Medical Needs and Therapeutic Options for Melanoma Patients Resistant to Anti-PD-1-Directed Immune Checkpoint Inhibition
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
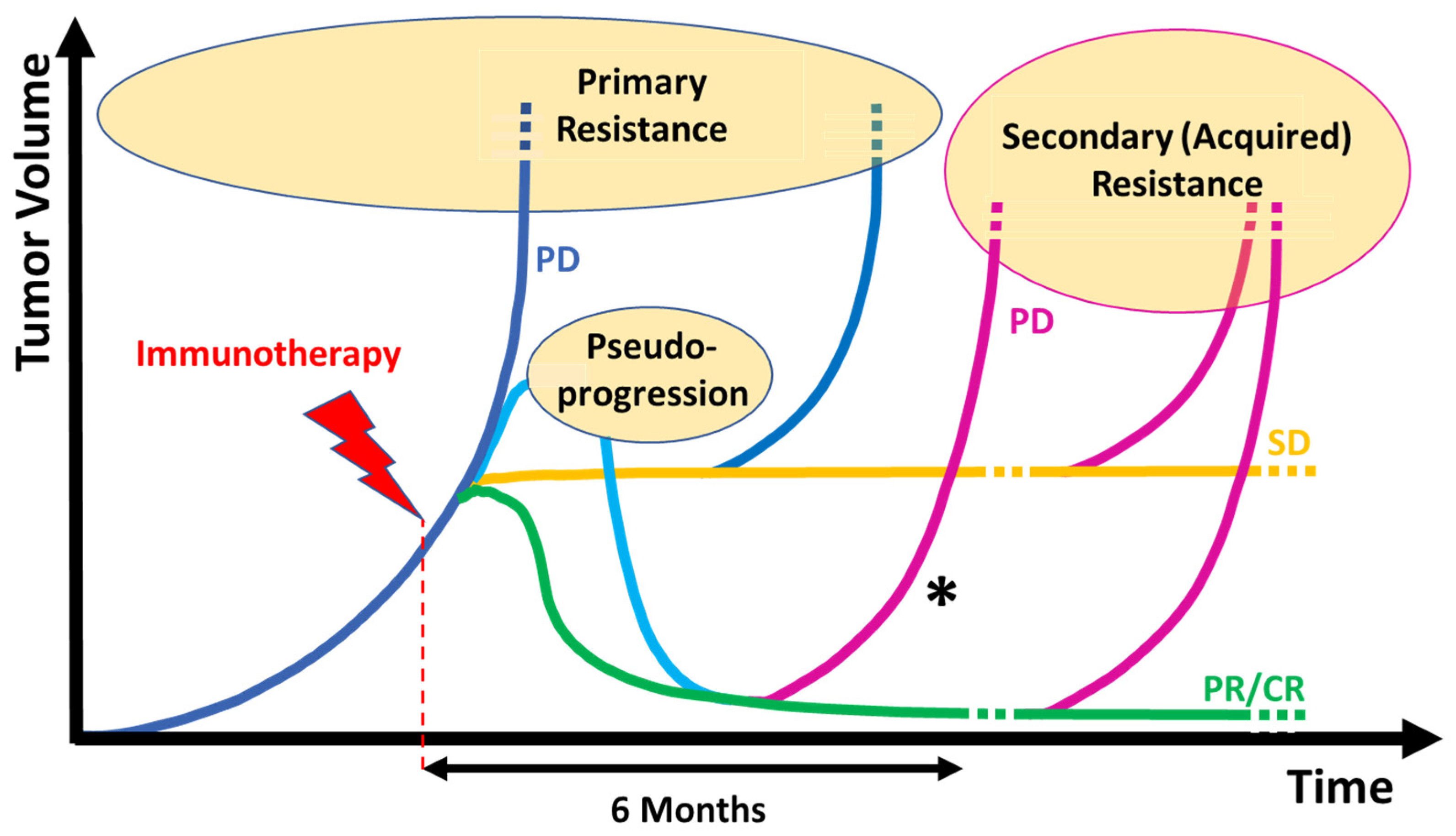
3. Definitions and Patterns of Response and Resistance to Immunotherapy in Melanoma
4. Potential Mechanisms of Resistance to Systemic ICI Therapy
5. Resistance in the Metastatic Setting
5.1. Systemic Approaches
5.2. Local Approaches
5.3. Rechallenge
6. Resistance in Melanoma Brain Metastases
7. Resistance in the Adjuvant Setting
8. Lessons from the Neoadjuvant Studies
9. PD-1/PD-L1 Resistant Melanoma: Limitations, Perspectives, Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Michielin, O.; van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; Keilholz, U.; The ESMO Guidelines Committee. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dreno, B.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment—Update 2019. Eur. J. Cancer 2020, 126, 159–177. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Sosman, J.A.; Atkins, M.B.; Leming, P.D.; et al. Five-Year Survival and Correlates Among Patients with Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated with Nivolumab. JAMA Oncol. 2019, 5, 1411–1420. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Ugurel, S.; Rohmel, J.; Ascierto, P.A.; Becker, J.C.; Flaherty, K.T.; Grob, J.J.; Hauschild, A.; Larkin, J.; Livingstone, E.; Long, G.V.; et al. Survival of patients with advanced metastatic melanoma: The impact of MAP kinase pathway inhibition and immune checkpoint inhibition—Update 2019. Eur. J. Cancer 2020, 130, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Hodi, F.S.; Lipson, E.J.; Schadendorf, D.; Ascierto, P.A.; Matamala, L.; Salman, P.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.; et al. Relatlimab and nivolumab versus nivolumab in previously untreated metastatic or unresectable melanoma: Overall survival and response rates from RELATIVITY-047 (CA224-047). J. Clin. Oncol. 2022, 40, 360385. [Google Scholar] [CrossRef]
- Long, G.V.; Luke, J.J.; Khattak, M.; de la Cruz Merino, L.; Del Vecchio, M.; Rutkowski, P.; Spagnolo, F.; Mackiewicz, J.; Chiarion-Sileni, V.; Kirkwood, J.M.; et al. Distant metastasis-free survival with pembrolizumab versus placebo as adjuvant therapy in stage IIB or IIC melanoma: The phase 3 KEYNOTE-716 study. J. Clin. Oncol. 2022, 40, LBA9500. [Google Scholar] [CrossRef]
- Long, G.V.D.V.; Weber, J.M.; Hoeller, C.; Grob, J.J.; Mohr, P.; Grabbe, S.; Dutriaux, C.; Chiarion-Sileni, V.; Mackiewicz, J. Adjuvant therapy with nivolumab versus placebo in patients with resected stage IIB/C melanoma (CheckMate 76K). In Proceedings of the SMR Meeting 2022, Edinburgh, UK, 17–20 October 2022. [Google Scholar]
- Grossmann, K.F.; Othus, M.; Patel, S.P.; Tarhini, A.A.; Sondak, V.K.; Knopp, M.V.; Petrella, T.M.; Truong, T.G.; Khushalani, N.I.; Cohen, J.V.; et al. Adjuvant Pembrolizumab versus IFNalpha2b or Ipilimumab in Resected High-Risk Melanoma. Cancer Discov. 2022, 12, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Del Vecchio, M.; Mandala, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant Nivolumab Versus Ipilimumab in Resected Stage III/IV Melanoma: 5-Year Efficacy and Biomarker Results from CheckMate 238. Clin. Cancer Res. 2023, OF1. [Google Scholar] [CrossRef]
- Livingstone, E.; Zimmer, L.; Hassel, J.C.; Fluck, M.; Eigentler, T.K.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant nivolumab plus ipilimumab or nivolumab alone versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): Final results of a randomised, double-blind, phase 2 trial. Lancet 2022, 400, 1117–1129. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Meshcheryakov, A.; Khattak, A.; et al. Five-Year Analysis of Adjuvant Pembrolizumab or Placebo in Stage III Melanoma. NEJM Evid. 2022, 1, EVIDoa2200214. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Gajewski, T. Mechanisms of required resistance. In Proceedings of the ESMO Annual Meeting, Lugano, Switzerland, 9–13 September 2022. [Google Scholar]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litiere, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Galldiks, N.; Kocher, M.; Ceccon, G.; Werner, J.M.; Brunn, A.; Deckert, M.; Pope, W.B.; Soffietti, R.; Le Rhun, E.; Weller, M.; et al. Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: Response, progression, and pseudoprogression. Neuro Oncol. 2020, 22, 17–30. [Google Scholar] [CrossRef]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, K.W.; Pyo, J.; Suh, C.H.; Yoon, S.; Hatabu, H.; Nishino, M. Incidence of Pseudoprogression during Immune Checkpoint Inhibitor Therapy for Solid Tumors: A Systematic Review and Meta-Analysis. Radiology 2020, 297, 87–96. [Google Scholar] [CrossRef]
