Enzalutamide Prior to Radium-223 Is Associated with Better Overall Survival in Metastatic Castration-Resistant Prostate Cancer Patients Compared to Abiraterone—A Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freedland, S.J.; Sandin, R.; Sah, J.; Emir, B.; Mu, Q.; Ratiu, A.; Hong, A.; Serfass, L.; Tagawa, S.T. Treatment patterns and survival in metastatic castration-sensitive prostate cancer in the US Veterans Health Administration. Cancer Med. 2021, 10, 8570–8580. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.P.; Higano, C.S.; Keane, T.; Andriole, G.; Saad, F.; Iversen, P.; Miller, K.; Kim, C.S.; Kimura, G.; Armstrong, A.J.; et al. The PREVAIL Study: Primary Outcomes by Site and Extent of Baseline Disease for Enzalutamide-treated Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2016, 70, 675–683. [Google Scholar] [CrossRef]
- Lipton, A. Implications of bone metastases and the benefits of bone-targeted therapy. Semin. Oncol. 2010, 37 (Suppl. 2), S15–S29. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Hong, J.-H.; Lu, Y.-C.; Pu, Y.-S.; Huang, C.-Y.; Huang, K.-H.; Liu, S.; Chen, C.-H. Impact of high-volume disease in Asian population with newly diagnosed metastatic prostate cancer. Urol. Sci. 2018, 29, 136–144. [Google Scholar] [CrossRef]
- Cattrini, C.; España, R.; Mennitto, A.; Bersanelli, M.; Castro, E.; Olmos, D.; Lorente, D.; Gennari, A. Optimal Sequencing and Predictive Biomarkers in Patients with Advanced Prostate Cancer. Cancers 2021, 13, 4522. [Google Scholar] [CrossRef]
- Jarvis, P.; Ho, A.; Sundram, F. Radium-223 therapy for metastatic castration-resistant prostate cancer: Survival benefit when used earlier in the treatment pathway. Nucl. Med. Commun. 2021, 42, 332–336. [Google Scholar] [CrossRef]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- George, D.J.; Agarwal, N.; Sartor, O.; Sternberg, C.N.; Tombal, B.; Saad, F.; Miller, K.; Constantinovici, N.; Guo, H.; Reeves, J.; et al. Real-world patient characteristics associated with survival of 2 years or more after radium-223 treatment for metastatic castration-resistant prostate cancer (EPIX study). Prostate Cancer Prostatic. Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Atkinson, S.; Pearson, R.; Leaning, D.; Cumming, S.; Burns, A.; Azzabi, A.; Frew, J.; McMenemin, R.; Pedley, I.D. Optimising Radium 223 Therapy for Metastatic Castration-Resistant Prostate Cancer -5-year Real-World Outcome: Focusing on Treatment Sequence and Quality of Life. Clin. Oncol. 2020, 32, e177–e187. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.E.; Joshi, V.B.; Badawy, M.; Pagliaro, L.C.; Karnes, R.J.; Lowe, V.; Thorpe, M.P.; Kwon, E.D.; Kendi, A.T. Radium-223 in the Third-Line Setting in Metastatic Castration-Resistant Prostate Cancer: Impact of Concomitant Use of Enzalutamide on Overall Survival (OS) and Predictors of Improved OS. Clin. Genitourin Cancer 2021, 19, 223–229. [Google Scholar] [CrossRef]
- Rizzo, S.; Galvano, A.; Pantano, F.; Iuliani, M.; Vincenzi, B.; Passiglia, F.; Spoto, S.; Tonini, G.; Bazan, V.; Russo, A.; et al. The effects of enzalutamide and abiraterone on skeletal related events and bone radiological progression free survival in castration resistant prostate cancer patients: An indirect comparison of randomized controlled trials. Crit. Rev. Oncol. Hematol. 2017, 120, 227–233. [Google Scholar] [CrossRef]
- Cha, T.-L.; Wu, T.T.-L.; Vogelzang, N.J.; Huang, C.-Y.; Huang, S.-P.; Lin, C.-C.; Ou, Y.-C.; Pang, S.-T.; Shen, D.H.-Y.; Wu, W.-J.; et al. Optimal usage of radium-223 in metastatic castration-resistant prostate cancer. J. Formos. Med. Assoc. 2017, 116, 825–836. [Google Scholar] [CrossRef]
- Piemontese, M.; Xiong, J.; Fujiwara, Y.; Thostenson, J.D.; O’Brien, C.A. Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E587–E593. [Google Scholar] [CrossRef]
- Chmielnicka, M.; Woźniacka, A.; Torzecka, J.D. The influence of corticosteroid treatment on the OPG/RANK/RANKL pathway and osteocalcin in patients with pemphigus. Postepy Dermatol. Alergol. 2014, 31, 281–288. [Google Scholar] [CrossRef]
