Osseous Metastases in Thyroid Cancer: Unveiling Risk Factors, Disease Outcomes, and Treatment Impact
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Study Variables
2.4. Primary Outcomes
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Risk Factors Predicting Bone Metastasis at the Time of Diagnosis
3.3. Disease Outcomes
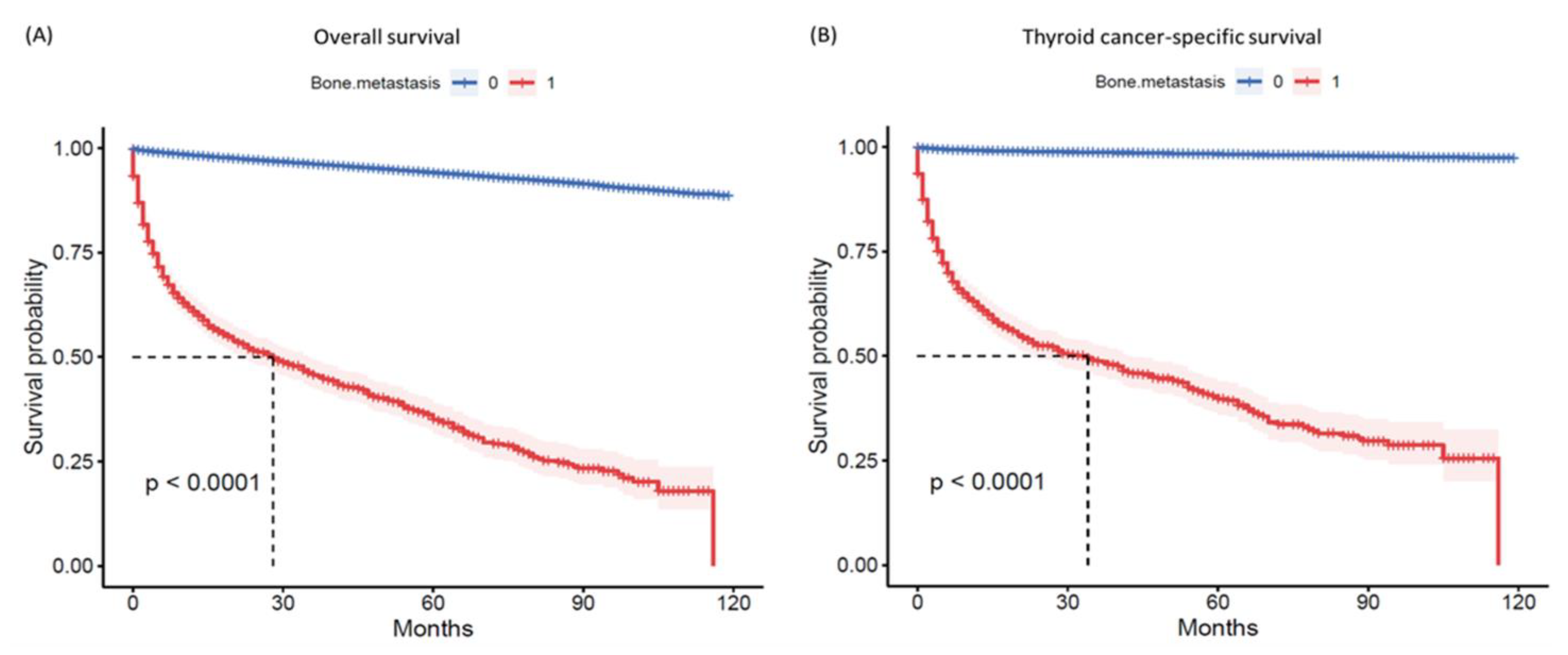
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pstrąg, N.; Ziemnicka, K.; Bluyssen, H.; Wesoły, J. Thyroid cancers of follicular origin in a genomic light: In-depth overview of common and unique molecular marker candidates. Mol. Cancer 2018, 17, 116. [Google Scholar] [CrossRef]
- Liu, F.C.; Lin, H.T.; Lin, S.F.; Kuo, C.F.; Chung, T.T.; Yu, H.P. Nationwide cohort study on the epidemiology and survival outcomes of thyroid cancer. Oncotarget 2017, 8, 78429–78451. [Google Scholar] [CrossRef]
- Toraih, E.A.; Hussein, M.H.; Zerfaoui, M.; Attia, A.S.; Marzouk Ellythy, A.; Mostafa, A.; Ruiz, E.M.L.; Shama, M.A.; Russell, J.O.; Randolph, G.W.; et al. Site-Specific Metastasis and Survival in Papillary Thyroid Cancer: The Importance of Brain and Multi-Organ Disease. Cancers 2021, 13, 1625. [Google Scholar] [CrossRef]
- Iñiguez-Ariza, N.M.; Bible, K.C.; Clarke, B.L. Bone metastases in thyroid cancer. J. Bone Oncol. 2020, 21, 100282. [Google Scholar] [CrossRef]
- Wu, D.; Gomes Lima, C.J.; Moreau, S.L.; Kulkarni, K.; Zeymo, A.; Burman, K.D.; Wartofsky, L.; Van Nostrand, D. Improved Survival after Multimodal Approach with 131I Treatment in Patients with Bone Metastases Secondary to Differentiated Thyroid Cancer. Thyroid 2019, 29, 971–978. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Wu, C.; Jia, Q.; Chai, J.; Meng, Z.; Zheng, W.; Tan, J. Bone metastases in newly diagnosed patients with thyroid cancer: A large population-based cohort study. Front. Oncol. 2022, 12, 955629. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Shen, C.T.; Sun, Z.K.; Song, H.J.; Zhang, G.Q.; Luo, Q.Y. Lung Metastases from Papillary Thyroid Cancer with Persistently Negative Thyroglobulin and Elevated Thyroglobulin Antibody Levels During Radioactive Iodine Treatment and Follow-Up: Long-Term Outcomes and Prognostic Indicators. Front. Endocrinol. 2019, 10, 903. [Google Scholar] [CrossRef]
