Association of Circulating Tumor Cells, Megakaryocytes and a High Immune-Inflammatory Environment in Metastatic Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Population
3.1.1. CTCs
3.1.2. Megakaryocytes
3.1.3. Blood-Based Immune-Inflammatory Markers SII, PIV, ΔSII%, and ΔPIV%
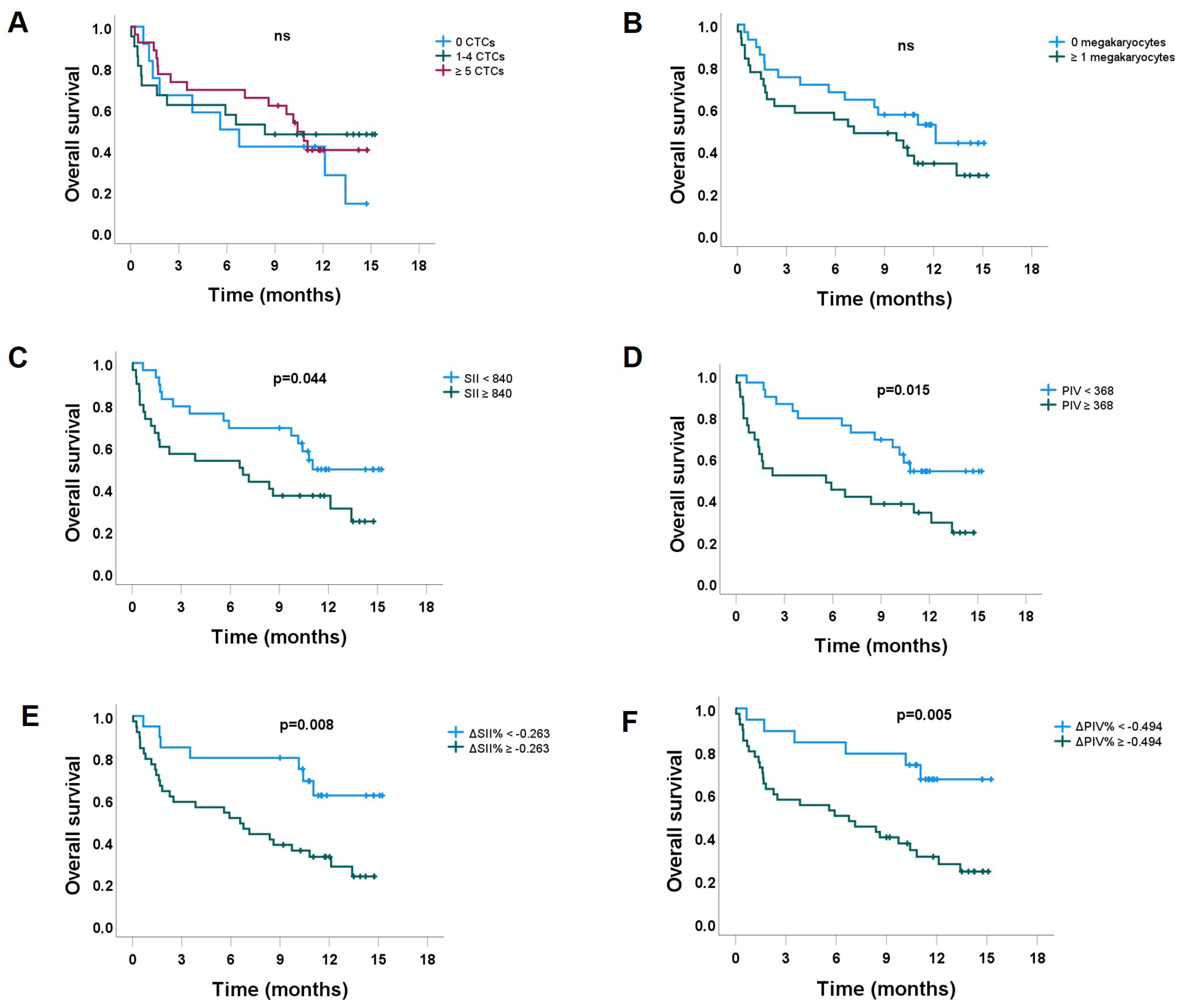
3.2. Overall Survival
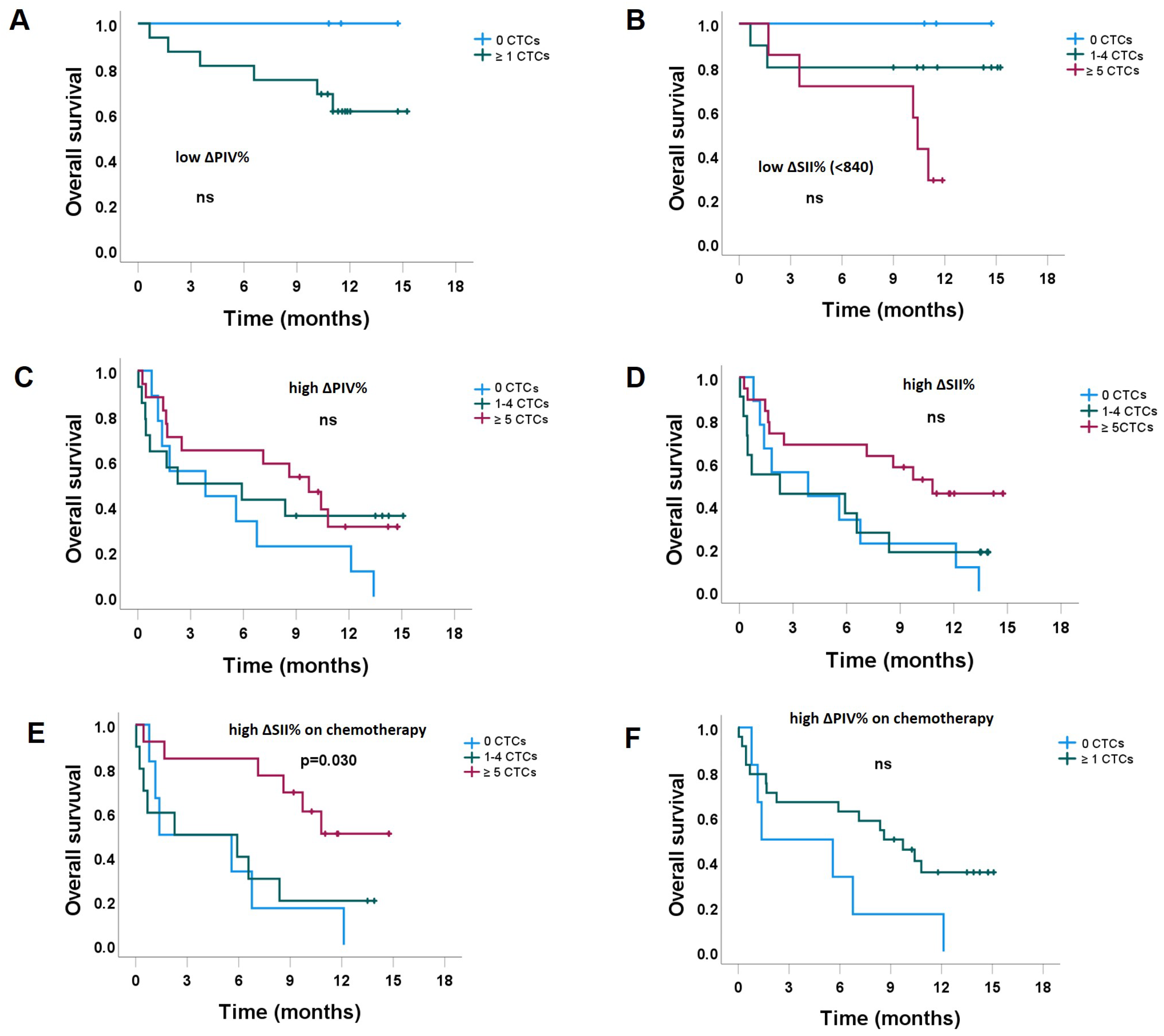
3.2.1. The Impact of CTC Counts, Stratified by the Level of ΔSII% and ΔPIV%
3.2.2. Prognostic Factors for OS
| Variable | HR (95% CI) | p Value |
|---|---|---|
| Molecular subtype | 1.07 (0.87–1.32) | 0.530 |
| CTCs 0 CTC vs. ≥1 CTC | 0.71 (0.33–1.51) | 0.373 |
| Clusters Absent vs. present | 1.05 (0.32–3.46) | 0.931 |
| Length of metastatic disease | 1.38 (1.04–1.85) | 0.028 |
| Infection in the last month Yes vs. no | 4.26 (1.77–10.22) | 0.001 |
| ΔSII% High vs. low | 2.92 (1.27–6.719 | 0.012 |
| SII High vs. low | 1.98 (1.01–3.91) | 0.048 |
| ΔPIV% High vs. low | 3.30 (1.36–7.99) | 0.008 |
| PIV High vs. low | 2.32 (1.16–4.66) | 0.018 |
| Megakaryocytes Yes vs. no | 1.60 (0.81–3.15) | 0.174 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019, 30, 1194–1220, Erratrum in: Ann. Oncol. 2019, 30, 1674; Erratrum in: Ann. Oncol. 2021, 32, 284. [Google Scholar] [CrossRef] [PubMed]
- Morse, B.; Jeong, D.; Ihnat, G.; Silva, A.C. Pearls and pitfalls of response evaluation criteria in solid tumors (RECIST) v1.1 non-target lesion assessment. Abdom. Imaging 2019, 44, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Castro-Giner, F.; Aceto, N. Tracking cancer progression: From circulating tumor cells to metastasis. Genome Med. 2020, 12, 31. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Pierga, J.-Y.; Reuben, J.; Rademaker, A.; Davis, A.A.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit. Rev. Oncol. 2019, 134, 39–45. [Google Scholar] [CrossRef]
