Association between Computed Tomography-Determined Loss of Muscle Mass and Impaired Three-Month Survival in Frail Older Adults with Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Ethics Statement
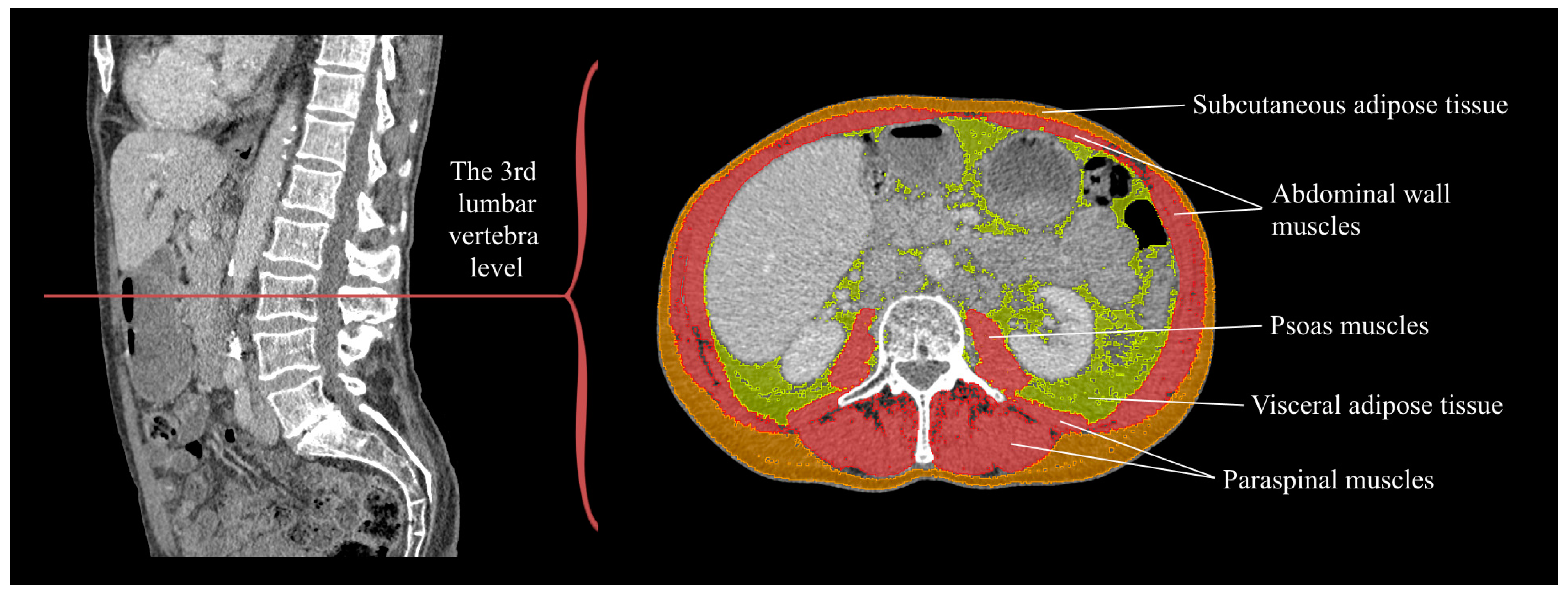
2.3. Body Composition Analysis
2.4. Clinical Data Acquisition
2.5. Statistical Analyses
3. Results
3.1. Patient Demographics
3.2. Initiated Treatments
3.3. Muscle Index Cut-Offs
3.4. Associations between Low Muscle Mass, Oncological Treatments, and Patient Characteristics
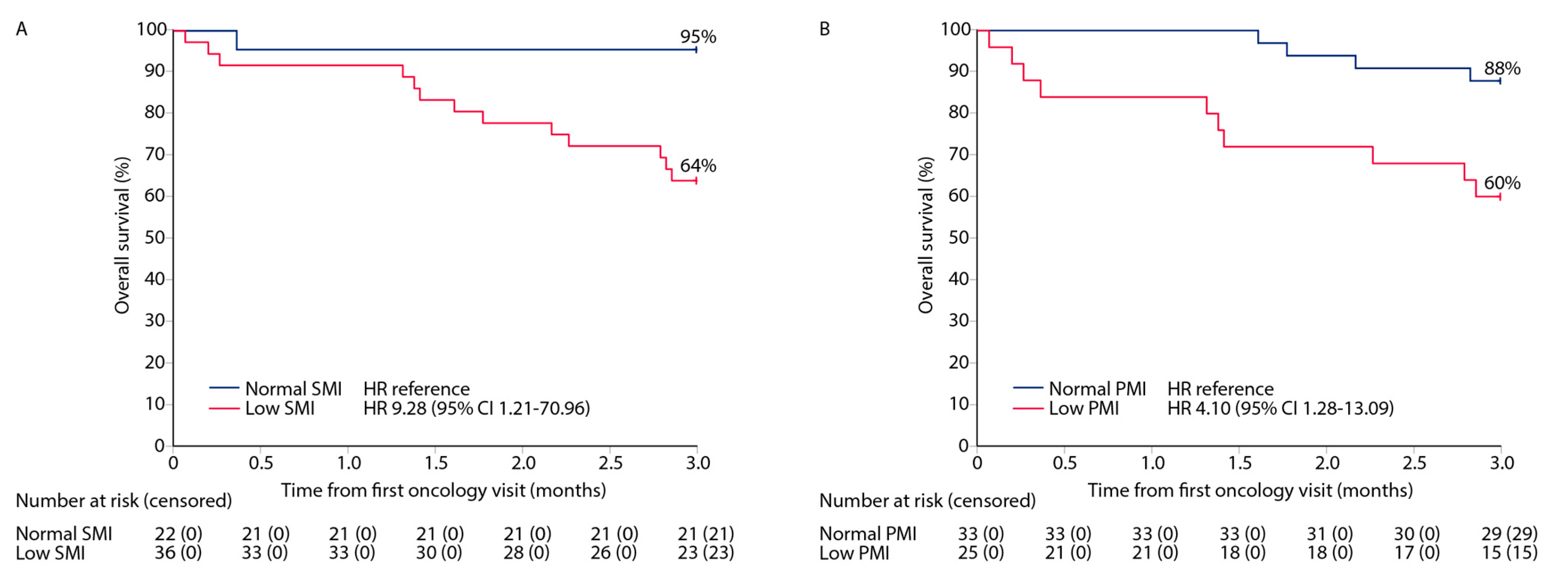
3.5. Three-Month Survival Analyses
3.6. Univariate and Multivariable Analyses for 3-Month Overall Survival in the Non-Curative Treatment Group
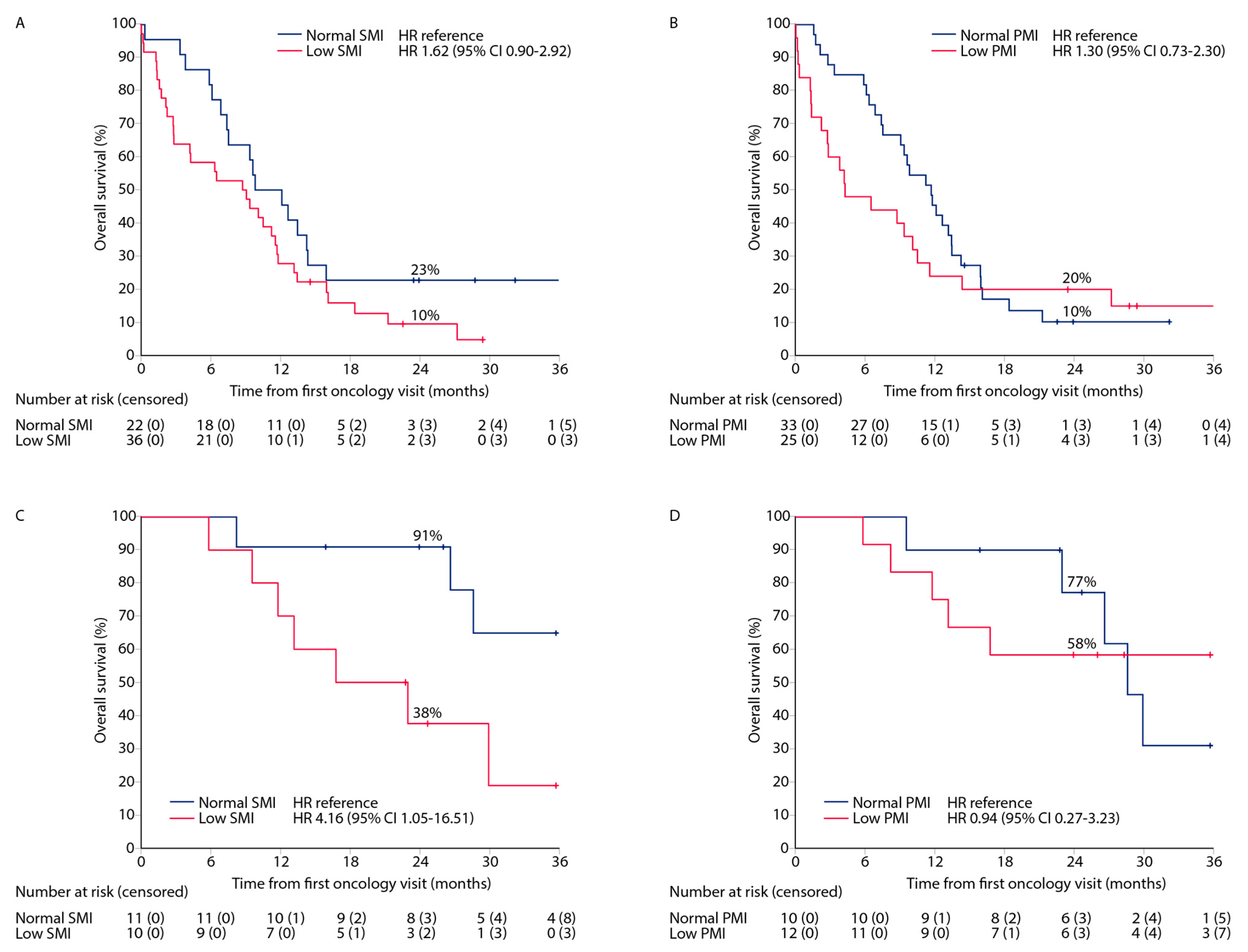
3.7. Long-Term Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balducci, L.; Aapro, M. Epidemiology of Cancer and Aging. Biol. Basis Geriatr. Oncol. 2005, 124, 1–15. [Google Scholar] [CrossRef]
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The Prevalence and Outcomes of Frailty in Older Cancer Patients: A Systematic Review. Ann. Oncol. 2015, 26, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Huynh, L.; Moore, J. Palliative and End-of-Life Care for the Older Adult with Cancer. Curr. Opin. Support. Palliat. Care 2021, 15, 23–28. [Google Scholar] [CrossRef]
- Veasey Rodrigues, H.; Baracos, V.E.; Wheler, J.J.; Parsons, H.A.; Hong, D.S.; Naing, A.; Fu, S.; Falchoock, G.; Tsimberidou, A.M.; Piha-Paul, S.; et al. Body Composition and Survival in the Early Clinical Trials Setting. Eur. J. Cancer 2013, 49, 3068–3075. [Google Scholar] [CrossRef]
- Extermann, M.; Aapro, M.; Topinkova, E.; Bernabei, R.; Cohen, H.J.; Droz, J.-P.; Lichtman, S.; Mor, V.; Monfardini, S.; Repetto, L.; et al. Use of Comprehensive Geriatric Assessment in Older Cancer Patients: Recommendations from the Task Force on CGA of the International Society of Geriatric Oncology (SIOG). Crit. Rev. Oncol. Hematol. 2005, 55, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.G.; Mohamed, M.R.; Culakova, E.; Xu, H.; Loh, K.P.; Magnuson, A.; Flannery, M.A.; Ramsdale, E.E.; Dunne, R.F.; Gilmore, N.; et al. A Geriatric Assessment (GA) Intervention to Reduce Treatment Toxicity in Older Patients with Advanced Cancer: A University of Rochester Cancer Center NCI Community Oncology Research Program Cluster Randomized Clinical Trial (CRCT). J. Clin. Oncol. 2020, 38, 12009. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.-L.; Kim, H.; Soto-Perez-de-Celis, E.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Cabrera Chien, L.; Charles, K.; et al. Geriatric Assessment-Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults with Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, e214158. [Google Scholar] [CrossRef]