- Moneke, I.; Kaifi, J.T.; Kloeser, R.; Samson, P.; Haager, B.; Wiesemann, S.; Diederichs, S.; Passlick, B. Pulmonary metastasectomy for thyroid cancer as salvage therapy for radioactive iodine-refractory metastases. Eur. J. Cardiothorac. Surg. 2018, 53, 625–630. [Google Scholar] [CrossRef]
- Fragnaud, H.; Mattei, J.C.; Le Nail, L.R.; Nguyễn, M.V.; Schubert, T.; Griffin, A.; Wunder, J.; Biau, D.; Gouin, F.; Bonnevialle, P.; et al. Mid and long-term overall survival after carcinologic resections of thyroid cancer bone metastases. Front. Surg. 2022, 9, 965951. [Google Scholar] [CrossRef]
- Kondraciuk, J.D.; Rice, S.L.; Zhou, X.; Gharzeddine, K.; Knezevic, A.; Spratt, D.E.; Sabra, M.; Larson, S.M.; Grewal, R.K.; Osborne, J.R. Thyroid Cancer Bone Metastasis: Survival and Genomic Characteristics of a Large Tertiary Care Cohort. Clin. Nucl. Med. 2019, 44, e465–e471. [Google Scholar] [CrossRef]
- Tong, Y.; Hu, C.; Huang, Z.; Fan, Z.; Zhu, L.; Song, Y. Novel nomogram to predict risk of bone metastasis in newly diagnosed thyroid carcinoma: A population-based study. BMC Cancer 2020, 20, 1055. [Google Scholar] [CrossRef]
- Farooki, A.; Leung, V.; Tala, H.; Tuttle, R.M. Skeletal-related events due to bone metastases from differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2012, 97, 2433–2439. [Google Scholar] [CrossRef]
- Matta-Coelho, C.; Simões-Pereira, J.; Vilar, H.; Leite, V. Bone Metastases from Thyroid Carcinoma of Follicular Origin: A Single Institutional Experience. Eur. Thyroid J. 2019, 8, 96–101. [Google Scholar] [CrossRef]
- Orita, Y.; Sugitani, I.; Toda, K.; Manabe, J.; Fujimoto, Y. Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid 2011, 21, 31–35. [Google Scholar] [CrossRef]
- Albano, D.; Bertagna, F.; Bonacina, M.; Durmo, R.; Cerudelli, E.; Gazzilli, M.; Panarotto, M.B.; Formenti, A.M.; Mazziotti, G.; Giustina, A.; et al. Possible delayed diagnosis and treatment of metastatic differentiated thyroid cancer by adopting the 2015 ATA guidelines. Eur. J. Endocrinol. 2018, 179, 143–151. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, W.; Ren, X.; Xu, R.; Liu, C.; Tu, C.; Li, Z. Incidence and Predictors of Synchronous Bone Metastasis in Newly Diagnosed Differentiated Thyroid Cancer: A Real-World Population-Based Study. Front. Surg. 2022, 9, 778303. [Google Scholar] [CrossRef]