- Borcoman, E.; Kanjanapan, Y.; Champiat, S.; Kato, S.; Servois, V.; Kurzrock, R.; Goel, S.; Bedard, P.; Le Tourneau, C. Novel patterns of response under immunotherapy. Ann. Oncol. 2019, 30, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Tawbi, H.A.; Ascierto, M.L.; Bowden, M.; Callahan, M.K.; Cha, E.; Chen, H.X.; Drake, C.G.; Feltquate, D.M.; Ferris, R.L.; et al. Defining tumor resistance to PD-1 pathway blockade: Recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J. Immunother. Cancer 2020, 8, e000398. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Hochmair, M.; Haug, A.R.; Schwabel, B.; Kifjak, D.; Wadsak, W.; Fuereder, T.; Fabikan, H.; Fazekas, A.; Schwab, S.; et al. Comparison of RECIST, iRECIST, and PERCIST for the Evaluation of Response to PD-1/PD-L1 Blockade Therapy in Patients with Non-Small Cell Lung Cancer. Clin. Nucl. Med. 2019, 44, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, M.T.; Messina, J.L.; Stein, J.E.; Xu, X.; Amaria, R.N.; Blank, C.U.; van de Wiel, B.A.; Ferguson, P.M.; Rawson, R.V.; Ross, M.I.; et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann. Oncol. 2018, 29, 1861–1868. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Jacquelot, N.; Yamazaki, T.; Roberti, M.P.; Duong, C.P.M.; Andrews, M.C.; Verlingue, L.; Ferrere, G.; Becharef, S.; Vetizou, M.; Daillere, R.; et al. Sustained Type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell. Res. 2019, 29, 846–861. [Google Scholar] [CrossRef]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef]
- Zou, J.; Du, K.; Li, S.; Lu, L.; Mei, J.; Lin, W.; Deng, M.; Wei, W.; Guo, R. Glutamine Metabolism Regulators Associated with Cancer Development and the Tumor Microenvironment: A Pan-Cancer Multi-Omics Analysis. Genes 2021, 12, 1305. [Google Scholar] [CrossRef]
- Varghese, S.; Pramanik, S.; Williams, L.J.; Hodges, H.R.; Hudgens, C.W.; Fischer, G.M.; Luo, C.K.; Knighton, B.; Tan, L.; Lorenzi, P.L.; et al. The Glutaminase Inhibitor CB-839 (Telaglenastat) Enhances the Antimelanoma Activity of T-Cell-Mediated Immunotherapies. Mol. Cancer Ther. 2021, 20, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.A.; Luke, J.J.; Zha, Y.; Segal, J.P.; Ritterhouse, L.L.; Spranger, S.; Matijevich, K.; Gajewski, T.F. Secondary resistance to immunotherapy associated with beta-catenin pathway activation or PTEN loss in metastatic melanoma. J. Immunother. Cancer 2019, 7, 295. [Google Scholar] [CrossRef]
- Lee, J.H.; Shklovskaya, E.; Lim, S.Y.; Carlino, M.S.; Menzies, A.M.; Stewart, A.; Pedersen, B.; Irvine, M.; Alavi, S.; Yang, J.Y.H.; et al. Transcriptional downregulation of MHC class I and melanoma de- differentiation in resistance to PD-1 inhibition. Nat. Commun. 2020, 11, 1897. [Google Scholar] [CrossRef]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef]
- Aldea, M.; Andre, F.; Marabelle, A.; Dogan, S.; Barlesi, F.; Soria, J.C. Overcoming Resistance to Tumor-Targeted and Immune-Targeted Therapies. Cancer Discov. 2021, 11, 874–899. [Google Scholar] [CrossRef] [PubMed]
- Wulfken, L.M.; Becker, J.C.; Hayajneh, R.; Wagner, A.D.; Schaper-Gerhardt, K.; Flatt, N.; Grimmelmann, I.; Gutzmer, R. Case Report: Sustained Remission Due to PD-1-Inhibition in a Metastatic Melanoma Patient with Depleted B Cells. Front. Immunol. 2021, 12, 733961. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Haanen, J.B.; Ribas, A.; Schumacher, T.N. Cancer Immunology. The “cancer immunogram”. Science 2016, 352, 658–660. [Google Scholar] [CrossRef]
- Wolchok, J.; Chiarion-Sileni, V.; Gonzalez, R.G.; Grob, J.J.; Rutkowski, P.; Lao, C.; Cowey, C.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J. Clin. Oncol. 2021, 39, 9506. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Rutkowski, P.; Hassel, J.C.; McNeil, C.M.; Kalinka, E.A.; et al. Five-Year Outcomes with Nivolumab in Patients with Wild-Type BRAF Advanced Melanoma. J. Clin. Oncol. 2020, 38, 3937–3946. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Hodi, F.S.; Algazi, A.P.; Hamid, O.; Lao, C.D.; Moschos, S.J.; Atkins, M.B.; Lewis, K.; Postow, M.A.; et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): Final results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 1692–1704. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Guminski, A.D.; Sandhu, S.K.; Brown, M.P.; Gonzalez, M.; Scolyer, R.A.; Emmett, L.; McArthur, G.A.; et al. Five-year overall survival from the anti-PD1 brain collaboration (ABC Study): Randomized phase 2 study of nivolumab (nivo) or nivo+ipilimumab (ipi) in patients (pts) with melanoma brain metastases (mets). J. Clin. Oncol. 2021, 39, 9508. [Google Scholar] [CrossRef]
- Weide, B.M.A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; Di Giacomo, A.M.; Brenner, N. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. [Google Scholar] [CrossRef]
- Madonna, G.; Masucci, G.V.; Capone, M.; Mallardo, D.; Grimaldi, A.M.; Simeone, E.; Vanella, V.; Festino, L.; Palla, M.; Scarpato, L.; et al. Clinical Categorization Algorithm (CLICAL) and Machine Learning Approach (SRF-CLICAL) to Predict Clinical Benefit to Immunotherapy in Metastatic Melanoma Patients: Real-World Evidence from the Istituto Nazionale Tumori IRCCS Fondazione Pascale, Napoli, Italy. Cancers 2021, 13, 4164. [Google Scholar] [CrossRef]
- Fattore, L.; Ruggiero, C.F.; Liguoro, D.; Castaldo, V.; Catizone, A.; Ciliberto, G.; Mancini, R. The Promise of Liquid Biopsy to Predict Response to Immunotherapy in Metastatic Melanoma. Front. Oncol. 2021, 11, 645069. [Google Scholar] [CrossRef]