- Aceto, N. Fluctuating numbers of circulating tumor cells in cancer patients and the meaning of zero counts. Oncotarget 2019, 10, 2658–2659. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Castro-Giner, F.; Schwab, F.D.; Foerster, C.; Saini, M.; Budinjas, S.; Strittmatter, K.; Krol, I.; Seifert, B.; Heinzelmann-Schwarz, V.; et al. The metastatic spread of breast cancer accelerates during sleep. Nature 2022, 607, 156–162. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Chen, J.; Deng, Q.; Pan, Y.; He, B.; Ying, H.; Sun, H.; Liu, X.; Wang, S. Prognostic value of neutrophil-to-lymphocyte ratio in breast cancer. FEBS Open Bio 2015, 5, 502–507. [Google Scholar] [CrossRef]
- Iwase, T.; Sangai, T.; Sakakibara, M.; Sakakibara, J.; Ishigami, E.; Hayama, S.; Nakagawa, A.; Masuda, T.; Tabe, S.; Nagashima, T. An increased neutrophil-to-lymphocyte ratio predicts poorer survival following recurrence for patients with breast cancer. Mol. Clin. Oncol. 2017, 6, 266–270. [Google Scholar] [CrossRef]
- Zhong, J.-H.; Huang, D.-H.; Chen, Z.-Y. Prognostic role of systemic immune-inflammation index in solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 75381–75388. [Google Scholar] [CrossRef]
- Chen, L.; Kong, X.; Wang, Z.; Wang, X.; Fang, Y.; Wang, J. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J. Cell. Mol. Med. 2020, 24, 2993–3021. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, L.-P.; Xie, S.-Y.; Huang, H.-Y.; Chen, X.-Y.; Jiang, T.-C.; Guo, L.; Lin, H.-X. Pan-Immune-Inflammation Value: A New Prognostic Index in Operative Breast Cancer. Front. Oncol. 2022, 12, 830138. [Google Scholar] [CrossRef]
- Gkountela, S.; Szczerba, B.; Donato, C.; Aceto, N. Recent advances in the biology of human circulating tumour cells and metastasis. ESMO Open 2016, 1, e000078. [Google Scholar] [CrossRef]
- Fabisiewicz, A.; Szostakowska-Rodzos, M.; Zaczek, A.J.; Grzybowska, E.A. Circulating Tumor Cells inEarly and Advanced Breast Cancer; Biology and Prognostic Value. Int. J. Mol. Sci. 2020, 21, 1671. [Google Scholar] [CrossRef]
- Schuster, E.; Taftaf, R.; Reduzzi, C.; Albert, M.K.; Romero-Calvo, I.; Liu, H. Better together: Circulating tumor cell clustering in metastatic cancer. Trends Cancer 2021, 7, 1020–1032. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, W.; Wang, B. Megakaryocytes in peripheral blood smears of non-hematological diseases. Int. J. Hematol. 2020, 112, 128–130. [Google Scholar] [CrossRef]
