Multi-Omic Biomarkers Improve Indeterminate Pulmonary Nodule Malignancy Risk Assessment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
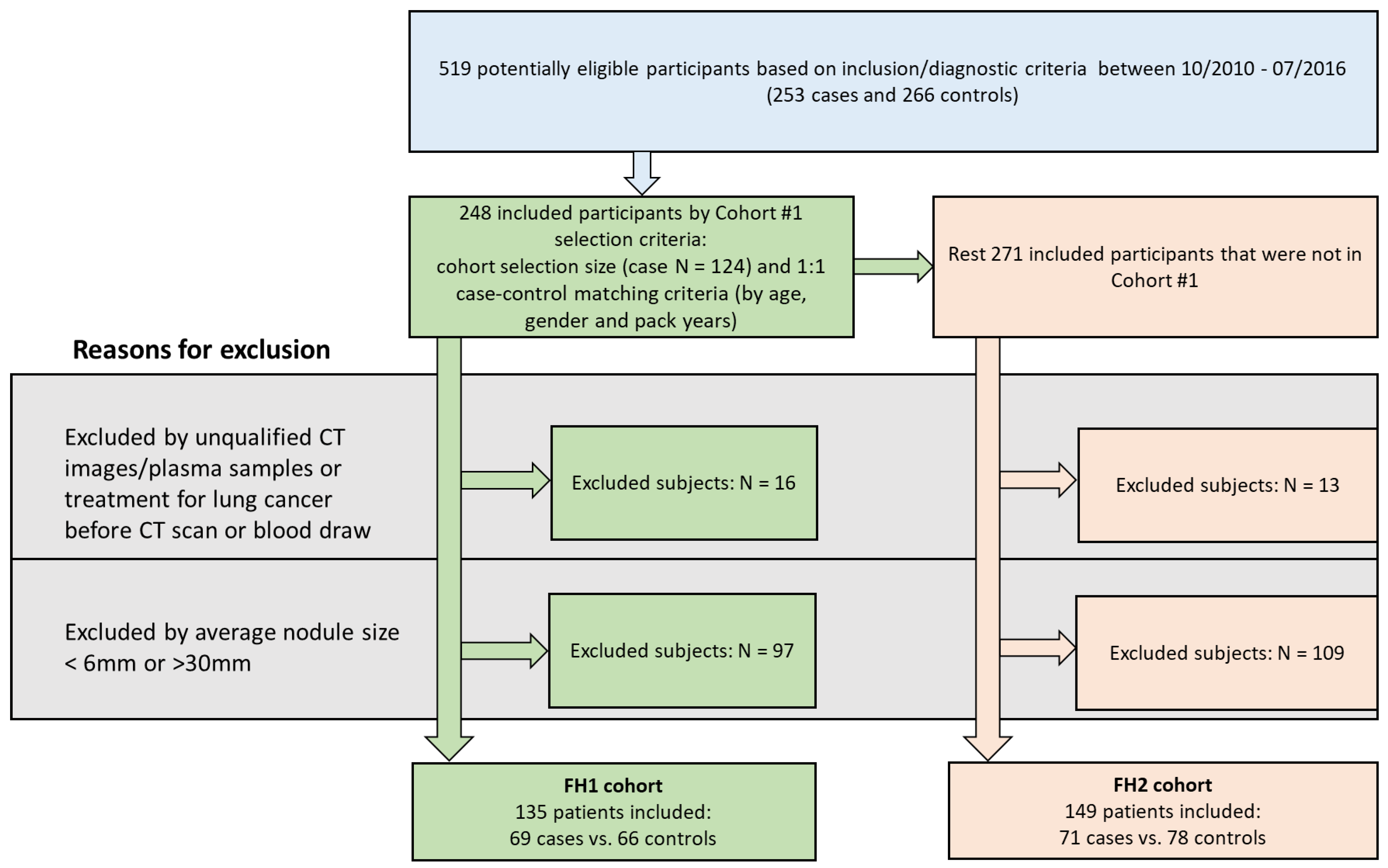
2.1. Study Cohorts
2.2. Multidimensional Array Analysis of Plasma Samples
2.3. Protein Analysis
2.4. Sialyl Lewis-X- and -A-Modified Protein Analysis
2.5. Autoantibody–Antigen Complex Analysis
2.6. Semantic and Quantitative Imaging Feature Analysis
2.7. Model and Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics
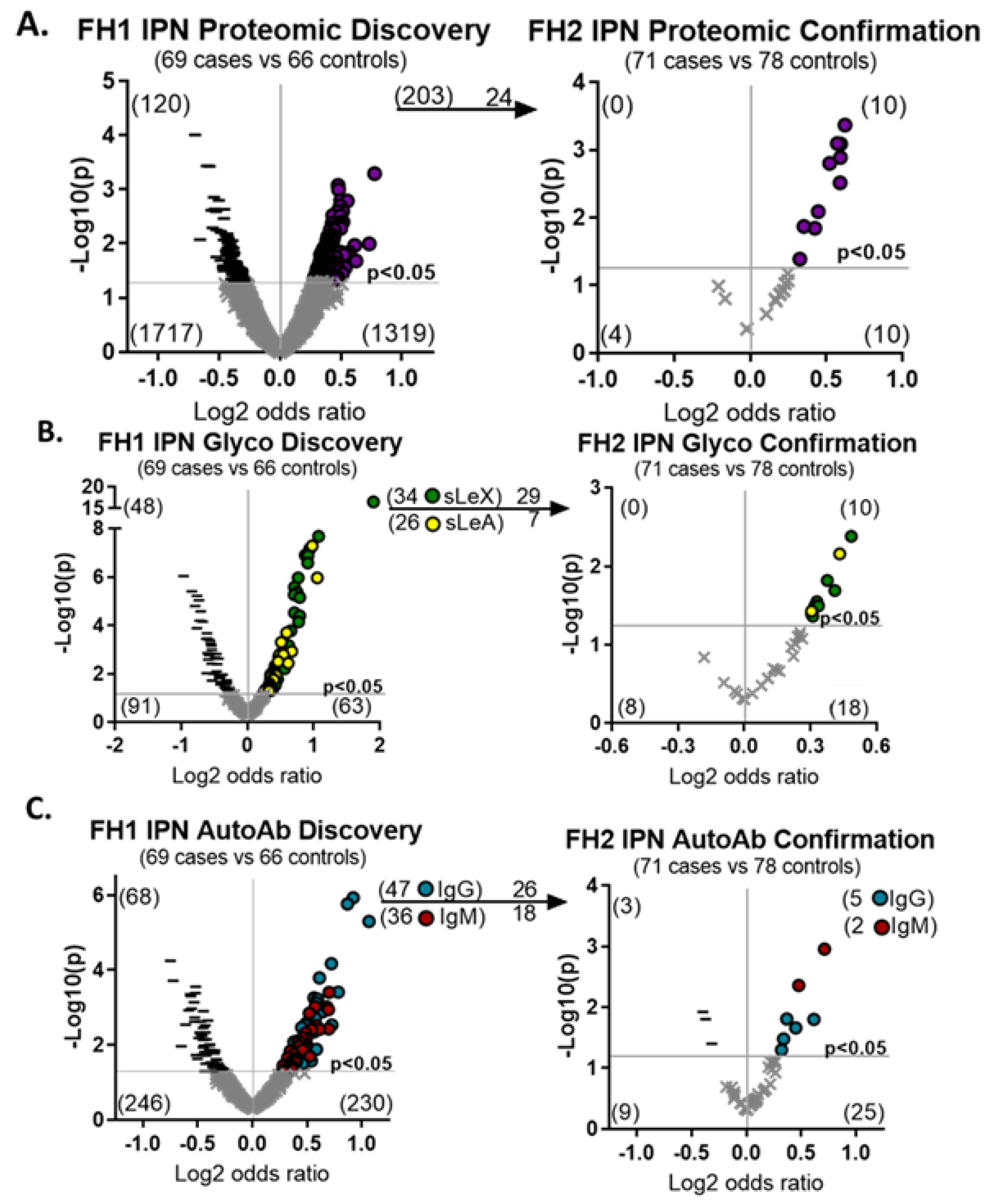
3.2. Specific Semantic Imaging Features and Upregulated Molecular Biomarkers Are Associated with Malignant IPNs
3.3. A Combination Risk Prediction Model Can Accurately Assess Indeterminate Pulmonary Nodules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.A.; Goldman, L.; Tanner, N.T.; Burleson, J.; Gould, M.; Kazerooni, E.A.; Mazzone, P.J.; Rivera, M.P.; Doria-Rose, V.P.; Rosenthal, L.S.; et al. Outcomes from More Than 1 Million People Screened for Lung Cancer With Low-Dose CT Imaging. Chest 2023, in press. [Google Scholar] [CrossRef]
- Gould, M.K.; Tang, T.; Liu, I.-L.A.; Lee, J.; Zheng, C.; Danforth, K.N.; Kosco, A.E.; Di Fiore, J.L.; Suh, D.E. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am. J. Respir. Crit. Care Med. 2015, 192, 1208–1214. [Google Scholar] [CrossRef]
- Swensen, S.J.; Silverstein, M.D.; Ilstrup, D.M.; Schleck, C.D.; Edell, E.S. The Probability of Malignancy in Solitary Pulmonary Nodules: Application to Small Radiologically Indeterminate Nodules. Arch. Intern. Med. 1997, 157, 849–855. [Google Scholar] [CrossRef]
- McWilliams, A.; Tammemagi, M.C.; Mayo, J.R.; Roberts, H.; Liu, G.; Soghrati, K.; Yasufuku, K.; Martel, S.; Laberge, F.; Gingras, M.; et al. Probability of Cancer in Pulmonary Nodules Detected on First Screening CT. N. Engl. J. Med. 2013, 369, 910–919. [Google Scholar] [CrossRef]
- Gould, M.K.; Ananth, L.; Barnett, P.G.; Veterans Affairs SNAP Cooperative Study Group. A Clinical Model to Estimate the Pretest Probability of Lung Cancer in Patients with Solitary Pulmonary Nodules. Chest 2007, 131, 383–388. [Google Scholar] [CrossRef]