- Van der Zande, K.; Oyen, W.J.G.; Zwart, W.; Bergman, A.M. Radium-223 Treatment of Patients with Metastatic Castration Resistant Prostate Cancer: Biomarkers for Stratification and Response Evaluation. Cancers 2021, 13, 4346. [Google Scholar] [CrossRef] [PubMed]
- Briot, K.; Roux, C. Glucocorticoid-induced osteoporosis. RMD Open 2015, 1, e000014. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Zhang, X.; Zhao, J.; Ni, Y.; Zhu, S.; He, B.; Dai, J.; Wang, Z.; Wang, Z.; et al. Comparison of Systemic Treatments for Metastatic Castration-Resistant Prostate Cancer After Docetaxel Failure: A Systematic Review and Network Meta-analysis. Front. Pharmacol. 2021, 12, 789319. [Google Scholar] [CrossRef]
- Sun, Y.; Zou, Q.; Sun, Z.; Li, C.; Du, C.; Chen, Z.; Shan, Y.; Huang, Y.; Jin, J.; Ye, Z.Q.; et al. Abiraterone acetate for metastatic castration-resistant prostate cancer after docetaxel failure: A randomized, double-blind, placebo-controlled phase 3 bridging study. Int. J. Urol. 2016, 23, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Chiang, P.C.; Chiang, P.H.; Chen, I.A.; Chen, Y.T.; Wang, H.J.; Cheng, Y.T.; Kang, C.H.; Chen, C.H.; Liu, Y.Y.; Su, Y.L.; et al. Treatment outcomes with radium-223 in docetaxel-naïve versus docetaxel-treated metastatic castration-resistant prostate cancer patients: Real-world evidence from Taiwan. Medicine 2023, 102, e32671. [Google Scholar] [CrossRef]
- Oguma, Y.; Hosono, M.; Okajima, K.; Inoue, E.; Nakamatsu, K.; Doi, H.; Matsuura, T.; Inada, M.; Uehara, T.; Wada, Y.; et al. Investigation into the Optimal Strategy of Radium-223 Therapy for Metastatic Castration-Resistant Prostate Cancer. Radiation 2022, 2, 273–284. [Google Scholar] [CrossRef]
- McDermott, R.S.; Greene, J.; McCaffrey, J.; Parker, I.; Helanova, S.; Baird, A.M.; Teiserskiene, A.; Lim, M.; Matthews, H.; Deignan, O.; et al. Radium-223 in combination with enzalutamide in metastatic castration-resistant prostate cancer: A multi-centre, phase II open-label study. Ther. Adv. Med. Oncol. 2021, 13, 17588359211042691. [Google Scholar] [CrossRef]
