Analysis of Expression of Programmed Cell Death Ligand 1 (PD-L1) and BRAFV600E Mutation in Thyroid Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
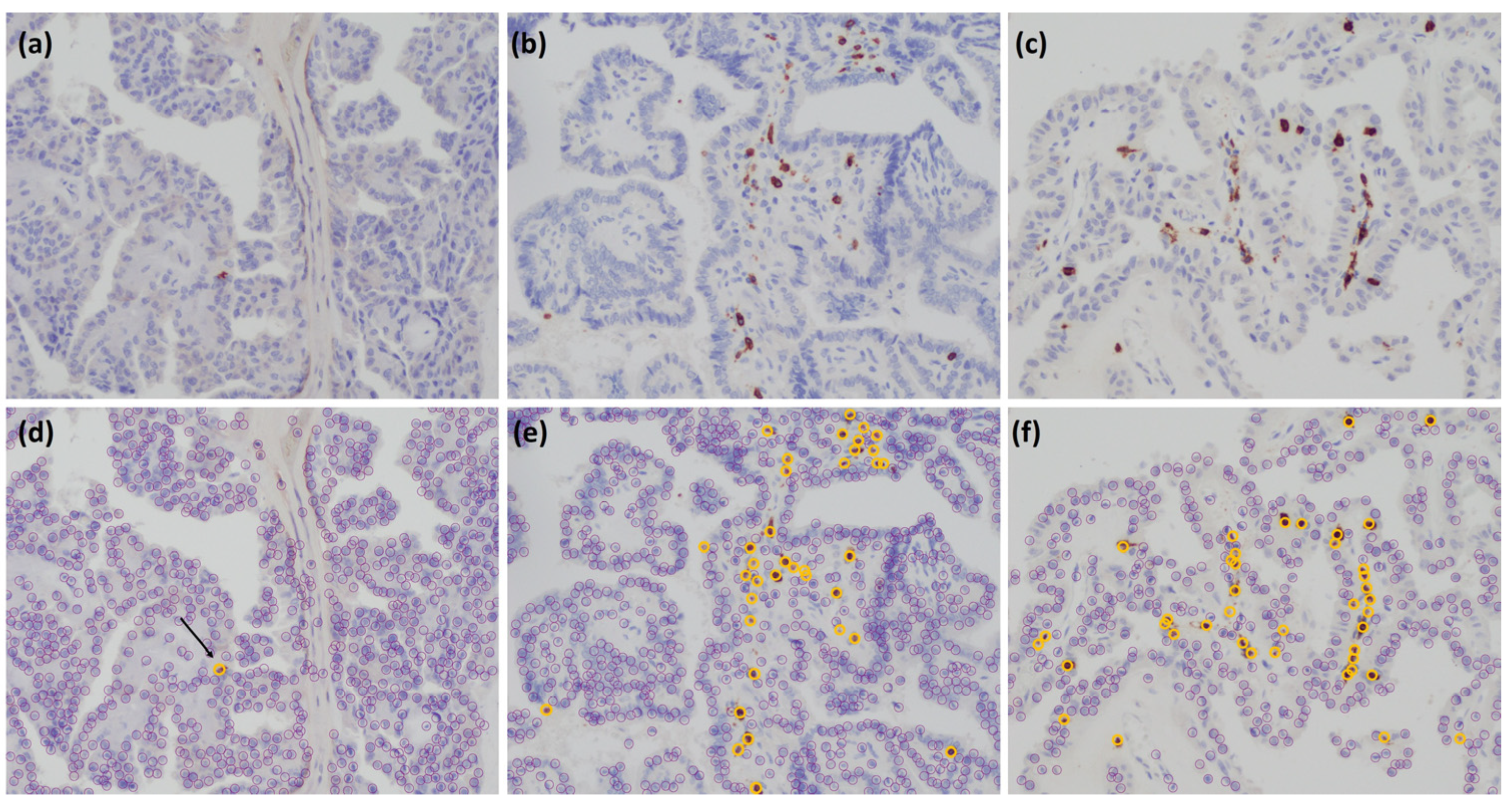
2.2. Immunohistochemistry
2.3. Scoring of Immune Markers
2.4. Flow Cytometry
2.5. Cytokine Production by PBMCs
2.6. Lymphocyte Proliferation Assay
2.7. Markers of Inflammation and Thyroid Status
2.8. Statistical Analysis
3. Results
3.1. Correlation between PD-L1 Expression in Cancer Tissues and Clinicopathologic Factors
3.2. Correlation between PD-L1 Expression and BRAFV600E Mutation
3.3. Correlation between PD-L1 Expression and CD8+ Expression
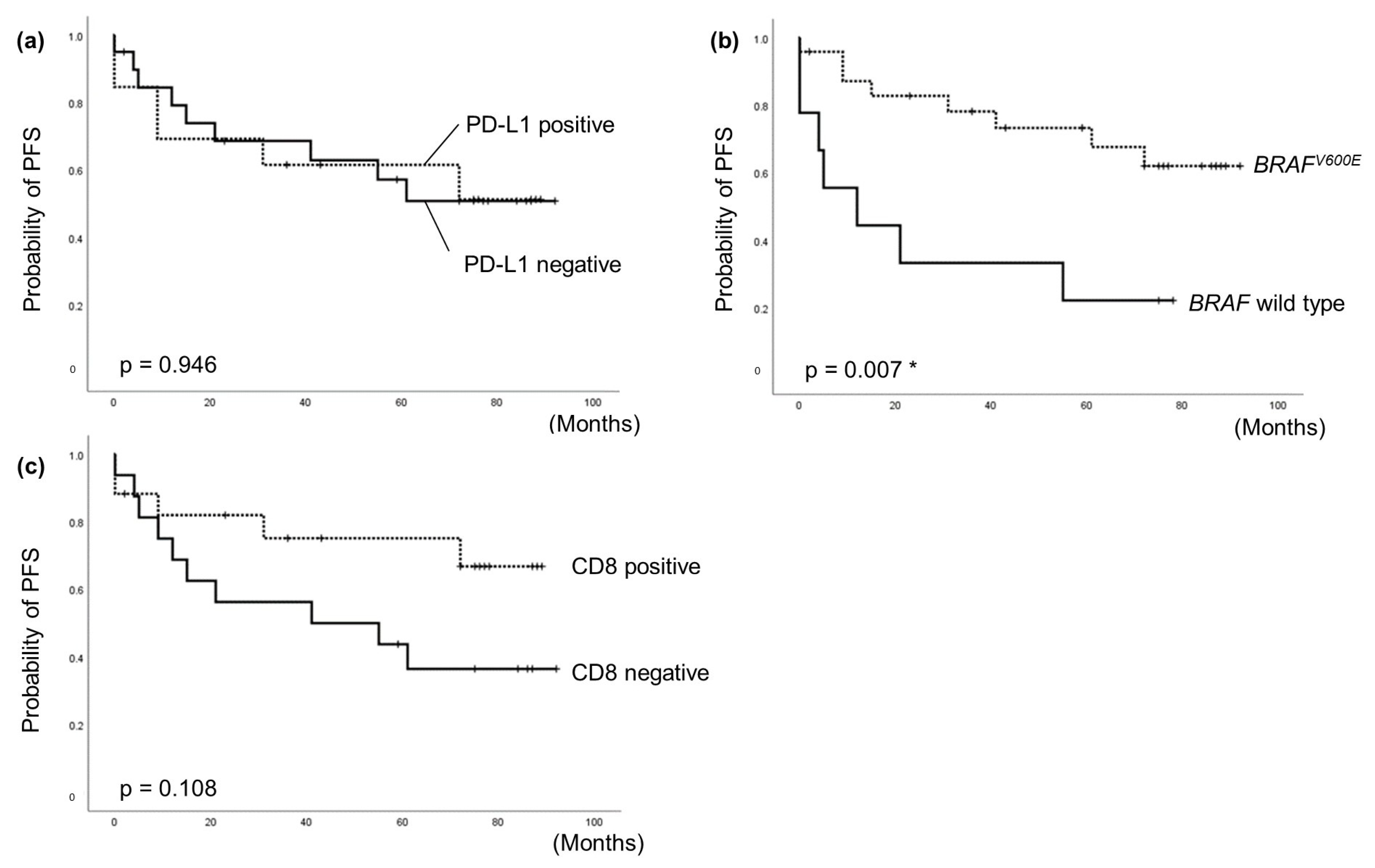
3.4. Correlation between PD-L1 Expression in Thyroid Cancer and Patient Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugitani, I.; Miyauchi, A.; Sugino, K.; Okamoto, T.; Yoshida, A.; Suzuki, S. Prognostic Factors and Treatment Outcomes for Anaplastic Thyroid Carcinoma: ATC Research Consortium of Japan Cohort Study of 677 Patients. World J. Surg. 2012, 36, 1247–1254. [Google Scholar] [CrossRef]
- Ozmen, S.; Timur, O.; Calik, I.; Altinkaynak, K.; Simsek, E.; Gozcu, H.; Arslan, A.; Carlioglu, A. Neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) may be superior to C-reactive protein (CRP) for predicting the occurrence of differentiated thyroid cancer. Endocr. Regul. 2017, 51, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Shibata, M.; Gonda, K.; Matsumoto, Y.; Nakano, K.; Iwadate, M.; Suzuki, S.; Suzuki, S. Prognostic impact of elevated preoperative C-reactive protein on patients with differentiated thyroid carcinoma. J. Surg. Res. 2018, 231, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; Varga, A.; Brose, M.S.; Aggarwal, R.R.; Lin, C.C.; Prawira, A.; DeBraud, F.; Tamura, K.; Doi, T.; Piha-Paul, S.A.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer 2019, 19, 196. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Andrea Fülöp, A.; Maya Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Karasaki, T.; Nagayama, K.; Kuwano, H.; Nitadori, J.I.; Sato, M.; Anraku, M.; Hosoi, A.; Matsushita, H.; Morishita, Y.; Kashiwabara, K.; et al. An Immunogram for the Cancer- Immunity Cycle: Towards Personalized Immunotherapy of Lung Cancer. J. Thorac. Oncol. 2017, 12, 791–803. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Ohigashi, Y.; Sho, M.; Yamada, Y.; Tsurui, Y.; Hamada, K.; Ikeda, N.; Mizuno, T.; Yoriki, R.; Kashizuka, H.; Yane, K.; et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer Res. 2005, 11, 2947–2953. [Google Scholar] [CrossRef]
- Konishi, J.; Yamazaki, K.; Azuma, M.; Kinoshita, I.; Dosaka-Akita, H.; Nishimura, M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin. Cancer Res. 2004, 10, 5094–5100. [Google Scholar] [CrossRef] [PubMed]
- Hino, R.; Kabashima, K.; Kato, Y.; Yagi, H.; Nakamura, M.; Honjo, T.; Okazaki, T.; Tokura, Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010, 116, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Aghajani, M.; Graham, S.; McCafferty, C.; Shaheed, C.A.; Roberts, T.; DeSouza, P.; Yang, T.; Niles, N. Clinicopathologic and Prognostic Significance of Programmed Cell Death Ligand 1 Expression in Patients with Non-Medullary Thyroid Cancer: A Systematic Review and Meta-Analysis. Thyroid 2018, 28, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Veyhl, J.; Jessa, F.; Polyakova, O.; Alenzi, A.; MacMillan, C.; Ralhan, R.; Walfish, P.G. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget 2016, 7, 32318–32328. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.L.; Marcello, M.A.; Vassallo, J.; Ward, L.S. Differentiated thyroid carcinomas and their B7H1 shield. Future Oncol. 2013, 9, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.L.; Marcello, M.A.; Morari, E.C.; Nonogaki, S.; Conte, F.F.; Gerhard, R.; Soares, F.A.; Vassallo, J.; Ward, L.S. Differentiated thyroid carcinomas may elude the immune system by B7H1 upregulation. Endocr. Relat. Cancer 2013, 20, 103–110. [Google Scholar] [CrossRef]