- Liu, W.C.; Li, Z.Q.; Luo, Z.W.; Liao, W.J.; Liu, Z.L.; Liu, J.M. Machine learning for the prediction of bone metastasis in patients with newly diagnosed thyroid cancer. Cancer Med. 2021, 10, 2802–2811. [Google Scholar] [CrossRef]
- Slook, O.; Levy, S.; Slutzky-Shraga, I.; Tsvetov, G.; Robenshtok, E.; Shimon, I.; Benbassat, C.; Hirsch, D. Long-Term Outcomes and Prognostic Factors in Patients with Differentiated Thyroid Carcinoma and Bone Metastases. Endocr. Pract. 2019, 25, 427–437. [Google Scholar] [CrossRef]
- Vuong, H.G.; Duong, U.N.P.; Pham, T.Q.; Tran, H.M.; Oishi, N.; Mochizuki, K.; Nakazawa, T.; Hassell, L.; Katoh, R.; Kondo, T. Clinicopathological Risk Factors for Distant Metastasis in Differentiated Thyroid Carcinoma: A Meta-Analysis. World J. Surg. 2018, 42, 1005–1017. [Google Scholar] [CrossRef]
- Do, M.Y.; Rhee, Y.; Kim, D.J.; Kim, C.S.; Nam, K.H.; Ahn, C.W.; Cha, B.S.; Kim, K.R.; Lee, H.C.; Park, C.S.; et al. Clinical features of bone metastases resulting from thyroid cancer: A review of 28 patients over a 20-year period. Endocr. J. 2005, 52, 701–707. [Google Scholar] [CrossRef]
- Wexler, J.A. Approach to the thyroid cancer patient with bone metastases. J. Clin. Endocrinol. Metab. 2011, 96, 2296–2307. [Google Scholar] [CrossRef]
- Marcocci, C.; Pacini, F.; Elisei, R.; Schipani, E.; Ceccarelli, C.; Miccoli, P.; Arganini, M.; Pinchera, A. Clinical and biologic behavior of bone metastases from differentiated thyroid carcinoma. Surgery 1989, 106, 960–966. [Google Scholar]
- Wu, K.; Hou, S.M.; Huang, T.S.; Yang, R.S. Thyroid carcinoma with bone metastases: A prognostic factor study. Clin. Med. Oncol. 2008, 2, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Mazziotti, G.; Formenti, A.M.; Panarotto, M.B.; Arvat, E.; Chiti, A.; Cuocolo, A.; Dottorini, M.E.; Durante, C.; Agate, L.; Filetti, S.; et al. Real-life management and outcome of thyroid carcinoma-related bone metastases: Results from a nationwide multicenter experience. Endocrine 2018, 59, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Kim, W.G.; Kwon, H.; Jeon, M.J.; Lee, J.J.; Ryu, J.S.; Hong, E.G.; Kim, T.Y.; Shong, Y.K.; Kim, W.B. Early prognostic factors at the time of diagnosis of bone metastasis in patients with bone metastases of differentiated thyroid carcinoma. Eur. J. Endocrinol. 2016, 175, 165–172. [Google Scholar] [CrossRef]
