Early Onset Metastatic Colorectal Cancer: Current Insights and Clinical Management of a Rising Condition
Abstract
Simple Summary
Abstract
1. Introduction
2. Epidemiology
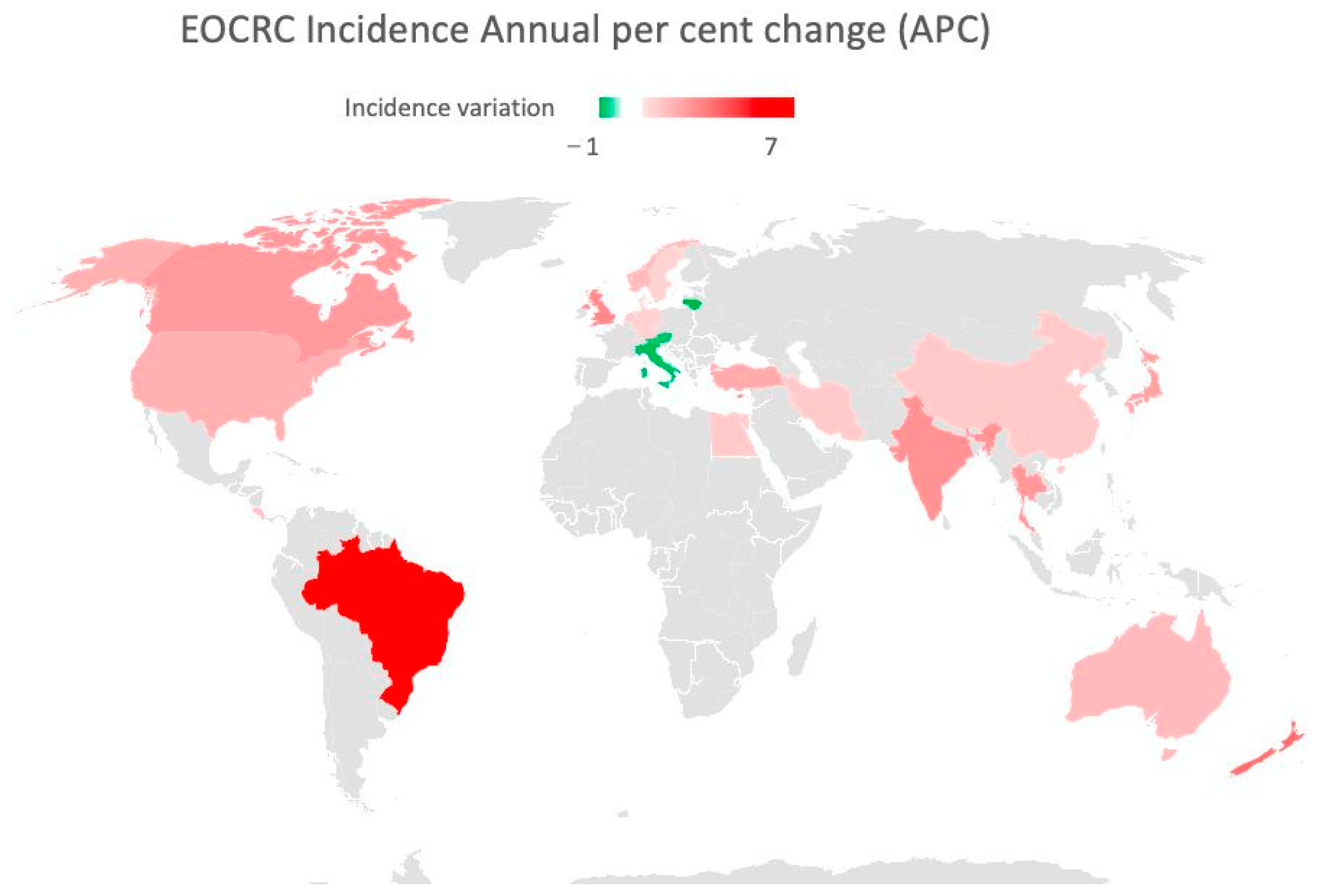
2.1. Geographic Differences
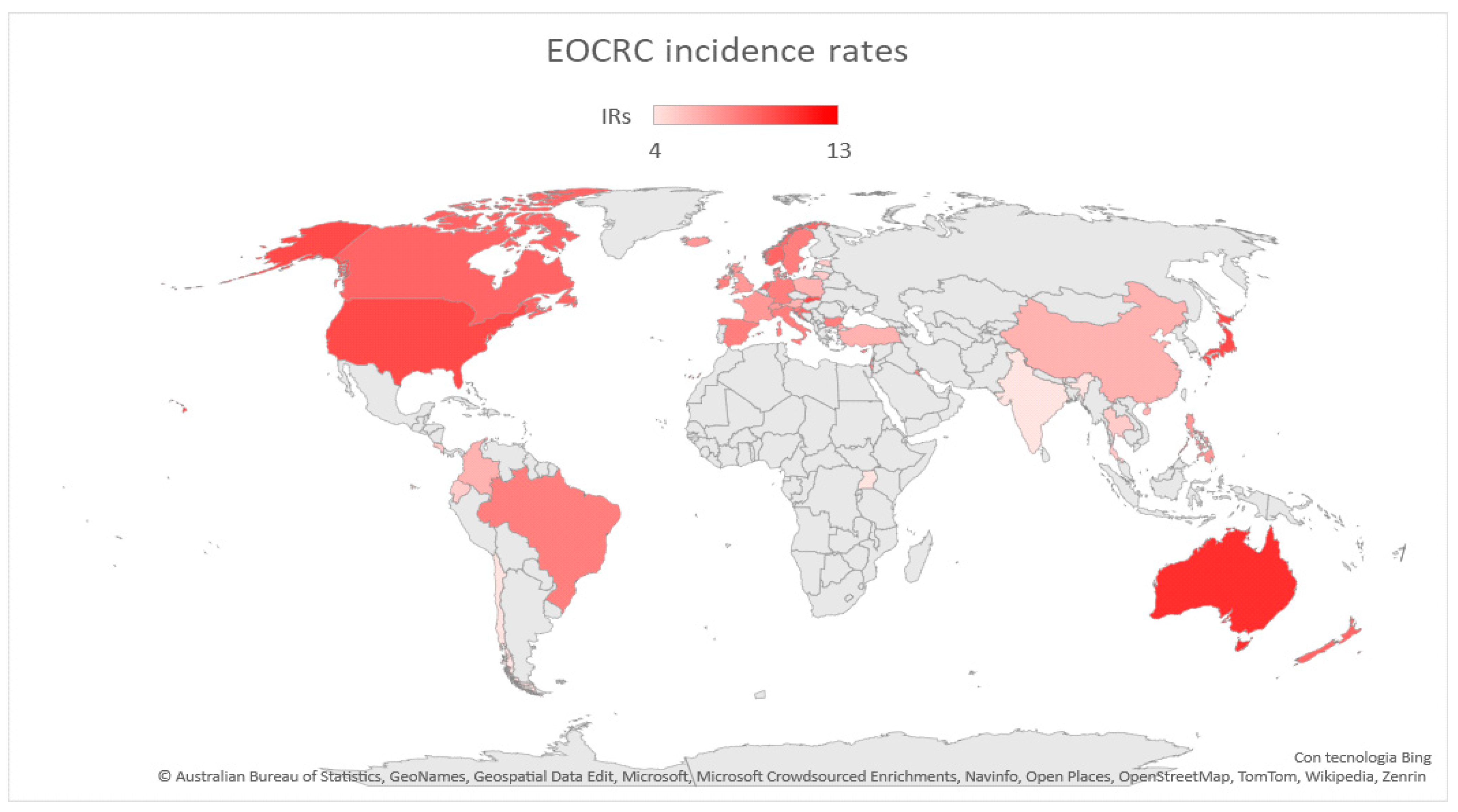
2.2. Screening Programs
3. Risk Factors
3.1. Hereditary EOCRC
3.1.1. Hereditary Nonpolyposis CRC or Lynch Syndrome (LS)
3.1.2. Adenomatous Polyposis Syndromes (APSs)
3.1.3. Other Germline Alterations Associated with EOCRC
3.1.4. Cystic Fibrosis (CF)
3.2. Sporadic EOCRC
3.2.1. Metabolic Syndrome
3.2.2. Diet
3.2.3. Physical Activity
3.2.4. Alcohol and Tobacco Use
3.2.5. Aspirin and Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
3.2.6. Chronic Inflammation
3.2.7. Intestinal Dysbiosis
3.2.8. Pollution
3.2.9. Acute Infections
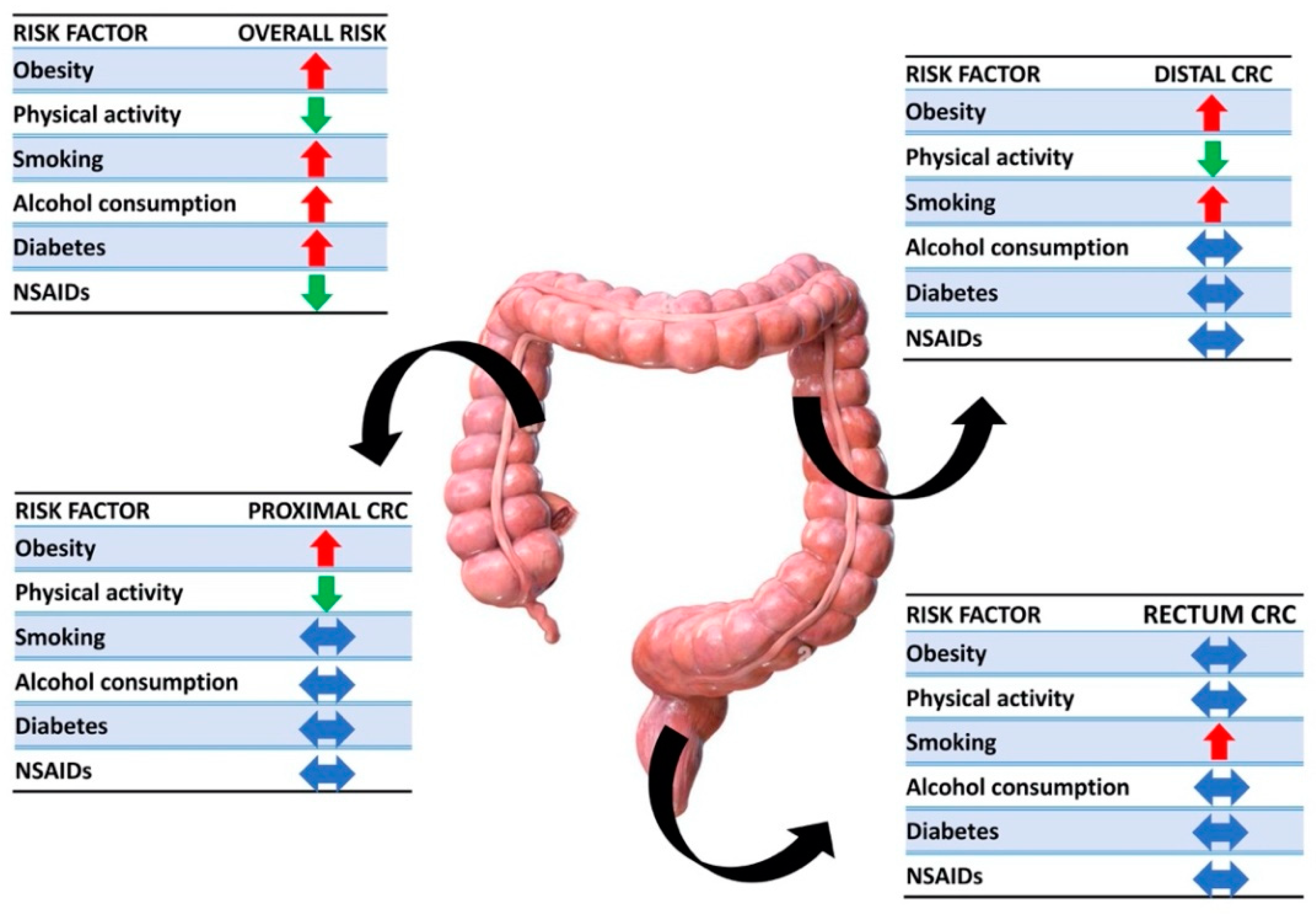
3.2.10. Association between Modifiable Risk Factors and Anatomical Sites
4. Prognosis
5. Clinicopathological Features
6. Molecular Signatures
- (1)
- Chromosomal instability (CIN), which is the classic pathway and causes mutations of multiple oncogenes, such as KRAS, and deletions of suppressor genes, such as APC and p53;
- (2)
- Microsatellite instability (MSI), which is usually sporadic, but there are also hereditary causes. MSI-CRC can be caused by hereditary germline mutations occurring in the MMR genes (MLH1, MSH2, MSH6, and PMS2) and tumor somatic hypermethylation of MLH1. Nearly 10% of CRCs are caused by MLH1 promoter hypermethylation and are often in association with the BRAF V600E pathogenetic variant in sporadic CRC [98];
- (3)
6.1. CIN
6.2. MSI and MSS
6.3. MACS (Microsatellite and Chromosome Stable)
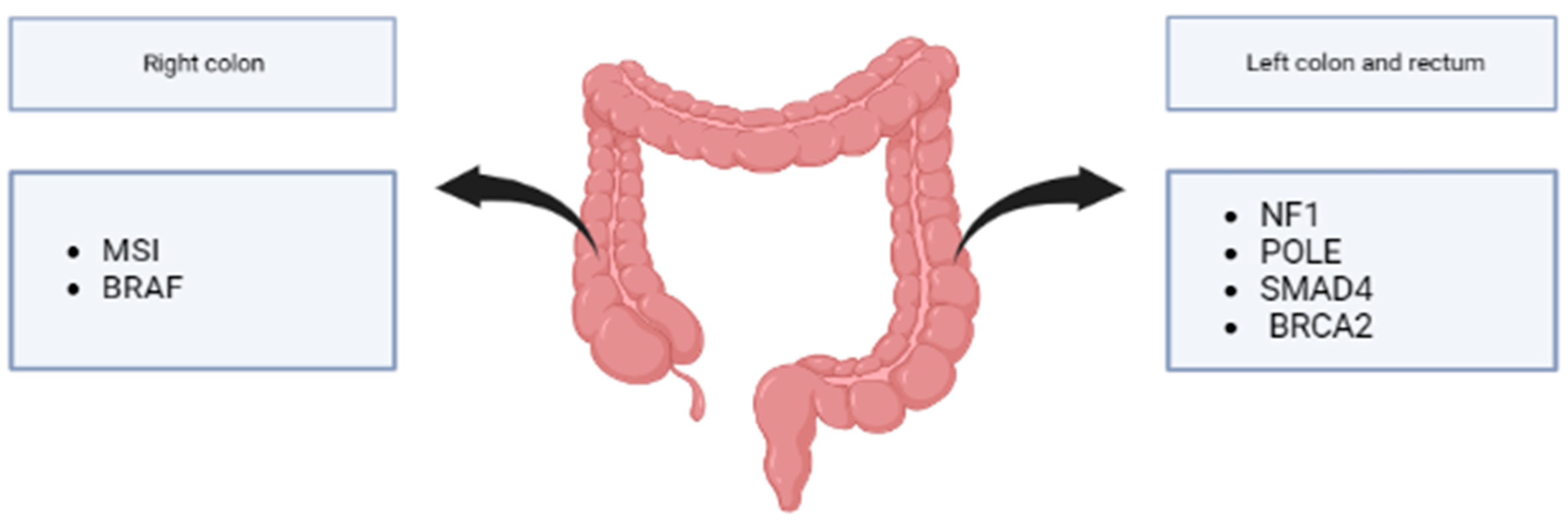
6.4. Molecular Differences Depending on Primary Tumor Location
7. Treatment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saad El Din, K.; Loree, J.M.; Sayre, E.C.; Gill, S.; Brown, C.J.; Dau, H.; De Vera, M.A. Trends in the epidemiology of young-onset colorectal cancer: A worldwide systematic review. BMC Cancer 2020, 20, 288. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Liu, P.-H.; Zheng, X.; Keum, N.; Zong, X.; Li, X.; Wu, K.; Fuchs, C.S.; Ogino, S.; Ng, K.; et al. Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr. 2018, 2, pky073. [Google Scholar] [CrossRef] [PubMed]
- Gausman, V.; Dornblaser, D.; Anand, S.; Hayes, R.B.; O’Connell, K.; Du, M.; Liang, P.S. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 2752–2759.e2. [Google Scholar] [CrossRef]
- Uy, G.B.; Kaw, L.L.; Punzalan, C.K.; Querol, R.I.L.C.; Koustova, E.V.; Bowyer, M.W.; Hobbs, C.M.; Sobin, L.H.; Wherry, D.C. Clinical and molecular biologic characteristics of early-onset versus late-onset colorectal carcinoma in Filipinos. World J. Surg. 2004, 28, 117–123. [Google Scholar] [CrossRef]
- Silla, I.O. Early-onset colorectal cancer: A separate subset of colorectal cancer. WJG 2014, 20, 17288. [Google Scholar] [CrossRef]
- Dozois, E.J.; Boardman, L.A.; Suwanthanma, W.; Limburg, P.J.; Cima, R.R.; Bakken, J.L.; Vierkant, R.A.; Aakre, J.A.; Larson, D.W. Young-Onset Colorectal Cancer in Patients With No Known Genetic Predisposition: Can We Increase Early Recognition and Improve Outcome? Medicine 2008, 87, 259–263. [Google Scholar] [CrossRef]
- You, Y.N.; Xing, Y.; Feig, B.W.; Chang, G.J.; Cormier, J.N. Young-Onset Colorectal Cancer: Is It Time to Pay Attention? Arch. Intern. Med. 2012, 172, 287–289. [Google Scholar] [CrossRef]
- Willauer, A.N.; Liu, Y.; Pereira, A.A.L.; Lam, M.; Morris, J.S.; Raghav, K.P.S.; Morris, V.K.; Menter, D.; Broaddus, R.; Meric-Bernstam, F.; et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 2019, 125, 2002–2010. [Google Scholar] [CrossRef]
- Liang, J.T.; Huang, K.C.; Cheng, A.L.; Jeng, Y.M.; Wu, M.S.; Wang, S.M. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br. J. Surg. 2003, 90, 205–214. [Google Scholar] [CrossRef]
- Siegel, R.L.; Jakubowski, C.D.; Fedewa, S.A.; Davis, A.; Azad, N.S. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e75–e88. [Google Scholar] [CrossRef]
- Van Der Heide, D.M.; Turaga, K.K.; Chan, C.H.F.; Sherman, S.K. Mismatch Repair Status Correlates with Survival in Young Adults with Metastatic Colorectal Cancer. J. Surg. Res. 2021, 266, 104–112. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Edwards, B.K.; Ward, E.; Kohler, B.A.; Eheman, C.; Zauber, A.G.; Anderson, R.N.; Jemal, A.; Schymura, M.J.; Lansdorp-Vogelaar, I.; Seeff, L.C.; et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010, 116, 544–573. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970–2014. JAMA 2017, 318, 572. [Google Scholar] [CrossRef]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. JNCI J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, A.; Carethers, J.M. Epidemiology and biology of early onset colorectal cancer. EXCLI J. 2022, 21, 162. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017: Colorectal Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef]