- Soo, W.-K.; King, M.; Pope, A.; Parente, P.; Darzins, P.; Davis, I.D. Integrated Geriatric Assessment and Treatment (INTEGERATE) in Older People with Cancer Planned for Systemic Anticancer Therapy. J. Clin. Oncol. 2020, 38, 12011. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Chen, J.-Y.; Tsai, W.-W.; Lee, C.-W.; Lee, M.-C.; Tseng, W.-H.; Hung, K.-C. Impact of Comprehensive Geriatric Assessment on the Risk of Adverse Events in the Older Patients Receiving Anti-Cancer Therapy: A Systematic Review and Meta-Analysis. Age Ageing 2022, 51, afac145. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer Cachexia in Adult Patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of the Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Narita, Y.; Mitani, S.; Honda, K.; Masuishi, T.; Taniguchi, H.; Kadowaki, S.; Ura, T.; Ando, M.; Tajika, M.; et al. Baseline Sarcopenia and Skeletal Muscle Loss During Chemotherapy Affect Survival Outcomes in Metastatic Gastric Cancer. Anticancer Res. 2018, 38, 5859–5866. [Google Scholar] [CrossRef]
- van Rijn-Dekker, M.I.; van den Bosch, L.; van den Hoek, J.G.M.; Bijl, H.P.; van Aken, E.S.M.; van der Hoorn, A.; Oosting, S.F.; Halmos, G.B.; Witjes, M.J.H.; van der Laan, H.P.; et al. Impact of Sarcopenia on Survival and Late Toxicity in Head and Neck Cancer Patients Treated with Radiotherapy. Radiother. Oncol. 2020, 147, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Cushen, S.J.; Power, D.G.; Murphy, K.P.; McDermott, R.; Griffin, B.T.; Lim, M.; Daly, L.; MacEneaney, P.; O’ Sullivan, K.; Prado, C.M.; et al. Impact of Body Composition Parameters on Clinical Outcomes in Patients with Metastatic Castrate-Resistant Prostate Cancer Treated with Docetaxel. Clin. Nutr. ESPEN 2016, 13, e39–e45. [Google Scholar] [CrossRef]
- Daly, L.E.; Ní Bhuachalla, É.B.; Power, D.G.; Cushen, S.J.; James, K.; Ryan, A.M. Loss of Skeletal Muscle during Systemic Chemotherapy Is Prognostic of Poor Survival in Patients with Foregut Cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 315–325. [Google Scholar] [CrossRef]
- Han, J.S.; Ryu, H.; Park, I.J.; Kim, K.W.; Shin, Y.; Kim, S.O.; Lim, S.-B.; Kim, C.W.; Yoon, Y.S.; Lee, J.L.; et al. Association of Body Composition with Long-Term Survival in Non-Metastatic Rectal Cancer Patients. Cancer Res. Treat. 2020, 52, 563–572. [Google Scholar] [CrossRef]