- Nervo, A.; Ragni, A.; Retta, F.; Gallo, M.; Piovesan, A.; Liberini, V.; Gatti, M.; Ricardi, U.; Deandreis, D.; Arvat, E. Bone metastases from differentiated thyroid carcinoma: Current knowledge and open issues. J. Endocrinol. Investig. 2021, 44, 403–419. [Google Scholar] [CrossRef]
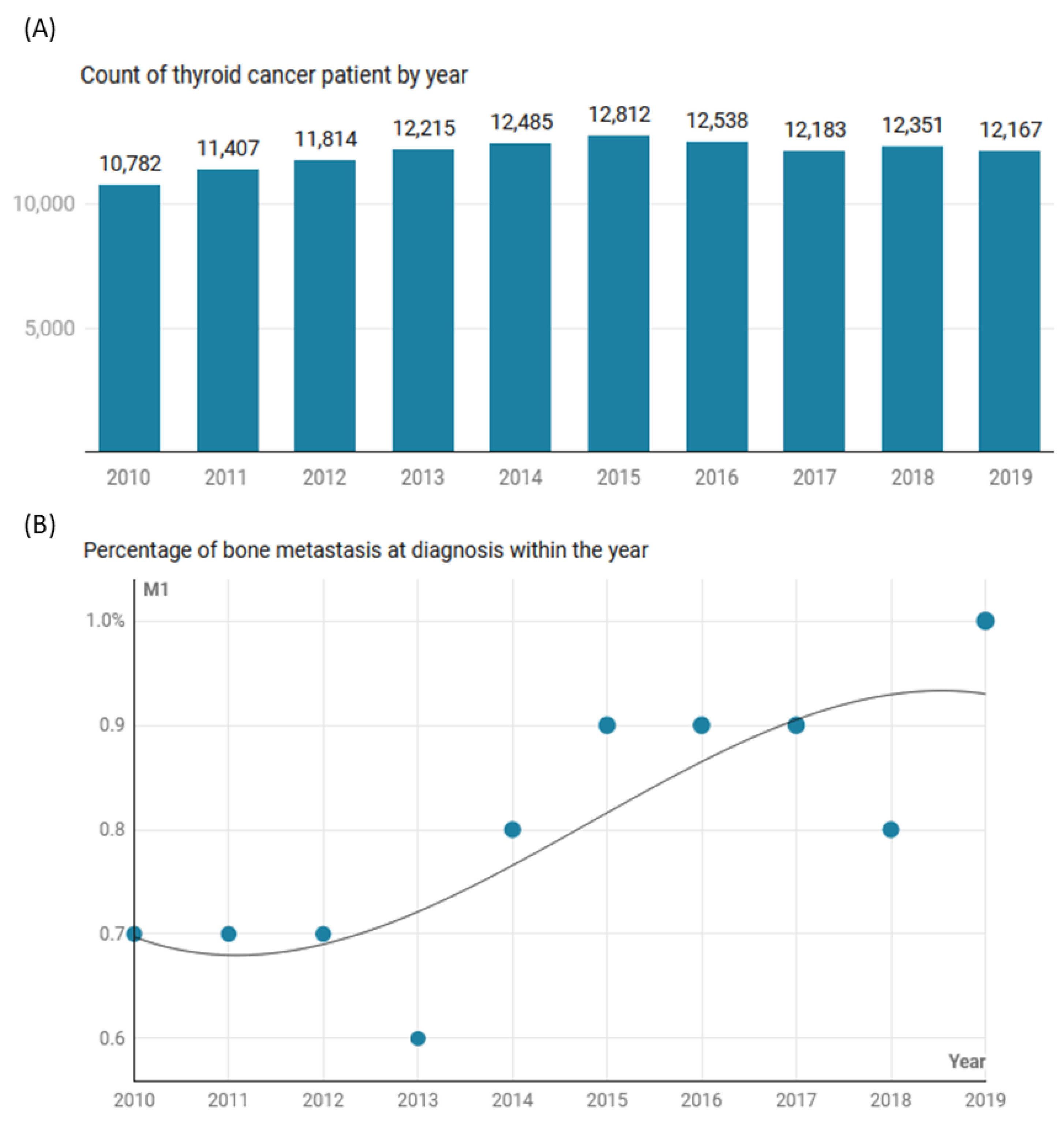
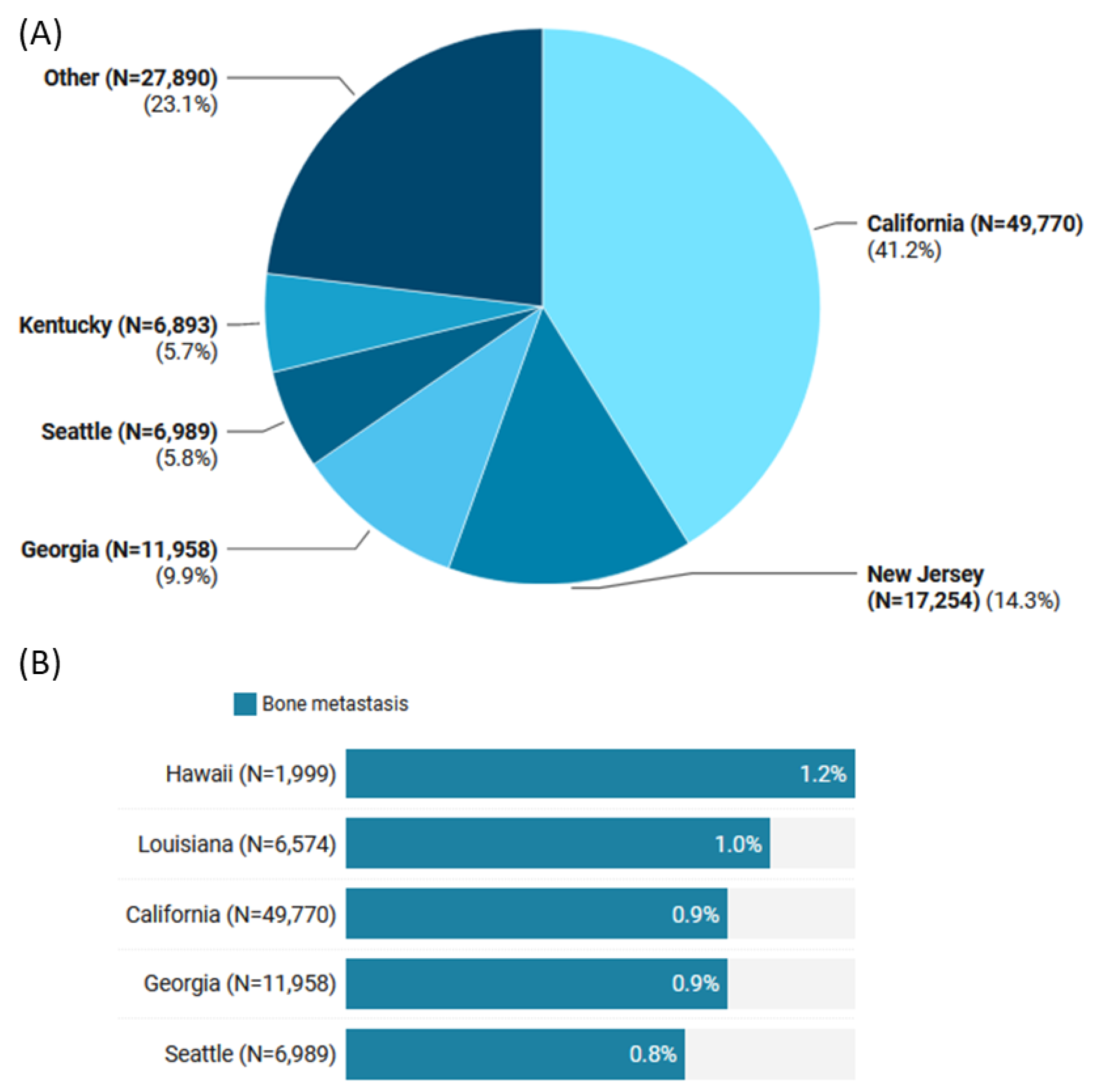
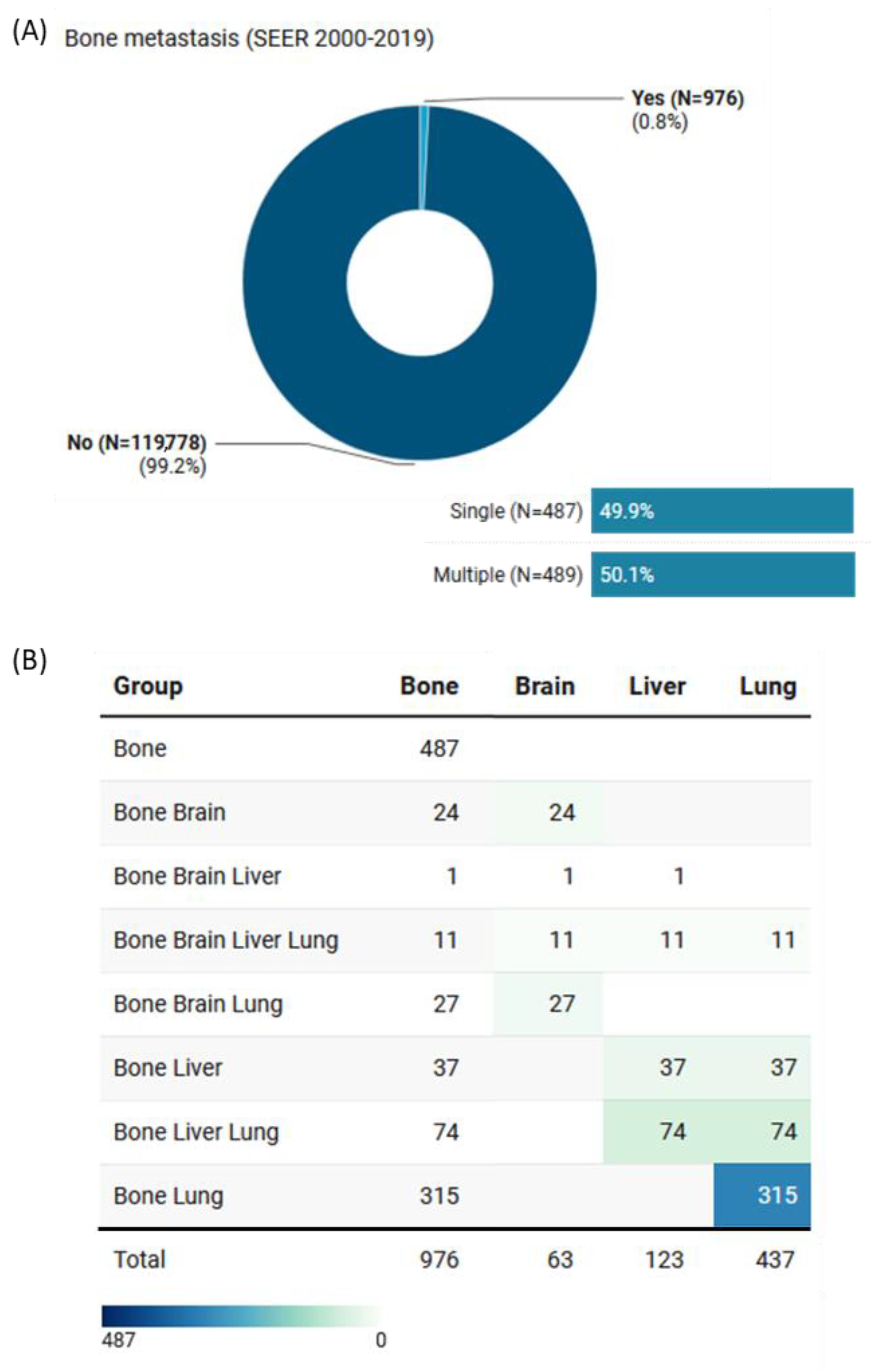
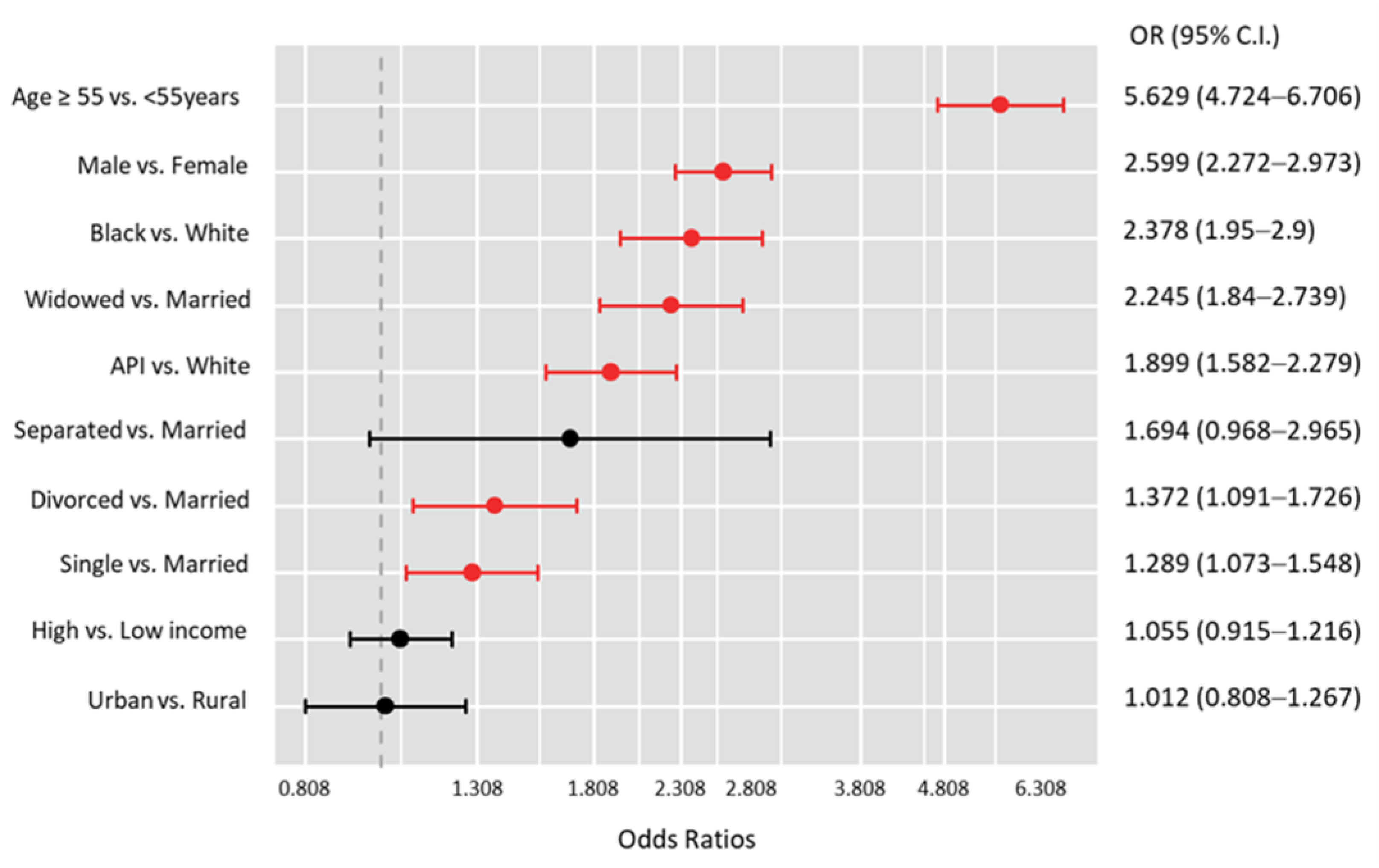
| Characteristics | Levels | Total (N = 120,754) | No Metastasis (N = 119,778) | Bone Metastasis (N = 976) | p-Value |
|---|---|---|---|---|---|
| Age | <55 years | 71,101 (58.9) | 70,927 (59.2) | 174 (17.8) | <0.001 |
| ≥55 years | 49,653 (41.1) | 48,851 (40.8) | 802 (82.2) | ||
| Gender | Female | 90,822 (75.2) | 90,306 (75.4) | 516 (52.9) | <0.001 |
| Male | 29,932 (24.8) | 29,472 (24.6) | 460 (47.1) | ||
| Race | White | 96,233 (81) | 95,546 (81.1) | 687 (70.6) | <0.001 |
| Black | 7921 (6.7) | 7792 (6.6) | 129 (13.3) | ||
| API | 13,702 (11.5) | 13,547 (11.5) | 155 (15.9) | ||
| AI/AN | 882 (0.7) | 880 (0.7) | 2 (0.2) | ||
| Ethnicity | Not Hispanic/Latino | 98,822 (81.8) | 97,993 (81.8) | 829 (84.9) | 0.012 |
| Hispanic/Latino | 21,932 (18.2) | 21,785 (18.2) | 147 (15.1) | ||
| Marital Status | Married/Common law | 71,142 (62.9) | 70,610 (62.9) | 532 (56.6) | <0.001 |
| Domestic Partner | 501 (0.4) | 497 (0.4) | 4 (0.4) | ||
| Separated | 1157 (1) | 1144 (1) | 13 (1.4) | ||
| Divorced | 8428 (7.4) | 8337 (7.4) | 91 (9.7) | ||
| Widowed | 5870 (5.2) | 5733 (5.1) | 137 (14.6) | ||
| Single (never married) | 26,077 (23) | 25,914 (23.1) | 163 (17.3) | ||
| Metropolitan | Metropolitan > 1M pop | 73,699 (61.1) | 73,094 (61.1) | 605 (62.1) | 0.53 |
| Metropolitan > 250K–1M | 27,116 (22.5) | 26901 (22.5) | 215 (22.1) | ||
| Metropolitan of < 250K | 8386 (7) | 8324 (7) | 62 (6.4) | ||
| Non-Metropolitan adj to a Metropolitan | 6326 (5.2) | 6267 (5.2) | 59 (6.1) | ||
| Non-Metropolitan not adj to a Metropolitan | 5072 (4.2) | 5038 (4.2) | 34 (3.5) | ||
