The Effect of Aspirin Use on Incident Hepatocellular Carcinoma—An Updated Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Selection Process
2.4. Data Collection and Items and Bias Assessment
2.5. Statistical Analysis
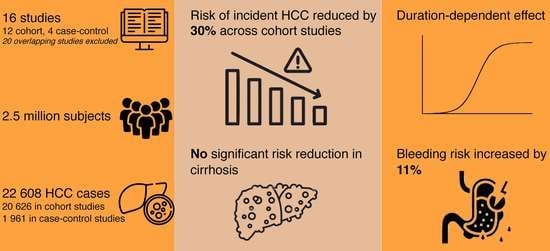
3. Results
3.1. Study Selection
3.2. Study Characteristics and Risk of Bias
3.3. Association between Aspirin Use and Risk of Incident HCC
3.3.1. Cohort Studies
3.3.2. Case-Control Studies
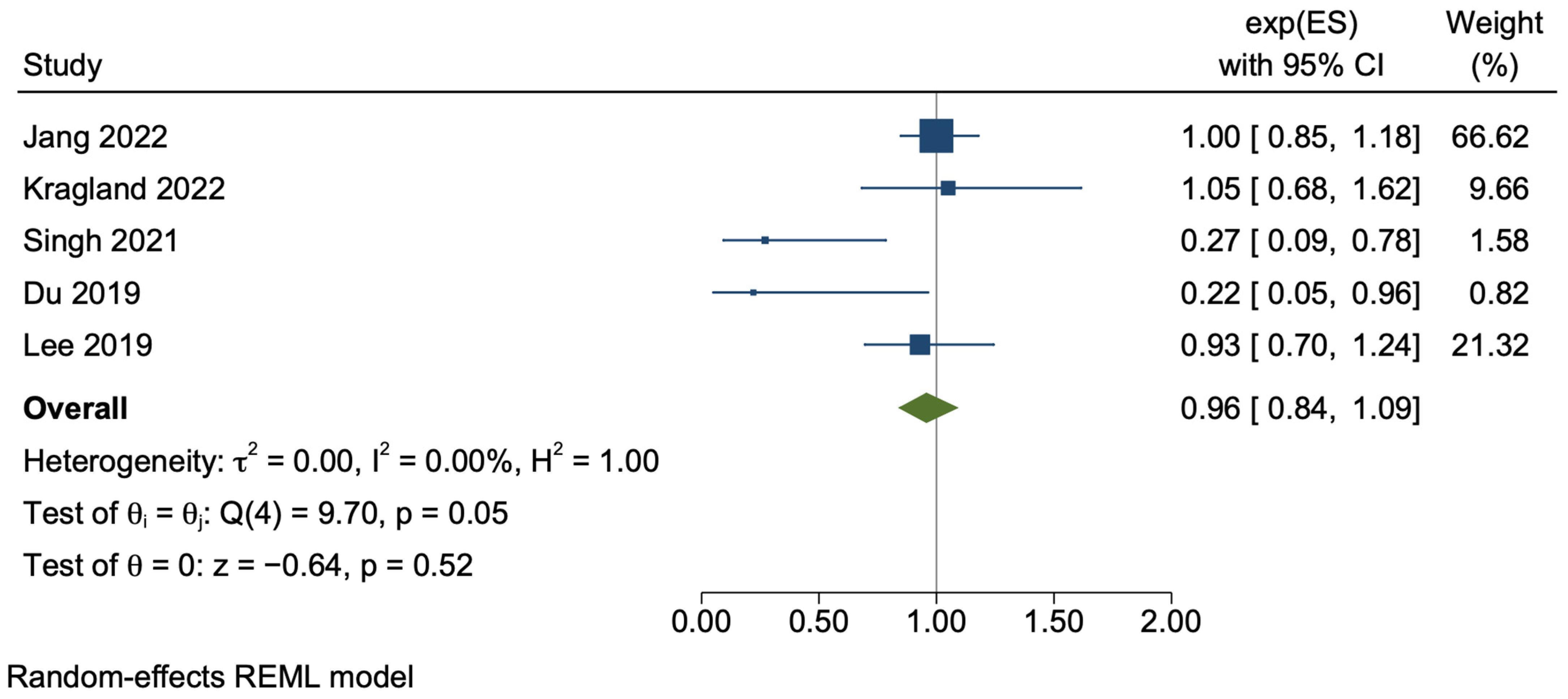
3.3.3. Cirrhosis
3.4. Dose and Duration Dependent Effects
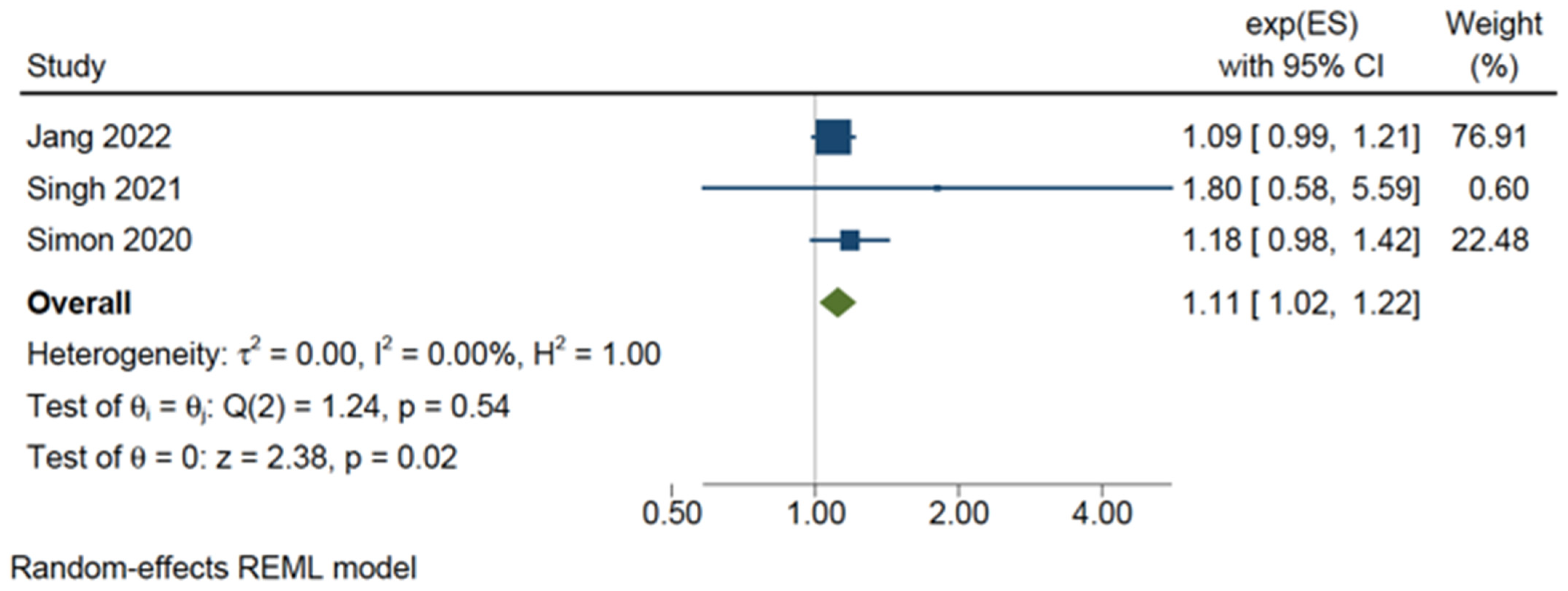
3.5. Bleeding Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Search Inputs and Results
Appendix A.1. PubMed Search
Appendix A.2. Scopus Search
Appendix A.3. MEDLINE Search
Appendix A.4. Embase Search
References
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e422. [Google Scholar] [CrossRef]
- Ryerson, A.B.; Eheman, C.R.; Altekruse, S.F.; Ward, J.W.; Jemal, A.; Sherman, R.L.; Henley, S.J.; Holtzman, D.; Lake, A.; Noone, A.-M.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016, 122, 1312–1337. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef] [PubMed]
- Lubel, J.S.; Roberts, S.K.; Strasser, S.I.; Thompson, A.J.; Philip, J.; Goodwin, M.; Clarke, S.; Crawford, D.H.; Levy, M.T.; Shackel, N. Australian recommendations for the management of hepatocellular carcinoma: A consensus statement. Med. J. Aust. 2021, 214, 475–483. [Google Scholar] [CrossRef]
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef]
- Singal, A.G.; Pillai, A.; Tiro, J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef]
- Iannacone, M.; Sitia, G.; Isogawa, M.; Marchese, P.; Castro, M.G.; Lowenstein, P.R.; Chisari, F.V.; Ruggeri, Z.M.; Guidotti, L.G. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat. Med. 2005, 11, 1167–1169. [Google Scholar] [CrossRef]
- Sitia, G.; Iannacone, M.; Guidotti, L.G. Anti-platelet therapy in the prevention of hepatitis B virus-associated hepatocellular carcinoma. J. Hepatol. 2013, 59, 1135–1138. [Google Scholar] [CrossRef]
- Cervello, M.; Montalto, G. Cyclooxygenases in hepatocellular carcinoma. World J. Gastroenterol. 2006, 12, 5113–5121. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.A.; Schoneweiss, M.M.; Sahi, D.; Bahlo, M.; Haugg, A.M.; Kasper, H.U.; Dienes, H.P.; Kaferstein, H.; Breuhahn, K.; Schirmacher, P. Cyclooxygenase-2 inhibitors suppress the growth of human hepatocellular carcinoma implants in nude mice. Carcinogenesis 2004, 25, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Flossmann, E.; Rothwell, P.M. Effect of aspirin on long-term risk of colorectal cancer: Consistent evidence from randomised and observational studies. Lancet 2007, 369, 1603–1613. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Elwin, C.E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef]