- Murphy, C.C.; Wallace, K.; Sandler, R.S.; Baron, J.A. Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology 2019, 156, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, A.A.; Jumuah, W.A.; Ebied, E.F.; Abd El Samee Atia, K.S.; El Ghamrini, Y.; Somaie, D.A. Hereditary factors are unlikely behind unusual pattern of early—Onset colorectal cancer in Egyptians: A study of family history and pathology features in Egyptians with large bowel cancer (cross-sectional study). Int. J. Surg. 2017, 44, 71–75. [Google Scholar] [CrossRef]
- Mauri, G.; Sartore-Bianchi, A.; Russo, A.; Marsoni, S.; Bardelli, A.; Siena, S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019, 13, 109–131. [Google Scholar] [CrossRef]
- Bailey, C.E.; Hu, C.-Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing Disparities in the Age-Related Incidences of Colon and Rectal Cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Murphy, C.C. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020, 158, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Lui, R.N.; Tsoi, K.K.F.; Ho, J.M.W.; Lo, C.M.; Chan, F.C.H.; Kyaw, M.H.; Sung, J.J.Y. Global Increasing Incidence of Young-Onset Colorectal Cancer Across 5 Continents: A Joinpoint Regression Analysis of 1,922,167 Cases. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1275–1282. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Wang, W.; Feng, W.; Shi, O.; Wang, Q. International incidence trends in early- and late-onset colorectal cancer: A population-based study. Int. J. Color. Dis. 2020, 35, 1077–1086. [Google Scholar] [CrossRef]
- Yeo, H.; Betel, D.; Abelson, J.S.; Zheng, X.E.; Yantiss, R.; Shah, M.A. Early-onset Colorectal Cancer is Distinct From Traditional Colorectal Cancer. Clin. Color. Cancer 2017, 16, 293–299.e6. [Google Scholar] [CrossRef]
- Theuer, C.P.; Wagner, J.L.; Taylor, T.H.; Brewster, W.R.; Tran, D.; McLaren, C.E.; Anton–Culver, H. Racial and ethnic colorectal cancer patterns affect the cost-effectiveness of colorectal cancer screening in the United States. Gastroenterology 2001, 120, 848–856. [Google Scholar] [CrossRef]
- Zahnd, W.E.; Gomez, S.L.; Steck, S.E.; Brown, M.J.; Ganai, S.; Zhang, J.; Arp Adams, S.; Berger, F.G.; Eberth, J.M. Rural-urban and racial/ethnic trends and disparities in early-onset and average-onset colorectal cancer. Cancer 2021, 127, 239–248. [Google Scholar] [CrossRef]
- Ahnen, D.J.; Wade, S.W.; Jones, W.F.; Sifri, R.; Mendoza Silveiras, J.; Greenamyer, J.; Guiffre, S.; Axilbund, J.; Spiegel, A.; You, Y.N. The Increasing Incidence of Young-Onset Colorectal Cancer: A Call to Action. Mayo Clin. Proc. 2014, 89, 216–224. [Google Scholar] [CrossRef]
- Al Zaabi, A.; Al Shehhi, A.; Sayed, S.; Al Adawi, H.; Al Faris, F.; Al Alyani, O.; Al Asmi, M.; Al-Mirza, A.; Panchatcharam, S.; Al-Shaibi, M. Early Onset Colorectal Cancer in Arabs, Are We Dealing with a Distinct Disease? Cancers 2023, 15, 889. [Google Scholar] [CrossRef]
- Cavestro, G.M.; Mannucci, A.; Zuppardo, R.A.; Di Leo, M.; Stoffel, E.; Tonon, G. Early onset sporadic colorectal cancer: Worrisome trends and oncogenic features. Dig. Liver Dis. 2018, 50, 521–532. [Google Scholar] [CrossRef]
- Hadjiliadis, D.; Khoruts, A.; Zauber, A.G.; Hempstead, S.E.; Maisonneuve, P.; Lowenfels, A.B.; Braid, A.L.; Cullina, J.; Daggett, A.; Fink, A.; et al. Cystic Fibrosis Colorectal Cancer Screening Consensus Recommendations. Gastroenterology 2018, 154, 736–745.e14. [Google Scholar] [CrossRef]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.-C.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society: ACS Colorectal Cancer Screening Guideline. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Carethers, J.M. Screening for Colorectal Cancer in African Americans: Determinants and Rationale for an Earlier Age to Commence Screening. Dig. Dis. Sci. 2015, 60, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Gini, A.; Meester, R.G.S.; Keshavarz, H.; Oeffinger, K.C.; Ahmed, S.; Hodgson, D.C.; Lansdorp-Vogelaar, I. Cost-Effectiveness of Colonoscopy-Based Colorectal Cancer Screening in Childhood Cancer Survivors. JNCI J. Natl. Cancer Inst. 2019, 111, 1161–1169. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Koeppe, E.; Everett, J.; Ulintz, P.; Kiel, M.; Osborne, J.; Williams, L.; Hanson, K.; Gruber, S.B.; Rozek, L.S. Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology 2018, 154, 897–905.e1. [Google Scholar] [CrossRef] [PubMed]
- Mork, M.E.; You, Y.N.; Ying, J.; Bannon, S.A.; Lynch, P.M.; Rodriguez-Bigas, M.A.; Vilar, E. High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer. J. Clin. Oncol. 2015, 33, 3544–3549. [Google Scholar] [CrossRef]