| Household Annual Income | USD 75,000+ | 40,527 (33.6) | 40,190 (33.6) | 337 (34.5) | 0.72 |
| USD 70,000–USD 74,999 | 8542 (7.1) | 8477 (7.1) | 65 (6.7) | ||
| USD 65,000–USD 69,999 | 19,964 (16.5) | 19,814 (16.5) | 150 (15.4) | ||
| USD 60,000–USD 64,999 | 20,013 (16.6) | 19,860 (16.6) | 153 (15.7) | ||
| USD 55,000–USD 59,999 | 7741 (6.4) | 7682 (6.4) | 59 (6) | ||
| USD 50,000–USD 54,999 | 10,048 (8.3) | 9965 (8.3) | 83 (8.5) | ||
| USD 45,000–USD 49,999 | 5511 (4.6) | 5455 (4.6) | 56 (5.7) | ||
| USD 40,000–USD 44,999 | 4211 (3.5) | 4173 (3.5) | 38 (3.9) | ||
| USD 35,000–USD 39,999 | 2462 (2) | 2438 (2) | 24 (2.5) | ||
| <USD 35,000 | 1725 (1.4) | 1714 (1.4) | 11 (1.1) |
| Characteristics | Levels | Total (N = 120,754) | No Metastasis (N = 119,778) | Bone Metastasis (N = 976) | p-Value |
|---|---|---|---|---|---|
| Presentation | |||||
| Histological Type | Papillary | 107,227 (88.8) | 106,858 (89.2) | 369 (37.8) | <0.001 |
| Follicular | 5718 (4.7) | 5487 (4.6) | 231 (23.7) | ||
| Anaplastic | 649 (0.5) | 534 (0.4) | 115 (11.8) | ||
| Medullary | 1993 (1.7) | 1895 (1.6) | 98 (10) | ||
| Mixed Med | 131 (0.1) | 126 (0.1) | 5 (0.5) | ||
| Others | 5036 (4.2) | 4878 (4.1) | 158 (16.2) | ||
| T Staging | T1 | 69,399 (57.5) | 69,285 (57.8) | 114 (11.7) | <0.001 |
| T2 | 20,454 (16.9) | 20,342 (17.0) | 112 (11.5) | ||
| T3 | 17,781 (14.7) | 17,607 (14.7) | 174 (17.8) | ||
| T4 | 4058 (3.4) | 3726 (3.1) | 332 (34.0) | ||
| N Staging | N0 | 85,544 (70.8) | 85,149 (71.1) | 395 (40.5) | <0.001 |
| N1 | 29,275 (24.2) | 28,830 (24.1) | 445 (45.6) | ||
| NA | 5935 (4.9) | 5799 (4.8) | 136 (13.9) | ||
| Extension to Nearby Structures | No Extension | 27,082 (22.4) | 26,685 (22.3) | 397 (40.7) | <0.001 |
| Ext into strap muscles | 37,440 (31) | 37,211 (31.1) | 229 (23.5) | ||
| Ext into the larynx or trachea | 56,232 (46.6) | 55,882 (46.7) | 350 (35.9) | ||
| Management | |||||
| Cancer-directed Surgery | Positive | 4568 (3.8) | 4154 (3.5) | 414 (42.5) | <0.001 |
| Radiotherapy | Positive | 115,956 (96.2) | 115,395 (96.5) | 561 (57.5) | |
| Radioactive iodine | Positive | 70,356 (58.3) | 70,029 (58.5) | 327 (33.5) | <0.001 |
| Systemic therapy | Positive | 50,398 (41.7) | 49,749 (41.5) | 649 (66.5) |