- Portal, D.; Hofstetter, L.; Eshed, I.; Dan-Lantsman, C.; Sella, T.; Urban, D.; Onn, A.; Bar, J.; Segal, G. L3 Skeletal Muscle Index (L3SMI) Is a Surrogate Marker of Sarcopenia and Frailty in Non-Small Cell Lung Cancer Patients. Cancer Manag. Res. 2019, 11, 2579–2588. [Google Scholar] [CrossRef]
- Couderc, A.-L.; Liuu, E.; Boudou-Rouquette, P.; Poisson, J.; Frelaut, M.; Montégut, C.; Mebarki, S.; Geiss, R.; Ap Thomas, Z.; Noret, A.; et al. Pre-Therapeutic Sarcopenia among Cancer Patients: An Up-to-Date Meta-Analysis of Prevalence and Predictive Value during Cancer Treatment. Nutrients 2023, 15, 1193. [Google Scholar] [CrossRef]
- Wendrich, A.W.; Swartz, J.E.; Bril, S.I.; Wegner, I.; de Graeff, A.; Smid, E.J.; de Bree, R.; Pothen, A.J. Low Skeletal Muscle Mass Is a Predictive Factor for Chemotherapy Dose-Limiting Toxicity in Patients with Locally Advanced Head and Neck Cancer. Oral Oncol. 2017, 71, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.L.; Brammer, K.; Randhawa, N.; Welch, N.T.; Parsons, S.L.; James, E.J.; Catton, J.A. Sarcopenia Is Associated with Toxicity in Patients Undergoing Neo-Adjuvant Chemotherapy for Oesophago-Gastric Cancer. Eur. J. Surg. Oncol. 2015, 41, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Nipp, R.D.; Fuchs, G.; El-Jawahri, A.; Mario, J.; Troschel, F.M.; Greer, J.A.; Gallagher, E.R.; Jackson, V.A.; Kambadakone, A.; Hong, T.S.; et al. Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. Oncologist 2018, 23, 97–104. [Google Scholar] [CrossRef]
- Tolonen, A.; Pakarinen, T.; Sassi, A.; Kyttä, J.; Cancino, W.; Rinta-Kiikka, I.; Pertuz, S.; Arponen, O. Methodology, Clinical Applications, and Future Directions of Body Composition Analysis Using Computed Tomography (CT) Images: A Review. Eur. J. Radiol. 2021, 145, 109943. [Google Scholar] [CrossRef]
- Soubeyran, P.; Bellera, C.; Goyard, J.; Heitz, D.; Curé, H.; Rousselot, H.; Albrand, G.; Servent, V.; Jean, O.S.; van Praagh, I.; et al. Screening for Vulnerability in Older Cancer Patients: The ONCODAGE Prospective Multicenter Cohort Study. PLoS ONE 2014, 9, e115060. [Google Scholar] [CrossRef]
- Osterlund, P.; Salminen, T.; Soveri, L.-M.; Kallio, R.; Kellokumpu, I.; Lamminmäki, A.; Halonen, P.; Ristamäki, R.; Lantto, E.; Uutela, A.; et al. Repeated Centralized Multidisciplinary Team Assessment of Resectability, Clinical Behavior, and Outcomes in 1086 Finnish Metastatic Colorectal Cancer Patients (RAXO): A Nationwide Prospective Intervention Study. Lancet Reg. Health Eur. 2021, 3, 100049. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- 3D Slicer. Available online: https://www.slicer.org/ (accessed on 13 April 2023).