- Yurgelun, M.B.; Kulke, M.H.; Fuchs, C.S.; Allen, B.A.; Uno, H.; Hornick, J.L.; Ukaegbu, C.I.; Brais, L.K.; McNamara, P.G.; Mayer, R.J.; et al. Cancer Susceptibility Gene Mutations in Individuals With Colorectal Cancer. J. Clin. Oncol. 2017, 35, 1086–1095. [Google Scholar] [CrossRef]
- Dinarvand, P.; Davaro, E.P.; Doan, J.V.; Ising, M.E.; Evans, N.R.; Phillips, N.J.; Lai, J.; Guzman, M.A. Familial Adenomatous Polyposis Syndrome: An Update and Review of Extraintestinal Manifestations. Arch. Pathol. Lab. Med. 2019, 143, 1382–1398. [Google Scholar] [CrossRef]
- Sieber, O.M.; Tomlinson, I.P.; Lamlum, H. The adenomatous polyposis coli (APC) tumour suppressor–genetics, function and disease. Mol. Med. Today 2000, 6, 462–469. [Google Scholar] [CrossRef]
- Ma, H.; Brosens, L.A.A.; Offerhaus, G.J.A.; Giardiello, F.M.; De Leng, W.W.J.; Montgomery, E.A. Pathology and genetics of hereditary colorectal cancer. Pathology 2018, 50, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Lynch, J.F.; Lynch, P.M.; Attard, T. Hereditary colorectal cancer syndromes: Molecular genetics, genetic counseling, diagnosis and management. Fam. Cancer 2008, 7, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Warrier, S.; Kalady, M. Familial Adenomatous Polyposis: Challenges and Pitfalls of Surgical Treatment. Clin. Colon Rectal Surg. 2012, 25, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Anderson, K.; Singhania, M.; Cormier, R. Cystic Fibrosis, CFTR, and Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 2891. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, X.; Zong, X.; Li, Z.; Li, N.; Hur, J.; Fritz, C.D.; Chapman Jr, W.; Nickel, K.B.; Tipping, A.; et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 2021, 70, 1147–1154. [Google Scholar] [CrossRef]
- Liu, P.-H.; Wu, K.; Ng, K.; Zauber, A.G.; Nguyen, L.H.; Song, M.; He, X.; Fuchs, C.S.; Ogino, S.; Willett, W.C.; et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019, 5, 37. [Google Scholar] [CrossRef]
- O’Sullivan, D.E.; Sutherland, R.L.; Town, S.; Chow, K.; Fan, J.; Forbes, N.; Heitman, S.J.; Hilsden, R.J.; Brenner, D.R. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1229–1240.e5. [Google Scholar] [CrossRef]
- Li, H.; Boakye, D.; Chen, X.; Jansen, L.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Associations of Body Mass Index at Different Ages With Early-Onset Colorectal Cancer. Gastroenterology 2022, 162, 1088–1097.e3. [Google Scholar] [CrossRef]
- Hidayat, K.; Yang, C.-M.; Shi, B.-M. Body fatness at an early age and risk of colorectal cancer: Early-life BMI and colorectal cancer. Int. J. Cancer 2018, 142, 729–740. [Google Scholar] [CrossRef]
- Low, E.E.; Demb, J.; Liu, L.; Earles, A.; Bustamante, R.; Williams, C.D.; Provenzale, D.; Kaltenbach, T.; Gawron, A.J.; Martinez, M.E.; et al. Risk Factors for Early-Onset Colorectal Cancer. Gastroenterology 2020, 159, 492–501.e7. [Google Scholar] [CrossRef]
- Yang, X.; Hennessy, S.; Lewis, J.D. Type 2 Diabetes Mellitus and the Risk of Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2005, 3, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Higurashi, T.; Hosono, K.; Takahashi, H.; Komiya, Y.; Umezawa, S.; Sakai, E.; Uchiyama, T.; Taniguchi, L.; Hata, Y.; Uchiyama, S.; et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016, 17, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, D.; Bordonaro, M. Multifactorial causation of early onset colorectal cancer. J. Cancer 2021, 12, 6825–6834. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Cao, Y.; Hur, J.; Mehta, R.S.; Sikavi, D.R.; Wang, Y.; Ma, W.; Wu, K.; Song, M.; Giovannucci, E.L.; et al. The Sulfur Microbial Diet Is Associated With Increased Risk of Early-Onset Colorectal Cancer Precursors. Gastroenterology 2021, 161, 1423–1432.e4. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.K.A.; Taylor, R.S.; Marshall, T.; Chapman, M.A.S. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Color. Dis. 2005, 7, 204–213. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Yan, Y.; Colditz, G.A.; Lee, I.-M. Physical activity and colon cancer prevention: A meta-analysis. Br. J. Cancer 2009, 100, 611–616. [Google Scholar] [CrossRef]
- Botteri, E.; Iodice, S.; Raimondi, S.; Maisonneuve, P.; Lowenfels, A.B. Cigarette Smoking and Adenomatous Polyps: A Meta-analysis. Gastroenterology 2008, 134, 388–395.e3. [Google Scholar] [CrossRef]
- Kaltenbach, T.R.; Soetikno, R.M.; DeVivo, R.; Laine, L.A.; Barkun, A.; McQuaid, K.R.; Cheifetz, A.; Chiorean, M.; East, J.E.; Farraye, F.A.; et al. Optimizing the quality of endoscopy in inflammatory bowel disease: Focus on surveillance and management of colorectal dysplasia using interactive image- and video-based teaching. Gastrointest. Endosc. 2017, 86, 1107–1117.e1. [Google Scholar] [CrossRef]
- McDowell, R.; Perrott, S.; Murchie, P.; Cardwell, C.; Hughes, C.; Samuel, L. Oral antibiotic use and early-onset colorectal cancer: Findings from a case-control study using a national clinical database. Br. J. Cancer 2022, 126, 957–967. [Google Scholar] [CrossRef]