- Cook, N.R.; Lee, I.M.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. Low-dose aspirin in the primary prevention of cancer: The Women’s Health Study: A randomized controlled trial. JAMA 2005, 294, 47–55. [Google Scholar] [CrossRef]
- Li, X.; Wu, S.; Yu, Y. Aspirin Use and the Incidence of Hepatocellular Carcinoma in Patients with Hepatitis B Virus or Hepatitis C Virus Infection: A Meta-Analysis of Cohort Studies. Front. Med. 2020, 7, 569759. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, Y.; Liu, L. Influence of aspirin use on clinical outcomes of patients with hepatocellular carcinoma: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101545. [Google Scholar] [CrossRef]
- Liao, K.F.; Lai, S.W. Aspirin Use and the Risk of Hepatocellular Carcinoma: A Meta-analysis. J. Clin. Gastroenterol. 2023, 57, 640–641. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, T.; Xu, X.; Jin, J. Association of aspirin and nonaspirin NSAIDs therapy with the incidence risk of hepatocellular carcinoma: A systematic review and meta-analysis on cohort studies. Eur. J. Cancer Prev. 2022, 31, 35–43. [Google Scholar] [CrossRef]
- Ma, S.; Qu, G.; Sun, C.; Liu, H.; Jiang, Y.; Li, N.; Wu, B.; Gao, J.; Feng, L.; Xie, P.; et al. Does aspirin reduce the incidence, recurrence, and mortality of hepatocellular carcinoma? A GRADE-assessed systematic review and dose-response meta-analysis. Eur. J. Clin. Pharmacol. 2023, 79, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Memel, Z.N.; Arvind, A.; Moninuola, O.; Philpotts, L.; Chung, R.T.; Corey, K.E.; Simon, T.G. Aspirin Use Is Associated with a Reduced Incidence of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Hepatol. Commun. 2021, 5, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Sidhu-Brar, S.; Woodman, R.; Chinnaratha, M.A. Regular Aspirin Use Is Associated with a Reduced Risk of Hepatocellular Carcinoma (HCC) in Chronic Liver Disease: A Systematic Review and Meta-analysis. J. Gastrointest. Cancer 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, Y.; Liu, X.; Deng, Q.; Yu, Y.; Yang, Z. Nonsteroidal anti-inflammatory drugs, especially aspirin, are linked to lower risk and better survival of hepatocellular carcinoma: A meta-analysis. Cancer Manag. Res. 2018, 10, 2695–2709. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Liu, C.; Wang, W.; Shi, J.; Dang, S. Aspirin Use and the Risk of Hepatocellular Carcinoma: A Meta-analysis. J. Clin. Gastroenterol. 2022, 56, e293–e302. [Google Scholar] [CrossRef]
- Yan, L.J.; Yao, S.Y.; Li, H.C.; Meng, G.X.; Liu, K.X.; Ding, Z.N.; Hong, J.G.; Chen, Z.Q.; Dong, Z.R.; Li, T. Efficacy and Safety of Aspirin for Prevention of Hepatocellular Carcinoma: An Updated Meta-analysis. J. Clin. Transl. Hepatol. 2022, 10, 835–846. [Google Scholar] [CrossRef]