- Kim, S.I.; Szeto, A.H.; Morgan, K.P.; Brower, B.; Dunn, M.W.; Khandani, A.H.; Godley, P.A.; Rose, T.L.; Basch, E.M.; Milowsky, M.I.; et al. A real-world evaluation of radium-223 in combination with abiraterone or enzalutamide for the treatment of metastatic castration-resistant prostate cancer. PLoS ONE 2021, 16, e0253021. [Google Scholar] [CrossRef]
- Maughan, B.L.; Kessel, A.; McFarland, T.R.; Sayegh, N.; Nussenzveig, R.; Hahn, A.W.; Hoffman, J.M.; Morton, K.; Sirohi, D.; Kohli, M.; et al. Radium-223 plus Enzalutamide Versus Enzalutamide in Metastatic Castration-Refractory Prostate Cancer: Final Safety and Efficacy Results. Oncologist 2021, 26, 1006-e2129. [Google Scholar] [CrossRef]
- Saad, F.; Carles, J.; Gillessen, S.; Heidenreich, A.; Heinrich, D.; Gratt, J.; Lévy, J.; Miller, K.; Nilsson, S.; Petrenciuc, O.; et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: An international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016, 17, 1306–1316. [Google Scholar] [CrossRef]
- Shore, N.; Higano, C.S.; George, D.J.; Sternberg, C.N.; Saad, F.; Tombal, B.; Miller, K.; Kalinovsky, J.; Jiao, X.; Tangirala, K.; et al. Concurrent or layered treatment with radium-223 and enzalutamide or abiraterone/prednisone: Real-world clinical outcomes in patients with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic. Dis. 2020, 23, 680–688. [Google Scholar] [CrossRef]
- Weng, W.C.; Huang, L.H.; Tseng, N.C.; Ou, Y.C. Radium-223 for metastatic, castration-resistant prostate cancer: A retrospective chart review study of real-world use in a tertiary hospital in Taiwan. J. Formos. Med. Assoc. 2022, 121, 1929–1937. [Google Scholar] [CrossRef]