- Weber, J.G.G.; Kudchadkar, R.; Yu, B.; Cheng, P.; Martinez, A.J.; Kroeger, J.; Richards, A.; McCormick, L.; Moberg, V.; Cronin, H. Phase I/II Study of Metastatic Melanoma Patients Treated with Nivolumab Who Had Progressed after Ipilimumab. Cancer Immunol. Res. 2016, 4, 345–353. [Google Scholar] [CrossRef]
- Ugurel, S.; Schadendorf, D.; Horny, K.; Sucker, A.; Schramm, S.; Utikal, J.; Pfohler, C.; Herbst, R.; Schilling, B.; Blank, C.; et al. Elevated baseline serum PD-1 or PD-L1 predicts poor outcome of PD-1 inhibition therapy in metastatic melanoma. Ann. Oncol. 2020, 31, 144–152. [Google Scholar] [CrossRef]
- Machiraju, D.; Wiecken, M.; Lang, N.; Hulsmeyer, I.; Roth, J.; Schank, T.E.; Eurich, R.; Halama, N.; Enk, A.; Hassel, J.C. Soluble immune checkpoints and T-cell subsets in blood as biomarkers for resistance to immunotherapy in melanoma patients. Oncoimmunology 2021, 10, 1926762. [Google Scholar] [CrossRef]
- Hassel, J.C.; Zucht, H.-D.; Mangana, J.; Dummer, R.; Pföhler, C.; Wistuba-Hamprecht, K.; Weide, B.; Hakim-Meibodi, L.E.; Meier, F.E.; Schulz, C.; et al. Autoantibodies as predictors for survival and immune-related adverse events in checkpoint inhibition therapy of metastasized melanoma. J. Clin. Oncol. 2020, 38, 10011. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutierrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.A.; Ribas, A.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined with Pembrolizumab for Advanced Melanoma. J. Clin. Oncol. 2022, 41, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Gogas, H.J.; Sandhu, S.K.; Long, G.V.; Ascierto, P.A.; Larkin, J.; Sznol, M.; Franke, F.A.; Ciuleanu, T.E.; Couselo, E.M.; et al. 785O—PIVOT IO 001: First disclosure of efficacy and safety of bempegaldesleukin (BEMPEG) plus nivolumab (NIVO) vs. NIVO monotherapy in advanced melanoma (MEL). Ann. Oncol. 2022, 33, S901. [Google Scholar] [CrossRef]
- Dummer, R.; Long, G.V.; Robert, C.; Tawbi, H.A.; Flaherty, K.T.; Ascierto, P.A.; Nathan, P.D.; Rutkowski, P.; Leonov, O.; Dutriaux, C.; et al. Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600-Mutant Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2022, 40, 1428–1438. [Google Scholar] [CrossRef]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1835–1844. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Overall survival with first-line atezolizumab in combination with vemurafenib and cobimetinib in BRAF(V600) mutation-positive advanced melanoma (IMspire150): Second interim analysis of a multicentre, randomised, phase 3 study. Lancet Oncol. 2022, 24, 33–44. [Google Scholar] [CrossRef]
- Sarnaik, A.; Khushalani, N.I.; Chesney, J.A.; Lewis, K.D.; Medina, T.M.; Kluger, H.M.; Thomas, S.S.; Domingo Musibay, E.; Pavlick, A.C.; Whitman, E.D.; et al. Long-term follow up of lifileucel (LN-144) cryopreserved autologous tumor infiltrating lymphocyte therapy in patients with advanced melanoma progressed on multiple prior therapies. J. Clin. Oncol. 2020, 38, 10006. [Google Scholar] [CrossRef]
- Arance, A.; de la Cruz-Merino, L.; Petrella, T.M.; Jamal, R.; Ny, L.; Carneiro, A.; Berrocal, A.; Marquez-Rodas, I.; Spreafico, A.; Atkinson, V.; et al. Phase II LEAP-004 Study of Lenvatinib Plus Pembrolizumab for Melanoma with Confirmed Progression on a Programmed Cell Death Protein-1 or Programmed Death Ligand 1 Inhibitor Given as Monotherapy or in Combination. J. Clin. Oncol. 2023, 41, 75–85. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Olson, D.; Luke, J.J.; Poklepovic, A.S.; Bajaj, M.; Higgs, E.; Carll, T.C.; Labadie, B.; Krausz, T.; Zha, Y.; Karrison, T.; et al. Significant antitumor activity for low-dose ipilimumab (IPI) with pembrolizumab (PEMBRO) immediately following progression on PD1 Ab in melanoma (MEL) in a phase II trial. J. Clin. Oncol. 2020, 38, 10004. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Bono, P.; Bhatia, S.; Melero, I.; Nyakas, M.S.; Svane, I.M.; Larkin, J.; Gomez-Roca, C.; Schadendorf, D.; Dummer, R.; et al. Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma who progressed during prior anti–PD-1/PD-L1 therapy (mel prior IO) in all-comer and biomarker-enriched populations. Ann. Oncol. 2017, 28, v611–v612. [Google Scholar]
- Amin, A.; Milhem, M.M.; Long, G.V.; Hoimes, C.J.; Medina, T.M.; Conry, R.M.; Lao, C.D.; Daniels, G.A.; Reddy, S.A.; Andtbacka, R.H.I.; et al. Phase 1b/2, open label, multicenter, study of the combination of SD-101 and pembrolizumab in patients with advanced/metastatic melanoma resistant to anti-PD-1/PD-L1 therapy. J. Clin. Oncol. 2019, 37. [Google Scholar] [CrossRef]
- Amaria, R.N.; Haymaker, C.L.; Forget, M.-A.; Bassett, R.; Cormier, J.N.; Davies, M.A.; Diab, A.; Gershenwald, J.E.; Glitza, I.C.; Lee, J.E.; et al. Lymphodepletion (LD), tumor-infiltrating lymphocytes (TIL) and high (HD-IL2) versus low-dose (LD-IL2) IL-2 followed by pembrolizumab (pembro) in patients (pts) with metastatic melanoma (MM). J. Clin. Oncol. 2019, 37, 9543. [Google Scholar] [CrossRef]
- Zager, J.S.; Sarnaik, A.S.; Pilon-Thomas, S.; Beatty, M.; Han, D.; Lu, G.; Agarwala, S.S.; Ross, M.; Shirai, K.; Essner, R.; et al. 1123P A phase Ib study of rose bengal disodium and anti-PD-1 in metastatic cutaneous melanoma: Initial results in patients refractory to checkpoint blockade. Ann. Oncol. 2020, 31, S755–S756. [Google Scholar] [CrossRef]
