Radioisotope-Guided Excision of Mediastinal Lymph Nodes in Patients with Non-Small Cell Lung Carcinoma: Feasibility and Clinical Impact
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
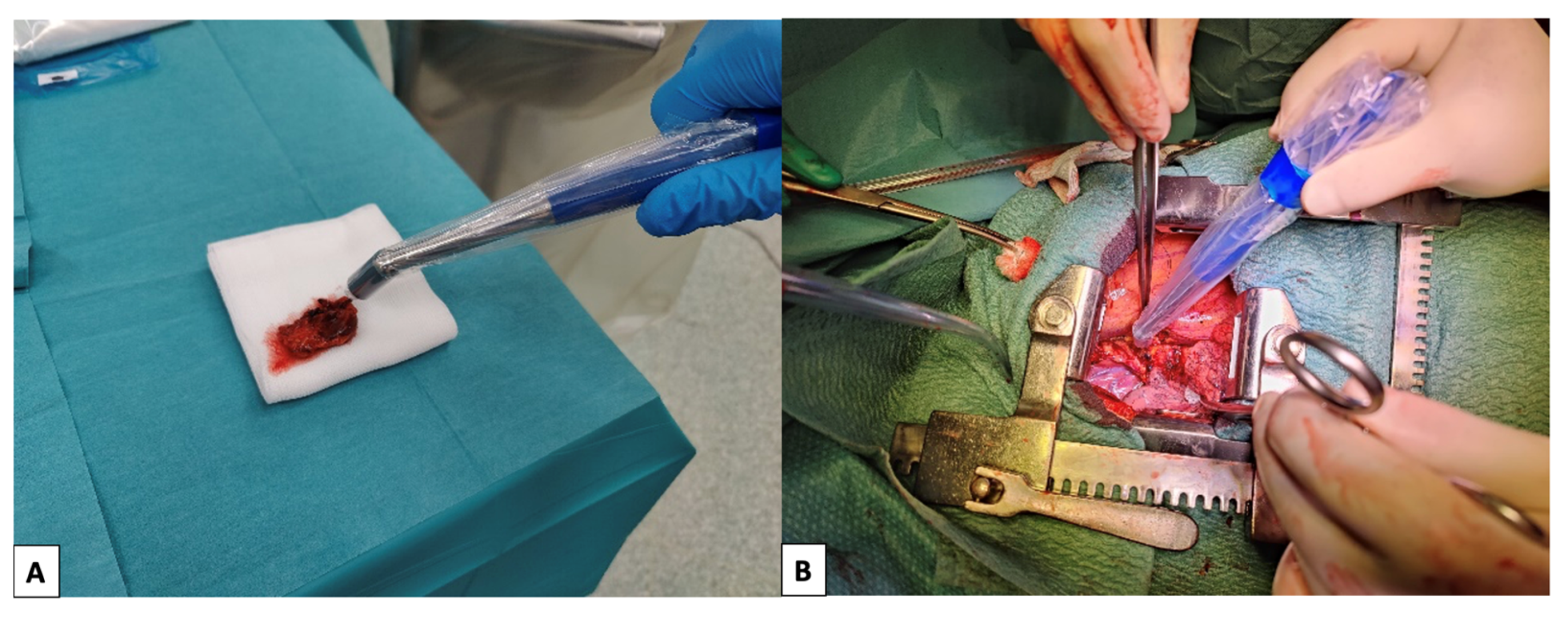
2.2. Lymphoscintigraphy and Surgery
2.3. Statistical Analysis
3. Results
3.1. Patients’ Population
3.2. Distribution of Metastases in SLNs and nSLNs
3.3. Lymphatic Pathing
3.4. Predictors of Lymph Nodes Localisations and of eSLNs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non–Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Balata, H.; Fong, K.M.; Hendriks, L.E.; Lam, S.; Ostroff, J.S.; Peled, N.; Wu, N.; Aggarwal, C. Prevention and Early Detection for NSCLC: Advances in Thoracic Oncology 2018. J. Thorac. Oncol. 2019, 14, 1513–1527. [Google Scholar] [CrossRef]
- Bao, F.; Yuan, P.; Yuan, X.; Lv, X.; Wang, Z.; Hu, J. Predictive Risk Factors for Lymph Node Metastasis in Patients with Small Size Non-Small Cell Lung Cancer. J. Thorac. Dis. 2014, 6, 1697. [Google Scholar] [CrossRef]
- Ost, D.E.; Yeung, S.C.J.; Tanoue, L.T.; Gould, M.K. Clinical and Organizational Factors in the Initial Evaluation of Patients With Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143, e121S–e141S. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, J.; Sawicki, L.M.; Nensa, F.; Schaarschmidt, B.M.; Reis, H.; Ingenwerth, M.; Bogner, S.; Aigner, C.; Buchbender, C.; Umutlu, L.; et al. Prospective Comparison of 18 F-FDG PET/MRI and 18 F-FDG PET/CT for Thoracic Staging of Non-Small Cell Lung Cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, P.; Zhang, H.; Shi, Y.; Wu, H.; Zhang, J.; Qian, Y.; Li, C.; Yang, J. Diagnostic Value of Fluorine 18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for the Detection of Metastases in Non-Small-Cell Lung Cancer Patients. Int. J. Cancer 2012, 132, E37–E47. [Google Scholar] [CrossRef]
- Akhurst, T. Staging of Non–Small-Cell Lung Cancer. PET Clin. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Yang, C.F.J.; Gu, L.; Shah, S.A.; Yerokun, B.A.; D’Amico, T.A.; Hartwig, M.G.; Berry, M.F. Long-Term Outcomes of Surgical Resection for Stage IV Non-Small-Cell Lung Cancer: A National Analysis. Lung Cancer 2018, 115, 75–83. [Google Scholar] [CrossRef]
- Raman, V.; Jawitz, O.K.; Yang, C.F.J.; Voigt, S.L.; Wang, H.; D’Amico, T.A.; Harpole, D.H.; Tong, B.C. Outcomes of Surgery versus Chemoradiotherapy in Patients with Clinical or Pathologic Stage N3 Non–Small Cell Lung Cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 1680–1692. [Google Scholar] [CrossRef]
- Dartevelle, P.G.; Mitilian, D.; Fadel, E. Extended Surgery for T4 Lung Cancer: A 30 Years’ Experience. Gen. Thorac. Cardiovasc. Surg. 2017, 65, 321–328. [Google Scholar] [CrossRef]