- Chelala, L.; Hossain, R.; Kazerooni, E.A.; Christensen, J.D.; Dyer, D.S.; White, C.S. Lung-RADS Version 1.1: Challenges and a Look Ahead, From the AJR Special Series on Radiology Reporting and Data Systems. Am. J. Roentgenol. 2021, 216, 1411–1422. [Google Scholar] [CrossRef]
- Wood, D.E.; Kazerooni, E.A.; Baum, S.L.; Eapen, G.A.; Ettinger, D.S.; Hou, L.; Jackman, D.M.; Klippenstein, D.; Kumar, R.; Lackner, R.P.; et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 412–441. [Google Scholar] [CrossRef]
- MacMahon, H.; Austin, J.H.M.; Gamsu, G.; Herold, C.J.; Jett, J.R.; Naidich, D.P.; Patz, E.F.; Swensen, S.J. Guidelines for Management of Small Pulmonary Nodules Detected on CT Scans: A Statement from the Fleischner Society. Radiology 2005, 237, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.; Powell, C.A.; Arenberg, D.; Bach, P.; Detterbeck, F.; Gould, M.K.; Jaklitsch, M.T.; Jett, J.; Naidich, D.; Vachani, A.; et al. Components Necessary for High-Quality Lung Cancer Screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest 2015, 147, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of Individuals with Pulmonary Nodules: When Is It Lung Cancer? Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143, e93S–e120S. [Google Scholar] [CrossRef] [PubMed]
- Ost, D.E.; Gould, M.K. Decision Making in Patients with Pulmonary Nodules. Am. J. Respir. Crit. Care Med. 2012, 185, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Lokhandwala, T.; Bittoni, M.A.; Dann, R.A.; D’Souza, A.O.; Johnson, M.; Nagy, R.J.; Lanman, R.B.; Merritt, R.E.; Carbone, D.P. Costs of Diagnostic Assessment for Lung Cancer: A Medicare Claims Analysis. Clin. Lung Cancer 2017, 18, e27–e34. [Google Scholar] [CrossRef]
- Freiman, M.R.; Clark, J.A.; Slatore, C.G.; Gould, M.K.; Woloshin, S.; Schwartz, L.M.; Wiener, R.S. Patients’ Knowledge, Beliefs, and Distress Associated with Detection and Evaluation of Incidental Pulmonary Nodules for Cancer: Results from a Multicenter Survey. J. Thorac. Oncol. 2016, 11, 700–708. [Google Scholar] [CrossRef]
- Shur, J.D.; Doran, S.J.; Kumar, S.; Ap Dafydd, D.; Downey, K.; O’Connor, J.P.B.; Papanikolaou, N.; Messiou, C.; Koh, D.-M.; Orton, M.R. Radiomics in Oncology: A Practical Guide. Radiographics 2021, 41, 1717–1732. [Google Scholar] [CrossRef]
