanoma: Outcomes based on tumor origin in 382 consecutive cases. Ophthalmology 2011, 118, 389–395.e1-2. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L.; Mashayekhi, A.; Marr, B.P.; Benavides, R.; Thangappan, A.; Phan, L.; Eagle, R.C., Jr. Primary acquired melanosis of the conjunctiva: Risks for progression to melanoma in 311 eyes. The 2006 Lorenz E. Zimmerman lecture. Ophthalmology 2008, 115, 511–519.e2. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Gunduz, K.; Cater, J.; Mercado, G.V.; Gross, N.; Lally, B. Conjunctival melanoma: Risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Arch. Ophthalmol. 2000, 118, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Finger, P.T.; Fili, M.; Damato, B.; Coupland, S.E.; Heimann, H.; Kenawy, N.; Brouwer, N.J.; Marinkovic, M.; Van Duinen, S.G.; et al. Conjunctival melanoma treatment outcomes in 288 patients: A multicentre international data-sharing study. Br. J. Ophthalmol. 2021, 105, 1358–1364. [Google Scholar] [CrossRef]
- Seregard, S. Cell growth and p53 expression in primary acquired melanosis and conjunctival melanoma. J. Clin. Pathol. 1996, 49, 338–342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, D.; Cismas, S.; Crudden, C.; Trocme, E.; Worrall, C.; Suleymanova, N.; Lin, T.; Zheng, H.; Seregard, S.; Girnita, A.; et al. IGF-1R is a molecular determinant for response to p53 reactivation therapy in conjunctival melanoma. Oncogene 2022, 41, 600–611. [Google Scholar] [CrossRef]
- Global Retinoblastoma Study Group; Fabian, I.D.; Abdallah, E.; Abdullahi, S.U.; Abdulqader, R.A.; Adamou Boubacar, S.; Ademola-Popoola, D.S.; Adio, A.; Afshar, A.R.; Aggarwal, P.; et al. Global Retinoblastoma Presentation and Analysis by National Income Level. JAMA Oncol. 2020, 6, 685–695. [Google Scholar] [CrossRef]
- Rodriguez-Galindo, C.; Orbach, D.B.; VanderVeen, D. Retinoblastoma. Pediatr. Clin. N. Am. 2015, 62, 201–223. [Google Scholar] [CrossRef]
- Fernandes, A.G.; Pollock, B.D.; Rabito, F.A. Retinoblastoma in the United States: A 40-Year Incidence and Survival Analysis. J. Pediatr. Ophthalmol. Strabismus 2018, 55, 182–188. [Google Scholar] [CrossRef]
- Zhang, J.; Benavente, C.A.; McEvoy, J.; Flores-Otero, J.; Ding, L.; Chen, X.; Ulyanov, A.; Wu, G.; Wilson, M.; Wang, J.; et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 2012, 481, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Knight, L.A.; Gardner, H.A.; Gallie, B.L. Segregation of chromosome 13 in retinoblastoma. Lancet 1978, 1, 989. [Google Scholar] [CrossRef] [PubMed]
- Sidle, A.; Palaty, C.; Dirks, P.; Wiggan, O.; Kiess, M.; Gill, R.M.; Wong, A.K.; Hamel, P.A. Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation. Crit. Rev. Biochem. Mol. Biol. 1996, 31, 237–271. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.A. Cancer biology: Second step to retinal tumours. Nature 2006, 444, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.A.; Tsao, H. Melanoma and genetics. Clin. Dermatol. 2009, 27, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A. Understanding signal transduction pathways to overcome targeted therapy resistance in glioblastoma. In Glioblastoma Resistance to Chemotherapy: Molecular Mechanisms and Innovative Reversal Strategies; Paulmurugan, R., Massoud, T.F., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 15, pp. 547–585. [Google Scholar]
- Sdek, P.; Ying, H.; Chang, D.L.; Qiu, W.; Zheng, H.; Touitou, R.; Allday, M.J.; Xiao, Z.X. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol. Cell 2005, 20, 699–708. [Google Scholar] [CrossRef]
- Tang, Y.A.; Lin, R.K.; Tsai, Y.T.; Hsu, H.S.; Yang, Y.C.; Chen, C.Y.; Wang, Y.C. MDM2 overexpression deregulates the transcriptional control of RB/E2F leading to DNA methyltransferase 3A overexpression in lung cancer. Clin. Cancer Res. 2012, 18, 4325–4333. [Google Scholar] [CrossRef][Green Version]
