Transperineal US-MRI Fusion-Guided Biopsy for the Detection of Clinical Significant Prostate Cancer: A Systematic Review and Meta-Analysis Comparing Cognitive and Software-Assisted Technique
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
2.3. Study Screening and Selection
2.4. Statistical Analysis
3. Results
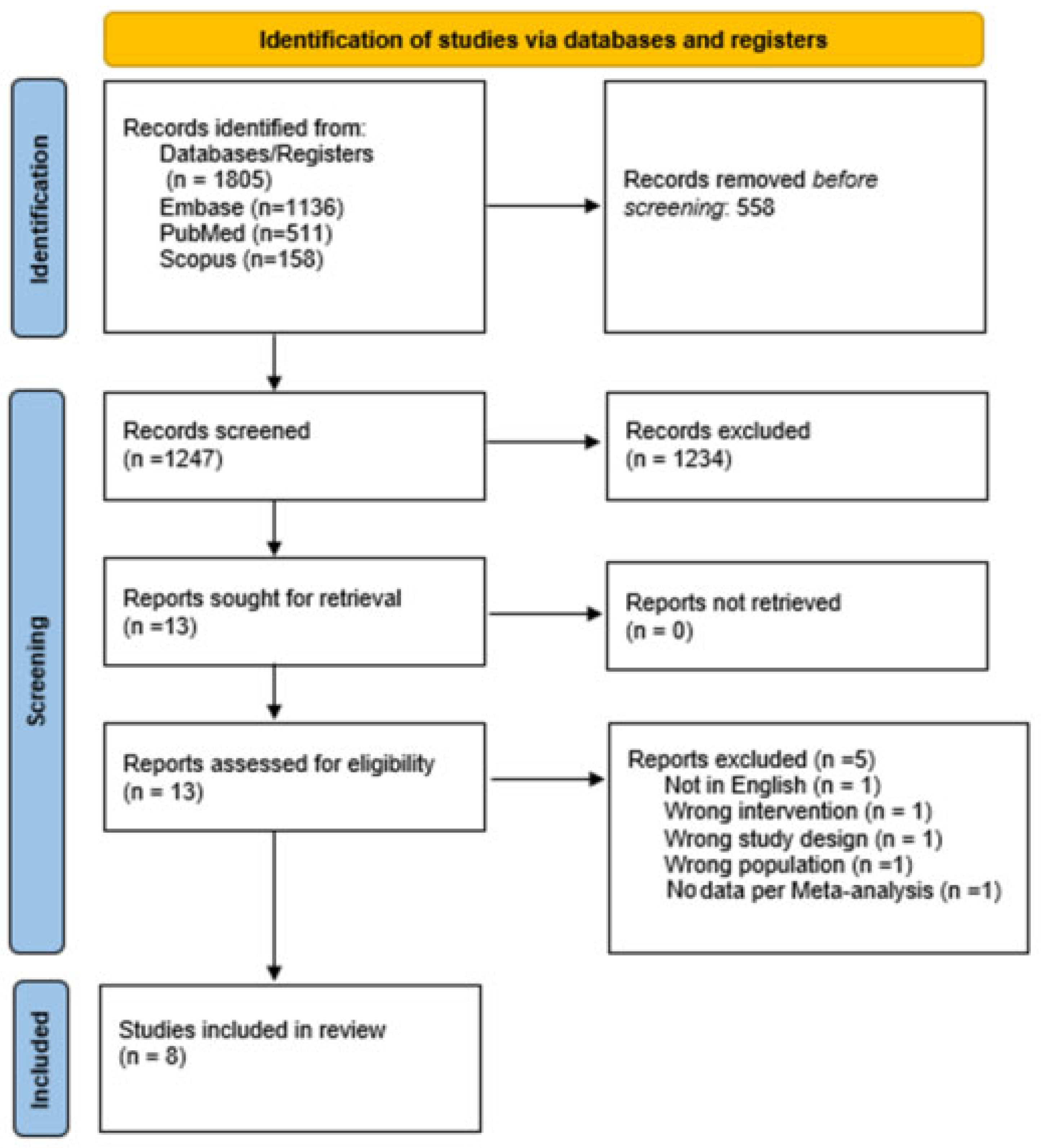
3.1. Literature Screening
3.2. Study Characteristics
3.3. Risk of Bias Assessment
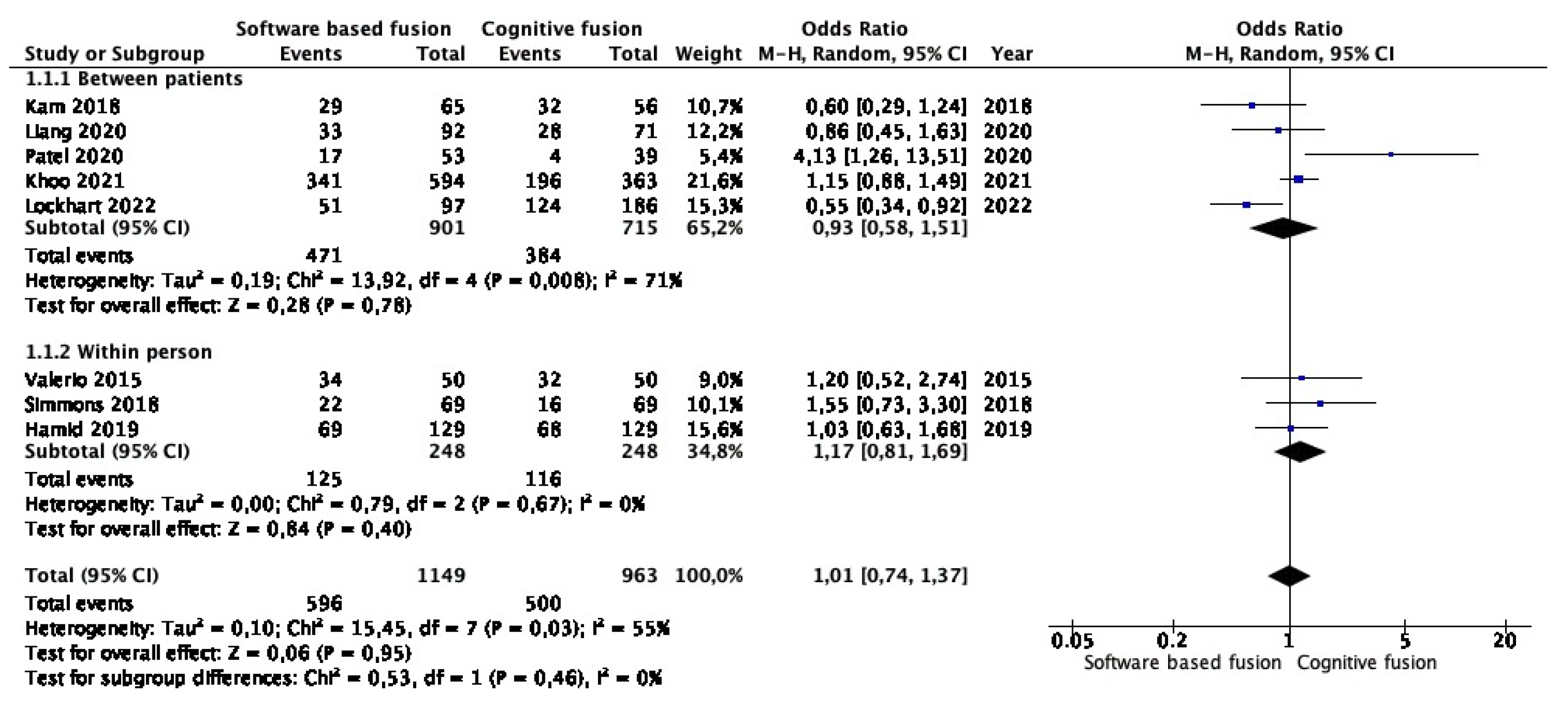
3.4. Meta-Analysis of Clinically Significant Prostate Cancer Detection Rates in Targeted Lesions
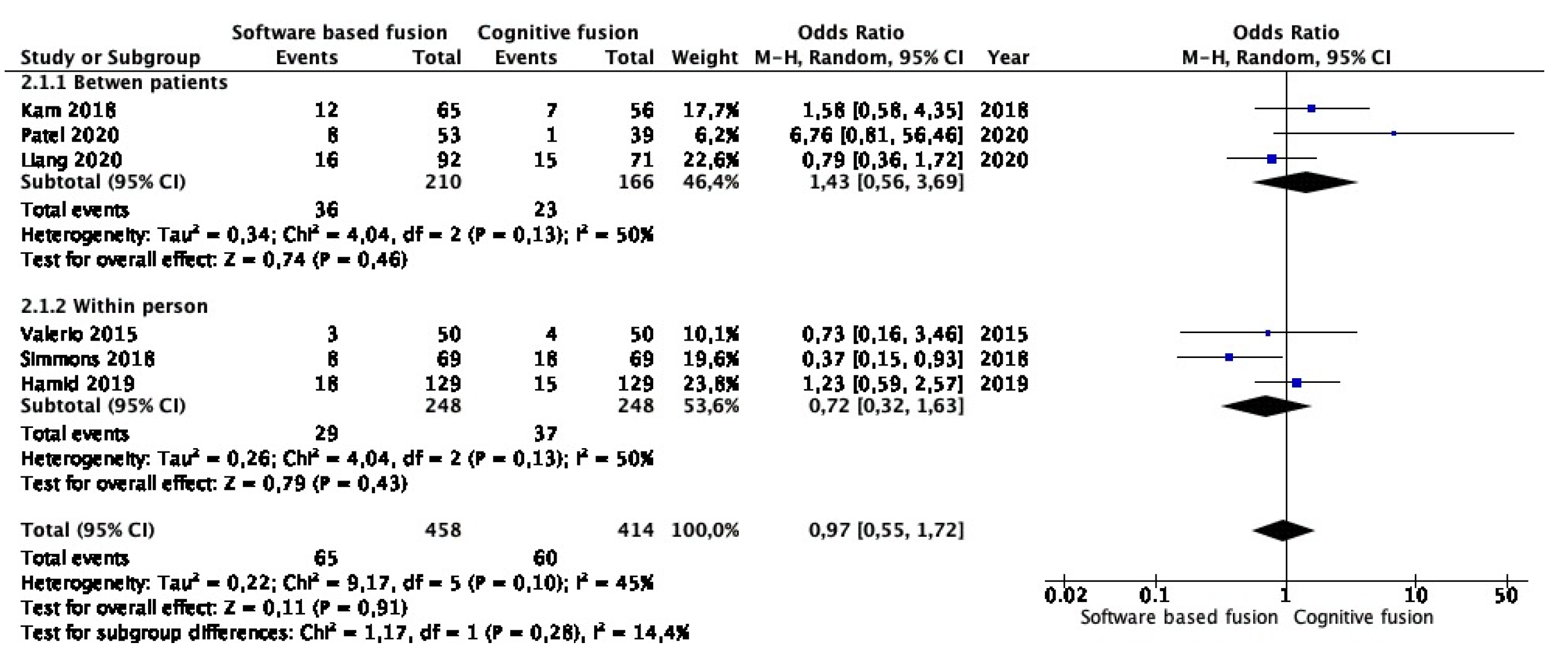
3.5. Meta-Analysis of Clinically Insignificant Prostate Cancer Detection Rates in Targeted Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cFB | cognitive fusion biopsy |
| csPCa | clinical significant prostate cancer |
| MA | meta-analysis |
| mpMRI | multiparametric magnetic resonance imaging |
| PB | prostate biopsy |
| PCa | prostate cancer |
| PIRADS | prostate imaging reporting and data system |
| saFB | software-assisted fusion biopsy |
| SB | systematic biopsy |
| SR | systematic review |
| TR | transrectal |
| TRUS | ultrasound |
| TP | transperineal |
References
- Arnold, M.; Karim-Kos, H.E.; Coebergh, J.W.; Byrnes, G.; Antilla, A.; Ferlay, J.; Renehan, A.G.; Forman, D.; Soerjomataram, I. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur. J. Cancer 2015, 51, 1164–1187. [Google Scholar] [CrossRef]
- Wei, G.; Ranasinghe, W.; Evans, M.; Bolton, D.; Dodds, L.; Frydenberg, M.; Kearns, P.; Lawrentschuk, N.; Murphy, D.G.; Millar, J.; et al. Decade-long trends in prostate cancer biopsy grade groups and treatment within a population-based registry. BJU Int. 2023, 131, 36–42. [Google Scholar] [CrossRef]
- Pepe, P.; Aragona, F. Prostate biopsy: Results and advantages of the transperineal approach—Twenty-year experience of a single center. World J. Urol. 2014, 32, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Ber, Y.; Segal, N.; Tamir, S.; Benjaminov, O.; Yakimov, M.; Sela, S.; Halstauch, D.; Baniel, J.; Kedar, D.; Margel, D. A noninferiority within-person study comparing the accuracy of transperineal to transrectal MRI–US fusion biopsy for prostate-cancer