- Cunin, P.; Nigrovic, P.A. Megakaryocytes as immune cells. J. Leukoc. Biol. 2019, 105, 1111–1121. [Google Scholar] [CrossRef]
- Lozar, T.; Jesenko, T.; Kloboves Prevodnik, V.; Cemazar, M.; Hosta, V.; Jericevic, A.; Nolde, N.; Grasic Kuhar, C. Preclinical and Clinical Evaluation of Magnetic-Activated Cell Separation Technology for CTC Isolation in Breast Cancer. Front Oncol. 2020, 10, 554554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jesenko, T.; Modic, Z.; Kuhar, C.G.; Cemazar, M.; Matkovic, U.; Miceska, S.; Varl, J.; Kuhar, A.; Kloboves-Prevodnik, V. Morphological features of breast cancer circulating tumor cells in blood after physical and biological type of isolation. Radiol. Oncol. 2021, 55, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Miceska, S.; Škof, E.; Novaković, S.; Stegel, V.; Jeričević, A.; Kuzmanov, B.G.; Smrkolj, Š.; Cvjetičanin, B.; Bebar, S.; Kukovica, M.G.; et al. Cytopathological assessment is an accurate method for identifying immunophenotypic features and BRCA1/2 mutations of high-grade serous carcinoma from ascites. Cancer Cytopathol. 2023, 131, 188–197. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, U.; Mego, M.; Scarpi, E.; Giordano, A.; Giuliano, M.; Valero, V.; Alvarez, R.H.; Ueno, N.T.; Cristofanilli, M.; Reuben, J.M. Association between circulating tumor cells and peripheral blood monocytes in metastatic breast cancer. Ther. Adv. Med. Oncol. 2019, 11, 1758835919866065. [Google Scholar] [CrossRef] [PubMed]
- Şahin, A.B.; Cubukcu, E.; Ocak, B.; Deligonul, A.; Orhan, S.O.; Tolunay, S.; Gokgoz, M.S.; Cetintas, S.; Yarbas, G.; Senol, K.; et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci. Rep. 2021, 11, 14662. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Han, X.; Zha, H.; Tao, H.; Li, X.; Yuan, F.; Chen, G.; Wang, L.; Ma, J.; Hu, Y. Systemic Immune-Inflammation Index and Changes of Neutrophil-Lymphocyte Ratio as Prognostic Biomarkers for Patients With Pancreatic Cancer Treated With Immune Checkpoint Blockade. Front. Oncol. 2021, 11, 585271. [Google Scholar] [CrossRef]