- Hassel, J.C.; Berking, C.; Schlaak, M.; Eigentler, T.; Gutzmer, R.; Ascierto, P.A.; Schilling, B.; Hamm, S.; Hermann, F.; Reimann, P.G.; et al. Results from the phase Ib of the SENSITIZE trial combining domatinostat with pembrolizumab in advanced melanoma patients refractory to prior checkpoint inhibitor therapy. J. Clin. Oncol. 2021, 39, 9545. [Google Scholar] [CrossRef]
- McCarter, M.; Tobin, R.P.; Cogswell, D.T.; Vorwald, V.M.; Davis, D.; Van Gulick, R.J.; Couts, K.L.; Jordan, K.R.; Nuanes, V.; Gao, D.; et al. Pembrolizumab and all-trans retinoic acid combination treatment of advanced melanoma. J. Clin. Oncol. 2021, 39, 9536. [Google Scholar] [CrossRef]
- Loquai, C.; Hassel, J.C.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Gold, M.; Schwarck-Kokarakis, D.; Attig, S.; Cuk, K.; Vogler, I.; et al. A shared tumor-antigen RNA-lipoplex vaccine with/without anti-PD1 in patients with checkpoint-inhibition experienced melanoma. J. Clin. Oncol. 2020, 38, 3136. [Google Scholar] [CrossRef]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Haymaker, C.; Johnson, D.H.; Murthy, R.; Bentebibel, S.E.; Uemura, M.I.; Hudgens, C.W.; Safa, H.; James, M.; Andtbacka, R.H.I.; Johnson, D.B.; et al. Tilsotolimod with Ipilimumab Drives Tumor Responses in Anti-PD-1 Refractory Melanoma. Cancer Discov. 2021, 11, 1996–2013. [Google Scholar] [CrossRef] [PubMed]
- Idera Pharmaceuticals. Idera Pharmaceuticals Announces Results from Illuminate-301 Trial of Tilsotolimod + Ipilimumab in Anti-Pd-1 Refractory Advanced Melanoma; Idera Pharmaceuticals, Inc.: Exton, PA, USA, 2021. [Google Scholar]
- Pires da Silva, I.; Ahmed, T.; Reijers, I.L.M.; Weppler, A.M.; Betof Warner, A.; Patrinely, J.R.; Serra-Bellver, P.; Allayous, C.; Mangana, J.; Nguyen, K.; et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: A multicentre, retrospective, cohort study. Lancet Oncol. 2021, 22, 836–847. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Borch, T.H.; van den Berg, J.H.; Met, O.; Kessels, R.; Geukes Foppen, M.H.; Stoltenborg Granhoj, J.; Nuijen, B.; Nijenhuis, C.; Jedema, I.; et al. Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2022, 387, 2113–2125. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134. J. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef]
- Kabiljo, J.; Harpain, F.; Carotta, S.; Bergmann, M. Radiotherapy as a Backbone for Novel Concepts in Cancer Immunotherapy. Cancers 2019, 12, 79. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; de Souza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef]
- Versluis, J.M.; Hendriks, A.M.; Weppler, A.M.; Brown, L.J.; de Joode, K.; Suijkerbuijk, K.P.M.; Zimmer, L.; Kapiteijn, E.W.; Allayous, C.; Johnson, D.B.; et al. The role of local therapy in the treatment of solitary melanoma progression on immune checkpoint inhibition: A multicentre retrospective analysis. Eur. J. Cancer 2021, 151, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Zaremba, A.; Eggermont, A.M.M.; Robert, C.; Dummer, R.; Ugurel, S.; Livingstone, E.; Ascierto, P.A.; Long, G.V.; Schadendorf, D.; Zimmer, L. The concepts of rechallenge and retreatment with immune checkpoint blockade in melanoma patients. Eur. J. Cancer 2021, 155, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.C.; Carlino, M.S.; McNeil, C.; Rebas, A.; Grob, J.J.; Schachter, J.; Nyakas, M.; Kee, D.; Petrella, T.; Blaustein, A.; et al. 7-year Follow-up of KEYNOTE-006: Pembrolizumab (pembro) Versus Ipilimumab (ipi) in Advanced Melanoma. Pigment. Cell. Melanoma Res. 2022, 35, 97–184. [Google Scholar]
- Hassel, J.C. 5-year results for pembrolizumab treatment of advanced melanoma. Lancet Oncol. 2019, 20, 1187–1189. [Google Scholar] [CrossRef]
- Betof Warner, A.; Palmer, J.S.; Shoushtari, A.N.; Goldman, D.A.; Panageas, K.S.; Hayes, S.A.; Bajwa, R.; Momtaz, P.; Callahan, M.K.; Wolchok, J.D.; et al. Long-Term Outcomes and Responses to Retreatment in Patients with Melanoma Treated With PD-1 Blockade. J. Clin. Oncol. 2020, 38, 1655–1663. [Google Scholar] [CrossRef]
- Reschke, R.; Simon, J.C.; Ziemer, M. Rechallenge of targeted therapy in metastatic melanoma. J. Dtsch. Dermatol. Ges. 2019, 17, 483–486. [Google Scholar] [CrossRef]
- Owen, C.N.; Shoushtari, A.N.; Chauhan, D.; Palmieri, D.J.; Lee, B.; Rohaan, M.W.; Mangana, J.; Atkinson, V.; Zaman, F.; Young, A.; et al. Management of early melanoma recurrence despite adjuvant anti-PD-1 antibody therapy. Ann. Oncol. 2020, 31, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Vordermark, D.; Hassel, J.C.; Krex, D.; Wendl, C.; Schadendorf, D.; Sickmann, T.; Rieken, S.; Pukrop, T.; Holler, C.; et al. Melanoma brain metastases—Interdisciplinary management recommendations 2020. Cancer Treat. Rev. 2020, 89, 102083. [Google Scholar] [CrossRef]
- Engelhardt, B.; Ransohoff, R.M. Capture, crawl, cross: The T cell code to breach the blood-brain barriers. Trends Immunol. 2012, 33, 579–589. [Google Scholar] [CrossRef]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.J.; Flaherty, K.T.; Arance, A.; Chiarion-Sileni, V.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef]