- Yi, M.; Feng, X.; Peng, W.; Teng, F.; Tang, Y.; Chen, Z. Aspirin for the prevention of hepatocellular carcinoma: An updated meta-analysis with particular focus on patients with chronic liver disease. Eur. J. Clin. Pharmacol. 2022, 78, 647–656. [Google Scholar] [CrossRef]
- Zeng, R.W.; Yong, J.N.; Tan, D.J.H.; Fu, C.E.; Lim, W.H.; Xiao, J.; Chan, K.E.; Tan, C.; Goh, X.L.; Chee, D.; et al. Meta-analysis: Chemoprevention of hepatocellular carcinoma with statins, aspirin and metformin. Aliment. Pharmacol. Ther. 2023, 57, 600–609. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Sun, Y.; Li, C.; Ding, X.; Zhu, Y.; Li, L.; Fan, Z. Systematic Review and Meta-analysis: Association of Aspirin with Incidence of Hepatocellular Carcinoma. Front. Pharmacol. 2022, 13, 764854. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Simpson, B.S.; Ball, R.; Freeman, A.; Kirkham, A.; Parry, M.A.; Moore, C.M.; Whitaker, H.C.; Emberton, M. A Modified Newcastle-Ottawa Scale for Assessment of Study Quality in Genetic Urological Research. Eur. Urol. 2021, 79, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.Q.; Zhao, J.Z.; Dong, J.; Bi, J.B.; Ren, Y.F.; Zhang, J.; Khalid, B.; Wu, Z.; Lv, Y.; Zhang, X.F.; et al. Effect of low-dose aspirin administration on long-term survival of cirrhotic patients after splenectomy: A retrospective single-center study. World J. Gastroenterol. 2019, 25, 3798–3807. [Google Scholar] [CrossRef]
- Friis, S.; Sorensen, H.T.; McLaughlin, J.K.; Johnsen, S.P.; Blot, W.J.; Olsen, J.H. A population-based cohort study of the risk of colorectal and other cancers among users of low-dose aspirin. Br. J. Cancer 2003, 88, 684–688. [Google Scholar] [CrossRef]
- Hwang, I.C.; Chang, J.; Kim, K.; Park, S.M. Aspirin Use and Risk of Hepatocellular Carcinoma in a National Cohort Study of Korean Adults. Sci. Rep. 2018, 8, 4968. [Google Scholar] [CrossRef]
- Jang, H.; Lee, Y.B.; Moon, H.; Chung, J.W.; Nam, J.Y.; Cho, E.J.; Lee, J.H.; Yu, S.J.; Kim, Y.J.; Lee, J.; et al. Aspirin use and risk of hepatocellular carcinoma in patients with chronic hepatitis B with or without cirrhosis. Hepatology 2022, 76, 492–501. [Google Scholar] [CrossRef]
- Kraglund, F.; Christensen, D.H.; Eiset, A.H.; Villadsen, G.E.; West, J.; Jepsen, P. Effects of statins and aspirin on HCC risk in alcohol-related cirrhosis: Nationwide emulated trials. Hepatol. Commun. 2023, 7, e0013. [Google Scholar] [CrossRef]
- Lee, T.Y.; Hsu, Y.C.; Tseng, H.C.; Yu, S.H.; Lin, J.T.; Wu, M.S.; Wu, C.Y. Association of Daily Aspirin Therapy with Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. JAMA Intern. Med. 2019, 179, 633–640. [Google Scholar] [CrossRef]
- Lee, T.Y.; Wu, J.C.; Yu, S.H.; Lin, J.T.; Wu, M.S.; Wu, C.Y. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int. J. Cancer 2017, 141, 1307–1314. [Google Scholar] [CrossRef]