detection. Prostate Cancer Prostatic Dis. 2020, 23, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Feng, Z.; Trock, B.J.; Pierorazio, P.M. Upgrading and Downgrading of Prostate Cancer from Biopsy to Radical Prostatectomy: Incidence and Predictive Factors Using the Modified Gleason Grading System and Factoring in Tertiary Grades. Eur. Urol. 2012, 61, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Hamoen, E.H.; de Rooij, M.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. Eur. Urol. 2015, 67, 1112–1121. [Google Scholar] [CrossRef]
- Schoots, I.G.; Roobol, M.J.; Nieboer, D.; Bangma, C.H.; Steyerberg, E.W.; Hunink, M.M. Magnetic Resonance Imaging–targeted Biopsy May Enhance the Diagnostic Accuracy of Significant Prostate Cancer Detection Compared to Standard Transrectal Ultrasound-guided Biopsy: A Systematic Review and Meta-analysis. Eur. Urol. 2015, 68, 438–450. [Google Scholar] [CrossRef]
- Martorana, E.; Pirola, G.M.; Scialpi, M.; Micali, S.; Iseppi, A.; Bonetti, L.R.; Kaleci, S.; Torricelli, P.; Bianchi, G. Lesion volume predicts prostate cancer risk and aggressiveness: Validation of its value alone and matched with prostate imaging reporting and data system score. BJU Int. 2017, 120, 92–103. [Google Scholar] [CrossRef]
- Sonn, G.A.; Margolis, D.J.; Marks, L.S. Target detection: Magnetic resonance imaging-ultrasound fusion–guided prostate biopsy. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 903–911. [Google Scholar] [CrossRef]
- Wegelin, O.; Exterkate, L.; van der Leest, M.; Kummer, J.A.; Vreuls, W.; de Bruin, P.C.; Bosch, J.; Barentsz, J.O.; Somford, D.M.; van Melick, H.H. The FUTURE Trial: A Multicenter Randomised Controlled Trial on Target Biopsy Techniques Based on Magnetic Resonance Imaging in the Diagnosis of Prostate Cancer in Patients with Prior Negative Biopsies. Eur. Urol. 2019, 75, 582–590. [Google Scholar] [CrossRef]