- Peng, Y.-C.; Wu, C.-H.; Tien, Y.-W.; Lu, T.-P.; Wang, Y.-H.; Chen, B.-B. Preoperative Sarcopenia Is Associated with Poor Overall Survival in Pancreatic Cancer Patients Following Pancreaticoduodenectomy. Eur. Radiol. 2021, 31, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and Response Criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- van Kan, G.A.; Rolland, Y.M.; Morley, J.E.; Vellas, B. Frailty: Toward a Clinical Definition. J. Am. Med. Dir. Assoc. 2008, 9, 71–72. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A Global Clinical Measure of Fitness and Frailty in Elderly People. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Finlayson, M.; Mallinson, T.; Barbosa, V.M. Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) Items Were Stable over Time in a Longitudinal Study on Aging. J. Clin. Epidemiol. 2005, 58, 338–349. [Google Scholar] [CrossRef]
- Aromaa, A.; Koskinen, S. Health and Functional Capacity in Finland. Baseline Results of the Health 2000 Health Examination Survey. National Public Health Institute. 2004. Available online: https://urn.fi/URN:NBN:fi-fe201204193452 (accessed on 13 April 2023).
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.J.; Morrison, D.S.; Talwar, D.; Balmer, S.M.; O’Rreilly, D.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. An Inflammation-Based Prognostic Score (MGPS) Predicts Cancer Survival Independent of Tumour Site: A Glasgow Inflammation Outcome Study. Br. J. Cancer 2011, 104, 726–734. [Google Scholar] [CrossRef]
- Sorbye, H.; Köhne, C.-H.; Sargent, D.J.; Glimelius, B. Patient Characteristics and Stratification in Medical Treatment Studies for Metastatic Colorectal Cancer: A Proposal for Standardization of Patient Characteristic Reporting and Stratification. Ann. Oncol. 2007, 18, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Baracos, V.E.; Rreiman, T.; Mourtzakis, M.; Gioulbasanis, I.; Antoun, S. Body Composition in Patients with Non-Small Cell Lung Cancer: A Contemporary View of Cancer Cachexia with the Use of Computed Tomography Image Analysis. Am. J. Clin. Nutr. 2010, 91, 1133S–1137S. [Google Scholar] [CrossRef]
- van Vledder, M.G.; Levolger, S.; Ayez, N.; Verhoef, C.; Tran, T.C.K.; IJzermans, J.N.M. Body Composition and Outcome in Patients Undergoing Resection of Colorectal Liver Metastases. Br. J. Surg. 2012, 99, 550–557. [Google Scholar] [CrossRef]
- Camus, V.; Lanic, H.; Kraut, J.; Modzelewski, R.; Clatot, F.; Picquenot, J.M.; Contentin, N.; Lenain, P.; Groza, L.; Lemasle, E.; et al. Prognostic Impact of Fat Tissue Loss and Cachexia Assessed by Computed Tomography Scan in Elderly Patients with Diffuse Large B-Cell Lymphoma Treated with Immunochemotherapy. Eur. J. Haematol. 2014, 93, 9–18. [Google Scholar] [CrossRef]
- Amini, N.; Spolverato, G.; Gupta, R.; Margonis, G.A.; Kim, Y.; Wagner, D.; Rezaee, N.; Weiss, M.J.; Wolfgang, C.L.; Makary, M.M.; et al. Impact Total Psoas Volume on Short- and Long-Term Outcomes in Patients Undergoing Curative Resection for Pancreatic Adenocarcinoma: A New Tool to Assess Sarcopenia. J. Gastrointest. Surg. 2015, 19, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, S.; Asghar, A.; Mott, S.L.; Johnson, B.E.; Button, A.M.; Clark, E.; Mezhir, J.J. Sarcopenia Is an Independent Predictor of Complications Following Pancreatectomy for Adenocarcinoma. J. Surg. Oncol. 2015, 111, 771–775. [Google Scholar] [CrossRef]
- Deluche, E.; Leobon, S.; Desport, J.C.; Venat-Bouvet, L.; Usseglio, J.; Tubiana-Mathieu, N. Impact of Body Composition on Outcome in Patients with Early Breast Cancer. Support Care Cancer 2018, 26, 861–868. [Google Scholar] [CrossRef]
- Su, H.; Ruan, J.; Chen, T.; Lin, E.; Shi, L. CT-Assessed Sarcopenia Is a Predictive Factor for Both Long-Term and Short-Term Outcomes in Gastrointestinal Oncology Patients: A Systematic Review and Meta-Analysis. Cancer Imaging 2019, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Shirdel, M.; Andersson, F.; Myte, R.; Axelsson, J.; Rutegård, M.; Blomqvist, L.; Riklund, K.; van Guelpen, B.; Palmqvist, R.; Gylling, B. Body Composition Measured by Computed Tomography Is Associated with Colorectal Cancer Survival, also in Early-Stage Disease. Acta Oncol. 2020, 59, 799–808. [Google Scholar] [CrossRef]