- Hernandez-Monge, J.; Rousset-Roman, A.B.; Medina-Medina, I.; Olivares-Illana, V. Dual function of MDM2 and MDMX toward the tumor suppressors p53 and RB. Genes Cancer 2016, 7, 278–287. [Google Scholar] [CrossRef][Green Version]
- Grace, C.R.; Ban, D.; Min, J.; Mayasundari, A.; Min, L.; Finch, K.E.; Griffiths, L.; Bharatham, N.; Bashford, D.; Kiplin Guy, R.; et al. Monitoring Ligand-Induced Protein Ordering in Drug Discovery. J. Mol. Biol. 2016, 428, 1290–1303. [Google Scholar] [CrossRef]
- Marine, J.C.; Jochemsen, A.G. Mdmx as an essential regulator of p53 activity. Biochem. Biophys. Res. Commun. 2005, 331, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Voltan, R.; Rimondi, E.; Melloni, E.; Rigolin, G.M.; Casciano, F.; Arcidiacono, M.V.; Celeghini, C.; Cuneo, A.; Zauli, G.; Secchiero, P. Ibrutinib synergizes with MDM-2 inhibitors in promoting cytotoxicity in B chronic lymphocytic leukemia. Oncotarget 2016, 7, 70623–70638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elison, J.R.; Cobrinik, D.; Claros, N.; Abramson, D.H.; Lee, T.C. Small molecule inhibition of HDM2 leads to p53-mediated cell death in retinoblastoma cells. Arch. Ophthalmol. 2006, 124, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Deo, D.; Xia, M.; Vassilev, L.T. Pharmacologic p53 activation blocks cell cycle progression but fails to induce senescence in epithelial cancer cells. Mol. Cancer Res. 2009, 7, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.C.; Federico, S.; Bradley, C.; Zhang, J.; Flores-Otero, J.; Wilson, M.; Stewart, C.; Zhu, F.; Guy, K.; Dyer, M.A. Targeting the p53 pathway in retinoblastoma with subconjunctival Nutlin-3a. Cancer Res. 2011, 71, 4205–4213. [Google Scholar] [CrossRef] [PubMed]
- Lenos, K.; de Lange, J.; Teunisse, A.F.; Lodder, K.; Verlaan-de Vries, M.; Wiercinska, E.; van der Burg, M.J.; Szuhai, K.; Jochemsen, A.G. Oncogenic functions of hMDMX in in vitro transformation of primary human fibroblasts and embryonic retinoblasts. Mol. Cancer 2011, 10, 111. [Google Scholar] [CrossRef]
- Laurie, N.A.; Donovan, S.L.; Shih, C.S.; Zhang, J.; Mills, N.; Fuller, C.; Teunisse, A.; Lam, S.; Ramos, Y.; Mohan, A.; et al. Inactivation of the p53 pathway in retinoblastoma. Nature 2006, 444, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, L.A.; Neriah, D.B.; Senecal, A.; Benard, L.; Thiruthuvanathan, V.; Yatsenko, T.; Narayanagari, S.R.; Wheat, J.C.; Todorova, T.I.; Mitchell, K.; et al. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci. Transl. Med. 2018, 10, eaao3003. [Google Scholar] [CrossRef] [PubMed]
- Mitobe, Y.; Nakagawa-Saito, Y.; Togashi, K.; Suzuki, S.; Sugai, A.; Matsuda, K.I.; Sonoda, Y.; Kitanaka, C.; Okada, M. CEP-1347 Targets MDM4 Protein Expression to Activate p53 and Inhibit the Growth of Glioma Cells. Anticancer Res. 2022, 42, 4727–4733. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Branisteanu, D.E.; Porumb-Andrese, E.; Starica, A.; Munteanu, A.C.; Toader, M.P.; Zemba, M.; Porumb, V.; Cozmin, M.; Moraru, A.D.; Nicolescu, A.C.; et al. Differences and Similarities in Epidemiology and Risk Factors for Cutaneous and Uveal Melanoma. Medicina 2023, 59, 943. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, D.A.; Brock, A.L. Radiation therapy for uveal melanoma: A review of treatment methods available in 2021. Curr. Opin. Ophthalmol. 2021, 32, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar]