- Stabile, A.; Dell’oglio, P.; Gandaglia, G.; Fossati, N.; Brembilla, G.; Cristel, G.; Dehò, F.; Scattoni, V.; Maga, T.; Losa, A.; et al. Not All Multiparametric Magnetic Resonance Imaging–targeted Biopsies Are Equal: The Impact of the Type of Approach and Operator Expertise on the Detection of Clinically Significant Prostate Cancer. Eur. Urol. Oncol. 2018, 1, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Watts, K.L.; Frechette, L.; Muller, B.; Ilinksy, D.; Kovac, E.; Sankin, A.; Aboumohamed, A. Systematic review and meta-analysis comparing cognitive vs. image-guided fusion prostate biopsy for the detection of prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 734.e19–734.e25. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Liang, L.; Cheng, Y.; Qi, F.; Zhang, L.; Cao, D.; Cheng, G.; Hua, L. A Comparative Study of Prostate Cancer Detection Rate Between Transperineal COG-TB and Transperineal FUS-TB in Patients with PSA ≤20 ng/mL. J. Endourol. 2020, 34, 1008–1014. [Google Scholar] [CrossRef]
- Khoo, C.C.; Eldred-Evans, D.; Peters, M.; van Son, M.; van Rossum, P.S.N.; Connor, M.J.; Hosking-Jervis, F.; Tanaka, M.B.; Reddy, D.; Bass, E.; et al. A Comparison of Prostate Cancer Detection between Visual Estimation (Cognitive Registration) and Image Fusion (Software Registration) Targeted Transperineal Prostate Biopsy. J. Urol. 2021, 205, 1075–1081. [Google Scholar] [CrossRef]
- Kam, J.; Yuminaga, Y.; Kim, R.; Aluwihare, K.; Macneil, F.; Ouyang, R.; Ruthven, S.; Louie-Johnsun, M. Does magnetic resonance imaging–guided biopsy improve prostate cancer detection? A comparison of systematic, cognitive fusion and ultrasound fusion prostate biopsy. Prostate Int. 2018, 6, 88–93. [Google Scholar] [CrossRef]
- Lockhart, K.; Martin, J.; White, M.; Raman, A.; Grant, A.; Chong, P. Fusion versus cognitive MRI-guided prostate biopsies in diagnosing clinically significant prostate cancer. J. Clin. Urol. 2022, 24, 1103–1109. [Google Scholar] [CrossRef]
- Patel, M.I.; Muter, S.; Vladica, P.; Gillatt, D. Robotic-assisted magnetic resonance imaging ultrasound fusion results in higher significant cancer detection compared to cognitive prostate targeting in biopsy naive men. Transl. Androl. Urol. 2020, 9, 601–608. [Google Scholar] [CrossRef]