- Dan, T.D.; Eldredge-Hindy, H.B.; Hoffman-Censits, J.; Lin, J.; Kelly, W.K.; Gomella, L.G.; Lallas, C.D.; Trabulsi, E.J.; Hurwitz, M.D.; Dicker, A.P.; et al. Hematologic Toxicity of Concurrent Administration of Radium-223 and Next-generation Antiandrogen Therapies. Am. J. Clin. Oncol. 2017, 40, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhou, F.; Guo, J.; Shi, H.; Yao, X.; Guo, H.; Yuan, J.; Tian, Y.; Zhang, X.; Wang, S.; et al. Radium-223 in Asian patients with castration-resistant prostate cancer with symptomatic bone metastases: A single-arm phase 3 study. Asia Pac. J. Clin. Oncol. 2021, 17, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.; Sartor, O.; O’Sullivan, J.M.; Johannessen, D.C.; Helle, S.I.; Logue, J.; Bottomley, D.; Nilsson, S.; Vogelzang, N.J.; Fang, F.; et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: A prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014, 15, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Uemura, H.; Uemura, H.; Nagamori, S.; Wakumoto, Y.; Kimura, G.; Kikukawa, H.; Yokomizo, A.; Mizokami, A.; Kosaka, T.; Masumori, N.; et al. Three-year follow-up of a phase II study of radium-223 dichloride in Japanese patients with symptomatic castration-resistant prostate cancer and bone metastases. Int. J. Clin. Oncol. 2019, 24, 557–566. [Google Scholar] [CrossRef]

| Parameters | Enzalutamide | Abiraterone | p-Value |
|---|---|---|---|
| Number of patients | 22 | 19 | |
| Age | |||
| Median(range)-year | 75.5(64–89) | 76(53–94) | 0.44 |
| >75 year (%) | 11(50) | 11(57.9) | 0.61 |
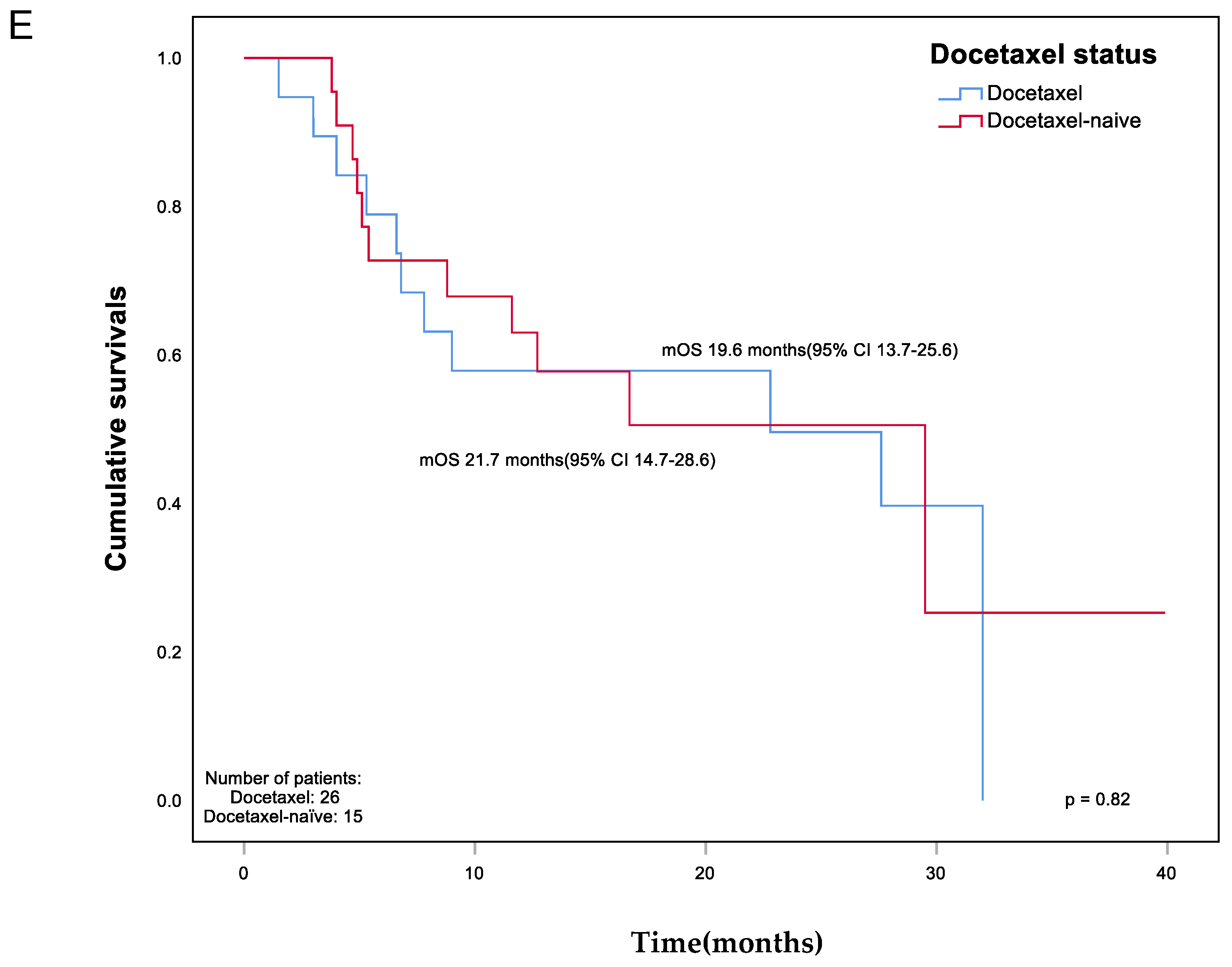
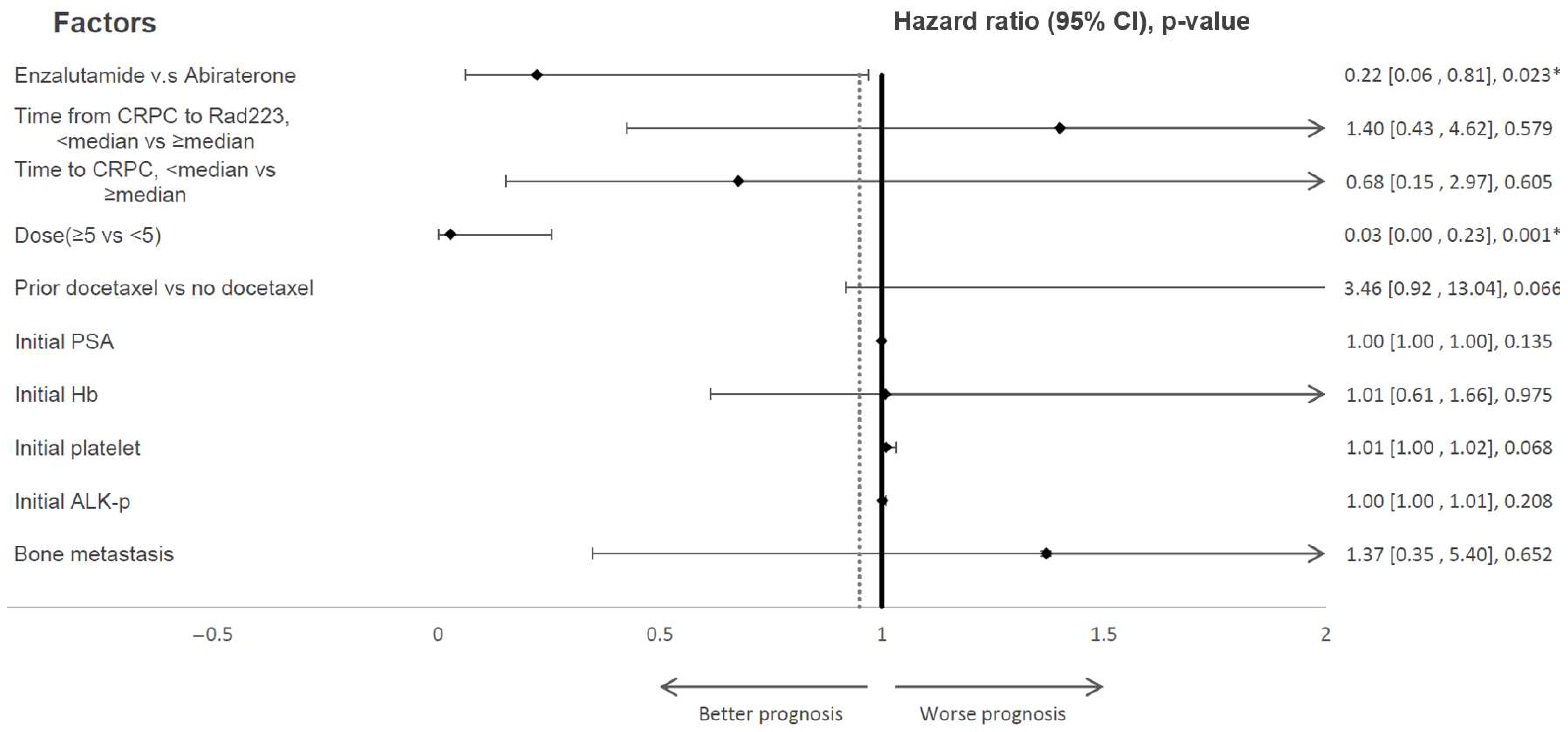
| Prior docetaxel (%) | 13(59.1%) | 13(68.4) | 0.55 |
| Early | 3(23.1) | 1(7.7) | |
| Late | 10(76.9) | 12(92.3) | |
| Dose of Radium-223 (%) | |||
| ≥5 | 17(77.3) | 11(57.9) | 0.24 |
| 3–4 | 4(18.2) | 4(21.1) | |
| ≤2 | 1(4.5) | 4(21.1) | |
| Prior denosumab (%) | 13(59.1) | 13(68.4) | |
| Median time to CRPC, mo. (range) | 24.6(7–164.9) | 26.9(4.5–78.4) | 0.56 |
| Median time from CRPC to Radium-223, mo. (range) | 25.3(1.5–75) | 24.9(2.9–85.7) | 0.88 |
| Median Duration of NHA | 13.1(5.0–61.2) | 11.0(0.2–50.2) | 0.78 |
| Total ALK-p | |||
| <220 | 21(95.5) | 16(84.2) | 0.23 |