- Turner, M.C.; Krewski, D.; Diver, W.R.; Pope, C.A.; Burnett, R.T.; Jerrett, M.; Marshall, J.D.; Gapstur, S.M. Ambient Air Pollution and Cancer Mortality in the Cancer Prevention Study II. Environ. Health Perspect 2017, 125, 087013. [Google Scholar] [CrossRef]
- López-Abente, G.; García-Pérez, J.; Fernández-Navarro, P.; Boldo, E.; Ramis, R. Colorectal cancer mortality and industrial pollution in Spain. BMC Public Health 2012, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Kleef, R.; Hager, E.D. Fever, Pyrogens and Cancer. In Hyperthermia in Cancer Treatment: A Primer; Medical Intelligence Unit; Springer: Boston, MA, USA, 2006; pp. 276–337. ISBN 978-0-387-33440-0. [Google Scholar]
- Murphy, N.; Ward, H.A.; Jenab, M.; Rothwell, J.A.; Boutron-Ruault, M.-C.; Carbonnel, F.; Kvaskoff, M.; Kaaks, R.; Kühn, T.; Boeing, H.; et al. Heterogeneity of Colorectal Cancer Risk Factors by Anatomical Subsite in 10 European Countries: A Multinational Cohort Study. Clin. Gastroenterol. Hepatol. 2019, 17, 1323–1331.e6. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Joo, J.; Bak, J.; Yang, H.-R.; Kim, J.; Park, S.; Nam, B.-H. Site-Specific Risk Factors for Colorectal Cancer in a Korean Population. PLoS ONE 2011, 6, e23196. [Google Scholar] [CrossRef]
- Carethers, J.M. Risk factors for colon location of cancer. Transl. Gastroenterol. Hepatol. 2018, 3, 76. [Google Scholar] [CrossRef]
- Wei, E.K.; Giovannucci, E.; Wu, K.; Rosner, B.; Fuchs, C.S.; Willett, W.C.; Colditz, G.A. Comparison of risk factors for colon and rectal cancer. Int. J. Cancer 2004, 108, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Varga, D.; Koenig, J.; Kuhr, K.; Strunz, K.; Geyer, V.; Kurzeder, C.; Atassi, Z.; Blettner, M.; Kreienberg, R.; Woeckel, A. Comparison of early onset breast cancer patients to older premenopausal breast cancer patients. Arch Gynecol Obs. 2010, 282, 427–432. [Google Scholar] [CrossRef]
- Tavares, A.; Gandra, A.; Viveiros, F.; Cidade, C.; Maciel, J. Analysis of Clinicopathologic Characteristics and Prognosis of Gastric Cancer in Young and Older Patients. Pathol. Oncol. Res. 2013, 19, 111–117. [Google Scholar] [CrossRef]
- Desandes, E.; Stark, D.P. Epidemiology of Adolescents and Young Adults with Cancer in Europe. In Progress in Tumor Research; Stark, D.P., Vassal, G., Eds.; S. Karger AG: Basel, Switzerland, 2016; Volume 43, pp. 1–15. ISBN 978-3-318-05911-3. [Google Scholar]
- Vatandoust, S.; Price, T.J.; Ullah, S.; Roy, A.C.; Beeke, C.; Young, J.P.; Townsend, A.; Padbury, R.; Roder, D.; Karapetis, C.S. Metastatic Colorectal Cancer in Young Adults: A Study From the South Australian Population-Based Registry. Clin. Color. Cancer 2016, 15, 32–36. [Google Scholar] [CrossRef]
- Hawk, N.N.; Long, T.-E.; Imam, M.H.; Mathew, B.M.; Kim, S.; Chen, Z.; Goodman, M.; Sullivan, P.; Brutcher, E.; Kauh, J.; et al. Clinicopathologic Features and Outcome of Young Adults With Stage IV Colorectal Cancer. Am. J. Clin. Oncol. 2015, 38, 543–549. [Google Scholar] [CrossRef]
- Blanke, C.D.; Bot, B.M.; Thomas, D.M.; Bleyer, A.; Kohne, C.-H.; Seymour, M.T.; de Gramont, A.; Goldberg, R.M.; Sargent, D.J. Impact of Young Age on Treatment Efficacy and Safety in Advanced Colorectal Cancer: A Pooled Analysis of Patients From Nine First-Line Phase III Chemotherapy Trials. J. Clin. Oncol. 2011, 29, 2781–2786. [Google Scholar] [CrossRef]
- Lieu, C.H.; Renfro, L.A.; de Gramont, A.; Meyers, J.P.; Maughan, T.S.; Seymour, M.T.; Saltz, L.; Goldberg, R.M.; Sargent, D.J.; Eckhardt, S.G.; et al. Association of Age With Survival in Patients With Metastatic Colorectal Cancer: Analysis From the ARCAD Clinical Trials Program. J. Clin. Oncol. 2014, 32, 2975–2982. [Google Scholar] [CrossRef] [PubMed]
- Rho, Y.S.; Gilabert, M.; Polom, K.; Aladashvili, A.; Kopeckova, K.; Megdanova, V.; Coleman, N.; Greally, M.; Marrelli, D.; Roviello, F.; et al. Comparing Clinical Characteristics and Outcomes of Young-onset and Late-onset Colorectal Cancer: An International Collaborative Study. Clin. Color. Cancer 2017, 16, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Bao, F.; Yan, J.; Liu, H.; Li, T.; Chen, H.; Li, G. Poor prognosis of young patients with colorectal cancer: A retrospective study. Int. J. Color. Dis. 2017, 32, 1147–1156. [Google Scholar] [CrossRef]
- Chan, K.; Dassanayake, B.; Deen, R.; Wickramarachchi, R.; Kumarage, S.; Samita, S.; Deen, K. Young patients with colorectal cancer have poor survival in the first twenty months after operation and predictable survival in the medium and long-term: Analysis of survival and prognostic markers. World J. Surg. Oncol. 2010, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Dixon, J.G.; Fiskum, J.M.; Parekh, H.D.; Sinicrope, F.A.; Yothers, G.; Allegra, C.J.; Wolmark, N.; Haller, D.; Schmoll, H.-J.; et al. Clinicopathological and Molecular Characteristics of Early-Onset Stage III Colon Adenocarcinoma: An Analysis of the ACCENT Database. JNCI J. Natl. Cancer Inst. 2021, 113, 1693–1704. [Google Scholar] [CrossRef]