- French, J.D.; Kotnis, G.R.; Said, S.; Raeburn, C.D.; McIntyre, R.C., Jr.; Klopper, J.P.; Haugen, B.R. Programmed death- 1+ T cells and regulatory T cells are enriched in tumor- involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2012, 97, E934–E943. [Google Scholar] [CrossRef]
- Severson, J.J.; Serracino, H.S.; Mateescu, V.; Raeburn, C.D.; McIntyre, R.C., Jr.; Sams, S.B.; Haugen, B.R.; French, J.D. PD-1+Tim-3+ CD8+ T Lymphocytes Display Varied Degrees of Functional Exhaustion in Patients with Regionally Metastatic Differentiated Thyroid Cancer. Cancer Immunol. Res. 2015, 3, 620–630. [Google Scholar] [CrossRef]
- Angell, T.E.; Lechner, M.G.; Jang, J.K.; Correa, A.J.; LoPresti, J.S.; Epstein, A.L. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid 2014, 24, 1385–1393. [Google Scholar] [CrossRef]
- Brown, J.A.; Dorfman, D.M.; Ma, F.R.; Sullivan, E.L.; Munoz, O.; Wood, C.R.; Greenfield, E.A.; Freeman, G.J. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003, 170, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Giancola, N.; Fugazzola, L. Personalized treatment for differentiated thyroid cancer: Current data and new perspectives. Minerva Endocrinol. 2021, 46, 62–89. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Ezzat, S.; Ada, S.L. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat. Rev. Cancer 2006, 6, 292–306. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, T.H.; Kim, S.W.; Ki, C.S.; Jang, H.W.; Kim, J.S.; Kim, J.H.; Choe, J.H.; Shin, J.H.; Hahn, S.Y. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr. Relat. Cancer 2017, 24, 97–106. [Google Scholar] [CrossRef]