| ≥220 | 1(4.5) | 3(15.8) | |
| Extent of disease | 0.855 | ||
| <6 metastases | 5(22.7) | 3(15.8) | |
| 6–20 metastases | 9(40.9) | 7(36.8) | |
| >20 metastases | 8(36.4) | 7(36.8) | |
| Superscan * | 0(0) | 2(10.5) | |
| Use of analgesic | 18(81.8) | 16(84.2) | 0.84 |
| Use of opioid | 8(36.4) | 10(52.6) | 0.29 |
| Baseline median Biochemical value (range) | |||
| Hb, g/dL | 12.4(8.2–14.6) | 11.6(7.8–12.9) | 0.12 |
| Platelet, 103/µL | 212(120–403) | 155.0(72.0–228.0) | 0.04 |
| ANC, 103/µL | 4035(1053–10896) | 4316(1938–4954) | 0.25 |
| Albumin, g/dL | 4(3.7–4.3) | 3.6(2.3–3.8) | 0.15 |
| Total ALK-P, U/L | 89(47–717) | 165(61–1109) | 0.32 |
| LDH, U/L | 171(111–372) | 205(127–346) | 0.54 |
| PSA, ng/mL | 76.1(7.08–2550.6) | 387.0(4.5–4557.6) | 0.19 |
| Endpoints | Enzalutamide | Abiraterone | p-Value |
|---|---|---|---|
| Primary | |||
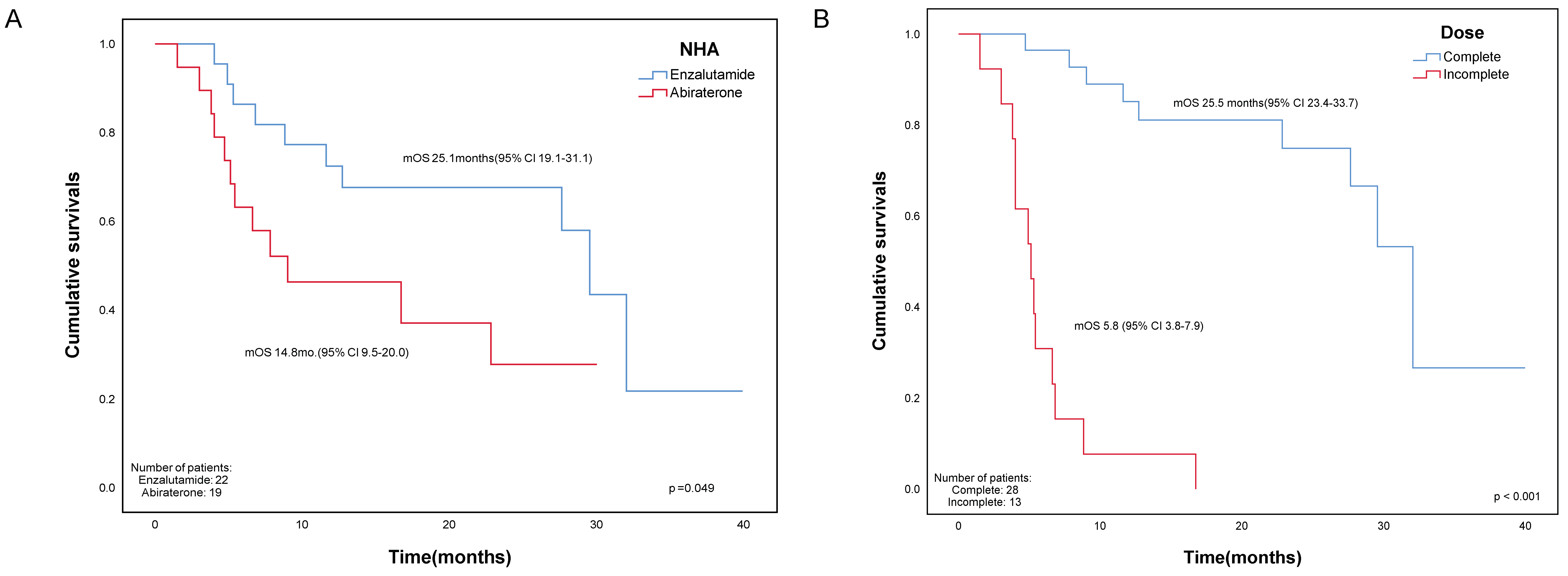
| mOS from the first dose of Rad223, mo. | 25.1 | 14.8 | 0.049 * |
| Secondary | |||
| mean Duration of exposure, mo. | 5.0 | 3.9 | 0.02 * |
| Alk-p-decrease | 10(45.4) | 8(42.1) | 0.83 |
| >30% | 8(80) | 8(100) | 0.48 |
| Normalization (%) | 5(50) | 7(87.5) | 0.93 |
| Median time to Alk-p increase, mo. | 3.1 | 2.6 | 0.92 |
| PSA decrease | 3 | 2 | 0.76 |
| PSA increase | 14 | 11 | 0.71 |
| Median time to PSA increase | 44 | 22 | 0.83 |
| SRE (%) | 3(18.7) | 2(13.3) | 0.76 |
| Median SRE-free survival | 8.5 | 6.6 | 0.96 |
| De-escalation of analgesic | 10(55.5) | 7(43.8) | 0.49 |
| Median dose to de-escalation | 2 | 2 | |
| Anemia | 21(95.4) | 19(100) | 1.0 |
| Blood transfusion | 2(9.1) | 5(26.3) | 0.16 |
| Values, Mean ± SD | Enzalutamide | Abiraterone | ||
|---|---|---|---|---|
| Cycle 1 | Last Cycle | Cycle 1 | Last Cycle | |
| Hemoglobin, g/dL | 12.1 ± 1.8 | 10.8 ± 1.7 | 11.4 ± 1.2 | 10.4 ± 1.8 |
| Platelet, 103/µL | 233.1 ± 71.8 | 202.0 ± 73.0 | 180.2 ± 62.5 | 154.9 ± 102.1 |
| ALK-P, U/L | 133.9 ± 149.9 | 117.0 ± 99.1 | 207.4 ± 255.9 | 128.2 ± 205.6 |