- Liao, Y.H.; Hsu, R.J.; Wang, T.H.; Wu, C.T.; Huang, S.Y.; Hsu, C.Y.; Su, Y.C.; Hsu, W.L.; Liu, D.W. Aspirin decreases hepatocellular carcinoma risk in hepatitis C virus carriers: A nationwide cohort study. BMC Gastroenterol. 2020, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Sahasrabuddhe, V.V.; Chan, A.T.; Alavanja, M.C.; Beane-Freeman, L.E.; Buring, J.E.; Chen, J.; Chong, D.Q.; Freedman, N.D.; Fuchs, C.S.; et al. Nsaid use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The liver cancer pooling project. Cancer Prev. Res. 2015, 8, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Duberg, A.S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef]
- Singh, J.; Wozniak, A.; Cotler, S.J.; Dhanarajan, A.; Aldrich, D.; Park, D.; Kasia, C.; Schmidt, B.; Scaglione, S. Combined Use of Aspirin and Statin is Associated with a Decreased Incidence of Hepatocellular Carcinoma. J. Clin. Gastroenterol. 2021, 56, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.K.F.; Ho, J.M.W.; Chan, F.C.H.; Sung, J.J.Y. Long-term use of low-dose aspirin for cancer prevention: A 10-year population cohort study in Hong Kong. Int. J. Cancer 2019, 145, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Coogan, P.F.; Rosenberg, L.; Palmer, J.R.; Strom, B.L.; Zauber, A.G.; Stolley, P.D.; Shapiro, S. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 119–123. [Google Scholar] [PubMed]
- Ramirez, A.G.; Munoz, E.; Parma, D.L.; Michalek, J.E.; Holden, A.E.C.; Phillips, T.D.; Pollock, B.H. Lifestyle and Clinical Correlates of Hepatocellular Carcinoma in South Texas: A Matched Case-control Study. Clin. Gastroenterol. Hepatol. 2017, 15, 1311–1312. [Google Scholar] [CrossRef]
- Shen, Y.; Risch, H.; Lu, L.; Ma, X.; Irwin, M.L.; Lim, J.K.; Taddei, T.; Pawlish, K.; Stroup, A.; Brown, R.; et al. Risk factors for hepatocellular carcinoma (HCC) in the northeast of the United States: Results of a case-control study. Cancer Causes Control 2020, 31, 321–332. [Google Scholar] [CrossRef]
- Yang, B.; Petrick, J.L.; Chen, J.; Hagberg, K.W.; Sahasrabuddhe, V.V.; Graubard, B.I.; Jick, S.; McGlynn, K.A. Associations of NSAID and paracetamol use with risk of primary liver cancer in the Clinical Practice Research Datalink. Cancer Epidemiol. 2016, 43, 105–111. [Google Scholar] [CrossRef]
- Hui, V.W.; Yip, T.C.; Wong, V.W.; Tse, Y.K.; Chan, H.L.; Lui, G.C.; Wong, G.L. Aspirin Reduces the Incidence of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B Receiving Oral Nucleos(t)ide Analog. Clin. Transl. Gastroenterol. 2021, 12, e00324. [Google Scholar] [CrossRef]
- Sung, J.J.; Ho, J.M.; Lam, A.S.; Yau, S.T.; Tsoi, K.K. Use of metformin and aspirin is associated with delayed cancer incidence. Cancer Epidemiol. 2020, 69, 101808. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.J.; Choi, W.M.; Kim, H.J.; Choi, S.H.; Han, S.; Ko, M.J.; Lim, Y.S. A risk scoring system to predict clinical events in chronic hepatitis B virus infection: A nationwide cohort study. J. Viral Hepat. 2022, 29, 115–123. [Google Scholar] [CrossRef]