| Characteristics | Levels | Total (N = 120,754) | No Metastasis (N = 119,778) | Bone Metastasis (N = 976) | p-Value |
|---|---|---|---|---|---|
| Recurrence | Negative | 120,354 (99.7) | 119,381 (99.7) | 973 (99.7) | 0.89 |
| Positive | 400 (0.3) | 397 (0.3) | 3 (0.3) | ||
| Second primary cancers | Negative | 98,681 (81.7) | 97,927 (81.8) | 754 (77.3) | <0.001 |
| Positive | 22,073 (18.3) | 21,851 (18.2) | 222 (22.7) | ||
| Survival status | Alive | 113,335 (93.9) | 112,928 (94.3) | 407 (41.7) | <0.001 |
| Dead | 7419 (6.1) | 6850 (5.7) | 569 (58.3) | ||
| Cause of death | Alive | 113,334 (93.9) | 112,927 (94.3) | 407 (41.7) | <0.001 |
| Dead, other cause | 5130 (4.2) | 5023 (4.2) | 107 (11) | ||
| Dead, this cancer | 2289 (1.9) | 1827 (1.5) | 462 (47.3) | ||
| Survival time (years) | Median (IQR) | 4.25 (1.8–6.8) | 4.2 (1.8–6.8) | 1.17 (0.25–3.5) | <0.001 |
| Risk Factor | No Bone Metastasis | Bone Metastasis | ||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Age ≥ 55 vs. <55 y | 3.91 (3.77–4.04) | <0.001 | 3.64 (1.75–7.57) | 0.001 |
| Male vs. Female | 1.32 (1.27–1.37) | <0.001 | 1.09 (0.69–1.72) | 0.71 |
| Black vs. White | 0.93 (0.87–1.00) | 0.046 | 1.4 (0.77–2.57) | 0.27 |
| API vs. White | 0.78 (0.73–0.82) | <0.001 | 0.46 (0.21–0.99) | 0.047 |
| AI/AN vs. White | 0.85 (0.68–1.07) | 0.17 | 0 (0.00–0.00) | 1.00 |
| Urban vs. Rural | 1 (0.95–1.06) | 0.91 | 1.65 (0.72–3.79) | 0.23 |
| High vs. Low income | 1.07 (1.03–1.11) | <0.001 | 0.95 (0.58–1.55) | 0.84 |
| FTC vs. PTC | 1 (0.92–1.08) | 0.96 | 1.04 (0.60–1.78) | 0.90 |
| ATC vs. PTC | 0.74 (0.59–0.93) | 0.009 | 1.32 (0.63–2.76) | 0.47 |
| MTC vs. PTC | 1.43 (1.27–1.60) | <0.001 | 1.28 (0.62–2.63) | 0.51 |
| N1 vs. N0 stage | 0.97 (0.93–1.01) | 0.19 | 0.91 (0.54–1.54) | 0.72 |
| Distant node metastasis vs. none | 0.59 (0.27–1.28) | 0.18 | 1.21 (0.49–2.99) | 0.68 |
| Surgery vs. none | 0.41 (0.37–0.46) | <0.001 | 2.45 (1.26–4.78) | 0.008 |
| Radiation vs. none | 1 (0.96–1.04) | 0.93 | 0.64 (0.38–1.09) | 0.10 |
| Systematic therapy vs. none | 1.03 (0.99–1.06) | 0.15 | 0.8 (0.47–1.36) | 0.41 |
| Risk Factor | PTC | FTC | ATC | MTC | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Age ≥ 55 vs. <55 y | 3.91 (3.77–4.06) | <0.001 | 4.14 (3.51–4.88) | <0.001 | 2.16 (1.01–4.63) | 0.048 | 3.08 (2.44–3.90) | <0.001 |