- Abbasian Ardakani, A.; Bureau, N.J.; Ciaccio, E.J.; Acharya, U.R. Interpretation of Radiomics Features-A Pictorial Review. Comput. Methods Programs Biomed. 2022, 215, 106609. [Google Scholar] [CrossRef]
- Yip, S.S.F.; Liu, Y.; Parmar, C.; Li, Q.; Liu, S.; Qu, F.; Ye, Z.; Gillies, R.J.; Aerts, H.J.W.L. Associations between Radiologist-Defined Semantic and Automatically Computed Radiomic Features in Non-Small Cell Lung Cancer. Sci. Rep. 2017, 7, 3519. [Google Scholar] [CrossRef]
- Loch, C.M.; Ramirez, A.B.; Liu, Y.; Sather, C.L.; Delrow, J.J.; Scholler, N.; Garvik, B.M.; Urban, N.D.; McIntosh, M.W.; Lampe, P.D. Use of High Density Antibody Arrays to Validate and Discover Cancer Serum Biomarkers. Mol. Oncol. 2007, 1, 313–320. [Google Scholar] [CrossRef]
- Rho, J.; Lampe, P.D. High-Throughput Screening for Native Autoantigen-Autoantibody Complexes Using Antibody Microarrays. J. Proteome Res. 2013, 12, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.; Mead, J.R.; Wright, W.S.; Brenner, D.E.; James, W.S.; Gildersleeve, J.C.; Lampe, P.D. Discovery of Sialyl Lewis A and Lewis X Modified Protein Cancer Biomarkers Using High Density Antibody Arrays. J. Proteom. 2014, 96, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.B.; Loch, C.M.; Zhang, Y.; Liu, Y.; Wang, X.; Wayner, E.A.; Sargent, J.E.; Sibani, S.; Hainsworth, E.; Mendoza, E.A.; et al. Use of a Single-Chain Antibody Library for Ovarian Cancer Biomarker Discovery. Mol. Cell. Proteom. 2010, 9, 1449–1460. [Google Scholar] [CrossRef]
- Ramirez, A.B.; Lampe, P.D. Discovery and Validation of Ovarian Cancer Biomarkers Utilizing High Density Antibody Microarrays. Cancer Biomark. 2010, 8, 293–307. [Google Scholar] [CrossRef]
- Li, C.I.; Mirus, J.E.; Zhang, Y.; Ramirez, A.B.; Ladd, J.J.; Prentice, R.L.; McIntosh, M.W.; Hanash, S.M.; Lampe, P.D. Discovery and Preliminary Confirmation of Novel Early Detection Biomarkers for Triple-Negative Breast Cancer Using Preclinical Plasma Samples from the Women’s Health Initiative Observational Study. Breast Cancer Res. Treat. 2012, 135, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Mirus, J.E.; Zhang, Y.; Hollingsworth, M.A.; Solan, J.L.; Lampe, P.D.; Hingorani, S.R. Spatiotemporal Proteomic Analyses during Pancreas Cancer Progression Identifies Serine/Threonine Stress Kinase 4 (STK4) as a Novel Candidate Biomarker for Early Stage Disease. Mol. Cell. Proteom. 2014, 13, 3484–3496. [Google Scholar] [CrossRef]