- Deutsch, T.M.; Riethdorf, S.; Fremd, C.; Feisst, M.; Nees, J.; Fischer, C.; Hartkopf, A.D.; Pantel, K.; Trumpp, A.; Schütz, F.; et al. HER2-targeted therapy influences CTC status in metastatic breast cancer. Breast Cancer Res. Treat. 2020, 182, 127–136. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating Tumor Cell Clusters Are Oligoclonal Precursors of Breast Cancer Metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. 2017, 161, 83–94. [Google Scholar] [CrossRef]
- Vetter, M.; Landin, J.; Szczerba, B.M.; Castro-Giner, F.; Gkountela, S.; Donato, C.; Krol, I.; Scherrer, R.; Balmelli, C.; Malinovska, A.; et al. Denosumab treatment is associated with the absence of circulating tumor cells in patients with breast cancer. Breast Cancer Res. 2018, 20, 141. [Google Scholar] [CrossRef]
- Piñeiro, R.; Martínez-Pena, I.; López-López, R. Relevance of CTC Clusters in Breast Cancer Metastasis. Adv. Exp. Med. Biol. 2020, 1220, 93–115. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, C.; Liu, X.; Yang, P.; Wang, J.; Chen, Y.; Liu, W.; Li, S.; Zhang, X.; Dong, G.; et al. Global characterization of megakaryocytes in bone marrow, peripheral blood, and cord blood by single-cell RNA sequencing. Cancer Gene Ther. 2022, 29, 1636–1647. [Google Scholar] [CrossRef]
- Dejima, H.; Nakanishi, H.; Kuroda, H.; Yoshimura, M.; Sakakura, N.; Ueda, N.; Ohta, Y.; Tanaka, R.; Mori, S.; Yoshida, T.; et al. Detection of abundant megakaryocytes in pulmonary artery blood in lung cancer patients using a microfluidic platform. Lung Cancer 2018, 125, 128–135. [Google Scholar] [CrossRef]
- Xu, L.; Mao, X.; Guo, T.; Chan, P.Y.; Shaw, G.; Hines JStankiewicz, E.; Wang, Y.; Oliver, R.T.D.; Ahmad, A.S. The novel association of circulating tumor cells and circulating megakaryocytes with prostate cancer prognosis. Clin. Cancer Res. 2017, 23, 5112–5122. [Google Scholar] [CrossRef]
- Lefrançais, E.; Ortiz-Muñoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef]