| Male vs. Female | 1.33 (1.28–1.39) | <0.001 | 1.17 (0.99–1.38) | 0.06 | 0.75 (0.50–1.12) | 0.15 | 1.35 (1.07–1.70) | 0.011 |
| Black vs. White | 0.96 (0.90–1.04) | 0.31 | 0.73 (0.56–0.95) | 0.019 | 0.75 (0.34–1.65) | 0.48 | 0.85 (0.56–1.30) | 0.45 |
| API vs. White | 0.78 (0.73–0.82) | <0.001 | 0.8 (0.60–1.07) | 0.13 | 0.69 (0.35–1.38) | 0.29 | 0.62 (0.38–1.03) | 0.06 |
| AI/AN vs. White | 0.8 (0.63–1.02) | 0.07 | 1.58 (0.61–4.12) | 0.34 | --- | --- | 0.98 (0.23–4.12) | 0.98 |
| Urban vs. Rural | 1.0 (0.94–1.06) | 0.95 | 1.09 (0.85–1.39) | 0.50 | 1.2 (0.68–2.13) | 0.52 | 0.85 (0.59–1.22) | 0.38 |
| High vs. Low income | 1.07 (1.03–1.11) | 0.001 | 1.07 (0.90–1.27) | 0.44 | 1.01 (0.65–1.56) | 0.96 | 1.07 (0.84–1.37) | 0.58 |
| N1 vs. N0 stage | 0.97 (0.92–1.01) | 0.17 | 1.2 (0.79–1.83) | 0.40 | 1.14 (0.76–1.71) | 0.52 | 0.9 (0.69–1.17) | 0.41 |
| Bone metastasis vs. none | 1.28 (0.52–3.14) | 0.58 | 0.58 (0.17–1.98) | 0.38 | 0.5 (0.05–4.94) | 0.55 | 0.47 (0.07–3.17) | 0.44 |
| Distant node metastasis vs. none | 0.71 (0.31–1.63) | 0.41 | 0.77 (0.08–7.63) | 0.82 | 0.85 (0.29–2.47) | 0.76 | 0.67 (0.13–3.49) | 0.63 |
| Multiple organs vs. bone only | 0.54 (0.29–1.01) | 0.054 | 1.03 (0.45–2.37) | 0.93 | 1.08 (0.30–3.85) | 0.90 | 1.35 (0.40–4.62) | 0.62 |
| Surgery vs. none | 0.39 (0.35–0.43) | <0.001 | 0.73 (0.43–1.25) | 0.25 | 1.19 (0.72–1.99) | 0.49 | 0.99 (0.52–1.88) | 0.98 |
| Radiation vs. none | 0.99 (0.95–1.03) | 0.62 | 1.04 (0.88–1.22) | 0.67 | 0.72 (0.47–1.10) | 0.12 | 1.99 (1.40–2.84) | <0.001 |
| Systematic therapy vs. none | 1.02 (0.99–1.06) | 0.22 | 1.03 (0.87–1.21) | 0.72 | 1.1 (0.65–1.87) | 0.71 | 1.18 (0.94–1.49) | 0.15 |
| Risk Factor | HR | LL | UL | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age at diagnosis: ≥55 vs. <55 years | 5.74 | 5.35 | 6.15 | <0.001 |
| Sex: Male vs. Female | 1.58 | 1.50 | 1.67 | <0.001 |
| Race: Black vs. White | 1.41 | 1.29 | 1.55 | <0.001 |
| Race: API vs. White | 0.94 | 0.86 | 1.03 | 0.19 |
| Race: AI/AN vs. White | 1.34 | 0.95 | 1.87 | 0.09 |
| Residency: Urban vs. Rural | 0.82 | 0.75 | 0.89 | <0.001 |
| Income: ≥$75K vs. <$75K | 0.85 | 0.80 | 0.90 | <0.001 |