- Xing, M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr. Rev. 2007, 28, 742–762. [Google Scholar] [CrossRef]
- Suzuki, S.; Shibata, M.; Gonda, K.; Kanke, Y.; Ashizawa, M.; Ujiie, D.; Suzushino, S.; Nakano, K.; Fukushima, T.; Sakurai, K. Immunosuppression involving increased myeloid derived suppressor cell levels, systemic inflammation and hypoalbuminemia are present in patients with anaplastic thyroid cancer. Mol. Clin. Oncol. 2013, 1, 959–964. [Google Scholar] [CrossRef]
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of the PD-1 pathway in the immune response. Am. J. Transplant. 2012, 12, 2575–2587. [Google Scholar] [CrossRef]
- Sheffield, B.S.; Fulton, R.; Kalloger, S.E.; Milne, K.; Geller, G.; Jones, M.; Jacquemont, C.; Zachara, S.; Zhao, E.; Pleasance, E.; et al. Investigation of PD-L1 Biomarker testing methods for PD-1 axis inhibition in non-squamous non-small cell lung cancer. J. Histochem. Cytochem. 2016, 64, 587–600. [Google Scholar] [CrossRef]
- Chintakuntlawar, A.V.; Rumilla, K.M.; Smith, C.Y.; Jenkins, S.M.; Foote, R.L.; Kasperbauer, J.L.; Morris, J.C.; Ryder, M.; Alsidawi, S.; Hilger, C.; et al. Expression of PD-1 and PD-L1 in anaplastic thyroid cancer patients treated with multimodal therapy: Results from a retrospective study. J. Clin. Endocrinol. Metab. 2017, 102, 1943–1950. [Google Scholar] [CrossRef]
- Ogiya, R.; Niikura, N.; Kumaki, N.; Bianchini, G.; Kitano, S.; Iwamoto, T.; Hayashi, N.; Yokoyama, K.; Oshitanai, R.; Terao, M.; et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016, 107, 1730–1735. [Google Scholar] [CrossRef]
- Tokoro, D.; Yane, Y.; Hida, J.; Okuno, K. Prognostic lmplications of Programmed Cell Death Ligand- 1 (PD-L1) Expression on Tumor- lnfiltrating lmmune Cells and CD8 + T Cells for Stage III Colorectal Cancer. Jpn. J. Cancer Chemother. 2018, 45, 1482–1485. Available online: http://ccpgan.com/ (accessed on 6 January 2020).