- Basile, D.; Parnofiello, A.; Vitale, M.G.; Cortiula, F.; Gerratana, L.; Fanotto, V.; Lisanti, C.; Pelizzari, G.; Ongaro, E.; Bartoletti, M.; et al. The IMPACT Study: Early Loss of Skeletal Muscle Mass in Advanced Pancreatic Cancer Patients. J. Cachexia Sarcopenia Muscle 2019, 10, 368–377. [Google Scholar] [CrossRef]
- Fukushima, H.; Nakanishi, Y.; Kataoka, M.; Tobisu, K.; Koga, F. Prognostic Significance of Sarcopenia in Patients with Metastatic Renal Cell Carcinoma. J. Urol. 2016, 195, 26–32. [Google Scholar] [CrossRef]
- Jung, A.R.; Roh, J.-L.; Kim, J.S.; Kim, S.-B.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Prognostic Value of Body Composition on Recurrence and Survival of Advanced-Stage Head and Neck Cancer. Eur. J. Cancer 2019, 116, 98–106. [Google Scholar] [CrossRef]
- Jiralerspong, S.; Kim, E.S.; Dong, W.; Feng, L.; Hortobagyi, G.N.; Giordano, S.H. Obesity, Diabetes, and Survival Outcomes in a Large Cohort of Early-Stage Breast Cancer Patients. Ann. Oncol. 2013, 24, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity with Survival Outcomes in Patients with Cancer: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e213520. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body Mass Index and Survival in Women with Breast Cancer-Systematic Literature Review and Meta-Analysis of 82 Follow-up Studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Chiu, C.-C.; Ho, C.-H.; Hung, C.-M.; Chao, C.-M.; Lai, C.-C.; Chen, C.-M.; Liao, K.-M.; Wang, J.-J.; Wu, Y.-C.; Shi, H.-Y.; et al. Correlation of Body Mass Index with Oncologic Outcomes in Colorectal Cancer Patients: A Large Population-Based Study. Cancers 2021, 13, 3592. [Google Scholar] [CrossRef]
- Takeyama, H.; Noura, S.; Suzuki, Y.; Odagiri, K.; Yanagimoto, Y.; Yamashita, M.; Shimizu, J.; Kawase, T.; Imamura, H.; Iwazawa, T.; et al. Higher Body Mass Index Is a Simple Favorable Non-Cancer Prognostic Marker for Japanese Elderly Colorectal Cancer Patients after Curative Resection. J. Anus Rectum Colon 2022, 6, 134–142. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Wu, Z.; Wang, N.; Yang, Z.; Li, Y.; Xu, B.; Sun, M. Association between Body Mass Index and Survival Outcomes for Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Transl. Med. 2020, 18, 235. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Alfano, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Burger, R.A.; Chlebowski, R.T.; Fabian, C.J.; Gucalp, A.; Hershman, D.L.; Hudson, M.M.; et al. American Society of Clinical Oncology Position Statement on Obesity and Cancer. J. Clin. Oncol. 2014, 32, 3568–3574. [Google Scholar] [CrossRef]
- Mullen, J.T.; Davenport, D.L.; Hutter, M.M.; Hosokawa, P.W.; Henderson, W.G.; Khuri, S.F.; Moorman, D.W. Impact of Body Mass Index on Perioperative Outcomes in Patients Undergoing Major Intra-Abdominal Cancer Surgery. Ann. Surg. Oncol. 2008, 15, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-S.; Wu, W.-G.; Li, M.-L.; Yang, J.-H.; Ding, Q.-C.; Zhang, L.; Mu, J.-S.; Gu, J.; Dong, P.; Lu, J.-H.; et al. Impact of Being Overweight on the Surgical Outcomes of Patients with Gastric Cancer: A Meta-Analysis. World J. Gastroenterol. 2013, 19, 4596–4606. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Neugebauer, R.; Meyerhardt, J.; Prado, C.M.; Weltzien, E.; Kwan, M.L.; Xiao, J.; Caan, B.J. Analysis of Body Mass Index and Mortality in Patients with Colorectal Cancer Using Causal Diagrams. JAMA Oncol. 2016, 2, 1137–1145. [Google Scholar] [CrossRef]
- Mayr, R.; Fritsche, H.-M.; Zeman, F.; Reiffen, M.; Siebertz, L.; Niessen, C.; Pycha, A.; van Rhijn, B.W.G.; Burger, M.; Gierth, M. Sarcopenia Predicts 90-Day Mortality and Postoperative Complications after Radical Cystectomy for Bladder Cancer. World J. Urol. 2018, 36, 1201–1207. [Google Scholar] [CrossRef]
- Yang, T.; Luo, K.; Deng, X.; Xu, L.; Wang, R.; Ji, P. Effect of Sarcopenia in Predicting Postoperative Mortality in Emergency Laparotomy: A Systematic Review and Meta-Analysis. World J. Emerg. Surg. 2022, 17, 36. [Google Scholar] [CrossRef]
- Morshed, R.A.; Young, J.S.; Casey, M.; Wang, E.J.; Aghi, M.K.; Berger, M.S.; Hervey-Jumper, S.L. Sarcopenia Diagnosed Using Masseter Muscle Diameter as a Survival Correlate in Elderly Patients with Glioblastoma. World Neurosurg. 2022, 161, e448–e463. [Google Scholar] [CrossRef] [PubMed]
- Berardi, G.; Antonelli, G.; Colasanti, M.; Meniconi, R.; Guglielmo, N.; Laurenzi, A.; Ferretti, S.; Levi Sandri, G.B.; Spagnoli, A.; Moschetta, G.; et al. Association of Sarcopenia and Body Composition with Short-Term Outcomes after Liver Resection for Malignant Tumors. JAMA Surg. 2020, 155, e203336. [Google Scholar] [CrossRef]