- Hamid, S.; Donaldson, I.A.; Hu, Y.; Rodell, R.; Villarini, B.; Bonmati, E.; Tranter, P.; Punwani, S.; Sidhu, H.S.; Willis, S.; et al. The SmartTarget Biopsy Trial: A Prospective, Within-person Randomised, Blinded Trial Comparing the Accuracy of Visual-registration and Magnetic Resonance Imaging/Ultrasound Image-fusion Targeted Biopsies for Prostate Cancer Risk Stratification. Eur. Urol. 2019, 75, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.A.; Kanthabalan, A.; Arya, M.; Briggs, T.; Barratt, D.; Charman, S.C.; Freeman, A.; Hawkes, D.; Hu, Y.; Jameson, C.; et al. Accuracy of Transperineal Targeted Prostate Biopsies, Visual Estimation and Image Fusion in Men Needing Repeat Biopsy in the PICTURE Trial. J. Urol. 2018, 200, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.; McCartan, N.; Freeman, A.; Punwani, S.; Emberton, M.; Ahmed, H.U. Visually directed vs. software-based targeted biopsy compared to transperineal template mapping biopsy in the detection of clinically significant prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 424.e9–424.e16. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Bergh, R.C.V.D.; et al. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clinically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef]
- Orecchia, L.; Nardi, A.; Fletcher, P.; Ippoliti, S.; Grounds, J.; Dokubo, I.; Spicchiale, C.F.; Miah, S.; Miano, R.; Barrett, T.; et al. Natural History of Patients with Prostate MRI Likert 1-3 and Development of RosCaP: A Multivariate Risk Score for Clinically Significant Cancer. Clin. Genitourin. Cancer 2023, 21, 162–170. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. ECISION Study Group Collaborators. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Zattoni, F.; Marra, G.; Kasivisvanathan, V.; Grummet, J.; Nandurkar, R.; Ploussard, G.; Olivier, J.; Chiu, P.K.; Valerio, M.; Gontero, P.; et al. The Detection of Prostate Cancer with Magnetic Resonance Imaging-Targeted Prostate Biopsies is Superior with the Transperineal vs the Transrectal Approach. A European Association of Urology-Young Academic Urologists Prostate Cancer Working Group Multi-Institutional Study. J. Urol. 2022, 208, 830–837. [Google Scholar] [CrossRef]
- Hogenhout, R.; Remmers, S.; van Leenders, G.J.L.H.; Roobol, M.J. The transition from transrectal to transperineal prostate biopsy without antibiotic prophylaxis: Cancer detection rates and complication rates. Prostate Cancer Prostatic Dis. 2023. ahead of print. [Google Scholar] [CrossRef]