- Ligorio, F.; Fucà, G.; Zattarin, E.; Lobefaro, R.; Zambelli, L.; Leporati, R.; Rea, C.; Mariani, G.; Bianchi, G.V.; Capri, G.; et al. The Pan-Immune-Inflammation-Value Predicts the Survival of Patients with Human Epidermal Growth Factor Receptor 2 (HER2)—Positive Advanced Breast Cancer Treated with First-Line Taxane-Trastuzumab-Pertuzumab. Cancers 2021, 13, 1964. [Google Scholar] [CrossRef]
- Mu, Z.; Wang, C.; Ye, Z.; Austin, L.; Civan, J.; Hyslop, T.; Palazzo, J.P.; Jaslow, R.; Li, B.; Myers, R.E.; et al. Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res Treat. 2015, 154, 563–571. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Larsson, A.-M.; Jansson, S.; Bendahl, P.-O.; Jörgensen, C.L.T.; Loman, N.; Graffman, C.; Lundgren, L.; Aaltonen, K.; Rydén, L. Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res. 2018, 20, 48. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; Grisanti, S.; Generali, D.; Garcia-Saenz, A.J.; Stebbing, J.; et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014, 15, 406–414. [Google Scholar] [CrossRef]
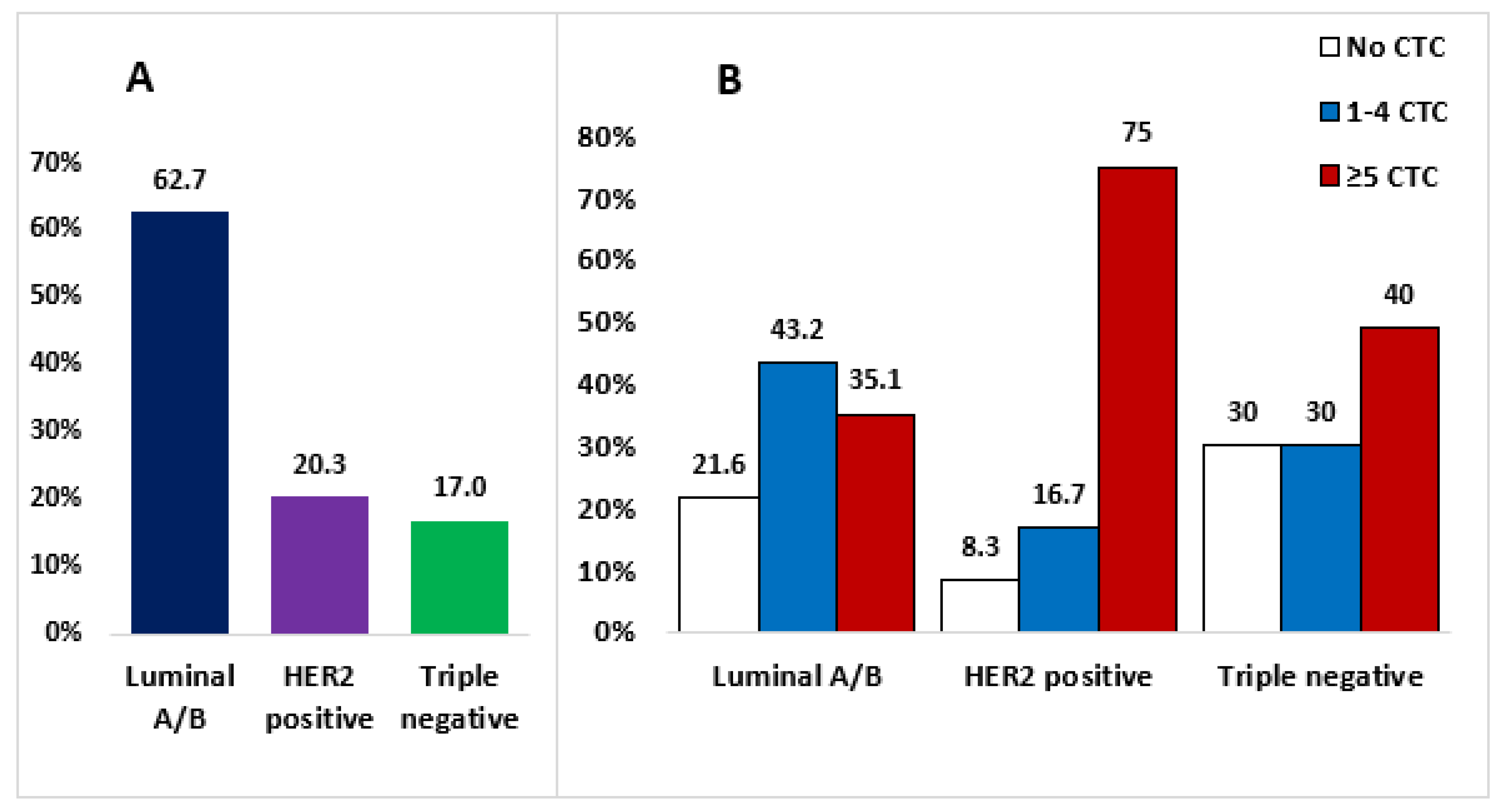
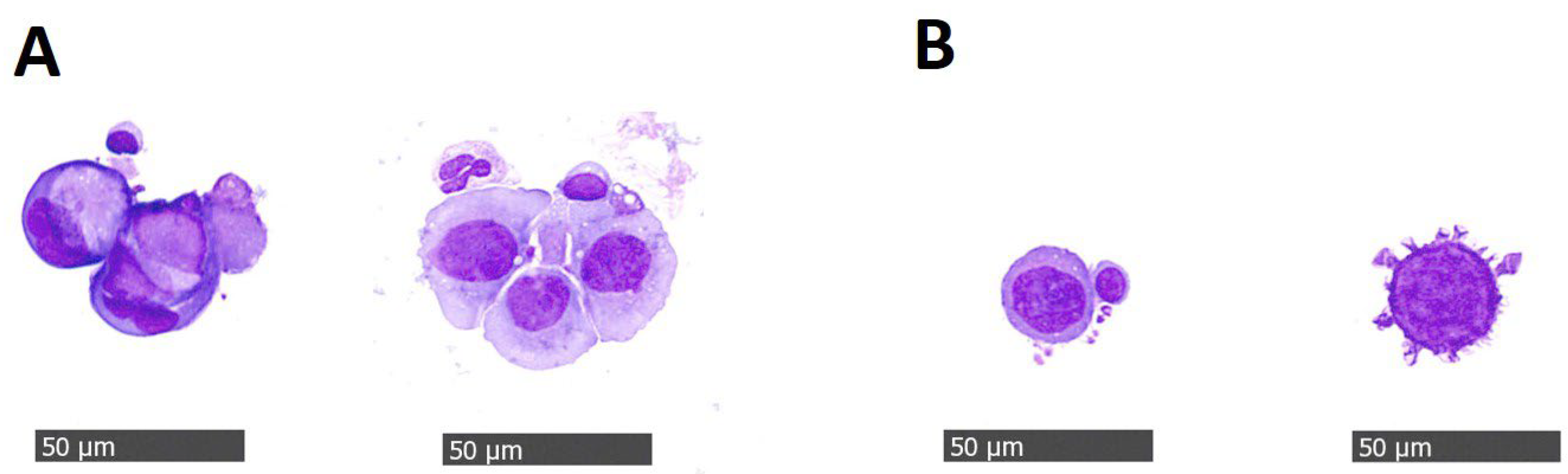
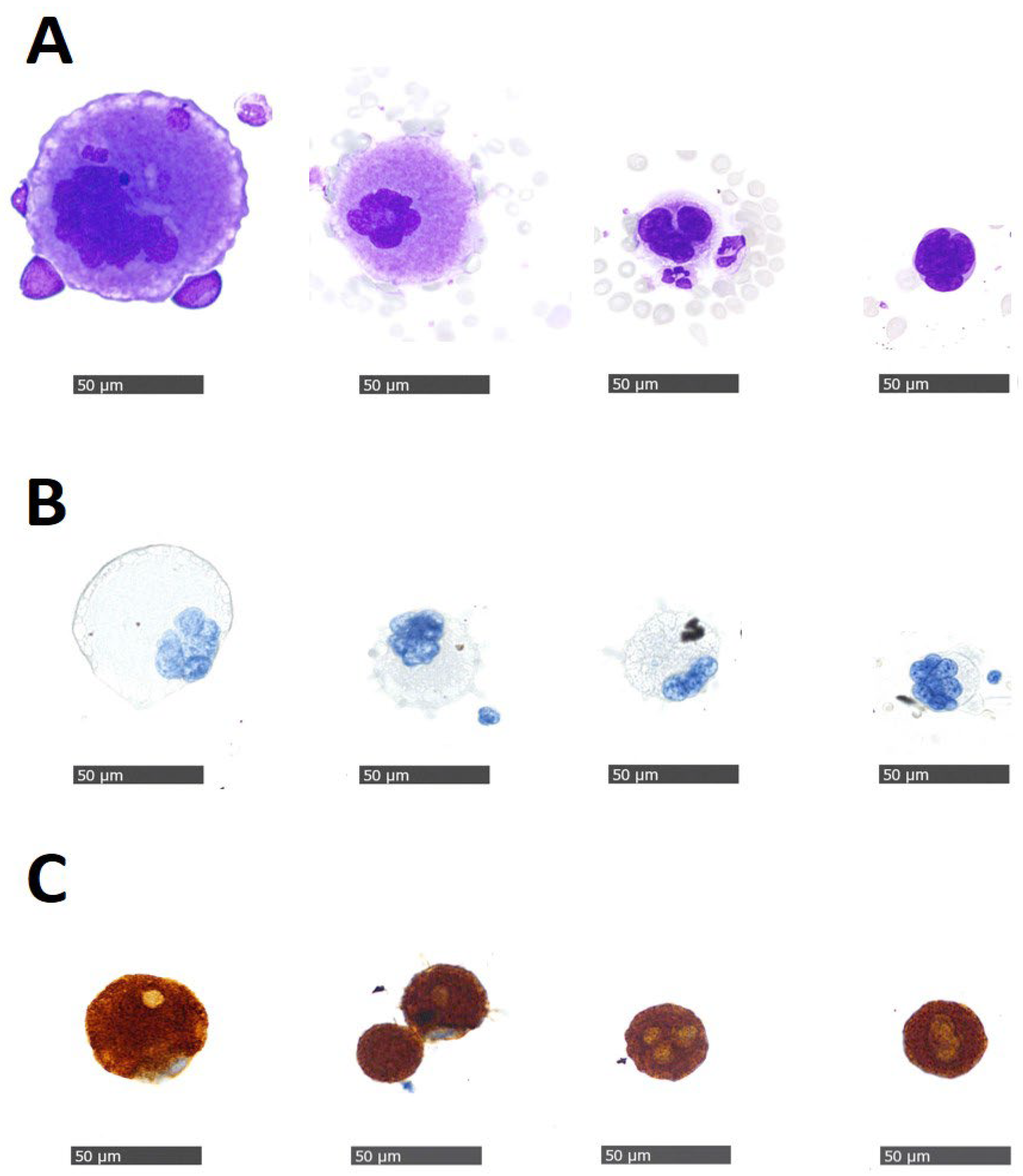
| Variable | No CTCs Number (%) | 1–4 CTCs Number (%) | ≥5 CTCs Number (%) | All Number (%) | p Value |
|---|---|---|---|---|---|
| Age at CTC collection Median (IQR) years | 64.9 (45.9–78.0) | 55.5 (49.4–67.3) | 61.3 (52.8–71.5) | 60.4 (52.3–71.2) | 0.255 |
| Subtype Luminal A/B HER2-positive Triple-negative | 8 (21.6) 1 (8.3) 3 (30) | 16 (43.3) 2 (16.7) 3 (30) | 13 (35.1) 9 (75) 4 (40) | 37 (62.7) 12 (20.3) 10 (17.0) | 0.161 |
| Type of therapy Endocrine Chemotherapy | 6 (31.6) 6 (15.4) | 7 (36.8) 14 (35.9) | 6 (31.6) 19 (48.7) | 19 (32.8) 39 (67.2) | 0.287 |
| Duration of relapse <1 year 1–2 years 2–3 years >3 years | 3 (18.8) 3 (21.4) 2 (20) 4 (21.1) | 3 (18.8) 6 (42.9) 4 (40) 8 (42.1) | 10 (62.4) 5 (35.7) 4 (40) 7 (36.8) | 16 (27.1) 14 (23.7) 10 (17.0) 19 (32.2) | 0.742 |
| Line of therapy 1st and 2nd line 3rd and 4th line ≥5th line | 5 (19.2) 3 (15.8) 4 (28.6) | 8 (30.8) 7 (36.8) 6 (42.8) | 13 (50) 9 (47.4) 4 (28.6) | 26 (44.1) 19 (32.2) 14 (23.7) | 0.721 |
| Cycle of therapy 1st or 2nd cycle Beyond 2nd cycle | 6 (25) 6 (17.2) | 10 (41.7) 11 (31.4) | 8 (33.3) 18 (51.4) | 24 (40.7) 35 (59.3) | 0.385 |