- Meister, F.A.; Lurje, G.; Verhoeven, S.; Wiltberger, G.; Heij, L.; Liu, W.-J.; Jiang, D.; Bruners, P.; Lang, S.A.; Ulmer, T.F.; et al. The Role of Sarcopenia and Myosteatosis in Short-and Long-Term Outcomes Following Curative-Intent Surgery for Hepatocellular Carcinoma in a European Cohort. Cancers 2022, 14, 720. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Nyunt, M.-S.-Z.; Gao, Q.; Wee, S.-L.; Yap, K.-B.; Ng, T.-P. Association of Frailty and Malnutrition With Long-Term Functional and Mortality Outcomes Among Community-Dwelling Older Adults: Results from the Singapore Longitudinal Aging Study 1. JAMA Netw. Open 2018, 1, e180650. [Google Scholar] [CrossRef]
- Shamliyan, T.; Talley, K.M.C.; Ramakrishnan, R.; Kane, R.L. Association of Frailty with Survival: A Systematic Literature Review. Ageing Res. Rev. 2013, 12, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Wu, C.; Odden, M.C.; Kim, D.H. Multimorbidity Patterns, Frailty, and Survival in Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, F.J.; Rossi, L.A.; Roberts, L.M.; Bosserman, L.D. Prospective Comparison of Medical Oncologists and a Machine Learning Model to Predict 3-Month Mortality in Patients with Metastatic Solid Tumors. JAMA Netw. Open 2022, 5, e2214514. [Google Scholar] [CrossRef]
- D’Journo, X.B.; Boulate, D.; Fourdrain, A.; Loundou, A.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; O’Neill, J.R.; Hoelscher, A.; Piessen, G.; van Lanschot, J.; et al. Risk Prediction Model of 90-Day Mortality after Esophagectomy for Cancer. JAMA Surg. 2021, 156, 836–845. [Google Scholar] [CrossRef]


| All | Non-Curative | Curative | ||||||
|---|---|---|---|---|---|---|---|---|
| N = 80 | % | n = 58 | % | n = 22 | % | OR (95% CI) | ||
| Age | 75–80 years | 39 | 49 | 28 | 48 | 11 | 50 | 1 |
| ≥80 years | 41 | 51 | 30 | 52 | 11 | 50 | 0.93 (0.4–2.5) | |
| Sex | Female | 37 | 46 | 28 | 48 | 9 | 41 | 1 |
| Male | 43 | 54 | 30 | 52 | 13 | 59 | 1.35 (0.5–3.6) | |
| Tumor site | Upper GI | 37 | 46 | 30 | 52 | 7 | 32 | 1 |
| Lower GI | 26 | 33 | 12 | 21 | 14 | 64 | 5.00 (1.6–15) * | |
| Other a | 17 | 21 | 16 | 28 | 1 | 5 | 0.27 (0.0–2.4) | |
| Tumor stage | Local | 13 | 16 | 0 | 0 | 13 | 59 | 1 |
| Metastatic or locally advanced | 67 | 84 | 58 | 100 | 9 | 41 | N/A | |
| ECOG performance status | 0 to 2 | 52 | 65 | 37 | 64 | 15 | 68 | 1 |
| 3 to 4 | 22 | 28 | 15 | 26 | 7 | 32 | 1.15 (0.4–3.4) | |
| Not available | 6 | 8 | 6 | 10 | 0 | 0 | ||
| Activities of daily living | Normal | 52 | 65 | 35 | 60 | 17 | 77 | 1 |
| Impaired | 24 | 30 | 19 | 33 | 5 | 23 | 0.54 (0.2–1.7) | |
| Not available | 4 | 5 | 4 | 7 | 0 | 0 | ||
| Clinical frailty scale | 1 to 4 | 48 | 60 | 35 | 60 | 13 | 59 | 1 |
| 5 to 9 | 26 | 33 | 20 | 35 | 6 | 27 | 0.81 (0.3–2.5) | |
| Not available | 6 | 8 | 3 | 5 | 3 | 14 | ||
| BMI | ≥22 kg/m2 | 61 | 76 | 42 | 72 | 19 | 86 | 1 |
| <22 kg/m2 | 19 | 24 | 16 | 28 | 3 | 14 | 0.41 (0.1–1.6) | |
| GLIM b | Normal | 31 | 39 | 19 | 33 | 12 | 55 | 1 |
| Malnourishment | 44 | 55 | 34 | 59 | 10 | 46 | 0.47 (0.2–1.39) | |
| Not available | 5 | 6 | 5 | 9 | 0 | 0 | ||
| Hand-grip strength test | Normal | 48 | 60 | 36 | 62 | 12 | 55 | 1 |
| Impaired | 22 | 28 | 13 | 22 | 9 | 41 | 2.08 (0.7–6.1) | |
| Not available | 10 | 13 | 9 | 16 | 1 | 5 | ||
| Albumin | ≥30 g/L | 41 | 51 | 28 | 48 | 13 | 59 | 1 |
| <30 g/L | 17 | 21 | 14 | 24 | 3 | 14 | 0.46 (0.1–1.9) | |
| Not available | 22 | 28 | 16 | 28 | 6 | 27 | ||
| SMI c | Normal | 33 | 42 | 22 | 38 | 11 | 52 | 1 |
| Low | 46 | 58 | 36 | 62 | 10 | 48 | 0.56 (0.2–1.5) | |
| PMI c | Normal | 43 | 54 | 33 | 57 | 10 | 46 | 1 |
| Low | 37 | 46 | 25 | 43 | 12 | 55 | 1.58 (0.6–4.3) | |
| Treatment decision | Oncological treatments | 41 | 51 | 31 | 53 | 10 | 46 | 1 |
| Follow-up or BSC d | 39 | 49 | 27 | 47 | 12 | 55 | 1.38 (0.5–3.7) | |
| Survival at 3 months | Alive | 66 | 83 | 44 | 76 | 22 | 100 | 1 |
| Deceased | 14 | 18 | 14 | 24 | 0 | 0 | N/A | |
| All Patients | Non-Curative Patients | Univariate | Multivariable a | ||
|---|---|---|---|---|---|
| Cut-Off | N | n Univariate/n Multivariable | HR 95% CI | HR 95% CI | |
| Study SMI (Youden) b | Normal | 33 | 22/22 | 1 | 1 |
| Low | 46 | 36/29 | 9.28 (1.2–71) * | 10.65 (1.0–110) * | |