- Ashizawa, M.; Okayama, H.; Ishigame, T.; Aung Kyi Thar, M.i.n.; Saito, K.; Ujiie, D.; Murakami, M.; Kikuchi, T.; Nakayama, Y.; Noda, M.; et al. miRNA-148a-3p regulates immunosuppression in DNA mismatch repair-deficient colorectal cancer by targeting PD-L1. Mol. Cancer Res. 2019, 17, 1403–1413. [Google Scholar] [CrossRef]
- Ogino, S.; Galon, J.; Fuchs, C.S.; Dranoff, G. Cancer immunology- analysis of host and tumor factors for personalized medicine. Nat. Rev. Clin. Oncol. 2011, 8, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Suzuki, S.; Mashiko, M.; Ohtake, T.; Endo, Y.; Takebayashi, Y.; Sekikawa, K.; Hagiwara, K.; Takenoshita, S. BRAF mutations in papillary carcinomas of the thyroid. Oncogene 2003, 22, 6455–6457. [Google Scholar] [CrossRef]
- Hirokawa, M.; Higuchi, M.; Suzuki, A.; Hayashi, T.; Kuma, A.; Miyauchi, A. Prevalence and diagnostic significance of noninvasive follicular thyroid neoplasm with papillary-like nuclear features among tumors previously diagnosed as follicular adenoma: A single-institutional study in Japan. Endocr. J. 2020, 67, 1071–1075. [Google Scholar] [CrossRef]
- Cunha, L.L.; Morari, E.C.; Guihen, A.C.; Razolli, D.; Gerhard, R.; Nonogaki, S.; Soares, F.A.; Vassallo, J.; Ward, L.S. Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clin. Endocrinol. 2012, 77, 918–925. [Google Scholar] [CrossRef]
- Ieni, A.; Vita, R.; Pizzimenti, C.; Benvenga, S.; Tuccari, G. Intratumoral heterogeneity in differentiated thyroid tumors: An intriguing reappraisal in the era of personalized medicine. J. Pers. Med. 2021, 11, 333. [Google Scholar] [CrossRef]
- Nicolson, N.G.; Paulsson, J.O.; Juhlin, C.C.; Carling, T.; Korah, R. Transcription factor profiling identifies spatially heterogenous mediators of follicular thyroid cancer invasion. Endocr. Pathol. 2020, 31, 367–376. [Google Scholar] [CrossRef]
- Pogliaghi, G. Liquid biopsy in thyroid cancer: From circulating biomarkers to a new prospective of tumor monitoring and therapy. Minerva Endocrinol. 2021, 46, 45–61. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | PD-L1 Expression Negative (n = 20) | PD-L1 Expression Positive (n = 13) | p-Value |
|---|---|---|---|
| Age | 53.9 ± 12.5 | 63.3 ± 14.5 | 0.071 |
| Sex | |||
| Male | 8 | 2 | 0.132 |
| Female | 12 | 11 | |
| Thyroid cancer | |||
| PTC | 14 | 10 | 0.315 |