- Welti, M.; Dimitriou, F.; Gutzmer, R.; Dummer, R. Triple Combination of Immune Checkpoint Inhibitors and BRAF/MEK Inhibitors in BRAFV600 Melanoma: Current Status and Future Perspectives. Cancers 2022, 14, 5489. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Shuto, T.; Yamamoto, M.; Yomo, S.; Kondoh, T.; Kobayashi, T.; Sato, M.; Okamoto, H.; Serizawa, T.; Nagano, O.; et al. Gamma Knife Radiosurgery for Metastatic Brain Tumors from Malignant Melanomas: A Japanese Multi-Institutional Cooperative and Retrospective Cohort Study (JLGK1501). Stereotact. Funct. Neurosurg. 2018, 96, 162–171. [Google Scholar] [CrossRef]
- Kessel, K.A.; Deichl, A.; Gempt, J.; Meyer, B.; Posch, C.; Diehl, C.; Zimmer, C.; Combs, S.E. Outcomes after stereotactic radiosurgery of brain metastases in patients with malignant melanoma and validation of the melanoma molGPA. Clin. Transl. Oncol. 2021, 23, 2020–2029. [Google Scholar] [CrossRef]
- Zhong, J.; Press, R.H.; Olson, J.J.; Oyesiku, N.M.; Shu, H.G.; Eaton, B.R. The use of Hypofractionated Radiosurgery for the Treatment of Intracranial Lesions Unsuitable for Single-Fraction Radiosurgery. Neurosurgery 2018, 83, 850–857. [Google Scholar] [CrossRef]
- Gaudy-Marqueste, C.; Dussouil, A.S.; Carron, R.; Troin, L.; Malissen, N.; Loundou, A.; Monestier, S.; Mallet, S.; Richard, M.A.; Regis, J.M.; et al. Survival of melanoma patients treated with targeted therapy and immunotherapy after systematic upfront control of brain metastases by radiosurgery. Eur. J. Cancer 2017, 84, 44–54. [Google Scholar] [CrossRef]
- Lehrer, E.J.; McGee, H.M.; Sheehan, J.P.; Trifiletti, D.M. Integration of immuno-oncology with stereotactic radiosurgery in the management of brain metastases. J. Neurooncol. 2021, 151, 75–84. [Google Scholar] [CrossRef]
- Martins, F.; Schiappacasse, L.; Levivier, M.; Tuleasca, C.; Cuendet, M.A.; Aedo-Lopez, V.; Gautron Moura, B.; Homicsko, K.; Bettini, A.; Berthod, G.; et al. The combination of stereotactic radiosurgery with immune checkpoint inhibition or targeted therapy in melanoma patients with brain metastases: A retrospective study. J. Neurooncol. 2020, 146, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Liermann, J.; Winkler, J.K.; Syed, M.; Neuberger, U.; Reuss, D.; Harrabi, S.; Naumann, P.; Ristau, J.; Weykamp, F.; El Shafie, R.A.; et al. Stereotactic Radiosurgery with Concurrent Immunotherapy in Melanoma Brain Metastases Is Feasible and Effective. Front. Oncol. 2020, 10, 592796. [Google Scholar] [CrossRef] [PubMed]
- Rauschenberg, R.; Bruns, J.; Brutting, J.; Daubner, D.; Lohaus, F.; Zimmer, L.; Forschner, A.; Zips, D.; Hassel, J.C.; Berking, C.; et al. Impact of radiation, systemic therapy and treatment sequencing on survival of patients with melanoma brain metastases. Eur. J. Cancer 2019, 110, 11–20. [Google Scholar] [CrossRef]
- Tetu, P.; Allayous, C.; Oriano, B.; Dalle, S.; Mortier, L.; Leccia, M.T.; Guillot, B.; Dalac, S.; Dutriaux, C.; Lacour, J.P.; et al. Impact of radiotherapy administered simultaneously with systemic treatment in patients with melanoma brain metastases within MelBase, a French multicentric prospective cohort. Eur. J. Cancer 2019, 112, 38–46. [Google Scholar] [CrossRef]
- Amaral, T.; Kiecker, F.; Schaefer, S.; Stege, H.; Kaehler, K.; Terheyden, P.; Gesierich, A.; Gutzmer, R.; Haferkamp, S.; Uttikal, J.; et al. Combined immunotherapy with nivolumab and ipilimumab with and without local therapy in patients with melanoma brain metastasis: A DeCOG* study in 380 patients. J. Immunother. Cancer 2020, 8, e000333. [Google Scholar] [CrossRef]
- Borius, P.Y.; Regis, J.; Carpentier, A.; Kalamarides, M.; Valery, C.A.; Latorzeff, I. Safety of radiosurgery concurrent with systemic therapy (chemotherapy, targeted therapy, and/or immunotherapy) in brain metastases: A systematic review. Cancer Metastasis Rev. 2021, 40, 341–354. [Google Scholar] [CrossRef]
- Jiang, J.M.; Kabarriti, R.; Brodin, N.P.; Ohri, N.; Guha, C.; Kalnicki, S.; Garg, M. Stereotactic radiosurgery with immunotherapy is associated with improved overall survival in patients with metastatic melanoma or non-small cell lung cancer: A National Cancer Database analysis. Clin. Transl. Oncol. 2022, 24, 104–111. [Google Scholar] [CrossRef]
- Chen, G.; Chakravarti, N.; Aardalen, K.; Lazar, A.J.; Tetzlaff, M.T.; Wubbenhorst, B.; Kim, S.B.; Kopetz, S.; Ledoux, A.A.; Gopal, Y.N.; et al. Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clin. Cancer Res. 2014, 20, 5537–5546. [Google Scholar] [CrossRef]
- Niessner, H.; Forschner, A.; Klumpp, B.; Honegger, J.B.; Witte, M.; Bornemann, A.; Dummer, R.; Adam, A.; Bauer, J.; Tabatabai, G.; et al. Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Med. 2013, 2, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Niessner, H.; Schmitz, J.; Tabatabai, G.; Schmid, A.M.; Calaminus, C.; Sinnberg, T.; Weide, B.; Eigentler, T.K.; Garbe, C.; Schittek, B.; et al. PI3K Pathway Inhibition Achieves Potent Antitumor Activity in Melanoma Brain Metastases In Vitro and In Vivo. Clin. Cancer Res. 2016, 22, 5818–5828. [Google Scholar] [CrossRef]