- Bertagnolli, M.M.; Niedzwiecki, D.; Compton, C.C.; Hahn, H.P.; Hall, M.; Damas, B.; Jewell, S.D.; Mayer, R.J.; Goldberg, R.M.; Saltz, L.B.; et al. Microsatellite Instability Predicts Improved Response to Adjuvant Therapy With Irinotecan, Fluorouracil, and Leucovorin in Stage III Colon Cancer: Cancer and Leukemia Group B Protocol 89803. J. Clin. Oncol. 2009, 27, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Svrcek, M.; Cohen, R.; Basile, D.; Tougeron, D.; Phelip, J.-M. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur. J. Cancer 2022, 175, 136–157. [Google Scholar] [CrossRef] [PubMed]
- Kanter, K.; Fish, M.; Mauri, G.; Horick, N.K.; Allen, J.N.; Blaszkowsky, L.S.; Clark, J.W.; Ryan, D.P.; Nipp, R.D.; Giantonio, B.J.; et al. Care Patterns and Overall Survival in Patients With Early-Onset Metastatic Colorectal Cancer. JCO Oncol. Pract. 2021, 17, e1846–e1855. [Google Scholar] [CrossRef]
- Kneuertz, P.J.; Chang, G.J.; Hu, C.-Y.; Rodriguez-Bigas, M.A.; Eng, C.; Vilar, E.; Skibber, J.M.; Feig, B.W.; Cormier, J.N.; You, Y.N. Overtreatment of Young Adults With Colon Cancer: More Intense Treatments With Unmatched Survival Gains. JAMA Surg. 2015, 150, 402. [Google Scholar] [CrossRef]
- Georgiou, A.; Khakoo, S.; Edwards, P.; Minchom, A.; Kouvelakis, K.; Kalaitzaki, E.; Starling, N. Outcomes of Patients with Early Onset Colorectal Cancer Treated in a UK Specialist Cancer Center. Cancers 2019, 11, 1558. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Yantiss, R.K.; Goodarzi, M.; Zhou, X.K.; Rennert, H.; Pirog, E.C.; Banner, B.F.; Chen, Y.-T. Clinical, Pathologic, and Molecular Features of Early-onset Colorectal Carcinoma. Am. J. Surg. Pathol. 2009, 33, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Meester, R.G.S.; Mannalithara, A.; Lansdorp-Vogelaar, I.; Ladabaum, U. Trends in Incidence and Stage at Diagnosis of Colorectal Cancer in Adults Aged 40 Through 49 Years, 1975–2015. JAMA 2019, 321, 1933. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.W.; Sundaram, V.; Chew, T.A.; Ladabaum, U. Advanced-Stage Colorectal Cancer in Persons Younger Than 50 Years Not Associated With Longer Duration of Symptoms or Time to Diagnosis. Clin. Gastroenterol. Hepatol. 2017, 15, 728–737.e3. [Google Scholar] [CrossRef]
- Cercek, A.; Chatila, W.K.; Yaeger, R.; Walch, H.; Fernandes, G.D.S.; Krishnan, A.; Palmaira, L.; Maio, A.; Kemel, Y.; Srinivasan, P.; et al. A Comprehensive Comparison of Early-Onset and Average-Onset Colorectal Cancers. JNCI J. Natl. Cancer Inst. 2021, 113, 1683–1692. [Google Scholar] [CrossRef]
- Riaz, R.; Masood, N.; Benish, A. Red flag symptoms: Detailed account of clinicopathological features in young-onset colorectal cancer. Intest. Res. 2017, 15, 203. [Google Scholar] [CrossRef]
- Burnett-Hartman, A.N.; Lee, J.K.; Demb, J.; Gupta, S. An Update on the Epidemiology, Molecular Characterization, Diagnosis, and Screening Strategies for Early-Onset Colorectal Cancer. Gastroenterology 2021, 160, 1041–1049. [Google Scholar] [CrossRef]
- Segev, L.; Kalady, M.F.; Church, J.M. Left-Sided Dominance of Early-Onset Colorectal Cancers: A Rationale for Screening Flexible Sigmoidoscopy in the Young. Dis. Colon Rectum 2018, 61, 897–902. [Google Scholar] [CrossRef]
- Chang, D.T.; Pai, R.K.; Rybicki, L.A.; Dimaio, M.A.; Limaye, M.; Jayachandran, P.; Koong, A.C.; Kunz, P.A.; Fisher, G.A.; Ford, J.M.; et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod. Pathol. 2012, 25, 1128–1139. [Google Scholar] [CrossRef]
- REACCT Collaborative; Zaborowski, A.M.; Abdile, A.; Adamina, M.; Aigner, F.; d’Allens, L.; Allmer, C.; Álvarez, A.; Anula, R.; Andric, M.; et al. Characteristics of Early-Onset vs. Late-Onset Colorectal Cancer: A Review. JAMA Surg. 2021, 156, 865. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Maggard, M.A.; Liu, J.H.; Etzioni, D.A.; Livingston, E.H.; Ko, C.Y. Do Young Colon Cancer Patients Have Worse Outcomes? World J. Surg. 2004, 28, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Antelo, M.; Balaguer, F.; Shia, J.; Shen, Y.; Hur, K.; Moreira, L.; Cuatrecasas, M.; Bujanda, L.; Giraldez, M.D.; Takahashi, M.; et al. A High Degree of LINE-1 Hypomethylation Is a Unique Feature of Early-Onset Colorectal Cancer. PLoS ONE 2012, 7, e45357. [Google Scholar] [CrossRef] [PubMed]