- Castellani, D.; Pirola, G.M.; Law, Y.X.T.; Gubbiotti, M.; Giulioni, C.; Scarcella, S.; Wroclawski, M.L.; Chan, E.; Chiu, P.K.-F.; Teoh, J.Y.-C.; et al. Infection Rate after Transperineal Prostate Biopsy with and without Prophylactic Antibiotics: Results from a Systematic Review and Meta-Analysis of Comparative Studies. J. Urol. 2022, 207, 25–34. [Google Scholar] [CrossRef]
- Pirola, G.M.; Gubbiotti, M.; Rubilotta, E.; Castellani, D.; Trabacchin, N.; Tafuri, A.; Princiotta, A.; Martorana, E.; Annino, F.; Antonelli, A. Is antibiotic prophylaxis still mandatory for transperineal prostate biopsy? Results of a comparative study. Prostate Int. 2022, 10, 34–37. [Google Scholar] [CrossRef]
- Ukimura, O.; Desai, M.M.; Palmer, S.; Valencerina, S.; Gross, M.; Abreu, A.L.; Aron, M.; Gill, I.S. 3-Dimensional Elastic Registration System of Prostate Biopsy Location by Real-Time 3-Dimensional Transrectal Ultrasound Guidance With Magnetic Resonance/Transrectal Ultrasound Image Fusion. J. Urol. 2012, 187, 1080–1086. [Google Scholar] [CrossRef]
- Valerio, M.; Donaldson, I.; Emberton, M.; Ehdaie, B.; Hadaschik, B.A.; Marks, L.S.; Mozer, P.; Rastinehad, A.R.; Ahmed, H.U. Detection of Clinically Significant Prostate Cancer Using Magnetic Resonance Imaging–Ultrasound Fusion Targeted Biopsy: A Systematic Review. Eur. Urol. 2015, 68, 8–19. [Google Scholar] [CrossRef]
- Ippoliti, S.; Fletcher, P.; Orecchia, L.; Miano, R.; Kastner, C.; Barrett, T. Optimal biopsy approach for detection of clinically significant prostate cancer. Br. J. Radiol. 2022, 95, 20210413. [Google Scholar] [CrossRef]
- Fletcher, P.; De Santis, M.; Ippoliti, S.; Orecchia, L.; Charlesworth, P.; Barrett, T.; Kastner, C. Vector Prostate Biopsy: A Novel Magnetic Resonance Imaging/Ultrasound Image Fusion Transperineal Biopsy Technique Using Electromagnetic Needle Tracking Under Local Anaesthesia. Eur. Urol. 2023, 83, 249–256. [Google Scholar] [CrossRef]
- Barrett, T.; Padhani, A.R.; Patel, A.; Ahmed, H.U.; Allen, C.; Bardgett, H.; Belfield, J.; Appayya, M.B.; Harding, T.; Hoch, O.; et al. Certification in reporting multiparametric magnetic resonance imaging of the prostate: Recommendations of a UK consensus meeting. BJU Int. 2021, 127, 304–306. [Google Scholar] [CrossRef]