- Tehranian, C.; Fankhauser, L.; Harter, P.N.; Ratcliffe, C.D.H.; Zeiner, P.S.; Messmer, J.M.; Hoffmann, D.C.; Frey, K.; Westphal, D.; Ronellenfitsch, M.W.; et al. The PI3K/Akt/mTOR pathway as a preventive target in melanoma brain metastasis. Neuro Oncol. 2022, 24, 213–225. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.M.; Jalali, A.; Kircher, D.A.; Lee, W.C.; McQuade, J.L.; Haydu, L.E.; Joon, A.Y.; Reuben, A.; de Macedo, M.P.; Carapeto, F.C.L.; et al. Molecular Profiling Reveals Unique Immune and Metabolic Features of Melanoma Brain Metastases. Cancer Discov. 2019, 9, 628–645. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.M.; Guerrieri, R.A.; Hu, Q.; Joon, A.Y.; Kumar, S.; Haydu, L.E.; McQuade, J.L.; Vashisht Gopal, Y.N.; Knighton, B.; Deng, W.; et al. Clinical, molecular, metabolic, and immune features associated with oxidative phosphorylation in melanoma brain metastases. Neurooncol. Adv. 2021, 3, vdaa177. [Google Scholar] [CrossRef] [PubMed]
- Amaral, T.; Niessner, H.; Sinnberg, T.; Thomas, I.; Meiwes, A.; Garbe, C.; Garzarolli, M.; Rauschenberg, R.; Eigentler, T.; Meier, F. An open-label, single-arm, phase II trial of buparlisib in patients with melanoma brain metastases not eligible for surgery or radiosurgery-the BUMPER study. Neurooncol. Adv. 2020, 2, vdaa140. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Del Vecchio, M.; Mandala, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results from the EORTC 1325-MG/KEYNOTE-054 Trial. J. Clin. Oncol. 2020, 38, 3925–3936. [Google Scholar] [CrossRef]
- Zimmer, L.; Livingstone, E.; Hassel, J.C.; Fluck, M.; Eigentler, T.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 395, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Schadendorf, D.; Vecchio, M.D.; Larkin, J.; Atkinson, V.; Schenker, M.; Pigozzo, J.; Gogas, H.J.; Dalle, S.; Meyer, N.; et al. Adjuvant therapy with nivolumab (NIVO) combined with ipilimumab (IPI) vs NIVO alone in patients (pts) with resected stage IIIB-D/IV melanoma (CheckMate 915). Cancer Res. 2021, 81, CT004. [Google Scholar] [CrossRef]
- Weber, J.S.; Schadendorf, D.; Del Vecchio, M.; Larkin, J.; Atkinson, V.; Schenker, M.; Pigozzo, J.; Gogas, H.; Dalle, S.; Meyer, N.; et al. Adjuvant Therapy of Nivolumab Combined with Ipilimumab Versus Nivolumab Alone in Patients with Resected Stage IIIB-D or Stage IV Melanoma (CheckMate 915). J. Clin. Oncol. 2022, 41, 517–527. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Sileni, V.C.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G.V.; et al. LBA3_PR—Pembrolizumab versus placebo after complete resection of high-risk stage II melanoma: Efficacy and safety results from the KEYNOTE-716 double-blind phase III trial. Ann. Oncol. 2021, 32, S1314–S1315. [Google Scholar] [CrossRef]
- Long, G.V.; Desai, K.; Tang, T.; Weber, J.S.; Dolfi, S.; Ritchings, C.; Huang, S.P.; Bolisetty, M.; Sausen, M.; Del Vecchio, M.; et al. 788O Association of pre-treatment ctDNA with disease recurrence and clinical and translational factors in patients with stage IIIB-D/IV melanoma treated with adjuvant immunotherapy (CheckMate 915). Ann. Oncol. 2022, 33, S904. [Google Scholar] [CrossRef]
- Weber, J.S.; Del Vecchio, M.; Mandala, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. 1310O—Adjuvant nivolumab (NIVO) versus ipilimumab (IPI) in resected stage III/IV melanoma: 3-year efficacy and biomarker results from the phase III CheckMate 238 trial. Ann. Oncol. 2019, 30, v533–v534. [Google Scholar] [CrossRef]
- Gebhardt, C.; Ascierto, P.; Atkinson, V.; Corrie, P.; Dummer, R.; Schadendorf, D. The concepts of rechallenge and retreatment in melanoma: A proposal for consensus definitions. Eur. J. Cancer 2020, 138, 68–76. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Meshcheryakov, A.; Atkinson, V.; Blank, C.U.; Mandala, M.; Long, G.V.; Barrow, C.; Di Giacomo, A.M.; Fisher, R.; Sandhu, S.; et al. Crossover and rechallenge with pembrolizumab in recurrent patients from the EORTC 1325-MG/Keynote-054 phase III trial, pembrolizumab versus placebo after complete resection of high-risk stage III melanoma. Eur. J. Cancer 2021, 158, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Menzies, A.M.; Amaria, R.N.; Rozeman, E.A.; Huang, A.C.; Tetzlaff, M.T.; van de Wiel, B.A.; Lo, S.; Tarhini, A.A.; Burton, E.M.; Pennington, T.E.; et al. Pathological response and survival with neoadjuvant therapy in melanoma: A pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat. Med. 2021, 27, 301–309. [Google Scholar] [CrossRef]
- Amaria, R.N.; Postow, M.A.; Tetzlaff, M.T.; Ross, M.I.; Glitza, I.C.; McQuade, J.L.; Wong, M.K.K.; Gershenwald, J.E.; Goepfert, R.; Keung, E.Z.-Y.; et al. Neoadjuvant and adjuvant nivolumab (nivo) with anti-LAG3 antibody relatlimab (rela) for patients (pts) with resectable clinical stage III melanoma. J. Clin. Oncol. 2021, 39, 9502. [Google Scholar] [CrossRef]
- Amaria, R.N.; Reddy, S.M.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.J.; Hanna, E.; et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Kendra, K.L.; Moon, J.; Eroglu, Z.; Hu-Lieskovan, S.; Carson, W.E.; Wada, D.A.; Plaza, J.A.; In, G.K.; Ikeguchi, A.; Hyngstrom, J.R.; et al. Neoadjuvant PD-1 blockade in patients with resectable desmoplastic melanoma (SWOG 1512). J. Clin. Oncol. 2022, 40, 9502. [Google Scholar] [CrossRef]