| Pathological presentation | ||||
| Follicular vs. Papillary | 1.11 | 0.99 | 1.25 | 0.07 |
| Anaplastic vs. Papillary | 7.03 | 6.17 | 8.02 | <0.001 |
| Medullary vs. Papillary | 1.36 | 1.18 | 1.57 | <0.001 |
| T2 vs. T1 stage | 1.27 | 1.17 | 1.38 | <0.001 |
| T3 vs. T1 stage | 1.76 | 1.63 | 1.89 | <0.001 |
| T4 vs. T1 stage | 4.48 | 4.06 | 4.94 | <0.001 |
| N1 vs. N0 | 1.25 | 1.17 | 1.34 | <0.001 |
| Bone metastasis | ||||
| Bone metastasis vs. none | 2.78 | 2.34 | 3.30 | <0.001 |
| Type of concomitant organ metastasis | ||||
| 1-Brain metastasis vs. Bone only | 1.62 | 1.03 | 2.55 | 0.035 |
| 2-Liver metastasis vs. Bone only | 0.98 | 0.71 | 1.34 | 0.88 |
| 3-Lung metastasis vs. Bone only | 0.81 | 0.64 | 1.02 | 0.07 |
| Distant LN metastasis vs. none | 0.80 | 0.56 | 1.15 | 0.23 |
| Management | ||||
| Primary cancer surgery vs. none | 0.20 | 0.18 | 0.22 | <0.001 |
| Radiation therapy vs. none | 0.66 | 0.62 | 0.70 | <0.001 |
| Systematic therapy vs. none | 0.91 | 0.86 | 0.96 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khired, Z.A.; Hussein, M.H.; Jishu, J.A.; Toreih, A.A.; Shaalan, A.A.M.; Ismail, M.M.; Fawzy, M.S.; Toraih, E.A. Osseous Metastases in Thyroid Cancer: Unveiling Risk Factors, Disease Outcomes, and Treatment Impact. Cancers 2023, 15, 3557. https://doi.org/10.3390/cancers15143557
Khired ZA, Hussein MH, Jishu JA, Toreih AA, Shaalan AAM, Ismail MM, Fawzy MS, Toraih EA. Osseous Metastases in Thyroid Cancer: Unveiling Risk Factors, Disease Outcomes, and Treatment Impact. Cancers. 2023; 15(14):3557. https://doi.org/10.3390/cancers15143557
Chicago/Turabian StyleKhired, Zenat Ahmed, Mohammad H. Hussein, Jessan A. Jishu, Ahmed A. Toreih, Aly A. M. Shaalan, Mohammed M. Ismail, Manal S. Fawzy, and Eman A. Toraih. 2023. "Osseous Metastases in Thyroid Cancer: Unveiling Risk Factors, Disease Outcomes, and Treatment Impact" Cancers 15, no. 14: 3557. https://doi.org/10.3390/cancers15143557
APA StyleKhired, Z. A., Hussein, M. H., Jishu, J. A., Toreih, A. A., Shaalan, A. A. M., Ismail, M. M., Fawzy, M. S., & Toraih, E. A. (2023). Osseous Metastases in Thyroid Cancer: Unveiling Risk Factors, Disease Outcomes, and Treatment Impact. Cancers, 15(14), 3557. https://doi.org/10.3390/cancers15143557