- Rodrigues, C.; Visram, K.; Sedghi, A.; Mousavi, P.; Siemens, D.R. Attitudes and experience of urology trainees in interpreting prostate magnetic resonance imaging. Can. Urol. Assoc. J. 2021, 15, E293–E298. [Google Scholar] [CrossRef]
- Ippoliti, S.; Orecchia, L.; Esperto, F.; Wroclawski, M.L.; Manenti, G.; Barrett, T.; Kastner, C.; Miano, R. Survey on prostate MRI reading and interpretation among urology residents in Italy, Brazil and the UK: A cry for help. Minerva Urol. Nephrol. 2022, 75, 297–307. [Google Scholar] [CrossRef]
- Petov, V.; Azilgareeva, C.; Shpikina, A.; Morozov, A.; Krupinov, G.; Kozlov, V.; Singla, N.; Rivas, J.G.; Jesús, M.-S.; Puliatti, S.; et al. Robot-Assisted Magnetic Resonance Imaging-Targeted versus Systematic Prostate Biopsy; Systematic Review and Meta-Analysis. Cancers 2023, 15, 1181. [Google Scholar] [CrossRef]
- Martorana, E.; Pirola, G.M.; Aisa, M.C.; Scialpi, P.; Di Blasi, A.; Saredi, G.; D’Andrea, A.; Signore, S.; Grisanti, R.; Scialpi, M.; et al. Prostate MRI and transperineal TRUS/MRI fusion biopsy for prostate cancer detection: Clinical practice updates. Urol. Res. Pract. 2019, 45, 237–244. [Google Scholar] [CrossRef]
- Porpiglia, F.; Checcucci, E.; DE Cillis, S.; Piramide, F.; Amparore, D.; Piana, A.; Volpi, G.; Granato, S.; Zamengo, D.; Stura, I.; et al. A prospective randomized controlled trial comparing target prostate biopsy alone approach vs. target plus standard in naïve patients with positive mpMRI. Minerva Urol. Nephrol. 2023, 75, 31–41. [Google Scholar] [CrossRef] [PubMed]
| Authors [REF] | Study Design | Fusion Type | saFB Cohort | cFB Cohort | Final Comment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients n | PSA ng/mL (%) | MRI Targets n (%) | Targeted Cores n | csPCa Target Biopsy n (%) | ciPCa Target Biopsy n (%) | Patients n | PSA ng/mL (%) | MRI Targets n (%) | Targeted Cores n | csPCa Target Biopsy n (%) | ciPCa Target Biopsy n (%) | ||||
| Liang et al. 2020 [16] | Retrospective | Rigid (Predictive Fusion Software; BK medical, Herlev, Denmark) | 92 | 8.03 | NR | 4 | 33 (35.87%) | 14 (15.2%) | 71 | 7.66 | NR | 4 | 28 (39.43) | 15 (21.1%) | Cognitive and fusion targeting detect similar rates of csPCa |