- Boardman, L.A.; Johnson, R.A.; Petersen, G.M.; Oberg, A.L.; Kabat, B.F.; Slusser, J.P.; Wang, L.; Morlan, B.W.; French, A.J.; Smyrk, T.C.; et al. Higher Frequency of Diploidy in Young-Onset Microsatellite-Stable Colorectal Cancer. Clin. Cancer Res. 2007, 13, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Kirzin, S.; Marisa, L.; Guimbaud, R.; De Reynies, A.; Legrain, M.; Laurent-Puig, P.; Cordelier, P.; Pradère, B.; Bonnet, D.; Meggetto, F.; et al. Sporadic Early-Onset Colorectal Cancer Is a Specific Sub-Type of Cancer: A Morphological, Molecular and Genetics Study. PLoS ONE 2014, 9, e103159. [Google Scholar] [CrossRef] [PubMed]
- Jandova, J.; Xu, W.; Nfonsam, V. Sporadic early-onset colon cancer expresses unique molecular features. J. Surg. Res. 2016, 204, 251–260. [Google Scholar] [CrossRef]
- Mastrodomenico, L.; Piombino, C.; Riccò, B.; Barbieri, E.; Venturelli, M.; Piacentini, F.; Dominici, M.; Cortesi, L.; Toss, A. Personalized Systemic Therapies in Hereditary Cancer Syndromes. Genes 2023, 14, 684. [Google Scholar] [CrossRef] [PubMed]
- Lao, V.V.; Grady, W.M. Epigenetics and colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.A.E.; Khalid-de Bakker, C.A.J.; Smits, K.M.; Van Den Brandt, P.A.; Jonkers, D.; Ahuja, N.; Herman, J.G.; Weijenberg, M.P.; Van Engeland, M. The CpG island methylator phenotype in colorectal cancer: Progress and problems. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2012, 1825, 77–85. [Google Scholar] [CrossRef]
- Kaneda, A.; Yagi, K. Two groups of DNA methylation markers to classify colorectal cancer into three epigenotypes. Cancer Sci. 2011, 102, 18–24. [Google Scholar] [CrossRef]
- Ågesen, T.H.; Berg, M.; Clancy, T.; Thiis-Evensen, E.; Cekaite, L.; Lind, G.E.; Nesland, J.M.; Bakka, A.; Mala, T.; Hauss, H.J.; et al. CLC and IFNAR1 are differentially expressed and a global immunity score is distinct between early- and late-onset colorectal cancer. Genes Immun. 2011, 12, 653–662. [Google Scholar] [CrossRef]
- Hong, Y.; Ho, K.S.; Eu, K.W.; Cheah, P.Y. A Susceptibility Gene Set for Early Onset Colorectal Cancer That Integrates Diverse Signaling Pathways: Implication for Tumorigenesis. Clin. Cancer Res. 2007, 13, 1107–1114. [Google Scholar] [CrossRef]
- Nam, S.; Park, T. Pathway-Based Evaluation in Early Onset Colorectal Cancer Suggests Focal Adhesion and Immunosuppression along with Epithelial-Mesenchymal Transition. PLoS ONE 2012, 7, e31685. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-H.; Liau, J.-Y.; Lin, Y.-L.; Tseng, L.-H.; Lin, L.-I.; Yeh, K.-H.; Jeng, Y.-M. Frequent BRAF mutation in early-onset colorectal cancer in Taiwan: Association with distinct clinicopathological and molecular features and poor clinical outcome. J. Clin. Pathol. 2016, 69, 319–325. [Google Scholar] [CrossRef]
- Alvarez, K.; Cassana, A.; De La Fuente, M.; Canales, T.; Abedrapo, M.; López-Köstner, F. Clinical, Pathological and Molecular Characteristics of Chilean Patients with Early-, Intermediate- and Late-Onset Colorectal Cancer. Cells 2021, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, Y.; Zhang, J.; Qi, C.; Liu, D.; Wang, Z.; Li, Y.; Ji, C.; Li, J.; Lin, X.; et al. Germline Profiling and Molecular Characterization of Early Onset Metastatic Colorectal Cancer. Front. Oncol. 2020, 10, 568911. [Google Scholar] [CrossRef]
- Tunca, B.; Tezcan, G.; Cecener, G.; Egeli, U.; Zorluoglu, A.; Yilmazlar, T.; Ak, S.; Yerci, O.; Ozturk, E.; Umut, G.; et al. Overexpression of CK20, MAP3K8 and EIF5A correlates with poor prognosis in early-onset colorectal cancer patients. J. Cancer Res. Clin. Oncol. 2013, 139, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Tariq, K.; Rafiq, S.; Raheem, A.; Ahmed, R.; Shabbir-Moosajee, M.; Ghias, K. Sporadic Early Onset Colorectal Cancer in Pakistan: A Case-Control Analysis of Microsatellite Instability. Asian Pac. J. Cancer Prev. 2016, 17, 2587. [Google Scholar] [PubMed]
- Southey, M.C.; Jenkins, M.A.; Mead, L.; Whitty, J.; Trivett, M.; Tesoriero, A.A.; Smith, L.D.; Jennings, K.; Grubb, G.; Royce, S.G.; et al. Use of Molecular Tumor Characteristics to Prioritize Mismatch Repair Gene Testing in Early-Onset Colorectal Cancer. J. Clin. Oncol. 2005, 23, 6524–6532. [Google Scholar] [CrossRef]
- Losi, L.; Di Gregorio, C.; Pedroni, M.; Ponti, G.; Roncucci, L.; Scarselli, A.; Genuardi, M.; Baglioni, S.; Marino, M.; Rossi, G.; et al. Molecular Genetic Alterations and Clinical Features in Early-Onset Colorectal Carcinomas and Their Role for the Recognition of Hereditary Cancer Syndromes. Off. J. Am. Coll. Gastroenterol.—ACG 2005, 100, 2280–2287. [Google Scholar] [CrossRef]
- Chan, T.L.; Curtis, L.C.; Leung, S.Y.; Farrington, S.M.; Ho, J.W.; Chan, A.S.; Lam, P.W.; Tse, C.W.; Dunlop, M.G.; Wyllie, A.H.; et al. Early-onset colorectal cancer with stable microsatellite DNA and near-diploid chromosomes. Oncogene 2001, 20, 4871–4876. [Google Scholar] [CrossRef]