- Eroglu, Z.; Zaretsky, J.M.; Hu-Lieskovan, S.; Kim, D.W.; Algazi, A.; Johnson, D.B.; Liniker, E.; Ben, K.; Munhoz, R.; Rapisuwon, S.; et al. High response rate to PD-1 blockade in desmoplastic melanomas. Nature 2018, 553, 347–350. [Google Scholar] [CrossRef]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; van de Wiel, B.; Kvistborg, P.; Krijgsman, O.; van den Braber, M.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef]
- Patel, S.O.; Othus, M.; Prieto, V.; Lowe, M.; Buchbinder, E.; Chen, Y.; Hyngstrom, J.; Lao, C.D.; Truong, T.; Chandra, S.; et al. LBA6—Neoadjvuant versus adjuvant pembrolizumab for resected stage III-IV melanoma (SWOG S1801). Ann. Oncol. 2022, 33, S1408. [Google Scholar] [CrossRef]
- Buder-Bakhaya, K.; Hassel, J.C. Biomarkers for Clinical Benefit of Immune Checkpoint Inhibitor Treatment-A Review from the Melanoma Perspective and Beyond. Front. Immunol. 2018, 9, 1474. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, P.B.; Dong, Z.; Gusenleitner, D.; Giobbie-Hurder, A.; Severgnini, M.; Zhou, J.; Manos, M.; Eastman, L.M.; Maecker, H.T.; Hodi, F.S. Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J. Immunother. Cancer 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, W.; van den Berg, M.; van der Niet, S.; Limpens, J.; Luiten, R.M. Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: A systematic review. Melanoma Res. 2019, 29, 453–464. [Google Scholar] [CrossRef]
- Amaria, R.N.; Menzies, A.M.; Burton, E.M.; Scolyer, R.A.; Tetzlaff, M.T.; Antdbacka, R.; Ariyan, C.; Bassett, R.; Carter, B.; Daud, A.; et al. Neoadjuvant systemic therapy in melanoma: Recommendations of the International Neoadjuvant Melanoma Consortium. Lancet Oncol. 2019, 20, e378–e389. [Google Scholar] [CrossRef]
- Mushti, S.L.; Mulkey, F.; Tang, S.; Singh, H.; Lemery, S.J.; Goldberg, K.B.; Sridhara, R.; Keegan, P.; Kluetz, P.G.; Pazdur, R.; et al. Immune Response Evaluation and Treatment with Immune Checkpoint Inhibitors Beyond Clinical Progression: Response Assessments for Cancer Immunotherapy. Curr. Oncol. Rep. 2020, 22, 116. [Google Scholar] [CrossRef] [PubMed]


| Study | Treatment Regimens | Trial Phase | Patients | N | ORR, % (CI) | PD Rate % (CI) | PFS Rate, 4 yrs % (CI) | PFS Rate, 5 yrs % (CI) | AR Rate %(For 4 yr/5 yr PFS) |
|---|---|---|---|---|---|---|---|---|---|
| 1st line ICI monotherapy (anti-PD-1) | |||||||||
| Keynote-001 [39] (NCT01295827) | (i) Pem (2 mg/kg q3w or 10 mg/kg q2w or 10 mg/kg q3w) | IB | Adv., treatment-naïve, BRAF± | (i) 151 | 52 (40–57) | 25 (22–29) | 35 | 29 | 40/44 |
| Keynote-006 [6] (NCT01866319) | (i) Pem 10 mg/kg q2w vs. 10 mg/kg q3w vs. (ii) Ipi 3 mg/kg q3w x4 | III | Adv., treatment-naïve, BRAF± | (i) 556 | 42 (38–44,58–60) | 29 | 23 (19–27) | nr | 48/nr |
| (ii) 278 | 17 (12–21) | 38 | 7 (3–13) | - | 55/nr | ||||
| CheckMate-066 [40] (NCT01721772) | (i) Nivo 3 mg/kg q2w vs. Dacarbazine 1000 mg/m² | III | Adv., treatment-naïve, BRAF wild type | (i) 210 | 42 (36–46,58–60) | 32 | 29 | 28 | 39/40 |
| (ii) 208 | 14 (10–20) | 50 | 3 | 3 | 47/47 | ||||
| CheckMate-067 [7] (NCT01844505) | (i) Nivo 3 mg/kg q2w (vs. Nivo + Ipi) vs. (ii) Ipi 3 mg/kg q3w x4 | III | Adv., treatment-naïve, BRAF± | (i) 316 | 45 (39–47,58–60) | 38 | nr | 29 | nr/48 |
| (ii) 315 | 19 (15–24) | 50 | nr | 8 | nr/42 | ||||
| 1st-line ICI combination therapy (anti-PD-1 + anti-CTLA-4) | |||||||||
| CheckMate-067 [7] (NCT01844505) | Nivo vs. (i) Nivo 1 mg/kg q2w + Ipi 3 mg/kg vs. (ii) Ipi 3 mg/kg q3w x4 | III | Adv., treatment-naïve, BRAF± | (i) 314 | 58 (50–57,61–64) | 24 | nr | 36 | nr/40 |
| (ii) 315 | 19 (15–24) | 50 | nr | 8 | nr/42 | ||||
| 1st-line ICI therapy in patients with asymptomatic brain metastasis (anti-PD-1 ± anti-CTLA-4) for intracranial outcome parameters | |||||||||
| CheckMate-204 [41] (NCT02320058) | (i) Nivo 1 mg/kg q2w + Ipi 3 mg/kg a | II | Adv., treatment-naïve b ABM, BRAF± | (i) 101 | 54 (40–57,61–64) | 30 | nr | nr | - |
| ABC [42] (NCT02374242) | Nivo 1 mg/kg q2w + Ipi 3 mg/kg vs. Nivo 3 mg/kg | II | Adv., treatment-naïve ABM, BRAF± | 27 | 59 | 30 | nr | 52 | nr/18 |
| 19 | 21 | 74 | nr | 14 | nr/12 | ||||
| Trial (NCT n°) | Treatment Regimens | Trial Phase | Patients | N | Primary Endpoint | ORR, % (CI) | PFS Median, mts (HR (CI)) | OS Med., mts (HR (CI)) |
|---|---|---|---|---|---|---|---|---|
| 2nd-line combination therapy (anti-PD-1 backbone) | ||||||||
| LEAP-004 [57] (NCT03776136) | Lenvatinib 20 od + Pem 200 mg q3w | II | Adv., PD-(L)1 pre-treated (PD upon/after therapy a) [58], BRAF± | 103 | ORR | 33 (17–53) b; 23 (13–35) c | 4.2 (3.8–7.1) | 14.0 (10.8-nr) |
| IRB17-0686 [59] (NCT02743819) | Ipi 1 mg/kg q3w × 4 + Pem 200 mg q3w | II | Adv., PD-(L)1 pre-treated (PD upon therapy d = Primary resistance), BRAF± | 70 | ORR | 31 (nr-nr) | 4.7 (2.8–8.3) | nr |
| CA224-020 [60] (NCT01968109) | Relatlimab 80 mg q2w + Nivo 240 mg q2w | I–II | Adv., PD-(L)1 pre-treated (PD upon ther. = Prim. resistance), BRAF± | 68 | Safety, ORR | 12 (nr-nr) | nr | nr |
| SYNERGY-001 [61] (NCT02521870) | SD-101 2 mg/kg q1-3w + Pem 200 mg q3w | I–II | Advanced, PD-(L)1-pre-treated (PD upon/after ther.), BRAF± | 23 | ORR | 20 (nr-nr) | nr | nr |