| Skeletal metastases No Yes | 4 (19) 8 (21.0) | 6 (28.6) 15 (39.5) | 11 (52.4) 15 (39.5) | 21 (35.6) 38 (64.4) | 0.610 |
| Liver metastases No Yes | 5 (10) 7 (20.6) | 7 (28) 14 (41.2) | 13 (51) 13 (38.2) | 25 (42.4) 34 (57.6) | 0.516 |
| Megakaryocytes No Yes | 8 (28.6) 4 (12.9) | 12 (42.8) 9 (29) | 8 (28.6) 18 (58.1) | 28 (47.5) 31 (52.5) | 0.065 |
| PIV at relapse Low (<311) High (≥311) | 5 (17.3) 7 (23.3) | 11 (37.9) 10 (33.3) | 13 (44.8) 13 (43.3) | 29 (49.2) 30 (50.8) | 0.834 |
| PIV at CTC collection Low (<368) High (≥368) | 5 (17.2) 7 (24.1) | 10 (34.5) 10 (34.5) | 14 (48.3) 12 (41.4) | 29 (50) 29 (50) | 0.784 |
| SII at relapse Low (<646) High (≥464) | 4 (13.8) 8 (26.7) | 11 (37.9) 10 (33.3) | 14 (48.3) 12 (40) | 29 (49.2) 30 (50.8) | 0.468 |
| SII at CTC collection Low (<840) High (≥840) | 4 (13.8) 8 (26.7) | 11 (37.9) 10 (33.3) | 14 (48.3) 12 (40) | 29 (49.2) 30 (50.8) | 0.468 |
| ΔPIV% <−0.494 ≥−0.494 | 3 (15) 9 (23.1) | 10 (50) 11 (28.2) | 7 (35) 19 (48.7) | 10 (33.9) 39 (66.1) | 0.252 |
| ΔSII% <−0.263 ≥−0.263 | 3 (15) 9 (23.1) | 10 (50) 11 (28.2) | 7 (35) 19 (48.7) | 20 (33.9) 39 (66.1) | 0.252 |
| Variable | No Megakaryocytes | Yes Megakaryocytes | All | p Value |
|---|---|---|---|---|
| Subtype Luminal A/B HER2-positive Triple-negative | 18 (48.6) 5 (41.7) 5 (50) | 19 (51.4) 7 (58.3) 5 (50) | 37 (62.7) 12 (20.3) 10 (17.0) | 0.901 |
| Line of therapy 1st–2nd line 3rd–4th line ≥5th line | 15 (57.7) 6 (31.6) 7 (50) | 11 (42.3) 13 (68.4) 7 (50) | 26 (44.1) 19 (32.2) 14 (23.7) | 0.208 |
| Type of therapy Endocrine Chemotherapy | 11 (57.9) 17 (43.6) | 8 (42.1) 22 (56.4) | 19 (32.8) 39 (67.2) | 0.306 |
| Skeletal metastases No Yes | 10 (47.6) 18 (47.4) | 11 (52.4) 20 (52.6) | 21 (35.6) 38 (64.4) | 0.985 |
| CTC clusters No Yes | 28 (51.9) 0 (0) | 26 (48.1) 5 (100) | 54 (91.5) 5 (8.5) | 0.026 |
| PIV at CTC collection Low High | 17 (58.6) 10 (34.5) | 12 (41.4) 19 (65.5) | 29 (50) 29 (50) | 0.065 |
| SII at CTC collection No Yes | 15 (51.7) 13 (43.3) | 14 (48.3) 17 (56.7) | 29 (49.2) 30 (50.8) | 0.519 |