- Banerjea, A.; Hands, R.E.; Powar, M.P.; Bustin, S.A.; Dorudi, S. Microsatellite and chromosomal stable colorectal cancers demonstrate poor immunogenicity and early disease recurrence. Color. Dis. 2009, 11, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Boardman, L.A.; Johnson, R.A.; Viker, K.B.; Hafner, K.A.; Jenkins, R.B.; Riegert-Johnson, D.L.; Smyrk, T.C.; Litzelman, K.; Seo, S.; Gangnon, R.E.; et al. Correlation of Chromosomal Instability, Telomere Length and Telomere Maintenance in Microsatellite Stable Rectal Cancer: A Molecular Subclass of Rectal Cancer. PLoS ONE 2013, 8, e80015. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Umar, A.; Polyak, K.; Graff, J.R.; Ahuja, N.; Issa, J.-P.J.; Markowitz, S.; Willson, J.K.V.; Hamilton, S.R.; Kinzler, K.W.; et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc. Natl. Acad. Sci. USA 1998, 95, 6870–6875. [Google Scholar] [CrossRef]
- Weinberg, B.A.; Marshall, J.L. Colon Cancer in Young Adults: Trends and Their Implications. Curr. Oncol. Rep. 2019, 21, 3. [Google Scholar] [CrossRef]
- Álvaro, E.; Cano, J.M.; García, J.L.; Brandáriz, L.; Olmedillas-López, S.; Arriba, M.; Rueda, D.; Rodríguez, Y.; Cañete, Á.; Arribas, J.; et al. Clinical and Molecular Comparative Study of Colorectal Cancer Based on Age-of-onset and Tumor Location: Two Main Criteria for Subclassifying Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 968. [Google Scholar] [CrossRef]
- Ogino, S.; Goel, A. Molecular Classification and Correlates in Colorectal Cancer. J. Mol. Diagn. 2008, 10, 13–27. [Google Scholar] [CrossRef]
- Salem, M.E.; Weinberg, B.A.; Xiu, J.; El-Deiry, W.S.; Hwang, J.J.; Gatalica, Z.; Philip, P.A.; Shields, A.F.; Lenz, H.-J.; Marshall, J.L. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 2017, 8, 86356–86368. [Google Scholar] [CrossRef]
- O’Sullivan, D.E.; Cheung, W.Y.; Boyne, D.J.; Jarada, T.N.; Tang, P.A.; Gill, S.; Hilsden, R.J.; Brenner, D.R. Treatment patterns and survival outcomes of early-onset colorectal cancer patients in Alberta, Canada: A population-based study. Cancer Treat. Res. Commun. 2022, 32, 100585. [Google Scholar] [CrossRef]
- Soto, J.; Gutiérrez Alonso, N.; Bringas Beranek, M.; Catoya Villa, J.L.; Gutierrez Ortiz de la Tabla, A.; López Jiménez, C.; Alva Bianchi, M.; Jiménez Rodríguez, R.; Martín Lozano, R.; Arregui Valles, M.; et al. Analysis of survival trends, clinical, and molecular characteristics of patients with early-onset colorectal cancer (EOCRC). J. Clin. Oncol. 2022, 40, 59. [Google Scholar] [CrossRef]
- Kim, T.J.; Kim, E.R.; Hong, S.N.; Chang, D.K.; Kim, Y.-H. Long-Term Outcome and Prognostic Factors of Sporadic Colorectal Cancer in Young Patients: A Large Institutional-Based Retrospective Study. Medicine 2016, 95, e3641. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008, 371, 1007–1016. [Google Scholar] [CrossRef]
- Jácome, A.A.; Vreeland, T.J.; Johnson, B.; Kawaguchi, Y.; Wei, S.H.; Nancy You, Y.; Vilar, E.; Vauthey, J.-N.; Eng, C. The prognostic impact of RAS on overall survival following liver resection in early versus late-onset colorectal cancer patients. Br. J. Cancer 2021, 124, 797–804. [Google Scholar] [CrossRef]
- Amado, R.G.; Wolf, M.; Peeters, M.; Van Cutsem, E.; Siena, S.; Freeman, D.J.; Juan, T.; Sikorski, R.; Suggs, S.; Radinsky, R.; et al. Wild-Type KRAS Is Required for Panitumumab Efficacy in Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2008, 26, 1626–1634. [Google Scholar] [CrossRef]
- Karapetis, C.S.; Khambata-Ford, S.; Jonker, D.J.; O’Callaghan, C.J.; Tu, D.; Tebbutt, N.C.; Simes, R.J.; Chalchal, H.; Shapiro, J.D.; Robitaille, S.; et al. K-ras Mutations and Benefit from Cetuximab in Advanced Colorectal Cancer. N. Engl. J. Med. 2008, 359, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.-Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, Phase III Trial of Panitumumab With Infusional Fluorouracil, Leucovorin, and Oxaliplatin (FOLFOX4) Versus FOLFOX4 Alone As First-Line Treatment in Patients With Previously Untreated Metastatic Colorectal Cancer: The PRIME Study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Kopetz, S.; Tie, J.; Gibbs, P.; Jiang, Z.-Q.; Lieu, C.H.; Agarwal, A.; Maru, D.M.; Sieber, O.; Desai, J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer: Metastatic Pattern in BRAF Mutant CRC. Cancer 2011, 117, 4623–4632. [Google Scholar] [CrossRef]
- Arnold, D.; Lueza, B.; Douillard, J.-Y.; Peeters, M.; Lenz, H.-J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.-P.; Tabernero, J.; et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef]
- Raimondi, A.; Randon, G.; Prisciandaro, M.; Pagani, F.; Lonardi, S.; Antoniotti, C.; Bozzarelli, S.; Sartore-Bianchi, A.; Tampellini, M.; Fanchini, L.; et al. Early onset metastatic colorectal cancer in patients receiving panitumumab-based upfront strategy: Overall and sex-specific outcomes in the Valentino trial. Int. J. Cancer 2022, 151, 1760–1769. [Google Scholar] [CrossRef]