- Tabchi, S.; Kassouf, E.; Rassy, E.; El Kourie, H.R.; Martin, J.; Campeau, M.P.; Tehfe, M.; Blais, N. Management of Stage III Non–Small Cell Lung Cancer. Semin. Oncol. 2017, 44, 163–177. [Google Scholar] [CrossRef]
- Fischer, B.M.; Olsen, M.W.B.; Ley, C.D.; Klausen, T.L.; Mortensen, J.; Højgaard, L.; Kristjansen, P.E.G. How Few Cancer Cells Can Be Detected by Positron Emission Tomography? A Frequent Question Addressed by an in Vitro Study. Eur. J. Nucl. Med. 2006, 33, 697–702. [Google Scholar] [CrossRef]
- Passlick, B.; Kubuschock, B.; Sienel, W.; Thetter, O.; Pantel, K.; Izbicki, J.R. Mediastinal Lymphadenectomy in Non-Small Cell Lung Cancer: Effectiveness in Patients with or without Nodal Micrometastases-Results of a Preliminary Study. Eur. J. Cardiothorac. Surg. 2002, 21, 520–526. [Google Scholar] [CrossRef]
- Taghizadeh Kermani, A.; Bagheri, R.; Tehranian, S.; Shojaee, P.; Sadeghi, R.; Krag, D.N. Accuracy of Sentinel Node Biopsy in the Staging of Non-Small Cell Lung Carcinomas: Systematic Review and Meta-Analysis of the Literature. Lung Cancer 2013, 80, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Somerfield, M.R.; Bosserman, L.D.; Perkins, C.L.; Weaver, D.L.; Giuliano, A.E. Sentinel Lymph Node Biopsy for Patients with Early-Stage Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 561–564. [Google Scholar] [CrossRef]
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.; et al. Guidelines of Care for the Management of Primary Cutaneous Melanoma. J. Am. Acad. Derm. 2019, 80, 208–250. [Google Scholar] [CrossRef]
- Handa, Y.; Tsutani, Y.; Mimae, T.; Miyata, Y.; Ito, H.; Shimada, Y.; Nakayama, H.; Ikeda, N.; Okada, M.; Okada, M.; et al. Systematic Versus Lobe-Specific Mediastinal Lymphadenectomy for Hypermetabolic Lung Cancer. Ann. Surg. Oncol. 2021, 28, 7162–7171. [Google Scholar] [CrossRef]
- Aokage, K.; Yoshida, J.; Ishii, G.; Hishida, T.; Nishimura, M.; Nagai, K. Subcarinal Lymph Node in Upper Lobe Non-Small Cell Lung Cancer Patients: Is Selective Lymph Node Dissection Valid? Lung Cancer 2010, 70, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Rami Porta, R.; Waller, D.; Passlick, B.; Zielinski, M.; Junker, K.; Rendina, A.; Ris, H.-B.; et al. Invited Paper ESTS Guidelines for Intraoperative Lymph Node Staging in Non-Small Cell Lung Cancer. Eur. J. Cardio-Thoracic Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Sugi, K.; Kaneda, Y.; Sudoh, M.; Sakano, H.; Hamano, K. Effect of Radioisotope Sentinel Node Mapping in Patients with CT1 N0 M0 Lung Cancer. J. Thorac. Cardiovasc. Surg. 2003, 126, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Liptay, M.J.; Masters, G.A.; Winchester, D.J.; Edelman, B.L.; Garrido, B.J.; Hirschtritt, T.R.; Perlman, R.M.; Fry, W.A. Intraoperative Radioisotope Sentinel Lymph Node Mapping in Non–Small Cell Lung Cancer. Ann. Thorac. Surg. 2000, 70, 384–389. [Google Scholar] [CrossRef]