- Mirus, J.E.; Zhang, Y.; Li, C.I.; Lokshin, A.E.; Prentice, R.L.; Hingorani, S.R.; Lampe, P.D. Cross-Species Antibody Microarray Interrogation Identifies a 3-Protein Panel of Plasma Biomarkers for Early Diagnosis of Pancreas Cancer. Clin. Cancer Res. 2015, 21, 1764–1771. [Google Scholar] [CrossRef]
- Rho, J.-H.; Ladd, J.J.; Li, C.I.; Potter, J.D.; Zhang, Y.; Shelley, D.; Shibata, D.; Coppola, D.; Yamada, H.; Toyoda, H.; et al. Protein and Glycomic Plasma Markers for Early Detection of Adenoma and Colon Cancer. Gut 2018, 67, 473–484. [Google Scholar] [CrossRef]
- Lastwika, K.J.; Kunihiro, A.; Solan, J.L.; Zhang, Y.; Taverne, L.R.; Shelley, D.; Rho, J.-H.; Randolph, T.W.; Li, C.I.; Grogan, E.L.; et al. Posttranslational Modifications Induce Autoantibodies with Risk Prediction Capability in Patients with Small Cell Lung Cancer. Sci. Transl. Med. 2023, 15, eadd8469. [Google Scholar] [CrossRef]
- Lastwika, K.J.; Kargl, J.; Zhang, Y.; Zhu, X.; Lo, E.; Shelley, D.; Ladd, J.J.; Wu, W.; Kinahan, P.; Pipavath, S.N.J.; et al. Tumor-Derived Autoantibodies Identify Malignant Pulmonary Nodules. Am. J. Respir. Crit. Care Med. 2019, 199, 1257–1266. [Google Scholar] [CrossRef]
- Rho, J.-H.; Lampe, P.D. High-Throughput Analysis of Plasma Hybrid Markers for Early Detection of Cancers. Proteomes 2014, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Society. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Hoerl, A.E.; Kennard, R.W. Ridge Regression: Biased Estimation for Nonorthogonal Problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Macheret, F.; Gabriel, R.A.; Ohno-Machado, L. A Tutorial on Calibration Measurements and Calibration Models for Clinical Prediction Models. J. Am. Med. Inform. Assoc. 2020, 27, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and Localization of Surgically Resectable Cancers with a Multi-Analyte Blood Test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.M.; Hatabu, H. Subsolid Pulmonary Nodules: Controversy and Perspective. Eur. J. Radiol. Open 2020, 7, 100267. [Google Scholar] [CrossRef]