- Falcone, A.; Ricci, S.; Brunetti, I.; Pfanner, E.; Allegrini, G.; Barbara, C.; Crinò, L.; Benedetti, G.; Evangelista, W.; Fanchini, L.; et al. Phase III Trial of Infusional Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan (FOLFOXIRI) Compared With Infusional Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) As First-Line Treatment for Metastatic Colorectal Cancer: The Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007, 25, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Antoniotti, C.; Stein, A.; Bendell, J.; Gruenberger, T.; Rossini, D.; Masi, G.; Ongaro, E.; Hurwitz, H.; Falcone, A.; et al. Individual Patient Data Meta-Analysis of FOLFOXIRI Plus Bevacizumab Versus Doublets Plus Bevacizumab as Initial Therapy of Unresectable Metastatic Colorectal Cancer. J. Clin. Oncol. 2020, 38, 3314–3324. [Google Scholar] [CrossRef] [PubMed]
- Moretto, R.; Elliott, A.; Rossini, D.; Intini, R.; Conca, V.; Pietrantonio, F.; Sartore-Bianchi, A.; Antoniotti, C.; Rasola, C.; Scartozzi, M.; et al. Benefit from upfront FOLFOXIRI and bevacizumab in BRAFV600E-mutated metastatic colorectal cancer patients: Does primary tumour location matter? Br. J. Cancer 2022, 127, 957–967. [Google Scholar] [CrossRef]
- Jácome, A.A.; Kee, B.; Fogelman, D.; Dasari, A.; Shureiqi, I.; Raghav, K.; Morris, V.; Johnson, B.; Overman, M.; Wolff, R.; et al. FOLFOXIRI Versus Doublet Regimens in Right-Sided Metastatic Colorectal Cancer: Focus on Subsequent Therapies and Impact on Overall Survival. Clin. Color. Cancer 2020, 19, 248–255.e6. [Google Scholar] [CrossRef] [PubMed]
- Antoniotti, C.; Germani, M.M.; Rossini, D.; Lonardi, S.; Pietrantonio, F.; Santini, D.; Marmorino, F.; Allegrini, G.; Daniel, F.; Raimondi, A.; et al. FOLFOXIRI and bevacizumab in patients with early-onset metastatic colorectal cancer. A pooled analysis of TRIBE and TRIBE2 studies. Eur. J. Cancer 2022, 167, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Grothey, A.; Cutsem, E.V.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Tabernero, J.; Taieb, J.; Prager, G.W.; Ciardiello, F.; Fakih, M.; Leger, C.; Fougeray, R.; Amellal, N.; Van Cutsem, E. Trifluridine/tipiracil plus bevacizumab for third-line management of metastatic colorectal cancer: SUNLIGHT study design. Future Oncol. 2021, 17, 1977–1985. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Lieu, C.H.; Golemis, E.A.; Serebriiskii, I.G.; Newberg, J.; Hemmerich, A.; Connelly, C.; Messersmith, W.A.; Eng, C.; Eckhardt, S.G.; Frampton, G.; et al. Comprehensive Genomic Landscapes in Early and Later Onset Colorectal Cancer. Clin. Cancer Res. 2019, 25, 5852–5858. [Google Scholar] [CrossRef]
| Risk Factors | |
|---|---|
| Hereditary factors | Lynch syndrome |
| Adenomatous polyposis syndromes | |
| Li-Fraumeni syndrome | |
| Cystic fibrosis | |
| Nonhereditary risk factors | Obesity |
| Type II diabetes mellitus | |
| Western diet | |
| Being sedentary | |
| Alcohol and tobacco use | |
| Chronic inflammation | |
| Intestinal dysbiosis | |
| Pollution |
| Differences | EOCRC | LOCRC |
|---|---|---|
| Stage at diagnosis | Advanced (III–IV) | Less advanced |
| Tumor location | Left | Right |
| Risk of synchronous or metachronous tumors | Higher | Lower |
| Histology | Mucinous and signet ring cell | |
| Histopathological features | Perineural, lymphovascular, and venous invasion |
| mEOCRC | mLOCRC | |
|---|---|---|
| PIK3CA | ↑ | |
| P53 | ↑ | |
| Wnt | ↑ | |
| APC | ↑ | |
| SMAD4 | ↑ | |
| DCC | ↑ | |
| Codons 12 and 13 of KRAS | = | = |
| NRAS | ? | ? |
| KRAS | ? | ? |
| BRAF V600E | ? | ? |
| BRAF | ? | ? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medici, B.; Riccò, B.; Caffari, E.; Zaniboni, S.; Salati, M.; Spallanzani, A.; Garajovà, I.; Benatti, S.; Chiavelli, C.; Dominici, M.; et al. Early Onset Metastatic Colorectal Cancer: Current Insights and Clinical Management of a Rising Condition. Cancers 2023, 15, 3509. https://doi.org/10.3390/cancers15133509
Medici B, Riccò B, Caffari E, Zaniboni S, Salati M, Spallanzani A, Garajovà I, Benatti S, Chiavelli C, Dominici M, et al. Early Onset Metastatic Colorectal Cancer: Current Insights and Clinical Management of a Rising Condition. Cancers. 2023; 15(13):3509. https://doi.org/10.3390/cancers15133509
Chicago/Turabian StyleMedici, Bianca, Beatrice Riccò, Eugenia Caffari, Silvia Zaniboni, Massimiliano Salati, Andrea Spallanzani, Ingrid Garajovà, Stefania Benatti, Chiara Chiavelli, Massimo Dominici, and et al. 2023. "Early Onset Metastatic Colorectal Cancer: Current Insights and Clinical Management of a Rising Condition" Cancers 15, no. 13: 3509. https://doi.org/10.3390/cancers15133509
APA StyleMedici, B., Riccò, B., Caffari, E., Zaniboni, S., Salati, M., Spallanzani, A., Garajovà, I., Benatti, S., Chiavelli, C., Dominici, M., & Gelsomino, F. (2023). Early Onset Metastatic Colorectal Cancer: Current Insights and Clinical Management of a Rising Condition. Cancers, 15(13), 3509. https://doi.org/10.3390/cancers15133509