| ATC | 2 | 3 | |
| FTC | 2 | 0 | |
| MTC | 2 | 0 | |
| Tumor size | 25.8 ± 20.0 | 34.2 ± 25.5 | 0.276 |
| Ex | |||
| Ex0 | 9 | 6 | 0.449 |
| EX1 | 8 | 3 | |
| EX2 | 3 | 4 | |
| pT | |||
| T1a | 3 | 4 | 0.794 |
| T1b | 6 | 2 | |
| T2 | 2 | 1 | |
| T3 | 5 | 2 | |
| T4a | 1 | 1 | |
| T4b | 3 | 3 | |
| pN | |||
| N0 | 6 | 7 | 0.201 |
| N1a | 3 | 0 | |
| N1b | 11 | 6 | |
| M | |||
| M0 | 15 | 11 | 0.419 |
| M1 | 5 | 2 | |
| STAGE | |||
| I | 5 | 5 | 0.678 |
| II | 1 | 0 | |
| III | 3 | 1 | |
| IV | 11 | 7 | |
| CRP (mg/dL) | 0.19 ± 0.28 | 1.5 ± 5.0 | 0.429 |
| WBC (103/μL) | 5.8 ± 3.4 | 7.4 ± 7.5 | 0.568 |
| NLR (%) | 6.9 ± 19.6 | 4.1 ± 4.1 | 0.2 |
| LMR (%) | 5.4 ± 3.4 | 3.9 ± 2.1 | 0.13 |
| SI | 694.8 ± 524.0 | 346.6 ± 219.0 | 0.046 * |
| IL-10 (µg/mL) | 2134.9 ± 2661.8 | 2488.3 ± 3113.3 | 0.815 |
| IL-17 (µg/mL) | 834.2 ± 1785.7 | 480.8 ± 606.7 | 0.741 |
| IL-12 (µg/mL) | 116.6 ± 93.8 | 91.4 ± 112.3 | 0.256 |
| IFN-γ (µg/mL) | 3943.1 ± 2695.9 | 3058 ± 2321.7 | 0.459 |
| VEGF (pg/mL) | 369.1 ± 380.1 | 2246.2 ± 4617.4 | 0.805 |
| MDSC (%PBMC) | 1.7 ± 1.7 | 1.5 ± 1.3 | 0.626 |
| BRAFV600E | |||
| Wild type | 8 | 1 | 0.047 * |
| Mutation | 12 | 12 | |
| CD8 | |||
| Negative | 14 | 2 | 0.003 * |
| Positive | 6 | 11 |
| Characteristics | HR | 95%CI Lower | 95%CI Upper | p-Value |
|---|---|---|---|---|
| Age | ||||
| <55 vs. ≥55 years | 2.37 | 0.75 | 7.49 | 0.14 |
| Sex | ||||
| Male vs. Female | 0.57 | 0.2 | 1.01 | 0.290 |
| Tumor size | ||||
| <20 vs. ≥20 (mm) | 7.79 | 1.74 | 34.78 | 0.007 * |
| Thyroid cancer | ||||
| DTC vs. ATC | 4.97 | 1.53 | 16.18 | 0.008 * |
| Ex | ||||
| Ex0 | 1 | / | / | / |
| Ex1 | 3.6 | 0.89 | 14.47 | 0.072 |
| Ex2 | 9.3 | 2.28 | 37.9 | 0.002 * |
| pT | ||||
| T1 | 1 | / | / | / |
| T2 | 1.28 | 0.13 | 12.38 | 0.829 |
| T3 | 3.8 | 0.84 | 17.22 | 0.084 |
| T4 | 8.12 | 2.07 | 31.86 | 0.003 * |
| pN | ||||
| N0 vs. N1 | 3.45 | 0.97 | 12.25 | 0.056 |
| M | ||||
| M0 vs. M1 | 5.73 | 1.98 | 16.58 | 0.001 * |
| Stage | ||||
| I–II vs. III–IV | 3.85 | 0.87 | 17.08 | 0.076 |
| CRP | ||||
| <0.15 vs. ≥0.15 (mg/dL) | 5.08 | 1.80 | 14.32 | 0.002 * |
| WBC | ||||
| <8.6 vs. ≥8.6 (103/μL) | 30.93 | 2.81 | 340.82 | 0.005 * |
| NLR | ||||
| Low vs. High | 2.08 | 0.69 | 6.23 | 0.191 |
| LMR | ||||
| Low vs. High | 0.58 | 0.20 | 1.69 | 0.321 |
| SI | ||||