- Ligero, M.; Torres, G.; Sanchez, C.; Diaz-Chito, K.; Perez, R.; Gil, D. Selection of Radiomics Features Based on Their Reproducibility. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 403–408. [Google Scholar]
- Bologna, M.; Corino, V.; Mainardi, L. Technical Note: Virtual Phantom Analyses for Preprocessing Evaluation and Detection of a Robust Feature Set for MRI-Radiomics of the Brain. Med. Phys. 2019, 46, 5116–5123. [Google Scholar] [CrossRef]
- Zhai, T.-T.; Wesseling, F.; Langendijk, J.A.; Shi, Z.; Kalendralis, P.; van Dijk, L.V.; Hoebers, F.; Steenbakkers, R.J.H.M.; Dekker, A.; Wee, L.; et al. External Validation of Nodal Failure Prediction Models Including Radiomics in Head and Neck Cancer. Oral Oncol. 2021, 112, 105083. [Google Scholar] [CrossRef]
- Liao, G.; Huang, L.; Wu, S.; Zhang, P.; Xie, D.; Yao, L.; Zhang, Z.; Yao, S.; Shanshan, L.; Wang, S.; et al. Preoperative CT-Based Peritumoral and Tumoral Radiomic Features Prediction for Tumor Spread through Air Spaces in Clinical Stage I Lung Adenocarcinoma. Lung Cancer 2022, 163, 87–95. [Google Scholar] [CrossRef]
- Shi, L.; Yang, M.; Yao, J.; Ni, H.; Shao, H.; Feng, W.; He, Z.; Ni, B. Application of Computed Tomography-Based Radiomics Combined with Clinical Factors in the Diagnosis of Malignant Degree of Lung Adenocarcinoma. J. Thorac. Dis. 2022, 14, 4435–4448. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in Cancer: Mechanisms and Clinical Implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Solassol, J.; Maudelonde, T.; Mange, A.; Pujol, J.-L. Clinical Relevance of Autoantibody Detection in Lung Cancer. J. Thorac. Oncol. 2011, 6, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, H.; Zhu, D. Wnt/β-Catenin Signaling Pathway in Lung Cancer. Med. Drug Discov. 2022, 13, 100113. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-Catenin Signaling in Cancers and Targeted Therapies. Signal Transduct. Target Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Jiang, D.; Ehlerding, E.; Ni, D.; Yu, B.; Barnhart, T.; Wang, R.; Cai, W. In Vivo Visualization of Brentuximab Vedotin and ImmunoPET of CD30 in Lung Cancer Murine Models. J. Nucl. Med. 2018, 59, 171. [Google Scholar]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Tissue Expression of RGL1—Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000143344-RGL1/tissue (accessed on 18 April 2023).