| Study SMI (median) c | Normal | 40 | 29/28 | 1 | 1 |
| Low | 39 | 29/23 | 2.06 (0.7–6.2) | 2.21 (0.6–8.4) | |
| Prado et al. SMI d [12] | Normal | 10 | 7/7 | 1 | 1 |
| Low | 69 | 51/44 | 25.21 (0.0–19,000) | N/A | |
| Martin et al. SMI e [39] | Normal | 13 | 9/8 | 1 | 1 |
| Low | 66 | 49/43 | 1.19 (0.3–5.3) | 1.00 (0.1–8.8) | |
| Baracos et al. SMI f [40] | Normal | 10 | 7/7 | 1 | 1 |
| Low | 69 | 51/44 | 25.21 (0.0–19,400) | N/A | |
| van Vledder et al. SMI g [41] | Normal | 21 | 14/13 | 1 | 1 |
| Low | 58 | 44/38 | 0.86 (0.3–2.7) | 1.01 (0.3–4.5) | |
| Camus et al. SMI h [42] | Normal | 9 | 7/7 | 1 | 1 |
| Low | 70 | 51/44 | 25.21 (0.3–19,000) | N/A | |
| Study PMI (Youden) i | Normal | 43 | 33/32 | 1 | 1 |
| Low | 37 | 25/18 | 4.10 (1.3–13) * | 2.23 (0.6–8.9) | |
| Study PMI (median) j | Normal | 41 | 31/30 | 1 | 1 |
| Low | 39 | 27/21 | 4.48 (1.1–11) * | 1.56 (0.4–6.5) | |
| Amini et al. PMI k [43] | Normal | 34 | 26/26 | 1 | 1 |
| Low | 46 | 32/25 | 3.46 (1.0–12) | 1.84 (0.4–7.7) | |
| Joglekar et al. PMI l [44] | Normal | 37 | 28/28 | 1 | 1 |
| Low | 43 | 30/23 | 2.74 (0.9–8.7) | 1.31 (0.3–5.2) |
| Non-Curative Treatment | Curative Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Normal SMI | Low SMI a | Normal SMI | Low SMI a | ||||
| n = 22 | n = 36 | OR (95% CI) | n = 11 | n = 10 | OR (95% CI) | ||
| n (%) | n (%) | n (%) | n (%) | ||||
| Age | 75–80 years | 8 (29) | 20 (71) | 1 | 8 (80) | 2 (20) | 1 |
| ≥80 years | 14 (47) | 16 (53) | 0.46 (0.2–1.4) | 3 (27) | 8 (73) | 10.67 (1.4–82) * | |
| Sex | Female | 19 (68) | 9 (32) | 1 | 6 (67) | 3 (33) | 1 |
| Male | 3 (10) | 27 (90) | 19.00 (4.5–80) * | 5 (42) | 7 (58) | 2.80 (0.5–17) | |
| Tumor site | Upper GI | 8 (27) | 22 (73) | 1 | 4 (57) | 3 (43) | 1 |
| Lower GI | 4 (33) | 8 (67) | 0.73 (0.2–3.1) | 7 (54) | 6 (46) | 1.14 (0.2–7.3) | |
| Other b | 10 (63) | 6 (38) | 0.22 (0.1–0.8) | 0 (0) | 1 (100) | N/A | |
| Tumor stage | Local | 0 (0) | 0 (0) | 1 | 7 (54) | 6 (46) | 1 |
| Metastatic or locally advanced | 22 (38) | 36 (62) | N/A | 4 (50) | 4 (50) | 1.17 (0.2–6.8) | |
| ECOG performance status | 0 to 2 | 16 (43) | 21 (57) | 1 | 9 (60) | 6 (40) | 1 |
| 3 to 4 | 6 (40) | 9 (60) | 1.14 (0.3–3.9) | 2 (33) | 4 (67) | 3.00 (0.4–22) | |
| Not available | 0 (0) | 6 (100) | 0 (0) | 0 (0) | |||
| Activities of daily living | Normal | 14 (40) | 21 (60) | 1 | 8 (50) | 8 (50) | 1 |
| Impaired | 7 (37) | 12 (63) | 1.14 (0.4–3.6) | 3 (60) | 2 (40) | 0.67 (0.1–5.1) | |
| Not available | 1 (25) | 3 (75) | 0 (0) | 0 (0) | |||
| Clinical frailty scale | 1 to 4 | 15 (43) | 20 (57) | 1 | 8 (62) | 5 (39) | 1 |
| 5 to 9 | 7 (35) | 13 (65) | 1.39 (0.4–4.3) | 2 (40) | 3 (60) | 2.40 (0.3–20) | |
| Not available | 0 (0) | 3 (100) | 1 (33) | 2 (67) | |||
| BMI | ≥22 kg/m2 | 17 (41) | 25 (60) | 1 | 10 (56) | 8 (44) | 1 |
| <22 kg/m2 | 5 (31) | 11 (69) | 1.50 (0.4–5.1) | 1 (33) | 2 (67) | 2.50 (0.2–33) | |
| GLIM c | Normal | 11 (58) | 8 (42) | 1 | 6 (55) | 5 (46) | 1 |
| Malnourishment | 9 (27) | 25 (74) | 3.82 (1.2–13) * | 5 (50) | 5 (50) | 1.20 (0.2–6.7) | |
| Not available | 2 (40) | 3 (60) | 0 (0) | 0 (0) | |||
| Hand-grip strength test | Normal | 16 (44) | 20 (56) | 1 | 6 (50) | 6 (50) | 1 |
| Impaired | 3 (23) | 10 (77) | 2.67 (0.6–11) | 4 (50) | 4 (50) | 1.00 (0.2–6.0) | |
| Not available | 3 (33) | 6 (67) | 1 (100) | 0 (0) | |||
| Albumin | ≥30 g/L | 12 (43) | 16 (57) | 1 | 8 (67) | 4 (33) | 1 |
| <30 g/L | 2 (14) | 12 (86) | 4.50 (0.8–24) | 1 (33) | 2 (67) | 4.00 (0.3–59) | |
| Not available | 8 (50) | 8 (50) | 2 (33) | 4 (67) | |||
| Treatment decision | Oncological treatment | 16 (52) | 15 (48) | 1 | 6 (60) | 4 (40) | 1 |
| Follow-up or BSC d | 6 (22) | 21 (78) | 3.73 (1.2–12) * | 5 (46) | 6 (55) | 1.80 (0.3–10) | |
| Survival at 3 months | Alive | 21 (48) | 23 (52) | 1 | 11 (52) | 10 (48) | 1 |
| Deceased | 1 (7) | 13 (93) | 11.87 (1.4–99) * | 0 (0) | 0 (0) | N/A | |
| Non-Curative Treatment | Curative Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Normal PMI | Low PMI a | Normal PMI | Low PMI a | ||||
| n = 33 | n = 25 | OR (95% CI) | n = 10 | n = 12 | OR (95% CI) | ||
| n (%) | n (%) | n (%) | n (%) | ||||
| Age | 75–80 years | 16 (57) | 12 (43) | 1 | 5 (46) | 6 (55) | 1 |
| ≥80 years | 17 (57) | 13 (43) | 1.02 (0.4–2.9) | 5 (46) | 6 (55) | 1.00 (0.2–5.4) | |
| Sex | Female | 16 (57) | 12 (43) | 1 | 3 (33) | 6 (67) | 1 |