- Yun, B.; Ahn, S.H.; Yoon, J.H.; Kim, B.K. Clinical Indication of Aspirin Associated with Reduced Risk of Liver Cancer in Chronic Hepatitis B: A Nationwide Cohort Study. Am. J. Gastroenterol. 2022, 117, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chung, G.E.; Lee, J.H.; Oh, S.; Nam, J.Y.; Chang, Y.; Cho, H.; Ahn, H.; Cho, Y.Y.; Yoo, J.J.; et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology 2017, 66, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, S.H.; Lee, M.; Kim, J.H.; Lee, W.; Lee, H.W.; Park, M.S.; Park, S.; Kim, T.S.; Choi, D.H. Aspirin and the risk of hepatocellular carcinoma development in patients with alcoholic cirrhosis. Medicine 2020, 99, e19008. [Google Scholar] [CrossRef]
- Goh, M.J.; Sinn, D.H.; Kim, S.; Woo, S.Y.; Cho, H.; Kang, W.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; et al. Statin Use and the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. Hepatology 2020, 71, 2023–2032. [Google Scholar] [CrossRef]
- Cho, K.S.; Sohn, W.; Lee, Y.C.; Chi, S.A.; Cho, J.Y.; Kim, K.; Paik, Y.H. Use of cyclooxygenase inhibitor and the risk of hepatocellular carcinoma in patients with chronic hepatitis B: A nested case-control study using a nationwide population-based data. J. Viral Hepat. 2019, 27, 68–73. [Google Scholar] [CrossRef]
- Choi, W.M.; Kim, H.J.; Jo, A.J.; Choi, S.H.; Han, S.; Ko, M.J.; Lim, Y.S. Association of aspirin and statin use with the risk of liver cancer in chronic hepatitis B: A nationwide population-based study. Liver Int. 2021, 41, 2777–2785. [Google Scholar] [CrossRef]
- Kim, G.; Jang, S.Y.; Han, E.; Lee, Y.H.; Park, S.Y.; Nam, C.M.; Kang, E.S. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: A nationwide nested case-control study. Int. J. Cancer 2017, 140, 798–806. [Google Scholar] [CrossRef]
- Brusselaers, N.; Lagergren, J. Maintenance use of non-steroidal anti-inflammatory drugs and risk of gastrointestinal cancer in a nationwide population-based cohort study in Sweden. BMJ Open 2018, 8, e021869. [Google Scholar] [CrossRef]
- Chen, V.C.; Lin, C.F.; Hsieh, Y.H.; Liang, H.Y.; Huang, K.Y.; Chiu, W.C.; Lee, Y.; McIntyre, R.S.; Chan, H.L. Hepatocellular carcinoma and antidepressants: A nationwide population-based study. Oncotarget 2017, 8, 30464–30470. [Google Scholar] [CrossRef]
- Chiu, H.F.; Ho, S.C.; Chen, C.C.; Yang, C.Y. Statin use and the risk of liver cancer: A population-based case-control study. Am. J. Gastroenterol. 2010, 106, 894–898. [Google Scholar] [CrossRef]