- Liang, W.; Chen, Z.; Li, C.; Liu, J.; Tao, J.; Liu, X.; Zhao, D.; Yin, W.; Chen, H.; Cheng, C.; et al. Accurate Diagnosis of Pulmonary Nodules Using a Noninvasive DNA Methylation Test. J. Clin. Investig. 2021, 131, e145973. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Marsh, T.; Irajizad, E.; Patel, N.; Murage, E.; Vykoukal, J.; Dennison, J.B.; Do, K.-A.; Ostrin, E.; Spitz, M.R.; et al. Blood-Based Biomarker Panel for Personalized Lung Cancer Risk Assessment. JCO 2022, 40, 876–883. [Google Scholar] [CrossRef]
- Paez, R.; Kammer, M.N.; Balar, A.; Lakhani, D.A.; Knight, M.; Rowe, D.; Xiao, D.; Heideman, B.E.; Antic, S.L.; Chen, H.; et al. Longitudinal Lung Cancer Prediction Convolutional Neural Network Model Improves the Classification of Indeterminate Pulmonary Nodules. Sci. Rep. 2023, 13, 6157. [Google Scholar] [CrossRef]
- Chabon, J.J.; Hamilton, E.G.; Kurtz, D.M.; Esfahani, M.S.; Moding, E.J.; Stehr, H.; Schroers-Martin, J.; Nabet, B.Y.; Chen, B.; Chaudhuri, A.A.; et al. Integrating Genomic Features for Non-Invasive Early Lung Cancer Detection. Nature 2020, 580, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.; Dotson, T.; Wahidi, M.M.; Bernstein, M.; Lee, H.J.; Feller Kopman, D.; Yarmus, L.; Whitney, D.; Stevenson, C.; Qu, J.; et al. Clinical Validation and Utility of Percepta GSC for the Evaluation of Lung Cancer. PLoS ONE 2022, 17, e0268567. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.B.; Boshier, P.R.; Markar, S.R.; Romano, A. Accuracy and Methodologic Challenges of Volatile Organic Compound-Based Exhaled Breath Tests for Cancer Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2019, 5, e182815. [Google Scholar] [CrossRef]
- Xing, L.; Su, J.; Guarnera, M.A.; Zhang, H.; Cai, L.; Zhou, R.; Stass, S.A.; Jiang, F. Sputum MicroRNA Biomarkers for Identifying Lung Cancer in Indeterminate Solitary Pulmonary Nodules. Clin. Cancer Res. 2015, 21, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Nolen, B.M.; Lomakin, A.; Marrangoni, A.; Velikokhatnaya, L.; Prosser, D.; Lokshin, A.E. Urinary Protein Biomarkers in the Early Detection of Lung Cancer. Cancer Prev. Res. 2015, 8, 111–119. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Tanner, N.T.; Kearney, P.; Vachani, A.; Massion, P.P.; Porter, A.; Springmeyer, S.C.; Fang, K.C.; Midthun, D.; Mazzone, P.J.; et al. Assessment of Plasma Proteomics Biomarker’s Ability to Distinguish Benign From Malignant Lung Nodules: Results of the PANOPTIC (Pulmonary Nodule Plasma Proteomic Classifier) Trial. Chest 2018, 154, 491–500. [Google Scholar] [CrossRef]