| PSA, ng/mL | 253.8 ± 574.6 | 903.0 ± 1519.4 | 672.6 ± 1196.3 | 1764.7 ± 3717.5 |
| Values, Mean ± SD | Enzalutamide | Abiraterone |
|---|---|---|
| Hemoglobin, g/dL | −1.4 ± 1.04 | −1.0 ± 1.7 |
| Platelet, 103/µL | −31.1 ± 67.1 | −25.2 ± 89.6 |
| ALK-P, U/L | −11.4 ± 96.7 | −55.0 ± 83.3 |
| PSA, ng/mL | 654.4 ± 1041.7 | 1126.8 ± 2746.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.X.; Tsai, L.-H.; Chang, C.-H.; Wu, H.-C.; Lin, C.-C.; Lin, C.-H.; Yeh, C.-C.; Yang, C.-R.; Lien, C.-S.; Chang, Y.-H.; et al. Enzalutamide Prior to Radium-223 Is Associated with Better Overall Survival in Metastatic Castration-Resistant Prostate Cancer Patients Compared to Abiraterone—A Retrospective Study. Cancers 2023, 15, 3516. https://doi.org/10.3390/cancers15133516
Chen HX, Tsai L-H, Chang C-H, Wu H-C, Lin C-C, Lin C-H, Yeh C-C, Yang C-R, Lien C-S, Chang Y-H, et al. Enzalutamide Prior to Radium-223 Is Associated with Better Overall Survival in Metastatic Castration-Resistant Prostate Cancer Patients Compared to Abiraterone—A Retrospective Study. Cancers. 2023; 15(13):3516. https://doi.org/10.3390/cancers15133516
Chicago/Turabian StyleChen, Hao Xiang, Li-Hsien Tsai, Chao-Hsiang Chang, Hsi-Chin Wu, Ching-Chan Lin, Che-Hung Lin, Chin-Chung Yeh, Chi-Rei Yang, Chi-Shun Lien, Yi-Huei Chang, and et al. 2023. "Enzalutamide Prior to Radium-223 Is Associated with Better Overall Survival in Metastatic Castration-Resistant Prostate Cancer Patients Compared to Abiraterone—A Retrospective Study" Cancers 15, no. 13: 3516. https://doi.org/10.3390/cancers15133516
APA StyleChen, H. X., Tsai, L.-H., Chang, C.-H., Wu, H.-C., Lin, C.-C., Lin, C.-H., Yeh, C.-C., Yang, C.-R., Lien, C.-S., Chang, Y.-H., Liang, J.-A., Chen, G.-H., Hsiao, P.-J., Hsieh, P.-F., & Huang, C.-P. (2023). Enzalutamide Prior to Radium-223 Is Associated with Better Overall Survival in Metastatic Castration-Resistant Prostate Cancer Patients Compared to Abiraterone—A Retrospective Study. Cancers, 15(13), 3516. https://doi.org/10.3390/cancers15133516