| Male | 17 (57) | 13 (43) | 1.02 (0.4–2.9) | 7 (54) | 6 (46) | 0.43 (0.1–2.5) | |
| Tumor site | Upper GI | 18 (60) | 12 (40) | 1 | 1 (14) | 6 (86) | 1 |
| Lower GI | 5 (42) | 7 (58) | 2.10 (0.5–8.2) | 8 (57) | 6 (43) | 0.13 (0.0–1.3) | |
| Other b | 10 (63) | 6 (38) | 0.9 (0.3–3.1) | 1 (100) | 0 (0) | N/A | |
| Tumor stage | Local | 0 (0) | 0 (0) | 1 | 7 (54) | 6 (46) | 1 |
| Metastatic or locally advanced | 33 (57) | 25 (43) | N/A | 3 (33) | 6 (67) | 2.33 (0.4–14) | |
| ECOG performance status | 0 to 2 | 26 (70) | 11 (30) | 1 | 8(53) | 7 (47) | 1 |
| 3 to 4 | 6 (40) | 9 (60) | 3.55 (1.0–12) * | 2 (29) | 5 (71) | 2.86 (0.4–20) | |
| Not available | 1 (17) | 5 (83) | 0 (0) | 0 (0) | |||
| Activities of daily living | Normal | 22 (63) | 13 (37) | 1 | 9 (53) | 8 (47) | 1 |
| Impaired | 10 (53) | 9 (47) | 1.52 (0.5–4.7) | 1 (20) | 4 (80) | 4.50 (0.4–49) | |
| Not available | 1 (25) | 3 (75) | 0 (0) | 0 (0) | |||
| Clinical frailty scale | 1 to 4 | 26 (74) | 9 (26) | 1 | 6 (46) | 7 (54) | 1 |
| 5 to 9 | 7 (35) | 13 (65) | 5.37 (1.6–18) * | 1 (17) | 5 (83) | 4.29 (0.4–48) | |
| Not available | 0 (0) | 3 (100) | 3 (100) | 0 (0) | |||
| BMI | ≥22 kg/m2 | 25 (60) | 17 (41) | 1 | 10 (53) | 9 (47) | 1 |
| <22 kg/m2 | 8 (50) | 8 (50) | 1.47 (0.5–4.7) | 0 (0) | 3 (100) | N/A | |
| GLIM c | Normal | 15 (79) | 4 (21) | 1 | 6 (50) | 6 (50) | 1 |
| Malnourishment | 16 (47) | 18 (53) | 4.22 (1.2–15) * | 4 (40) | 6 (60) | 1.50 (0.3–8.2) | |
| Not available | 2 (40) | 3 (60) | 0 (0) | 0 (0) | |||
| Hand-grip strength | Normal | 26 (72) | 10 (28) | 1 | 4 (33) | 8 (67) | 1 |
| Impaired | 4 (31) | 9 (69) | 5.85 (1.5–23) * | 5 (56) | 4 (44) | 0.40 (0.1–2.4) | |
| Not available | 3 (33) | 6 (67) | 1 (100) | 0 (0) | |||
| Albumin | ≥30 | 19 (68) | 9 (32) | 1 | 7 (54) | 6 (46) | 1 |
| <30 | 6 (43) | 8 (57) | 2.82 (0.8–11) | 0 (0) | 3 (100) | N/A | |
| Not available | 8 (50) | 8 (50) | 3 (50) | 3 (50) | |||
| Treatment decision | Oncological treatment | 23 (74) | 8 (26) | 1 | 3 (30) | 7 (70) | 1 |
| Follow-up or BSC d | 10 (37) | 17 (63) | 4.89 (1.6–15) * | 7 (58) | 5 (42) | 0.31 (0.1–1.8) | |
| Survival at 3 months | Alive | 29 (66) | 15 (34) | 1 | 10 (46) | 12 (55) | 1 |
| Deceased | 4 (29) | 10 (71) | 4.83 (1.3–18) * | 0 (0) | 0 (0) | N/A | |
| SMI | PMI | ||||
|---|---|---|---|---|---|
| Patient n | Univariate | Multivariable | Univariate | Multivariable | |
| Univariate/Multivariable | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Muscle mass | |||||
| Normal | SMI 22/22; PMI 33/32 | 1 | 1 | 1 | 1 |
| Low a | SMI 36/29; PMI 25/18 | 9.28 (1.2–71) * | 10.65 (1.0–110) * | 4.10 (1.3–13) * | 2.23 (0.6–8.9) |
| Sex | |||||
| Women | 28/25 | 1 | 1 | 1 | 1 |
| Men | 30/26 | 1.68 (0.6–5.0) | 0.57 (0.1–2.8) | 1.68 (0.6–5.0) | 2.1 (0.5–8.1) |
| ECOG PS | |||||
| 0 to 2 | 37/36 | 1 | 1 | 1 | 1 |
| 3 to 4 | 15/15 | 3.01 (0.9–10) | 2.34 (0.6–9.8) | 3.01 (0.9–10) | 1.80 (0.4–7.2) |
| Clinical frailty scale | |||||
| 1 to 4 | 35/33 | 1 | 1 | 1 | 1 |
| 5 to 9 | 20/18 | 3.35 (1.1–10) * | 2.11 (0.5–8.6) | 3.35 (1.1–10) * | 1.66 (0.4–7.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolonen, A.; Kerminen, H.; Lehtomäki, K.; Huhtala, H.; Bärlund, M.; Österlund, P.; Arponen, O. Association between Computed Tomography-Determined Loss of Muscle Mass and Impaired Three-Month Survival in Frail Older Adults with Cancer. Cancers 2023, 15, 3398. https://doi.org/10.3390/cancers15133398
Tolonen A, Kerminen H, Lehtomäki K, Huhtala H, Bärlund M, Österlund P, Arponen O. Association between Computed Tomography-Determined Loss of Muscle Mass and Impaired Three-Month Survival in Frail Older Adults with Cancer. Cancers. 2023; 15(13):3398. https://doi.org/10.3390/cancers15133398
Chicago/Turabian StyleTolonen, Antti, Hanna Kerminen, Kaisa Lehtomäki, Heini Huhtala, Maarit Bärlund, Pia Österlund, and Otso Arponen. 2023. "Association between Computed Tomography-Determined Loss of Muscle Mass and Impaired Three-Month Survival in Frail Older Adults with Cancer" Cancers 15, no. 13: 3398. https://doi.org/10.3390/cancers15133398
APA StyleTolonen, A., Kerminen, H., Lehtomäki, K., Huhtala, H., Bärlund, M., Österlund, P., & Arponen, O. (2023). Association between Computed Tomography-Determined Loss of Muscle Mass and Impaired Three-Month Survival in Frail Older Adults with Cancer. Cancers, 15(13), 3398. https://doi.org/10.3390/cancers15133398