- Sullivan, F.M.; Mair, F.S.; Anderson, W.; Armory, P.; Briggs, A.; Chew, C.; Dorward, A.; Haughney, J.; Hogarth, F.; Kendrick, D.; et al. Earlier Diagnosis of Lung Cancer in a Randomised Trial of an Autoantibody Blood Test Followed by Imaging. Eur. Respir. J. 2021, 57, 2000670. [Google Scholar] [CrossRef]
- Kammer, M.N.; Lakhani, D.A.; Balar, A.B.; Antic, S.L.; Kussrow, A.K.; Webster, R.L.; Mahapatra, S.; Barad, U.; Shah, C.; Atwater, T.; et al. Integrated Biomarkers for the Management of Indeterminate Pulmonary Nodules. Am. J. Respir. Crit. Care Med. 2021, 204, 1306–1316. [Google Scholar] [CrossRef]
- Aldrich, M.C.; Mercaldo, S.F.; Sandler, K.L.; Blot, W.J.; Grogan, E.L.; Blume, J.D. Evaluation of USPSTF Lung Cancer Screening Guidelines among African American Adult Smokers. JAMA Oncol. 2019, 5, 1318–1324. [Google Scholar] [CrossRef]
- Šutić, M.; Vukić, A.; Baranašić, J.; Försti, A.; Džubur, F.; Samaržija, M.; Jakopović, M.; Brčić, L.; Knežević, J. Diagnostic, Predictive, and Prognostic Biomarkers in Non-Small Cell Lung Cancer (NSCLC) Management. J. Pers. Med. 2021, 11, 1102. [Google Scholar] [CrossRef]
- Data from RIDER_Lung CT. The Cancer Imaging Archive. Available online: https://wiki.cancerimagingarchive.net/display/Public/RIDER+Lung+CT (accessed on 24 May 2023).
- Data from NSCLC-Radiomics-Interobserver1. The Cancer Imaging Archive. Available online: https://wiki.cancerimagingarchive.net/display/Public/NSCLC-Radiomics-Interobserver1 (accessed on 26 May 2023).
- Du, D.; Gu, J.; Chen, X.; Lv, W.; Feng, Q.; Rahmim, A.; Wu, H.; Lu, L. Integration of PET/CT Radiomics and Semantic Features for Differentiation between Active Pulmonary Tuberculosis and Lung Cancer. Mol. Imaging Biol. 2021, 23, 287–298. [Google Scholar] [CrossRef] [PubMed]


| Variable | FH1 (n = 135) | P † | FH2 (n = 149) | P † | ||
|---|---|---|---|---|---|---|
| Control (n = 66) | Case (n = 69) | Control (n = 78) | Case (n = 71) | |||
| Gender (M) | 33 (50.0%) | 31 (44.9%) | 0.61 | 47 (60.26%) | 38 (53.52%) | 0.41 |
| Age | 67 (40–91) | 67 (44–83) | 0.74 | 63 (33–87) | 68 (48–94) | <0.001 |
| Race | 0.37 | 0.72 | ||||
| African American | 0 (0.00%) | 1 (1.45%) | 4 (5.13%) | 1 (1.41%) | ||
| Asian | 1 (1.52%) | 4 (5.80%) | 3 (3.85%) | 5 (7.04%) | ||
| Caucasian | 63 (95.45%) | 62 (89.86%) | 68 (87.18%) | 62 (87.32%) | ||
| Hispanic or Latino | 0 (0.00%) | 1 (1.45%) | 1 (1.28%) | 0 (0.00%) | ||
| Native American | 0 (0.00%) | 1 (1.45%) | 1 (1.28%) | 1 (1.41%) | ||
| Native Hawaiian/Pacific Islander | 1 (1.52%) | 0 (0.00%) | 0 (0.00%) | 1 (1.41%) | ||
| Unknown/Not Reported | 1 (1.52%) | 0 (0.00%) | 1 (1.28%) | 1 (1.41%) | ||
| BMI * | 26.7 (18.1–58.7) | 26.6 (17.9–47.4) | 0.86 | 26.5 (18.3–52.9) | 26.1 (16.1–42.8) | 0.37 |
| BMI class * | 0.86 | 0.64 | ||||
| Normal | 24 (36.92%) | 28 (40.58%) | 31 (39.74%) | 30 (42.25%) | ||