- Wang, C.H.; Huang, C.W.; Nguyen, P.A.; Lin, M.C.; Yeh, C.Y.; Islam, M.M.; Rahmanti, A.R.; Yang, H.C. Chemopreventive Effects of Concomitant or Individual Use of Statins, Aspirin, Metformin, and Angiotensin Drugs: A Study Using Claims Data of 23 Million Individuals. Cancers 2022, 14, 1211. [Google Scholar] [CrossRef]
- Ho, C.M.; Lee, C.H.; Lee, M.C.; Zhang, J.F.; Wang, J.Y.; Hu, R.H.; Lee, P.H. Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in chemoprevention of hepatocellular carcinoma: A nationwide high-risk cohort study. BMC Cancer 2018, 18, 401. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Lu, T.W.; Liang, H.L.; Hsu, W.H.; Sung, Y.W.; Lee, M.Y. Antiplatelet agents aspirin and dipyridamole, and the risk of different carcinoma in patients with type 2 diabetes mellitus: A Taiwan retrospective cohort study. Medicine 2022, 101, e30468. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Yeh, C.C.; Huang, S.F.; Chou, Y.S.; Kuo, L.T.; Sung, F.C.; Muo, C.H.; Su, C.T.; Su, F.H. Aspirin associated with risk reduction of secondary primary cancer for patients with head and neck cancer: A population-based analysis. PLoS ONE 2018, 13, e0199014. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 2018, 38, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabuddhe, V.V.; Gunja, M.Z.; Graubard, B.I.; Trabert, B.; Schwartz, L.M.; Park, Y.; Hollenbeck, A.R.; Freedman, N.D.; McGlynn, K.A. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J. Natl. Cancer Inst. 2012, 104, 1808–1814. [Google Scholar] [CrossRef]
- Simon, T.G.; Ma, Y.; Ludvigsson, J.F.; Chong, D.Q.; Giovannucci, E.L.; Fuchs, C.S.; Meyerhardt, J.A.; Corey, K.E.; Chung, R.T.; Zhang, X.; et al. Association Between Aspirin Use and Risk of Hepatocellular Carcinoma. JAMA Oncol. 2018, 4, 1683–1690. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Yang, J.D.; Larson, J.J.; Watt, K.D.; Allen, A.M.; Wiesner, R.H.; Gores, G.J.; Roberts, L.R.; Heimbach, J.A.; Leise, M.D. Hepatocellular Carcinoma Is the Most Common Indication for Liver Transplantation and Placement on the Waitlist in the United States. Clin. Gastroenterol. Hepatol. 2017, 15, 767–775.e3. [Google Scholar] [CrossRef]
- Chan, A.T.; Giovannucci, E.L.; Meyerhardt, J.A.; Schernhammer, E.S.; Curhan, G.C.; Fuchs, C.S. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 2005, 294, 914–923. [Google Scholar] [CrossRef]
- Chan, A.T.; Giovannucci, E.L.; Meyerhardt, J.A.; Schernhammer, E.S.; Wu, K.; Fuchs, C.S. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology 2008, 134, 21–28. [Google Scholar] [CrossRef]
- DuPont, A.W.; Arguedas, M.R.; Wilcox, C.M. Aspirin chemoprevention in patients with increased risk for colorectal cancer: A cost-effectiveness analysis. Aliment. Pharmacol. Ther. 2007, 26, 431–441. [Google Scholar] [CrossRef]
- Giovannucci, E.; Egan, K.M.; Hunter, D.J.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C.; Speizer, F.E. Aspirin and the risk of colorectal cancer in women. N. Engl. J. Med. 1995, 333, 609–614. [Google Scholar] [CrossRef]