| Khoo et al. 2021 [17] | Retrospective | Elastic (BiopSee, Medcom, Darmstadt, Germany | 594 | 7.9 | NR | NR | 341 (57.4%) | NR | 363 | 8.4 | NR | NR | 196 (54.0%) | Cognitive and fusion targeting detect similar rates of csPCa, although fusion biopsy may be superior in experienced hands | |
| Kam et al. 2018 [18] | Prospective | Not specified (Biojet, D&K Technologies GmbH, Barum, Germany | 65 | 7.3 | PIRADS 3: 28 (43%); PIRADS4 or 5:37 (57%) | 4.6 | 29 (44.6%) | 12 (18.4%) | 56 | 7.5 | PIRADS 3: 18 (32%) PIRADS 4 or 5: 38 (68%) | 3.1 | 32 (57.1%) | 7 (12.5%) | Cognitive and fusion targeting detect similar rates of csPCa. Cognitive biopsy had a significantly higher core positivity rate than fusion biopsy. |
| Lockhart et al. 2022 [19] | Prospective | Not specified (MIM Bx, MIM Software Inc, Cleveland, OH, USA | 131 | 5.8 | NR | NR | 52 (39.70%) | NR | 224 | 7.64 | NR | NR | 120 (53.60%) | NR | Cognitive and fusion targeting detect similar rates of csPCa |
| Patel et al. 2020 [20] | Retrospective | Elastic (Urofusion, Biobot Surgical, Singapore, Singapore) | 53 | <4: 11 (20.8%) 4-10: 40 (75.5%) >10: 2 (3.8%) | PIRADS 3: 14 (26.4%) PIRADS 4: 28 (58.2%) PIRADS 5:11 (20.8%) | 4 | 17 (32.1%) | 8 (15.1%) | 39 | <4: 9 (23.1%) 4-10: 22 (56.4%) >10: 8 (20.5%) | PIRADS 3: 14 (38.5%) PIRADS 4: 13 (33.3%) PIRADS 5: 11 (28.2%) | 3 | 4 (10.3%) | 1 (2.6%) | Robot-assisted fusion targeting detects a significantly higher percentage of csPCa than cognitive targeting |
| Hamid et al. 2019 [21] | Randomized Controlled Trial | Elastic (SmartTarget software, London, UK) | 129 | 8.5 | Likert 3: 22 (17%) Likert 4: 67 (52%) Likert 5: 40 (31%) | 3 | 69 (54%) | 18 (14%) | 129 | 8.5 | Likert 3: 22 (17%) Likert 4: 67 (52%) Likert 5: 40 (31%) | 3 | 68 (53%) | 15 (12%) | Cognitive and fusion targeting detect similar rates of csPCa |
| Simmons et al. 2018 [22] | Prospective, Comparative Trial | Elastic (SmartTarget software, London, UK) | 69 | NR | Likert | 4 | 22 (31.8%) | 8 (11.6%) | 69 | NR | Likert | 4 | 16 (23.2%) | 18 (26.1%) | csPCa defined as any grade of cancer core with a length of 4 mm or greater and/or any length of cancer with a Gleason score of 3 + 4 = 7 or greater (UCL/Ahmed definition)—Cognitive and fusion targeting detect similar rates of csPCa |