| Overweight | 23 (35.38%) | 21 (30.43%) | 24 (30.77%) | 25 (35.21%) | ||
| Obese | 18 (27.69%) | 20 (28.99%) | 23 (29.49%) | 16 (22.54%) | ||
| Smoking status | 0.10 | <0.01 | ||||
| Current smoker | 17 (25.76%) | 28 (40.58%) | 15 (19.23%) | 14 (19.72%) | ||
| Former smoker | 34 (51.52%) | 33 (47.83%) | 31 (39.74%) | 44 (61.97%) | ||
| Never smoker | 15 (22.73%) | 8 (11.59%) | 32 (41.03%) | 13 (18.31%) | ||
| Years since quitting * | 1.5 (0–58) | 0 (0–53) | 0.59 | 0 (0–64) | 2 (0–52) | 0.23 |
| Prior cancer history (Y) | 26 (39.39%) | 21 (30.43%) | 0.29 | 19 (24.36%) | 14 (19.72%) | 0.56 |
| Family history of lung cancer * (Y) | 13 (20.00%) | 22 (31.88%) | 0.17 | 25 (32.89%) | 17 (23.94%) | 0.27 |
| Histology for NSCLC | ||||||
| AD | 56 (81.16%) | 51 (71.83%) | ||||
| AD, SCC | 0 (0.00%) | 1 (1.41%) | ||||
| Large-cell carcinoma | 1 (1.45%) | 0 (0.00%) | ||||
| SCC | 12 (17.39%) | 19 (26.76%) | ||||
| NSCLC cancer stage | ||||||
| 0 | 2 (2.90%) | 0 (0.00%) | ||||
| I | 40 (57.97%) | 55 (77.46%) | ||||
| II | 4 (5.80%) | 8 (11.27%) | ||||
| III | 9 (13.04%) | 4 (5.63%) | ||||
| IV | 14 (20.29%) | 4 (5.63%) | ||||
| PSR Model | # of Subjects (6~30 mm) | # of Variables Considered | Lasso Selected | 6~30 mm Nodule (AUC) |
|---|---|---|---|---|
| Training on FH1 | 69 case vs. 66 ctrl | 188 | 9 | 0.964 |
| Testing on FH2 | 71 case vs. 78 ctrl | 9 | 0.846 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lastwika, K.J.; Wu, W.; Zhang, Y.; Ma, N.; Zečević, M.; Pipavath, S.N.J.; Randolph, T.W.; Houghton, A.M.; Nair, V.S.; Lampe, P.D.; et al. Multi-Omic Biomarkers Improve Indeterminate Pulmonary Nodule Malignancy Risk Assessment. Cancers 2023, 15, 3418. https://doi.org/10.3390/cancers15133418
Lastwika KJ, Wu W, Zhang Y, Ma N, Zečević M, Pipavath SNJ, Randolph TW, Houghton AM, Nair VS, Lampe PD, et al. Multi-Omic Biomarkers Improve Indeterminate Pulmonary Nodule Malignancy Risk Assessment. Cancers. 2023; 15(13):3418. https://doi.org/10.3390/cancers15133418
Chicago/Turabian StyleLastwika, Kristin J., Wei Wu, Yuzheng Zhang, Ningxin Ma, Mladen Zečević, Sudhakar N. J. Pipavath, Timothy W. Randolph, A. McGarry Houghton, Viswam S. Nair, Paul D. Lampe, and et al. 2023. "Multi-Omic Biomarkers Improve Indeterminate Pulmonary Nodule Malignancy Risk Assessment" Cancers 15, no. 13: 3418. https://doi.org/10.3390/cancers15133418
APA StyleLastwika, K. J., Wu, W., Zhang, Y., Ma, N., Zečević, M., Pipavath, S. N. J., Randolph, T. W., Houghton, A. M., Nair, V. S., Lampe, P. D., & Kinahan, P. E. (2023). Multi-Omic Biomarkers Improve Indeterminate Pulmonary Nodule Malignancy Risk Assessment. Cancers, 15(13), 3418. https://doi.org/10.3390/cancers15133418