| Low vs. High | 0.50 | 0.15 | 1.70 | 0.266 |
| VEGF | ||||
| Low vs. High | 9.02 | 1.26 | 64.76 | 0.029 * |
| IL10 | ||||
| Low vs. High | 0.31 | 0.07 | 1.49 | 0.145 |
| IL17 | ||||
| Low vs. High | 0.38 | 0.05 | 2.97 | 0.353 |
| IL12 | ||||
| Low vs. High | 0.43 | 0.09 | 2.02 | 0.283 |
| IFNγ | ||||
| Low vs. High | 3.16 | 0.88 | 11.29 | 0.077 |
| MDSC | ||||
| Low vs. High | 1.48 | 0.50 | 4.35 | 0.476 |
| CD8 | ||||
| Negative vs. Positive | 0.43 | 0.15 | 1.26 | 0.122 |
| BRAFV600E | ||||
| Wild type vs. Mutation | 0.27 | 0.10 | 0.76 | 0.013 * |
| PD-L1 | ||||
| Negative vs. Positive | 1.04 | 0.37 | 2.91 | 0.947 |
| Characteristics | HR | 95%CI Lower | 95%CI Upper | p-Value |
|---|---|---|---|---|
| CRP | ||||
| <0.15 vs. ≥0.15 (mg/dL) | 14.34 | 1.14 | 179.75 | 0.039 * |
| WBC | ||||
| <8.6 vs. ≥8.6 (103/μL) | 9.56 | 0.63 | 144.46 | 0.103 |
| VEGF | ||||
| Low vs. high | 3.83 | 0.31 | 47.41 | 0.295 |
| BRAFV600E | ||||
| Wild type vs. Mutation | 0.04 | 0.00 | 0.62 | 0.022 * |
| PD-L1 | ||||
| Negative vs. positive | 2.09 | 0.32 | 13.87 | 0.445 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekino, M.; Iwadate, M.; Yamaya, Y.; Matsumoto, Y.; Suzuki, S.; Mizunuma, H.; Nakano, K.; Nakamura, I.; Suzuki, S. Analysis of Expression of Programmed Cell Death Ligand 1 (PD-L1) and BRAFV600E Mutation in Thyroid Cancer. Cancers 2023, 15, 3449. https://doi.org/10.3390/cancers15133449
Sekino M, Iwadate M, Yamaya Y, Matsumoto Y, Suzuki S, Mizunuma H, Nakano K, Nakamura I, Suzuki S. Analysis of Expression of Programmed Cell Death Ligand 1 (PD-L1) and BRAFV600E Mutation in Thyroid Cancer. Cancers. 2023; 15(13):3449. https://doi.org/10.3390/cancers15133449
Chicago/Turabian StyleSekino, Mizuki, Manabu Iwadate, Yukie Yamaya, Yoshiko Matsumoto, Satoshi Suzuki, Hiroshi Mizunuma, Keiichi Nakano, Izumi Nakamura, and Shinichi Suzuki. 2023. "Analysis of Expression of Programmed Cell Death Ligand 1 (PD-L1) and BRAFV600E Mutation in Thyroid Cancer" Cancers 15, no. 13: 3449. https://doi.org/10.3390/cancers15133449
APA StyleSekino, M., Iwadate, M., Yamaya, Y., Matsumoto, Y., Suzuki, S., Mizunuma, H., Nakano, K., Nakamura, I., & Suzuki, S. (2023). Analysis of Expression of Programmed Cell Death Ligand 1 (PD-L1) and BRAFV600E Mutation in Thyroid Cancer. Cancers, 15(13), 3449. https://doi.org/10.3390/cancers15133449