| Valerio et al. 2015 [23] | Prospective | Rigid (Biojet, D&K Technologies GmbH, Barum, Germany) | 50 | 7.9 | Likert 3: 27 (34%) Likert 4: 28 (35%) Likert 5: 24 (31%) | 3 | 34 (68%) | 3 (6%) | 50 | 7.9 | Likert 3: 27 (34%) Likert 4: 28 (35%) Likert 5: 24 (31%) | 4 | 32 (64%) | 4 (8%) | Cognitive and fusion targeting detect similar rates of csPCa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirola, G.M.; Castellani, D.; Orecchia, L.; Giulioni, C.; Gubbiotti, M.; Rubilotta, E.; Maggi, M.; Teoh, J.Y.-C.; Gauhar, V.; Naselli, A. Transperineal US-MRI Fusion-Guided Biopsy for the Detection of Clinical Significant Prostate Cancer: A Systematic Review and Meta-Analysis Comparing Cognitive and Software-Assisted Technique. Cancers 2023, 15, 3443. https://doi.org/10.3390/cancers15133443
Pirola GM, Castellani D, Orecchia L, Giulioni C, Gubbiotti M, Rubilotta E, Maggi M, Teoh JY-C, Gauhar V, Naselli A. Transperineal US-MRI Fusion-Guided Biopsy for the Detection of Clinical Significant Prostate Cancer: A Systematic Review and Meta-Analysis Comparing Cognitive and Software-Assisted Technique. Cancers. 2023; 15(13):3443. https://doi.org/10.3390/cancers15133443
Chicago/Turabian StylePirola, Giacomo Maria, Daniele Castellani, Luca Orecchia, Carlo Giulioni, Marilena Gubbiotti, Emanuele Rubilotta, Martina Maggi, Jeremy Yuen-Chun Teoh, Vineet Gauhar, and Angelo Naselli. 2023. "Transperineal US-MRI Fusion-Guided Biopsy for the Detection of Clinical Significant Prostate Cancer: A Systematic Review and Meta-Analysis Comparing Cognitive and Software-Assisted Technique" Cancers 15, no. 13: 3443. https://doi.org/10.3390/cancers15133443
APA StylePirola, G. M., Castellani, D., Orecchia, L., Giulioni, C., Gubbiotti, M., Rubilotta, E., Maggi, M., Teoh, J. Y.-C., Gauhar, V., & Naselli, A. (2023). Transperineal US-MRI Fusion-Guided Biopsy for the Detection of Clinical Significant Prostate Cancer: A Systematic Review and Meta-Analysis Comparing Cognitive and Software-Assisted Technique. Cancers, 15(13), 3443. https://doi.org/10.3390/cancers15133443