| 2014-0922 [62] (NCT02500576) | Cryopreserved TILe + IL-2 (Aldesleukin) (high dose/low dose) + Pem 200 mg q3w | I–II | Metastatic, un-/pre-treated (13 out of 14 pts were PD-1-pre-treated), BRAF± | 14 | ORR | 14 (nr-nr) | 3.9 (nr-nr)/2.1 (nr-nr) | 9.7 (nr-nr)/ 8.8 (nr-nr) |
| PV-10-MM-1201 [63] (NCT02557321) | PV-10 (intralesional) + Pem 2 mg/kg q3w | I | Metastatic, ICI-pre-treated (PD upon/after therapy), BRAF± | 13 | Safety | 31 (nr-nr) | nr | nr |
| 4SC-202-2-2017 [64] (NCT03278665) | Domatinostat + Pem 2 mg/kg q3w | I–II | Metastatic, ICI-pre-treated (PD upon/after therapy), BRAF± | 40 | Safety | 8 (nr-nr) | nr | nr |
| 16-1080.cc [65] (NCT03200847) | all-trans-Retinoic acid + Pem 200 mg q3w | I–II | Metastatic, ICI pre-treated (PD upon/after therapy), BRAF± | 24 | Safety | 67 (nr-nr) | 20.3 | nr |
| Lipo-MERIT [66,67] (NCT02410733) | FixVak (RNA vaccine) ± anti-PD-1 | I | Metastatic, ICI-pre-treated (PD on/after therapy), BRAF± | 42 | Safety | 16 (FV mono) 35 (FV + PD1) | nr | nr |
| 2nd-line combination therapy (anti-CTLA-4 backbone) | ||||||||
| ILLUMINATE-204 [68] (NCT02644967) | Tilsotolimod 8 mg/kg q1-6w + Ipi 3 m/kg q3w x4 | I–II | Advanced, PD-1-pre-treated (PD on/after therapy), BRAF± | 62 | Safety, ORR | 22 (12–37) | 5.1 (3.7–7.0) | 21.0 (9.8-nr) |
| ILLUMINATE-301 [69] (NCT03445533) | Tilsotolimod 8 mg/kg q1-6w + Ipi 3 m/kg q3w x4 vs. Ipi | III | Advanced, PD-1-pre-treated | 481 | OS and ORR | 9 (nr-nr) | nr | nr |
| 2nd-line monotherapy | ||||||||
| C144-01 [56] (NCT02360579) | Lifileucel (i.e., autologous, cryo-preserved TIL e) + IL-2 x6 | II | Advanced, PD-1-pre-treated, BRAF± | 66 | ORR | 36 (nr-nr) | nr | nr |
| Dutch [56] (NCT02278887) | TIL (i.e., autologous, cryo-preserved TIL e) vs. Ipi | III | Advanced, progression after the maximal one line of pre-treatment (no Ipi), BRAF±; approximately 90% of patients had PD-1 pre-treatment in both arms | 84 84 | PFS | 48.8 21.4 | 7.2 (4.2–13.1)/3.1 (3.0–4.3), HR 0.05, p < 0.001 | 25.8 (18.2-nr) /18.9 (13.8–32.6), HR 0.83, p = 0.39 |
| Study | Treatment Regimens | Trial Phase | Patients (Stage Distribution) | N | RFS rate, % (CI) | Recurrence Rate, % (5 yr/*3 yr/4 yr) a | Patterns of Recurrence: Local vs. (//) Distant Only % (n/ntotal) b |
|---|---|---|---|---|---|---|---|
| Stage III/IV | |||||||
| Keynote-054 [15] (NCT02362594) | (i) Pem 200 mg q3w vs. | III | 100% III | (i) 514 | 55 at 5 yrs (51–60) | 45 | 14 (74/514)//28 (143/514) |
| (ii) Placebo | 100% III | (ii) 505 | 38 at 5 yrs (34–43) | 62 | 19 (96/505//41 (206/505)) | ||
| CheckMate-238 [13] (NCT02388906) | (i) Nivo 3mg/kg q2w vs. | III | 81% III, 18% IV | (i) 453 | 50 at 5 yrs (not reported) | nr 50 | |
| (ii) Ipi 10mg/kg q3w x4 | 81% III, 19% IV | (ii) 453 | 39 at 5 yrs (not reported) | nr 61 | |||
| CheckMate-915 [110] (NCT03068455) | (i) Nivo 480 mg q2w vs. (ii) Nivo | III | 86% III, 13% IV | (i) 924 | 63 at 2 yrs A (60–66) | - | nr//nr |
| 240 mg q2w + Ipi 1 mg/kg q6w | 87% III, 13% IV | (ii) 920 | 65 at 2 yrs (61–68) | - | nr//nr | ||
| IMMUNED [14] (NCT02523313) | (i) Nivo 3mg/kg q2w vs. | II | 100% IV | (i) 59 | 31 at 3 /4 yrs (20–41,58–60) | 69*/69* | 14 (8/59)//(42 (25/59) |
| (ii) Nivo 1mg/kg q2w + Ipi | 100% IV | (ii) 56 | 64 at 3 /4 yrs (49–76) | 36*/36* | 4 (2/56)//16 (9/56) | ||
| 3 mg/kg q3w x4 vs. (iii) Placebo | 100% IV | (iii) 52 | 15 at 3 /4 yrs (7–27) | 85*/85* | 25 (13/52)//46 (24/52) | ||
| Stage II | |||||||
| Keynote-716 [10] (NCT03553836) | (i) Pem 200 mg q3w vs. | III | 63% IIB, 35% IIC | (i) 487 | 81 at 2 yr (not reported) | - | - |
| (ii) Placebo | 65% IIB, 35% IIC | (ii) 489 | 73 at 2 yr (not reported) | - | - | ||
| CheckMate-76K [11] (NCT04099251) | (i) Nivo 480 mg q4w vs. | III | 60% IIB, 40% IIC | (i) 526 | 89 at 1 year (86–92) | - | - |
| (ii) Placebo | 62% IIB, 38% IIC | (ii) 264 | 79 at 1 year (74–84) | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassel, J.C.; Zimmer, L.; Sickmann, T.; Eigentler, T.K.; Meier, F.; Mohr, P.; Pukrop, T.; Roesch, A.; Vordermark, D.; Wendl, C.; et al. Medical Needs and Therapeutic Options for Melanoma Patients Resistant to Anti-PD-1-Directed Immune Checkpoint Inhibition. Cancers 2023, 15, 3448. https://doi.org/10.3390/cancers15133448
Hassel JC, Zimmer L, Sickmann T, Eigentler TK, Meier F, Mohr P, Pukrop T, Roesch A, Vordermark D, Wendl C, et al. Medical Needs and Therapeutic Options for Melanoma Patients Resistant to Anti-PD-1-Directed Immune Checkpoint Inhibition. Cancers. 2023; 15(13):3448. https://doi.org/10.3390/cancers15133448
Chicago/Turabian StyleHassel, Jessica C., Lisa Zimmer, Thomas Sickmann, Thomas K. Eigentler, Friedegund Meier, Peter Mohr, Tobias Pukrop, Alexander Roesch, Dirk Vordermark, Christina Wendl, and et al. 2023. "Medical Needs and Therapeutic Options for Melanoma Patients Resistant to Anti-PD-1-Directed Immune Checkpoint Inhibition" Cancers 15, no. 13: 3448. https://doi.org/10.3390/cancers15133448
APA StyleHassel, J. C., Zimmer, L., Sickmann, T., Eigentler, T. K., Meier, F., Mohr, P., Pukrop, T., Roesch, A., Vordermark, D., Wendl, C., & Gutzmer, R. (2023). Medical Needs and Therapeutic Options for Melanoma Patients Resistant to Anti-PD-1-Directed Immune Checkpoint Inhibition. Cancers, 15(13), 3448. https://doi.org/10.3390/cancers15133448