- Gragoudas, E.S. Proton beam irradiation of uveal melanomas: The first 30 years. The Weisenfeld Lecture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4666–4673. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L.; Rojanaporn, D.; Badal, J.; Devisetty, L.; Emrich, J.; Komarnicky, L.; Shields, J.A. Scleral necrosis after plaque radiotherapy of uveal melanoma: A case-control study. Ophthalmology 2013, 120, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, K.; Shields, C.L.; Shields, J.A.; Cater, J.; Freire, J.E.; Brady, L.W. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch. Ophthalmol. 1999, 117, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, C.; Kim, D.W.; Gombos, D.S.; Oba, J.; Qin, Y.; Williams, M.D.; Esmaeli, B.; Grimm, E.A.; Wargo, J.A.; Woodman, S.E.; et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer 2016, 122, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P. Mechanisms of UV-induced mutations and skin cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Onyekwere, O.; Sidransky, D.; Vogelstein, B.; Craig, R.W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991, 51, 6304–6311. [Google Scholar] [CrossRef]
- Sun, Y.; Tran, B.N.; Worley, L.A.; Delston, R.B.; Harbour, J.W. Functional analysis of the p53 pathway in response to ionizing radiation in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1561–1564. [Google Scholar] [CrossRef]
- Chana, J.S.; Wilson, G.D.; Cree, I.A.; Alexander, R.A.; Myatt, N.; Neale, M.; Foss, A.J.; Hungerford, J.L. c-myc, p53, and Bcl-2 expression and clinical outcome in uveal melanoma. Br. J. Ophthalmol. 1999, 83, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Zemba, M.; Dumitrescu, O.M.; Gheorghe, A.G.; Radu, M.; Ionescu, M.A.; Vatafu, A.; Dinu, V. Ocular Complications of Radiotherapy in Uveal Melanoma. Cancers 2023, 15, 333. [Google Scholar] [CrossRef] [PubMed]
- Decaudin, D.; Frisch Dit Leitz, E.; Nemati, F.; Tarin, M.; Naguez, A.; Zerara, M.; Marande, B.; Vivet-Noguer, R.; Halilovic, E.; Fabre, C.; et al. Preclinical evaluation of drug combinations identifies co-inhibition of Bcl-2/XL/W and MDM2 as a potential therapy in uveal melanoma. Eur. J. Cancer 2020, 126, 93–103. [Google Scholar] [CrossRef]
- Aguilar, A.; Lu, J.; Liu, L.; Du, D.; Bernard, D.; McEachern, D.; Przybranowski, S.; Li, X.; Luo, R.; Wen, B.; et al. Discovery of 4-((3′R,4′S,5′R)-6″-Chloro-4′-(3-chloro-2-fluorophenyl)-1′-ethyl-2″-oxodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indoline]-5′-carboxamido)bicyclo[2.2.2]octane-1-carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development. J. Med. Chem. 2017, 60, 2819–2839. [Google Scholar]
- Fang, D.D.; Tang, Q.; Kong, Y.; Wang, Q.; Gu, J.; Fang, X.; Zou, P.; Rong, T.; Wang, J.; Yang, D.; et al. MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment. J. Immunother. Cancer 2019, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Reeves, J.A.; McKean, M.; Chmielowski, B.; Beck, J.T.; Shaheen, M.F.; Somaiah, N.; Wilson, M.; Spira, A.I.; Drabick, J.J.; et al. Preliminary results of a phase II study of alrizomadlin (APG-115), a novel, small-molecule MDM2 inhibitor, in combination with pembrolizumab in patients (pts) with unresectable or metastatic melanoma or advanced solid tumors that have failed immuno-oncologic (I-O) drugs. J. Clin. Oncol. 2021, 39, 2506. [Google Scholar]
- Attardi, L.D.; Reczek, E.E.; Cosmas, C.; Demicco, E.G.; McCurrach, M.E.; Lowe, S.W.; Jacks, T. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 2000, 14, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Spiller, D.; White, M.R.; Grierson, I.; Paraoan, L. PERP expression stabilizes active p53 via modulation of p53-MDM2 interaction in uveal melanoma cells. Cell Death Dis. 2011, 2, e136. [Google Scholar] [CrossRef] [PubMed]
- Paraoan, L.; Gray, D.; Hiscott, P.; Ebrahimi, B.; Damato, B.; Grierson, I. Expression of p53-induced apoptosis effector PERP in primary uveal melanomas: Downregulation is associated with aggressive type. Exp. Eye Res. 2006, 83, 911–919. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).