| ΔPIV <−0.494 ≥−0.494 | 11 (59.9) 17 (42.5) | 8 (42.1) 23 (57.5) | 19 (32.2) 40 (67.8) | 0.269 |
| ΔSII <−0.263 ≥−0.263 | 13 (65) 15 (38.5) | 7 (35) 24 (61.5) | 20 (33.9) 39 (66.1) | 0.053 |
| Variable | Low PIV (<368) | High PIV (≥368) | All | p Value |
|---|---|---|---|---|
| Subtype Luminal A/B HER2-positive Triple-negative | 20 (55.6) 5 (41.7) 4 (40) | 16 (44.4) 7 (58.3) 6 (60) | 36 (62.1) 12 (20.7) 10 (17.2) | 0.555 |
| Type of therapy Endocrine Chemotherapy | 13 (68.4) 16 (42.1) | 6 (31.6) 22 (57.9) | 19 (33.3) 38 (66.7) | 0.061 |
| Line of therapy 1st–2nd line 3rd–4th line ≥5th line | 14 (53.8) 10 (52.6) 5 (38.5) | 12 (46.2) 9 (47.4) 8 (61.5) | 26 (44.8) 19 (32.8) 13 (22.4) | 0.638 |
| Cycle of therapy 1st–2nd cycle ≥3rd cycle | 10 (41.7) 19 (55.9) | 14 (58.3) 15 (44.1) | 24 (41.4) 34 (58.6) | 0.286 |
| Skeletal metastases No Yes | 6 (28.6) 23 (62.2) | 15 (71.4) 14 (37.8) | 21 (36.2) 37 (63.8) | 0.014 |
| Liver metastases No Yes | 13 (52) 16 (48.5) | 12 (48) 17 (51.5) | 25 (43.1) 33 (56.9) | 0.791 |
| CTC clusters No Yes | 26 (49.1) 3 (60) | 27 (50.9) 2 (40) | 54 (91.4) 5 (8.6) | 0.640 |
| Megakaryocytes No Yes | 17 (63) 12 (38.7) | 10 (37) 19 (61.3) | 27 (46.6) 31 (53.4) | 0.065 |
| CTC group 0 CTC 1–4 CTC ≥5 CTC | 5 (41.7) 10 (50) 14 (53.8) | 7 (58.3) 10 (50) 12 (46.2) | 12 (20.7) 20 (34.5) 26 (44.8) | 0.784 |
| Variable | ΔPIV% < −0.494 | ΔPIV% ≥ −0.494 | All | p Value |
|---|---|---|---|---|
| Subtype Luminal A/B HER2-positive Triple-negative | 14 (37.8) 3 (25) 2 (20) | 23 (62.2) 9 (75) 8 (80) | 37 (62.7) 12 (20.3) 10 (17.0) | 0.471 |
| Type of therapy Endocrine Chemotherapy | 10 (52.6) 9 (23.1) | 9 (47.4) 30 (76.9) | 19 (32.8) 39 (67.2) | 0.024 |
| Line of therapy 1st–2nd line 3rd–4th line ≥5th line | 11 (42.3) 7 (36.8) 1 (7.1) | 15 (57.7) 12 (63.2) 13 (92.9) | 26 (44.1) 19 (32.2) 14 (23.7) | 0.066 |
| Cycle of therapy 1st–2nd cycle ≥3rd cycle | 3 (12.5) 16 (45.7) | 21 (87.5) 19 (54.3) | 24 (40.7) 35 (59.3) | 0.010 |
| Skeletal metastases No Yes | 4 (19) 15 (39.5) | 17 (81) 23 (60.5) | 21 (35.6) 38 (64.4) | 0.108 |
| Liver metastases No Yes | 11 (44) 8 (23.5) | 14 (56) 26 (76.5) | 25 (42.4) 34 (57.6) | 0.096 |