- Nishihara, R.; Lochhead, P.; Kuchiba, A.; Jung, S.; Yamauchi, M.; Liao, X.; Imamura, Y.; Qian, Z.R.; Morikawa, T.; Wang, M.; et al. Aspirin use and risk of colorectal cancer according to BRAF mutation status. JAMA 2013, 309, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.K.; Chan, F.C.; Hirai, H.W.; Sung, J.J. Risk of gastrointestinal bleeding and benefit from colorectal cancer reduction from long-term use of low-dose aspirin: A retrospective study of 612509 patients. J. Gastroenterol. Hepatol. 2018, 33, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Fu, J.; Yang, Y.; Chen, S. Dose-risk and duration-risk relationships between aspirin and colorectal cancer: A meta-analysis of published cohort studies. PLoS ONE 2013, 8, e57578. [Google Scholar] [CrossRef]
- Antithrombotic Trialists, C.; Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar]
- Zheng, S.L.; Roddick, A.J. Association of Aspirin Use for Primary Prevention with Cardiovascular Events and Bleeding Events: A Systematic Review and Meta-analysis. JAMA 2019, 321, 277–287. [Google Scholar] [CrossRef]
- Garcia Rodriguez, L.A.; Martin-Perez, M.; Hennekens, C.H.; Rothwell, P.M.; Lanas, A. Bleeding Risk with Long-Term Low-Dose Aspirin: A Systematic Review of Observational Studies. PLoS ONE 2016, 11, e0160046. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Yang, H.C.; Jack Li, Y.C. Statin Use and the Risk of Hepatocellular Carcinoma: A Meta-Analysis of Observational Studies. Cancers 2020, 12, 671. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Zhu, G.Q.; Liu, T.; Zheng, J.N.; Cheng, Z.; Zou, T.T.; Braddock, M.; Fu, S.W.; Zheng, M.H. Systematic Review with Network Meta-Analysis: Antidiabetic Medication and Risk of Hepatocellular Carcinoma. Sci. Rep. 2016, 6, 33743. [Google Scholar] [CrossRef] [PubMed]



| Population | Study | Years | Subjects (n) | Outcome |
|---|---|---|---|---|
| Hong Kong | Tsoi K 2019 [45] | 2000–2004 | 612,569 | Included |
| Hui V 2021 [50] | 2000–2018 | 35,111 | Excluded | |
| Sung JJY 2020 [51] | 2000–2004 | 405,533 | ||
| South Korea | Hwang C 2018 [36] | 2002–2006 | 460,775 | Included |
| Jang H 2022 [37] | 2007–2017 | 329,635 | ||
| Jo JVH 2021 [52] | 2005–2015 | 507,239 | Excluded | |
| Yun B 2022 [53] | 2010–2011 | 161,673 | ||
| Lee M 2017 [54] | 2002–2015 | 1674 | ||
| Shin S 2020 [55] | 2003–2016 | 949 | ||
| Goh M 2020 [56] | 2008–2012 | 7713 | ||
| Cho K 2019 [57] | 2003–2013 | 4980 | ||
| Choi W 2021 [58] | 2005–2015 | 32,695 | ||
| Kim G 2017 [59] | 2002–2013 | 1374 | ||
| Sweden | Simon T 2020 [43] | 2005–2015 | 50,275 | Included |
| Brusselaers N 2018 [60] | 2005–2012 | NA a | Excluded | |
| Taiwan | Lee TY 2017 * [40] | 1998–2012 | 18,080 | Included |
| Lee TY 2019 ** [39] | 1997–2012 | 10,615 | ||