| CTC clusters No Yes | 16 (29.6) 3 (60) | 38 (70.4) 2 (40) | 54 (91.5) 5 (8.5) | 0.316 |
| Megakaryocytes No Yes | 11 (39.3) 8 (25.8) | 17 (60.7) 23 (74.2) | 28 (47.5) 31 (52.5) | 0.296 |
| CTC group 0 CTC 1–4 CTCs ≥5 CTCs | 3 (25) 7 (33.3) 9 (34.6) | 9 (75) 14 (66.7) 17 (65.4) | 12 (20.3) 21 (35.6) 26 (44.1) | 0.832 |
| Variable | Multivariable HR (95% CI) | p Value |
|---|---|---|
| Length of metastatic disease | 1.59 (1.16–2.17) | 0.004 |
| Infection in the last month Yes vs. no | 7.88 (2.88–21.6) | <0.001 |
| ΔSII% High vs. low | 1.47 (0.59–3.67) | 0.406 |
| ΔPIV% High vs. low | 4.46 (1.52–13.07) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grašič Kuhar, C.; Silvester, J.; Mencinger, M.; Ovčariček, T.; Čemažar, M.; Miceska, S.; Modic, Ž.; Kuhar, A.; Jesenko, T.; Kloboves Prevodnik, V. Association of Circulating Tumor Cells, Megakaryocytes and a High Immune-Inflammatory Environment in Metastatic Breast Cancer. Cancers 2023, 15, 3397. https://doi.org/10.3390/cancers15133397
Grašič Kuhar C, Silvester J, Mencinger M, Ovčariček T, Čemažar M, Miceska S, Modic Ž, Kuhar A, Jesenko T, Kloboves Prevodnik V. Association of Circulating Tumor Cells, Megakaryocytes and a High Immune-Inflammatory Environment in Metastatic Breast Cancer. Cancers. 2023; 15(13):3397. https://doi.org/10.3390/cancers15133397
Chicago/Turabian StyleGrašič Kuhar, Cvetka, Jernej Silvester, Marina Mencinger, Tanja Ovčariček, Maja Čemažar, Simona Miceska, Živa Modic, Anamarija Kuhar, Tanja Jesenko, and Veronika Kloboves Prevodnik. 2023. "Association of Circulating Tumor Cells, Megakaryocytes and a High Immune-Inflammatory Environment in Metastatic Breast Cancer" Cancers 15, no. 13: 3397. https://doi.org/10.3390/cancers15133397
APA StyleGrašič Kuhar, C., Silvester, J., Mencinger, M., Ovčariček, T., Čemažar, M., Miceska, S., Modic, Ž., Kuhar, A., Jesenko, T., & Kloboves Prevodnik, V. (2023). Association of Circulating Tumor Cells, Megakaryocytes and a High Immune-Inflammatory Environment in Metastatic Breast Cancer. Cancers, 15(13), 3397. https://doi.org/10.3390/cancers15133397