| Liao Y 2020 *** [41] | 2000–2012 | 3822 | ||
| Chen VCH 2017 [61] | 1997–2008 | 294,234 | Excluded | |
| Chiu H 2010 [62] | 2005–2008 | 1166 | ||
| Wang CH 2022 [63] | 2001–2011 | 3,008,665 | ||
| Ho CM 2018 [64] | 2005–2014 | 15,597 | ||
| Huang HY 2022 [65] | 1998–2000 | 5308 | ||
| Lin Y 2018 [66] | 2000–2011 | 18,243 | ||
| Tseng C 2017 [67] | 1999–2005 | 43,800 | ||
| USA | Petrick J 2015 i [42] | 1980–2015 | 803,248 | Included |
| Singh J 2021 ii [44] | 2012–2017 | 521 | ||
| Coogan P 2000 ii [46] | 1977–1998 | 5884 | ||
| Ramirez A 2017 ii [47] | 2012–2014 | 84 | ||
| Shen Y 2020 ii [48] | 2011–2016 | 1839 | ||
| Sahasrabuddhe V 2012 iii [68] | 1996–2008 | 300,504 | Excluded | |
| Simon T 2018 iv [69] | 1980–2010 | 133,371 |
| Author | Year | Country | Population | Cohort (n) | Aspirin Users (n) | HCC Cases (n) | mNOS |
|---|---|---|---|---|---|---|---|
| Du [34] | 2019 | China | Viral cirrhosis | 264 | 59 | 41 | 9 |
| Friis S [35] | 2003 | Denmark | Public | 29,470 | 21 * | 6 | |
| Hwang C [36] | 2018 | South Korea | Public | 460,755 | 64,782 | 2336 | 9 |
| Jang H [37] | 2022 | South Korea | HBV | 329,635 | 20,200 | 2697 | 9 |
| Kraglund F [38] | 2023 | Denmark | Alcohol cirrhosis | 115,092 | 1449 | 2830 | 9 |
| Lee TY. [40] | 2017 | Taiwan | NAFLD | 18,080 | 5602 | 41 | 7 |
| Lee TY [39] | 2019 | Taiwan | HBV | 10,615 | 2123 | 697 | 8 |
| Liao Y [41] | 2020 | Taiwan | HCV | 3822 | 2980 | 278 | 9 |
| Petrick J [42] | 2015 | USA | Public | 803,248 | 477,470 | 679 | 7 |
| Simon T [43] | 2020 | Sweden | HBV/HCV | 50,275 | 14,205 | 1612 | 9 |
| Singh J [44] | 2021 | USA | Cirrhosis | 521 | 170 | 45 | 9 |
| Tsoi K [45] | 2019 | Hong Kong | Public | 612,569 | 204,170 | 9370 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmalak, J.; Tan, N.; Con, D.; Eslick, G.; Majeed, A.; Kemp, W.; Roberts, S.K. The Effect of Aspirin Use on Incident Hepatocellular Carcinoma—An Updated Systematic Review and Meta-Analysis. Cancers 2023, 15, 3518. https://doi.org/10.3390/cancers15133518
Abdelmalak J, Tan N, Con D, Eslick G, Majeed A, Kemp W, Roberts SK. The Effect of Aspirin Use on Incident Hepatocellular Carcinoma—An Updated Systematic Review and Meta-Analysis. Cancers. 2023; 15(13):3518. https://doi.org/10.3390/cancers15133518
Chicago/Turabian StyleAbdelmalak, Jonathan, Natassia Tan, Danny Con, Guy Eslick, Ammar Majeed, William Kemp, and Stuart K. Roberts. 2023. "The Effect of Aspirin Use on Incident Hepatocellular Carcinoma—An Updated Systematic Review and Meta-Analysis" Cancers 15, no. 13: 3518. https://doi.org/10.3390/cancers15133518
APA StyleAbdelmalak, J., Tan, N., Con, D., Eslick, G., Majeed, A., Kemp, W., & Roberts, S. K. (2023). The Effect of Aspirin Use on Incident Hepatocellular Carcinoma—An Updated Systematic Review and Meta-Analysis. Cancers, 15(13), 3518. https://doi.org/10.3390/cancers15133518