- Faries, M.B.; Bleicher, R.J.; Ye, X.; Essner, R.; Morton, D.L. Lymphatic Mapping and Sentinel Lymphadenectomy for Primary and Metastatic Pulmonary Malignant Neoplasms. Arch. Surg. 2004, 139, 870–877. [Google Scholar] [CrossRef]
- Lardinois, D.; Brack, T.; Gaspert, A.; Spahr, T.; Schneiter, D.; Steinert, H.C.; Weder, W. Bronchoscopic Radioisotope Injection for Sentinel Lymph-Node Mapping in Potentially Resectable Non-Small-Cell Lung Cancer. Eur. J. Cardio-Thoracic Surg. 2003, 23, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Nomori, H.; Watanabe, K.; Ohtsuka, T.; Naruke, T.; Suemasu, K. In Vivo Identification of Sentinel Lymph Nodes for Clinical Stage I Non-Small Cell Lung Cancer for Abbreviation of Mediastinal Lymph Node Dissection. Lung Cancer 2004, 46, 49–55. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.K.; Kang, D.-Y.; Jeong, J.M.; Choi, Y.H. Intra-Operative Sentinel Lymph Node Identification Using a Novel Receptor-Binding Agent (Technetium-99m Neomannosyl Human Serum Albumin, 99mTc-MSA) in Stage I Non-Small Cell Lung Cancer. Eur. J. Cardio-Thorac. Surg. 2010, 37, 1450–1456. [Google Scholar] [CrossRef]
- Suzuki, K.; Saji, H.; Aokage, K.; Watanabe, S.; Okada, M.; Mizusawa, J.; Nakajima, R.; Tsuboi, M.; Nakamura, S.; Nakamura, K.; et al. Comparison of Pulmonary Segmentectomy and Lobectomy: Safety Results of a Randomized Trial. J. Thorac. Cardiovasc. Surg. 2019, 158, 895–907. [Google Scholar] [CrossRef]
- Kanzaki, R.; Nagoya, A.; Kanou, T.; Ose, N.; Funaki, S.; Minami, M.; Okamoto, Y.; Tabuchi, H.; Hoshino, T.; Tajima, T.; et al. Risk Factors for Non-Cancer Death after Surgery in Patients with Stage I Non-Small-Cell Lung Cancer. Eur. J. Cardio-Thorac. Surg. 2021, 59, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, I.; Sánchez-Izquierdo, N.; Martínez, D.; Sánchez-Lorente, D.; Casanueva-Eliceiry, S.; Boada, M.; Guirao, Á.; Romero-Zayas, I.; Vidal-Sicart, S.; Paredes, P. Role of a Portable Gamma-Camera with Optical View for Margins Assessment of Pulmonary Nodules Resected by Radioguided Surgery. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Profeta, A.C.; Schilling, C.; McGurk, M. Augmented Reality Visualization in Head and Neck Surgery: An Overview of Recent Findings in Sentinel Node Biopsy and Future Perspectives. Br. J. Oral. Maxillofac. Surg. 2016, 54, 694–696. [Google Scholar] [CrossRef]
- Lecointre, L.; Verde, J.; Goffin, L.; Venkatasamy, A.; Seeliger, B.; Lodi, M.; Swanström, L.L.; Akladios, C.; Gallix, B. Robotically Assisted Augmented Reality System for Identification of Targeted Lymph Nodes in Laparoscopic Gynecological Surgery: A First Step toward the Identification of Sentinel Node. Surg. Endosc. 2022, 36, 9224–9233. [Google Scholar] [CrossRef] [PubMed]
| Patients, n | 48 | Mean injected activity, MBq (IQR) | 31.5 (5) | ||
| Sex, n | 17 F (35%) | 31 M (65%) | |||
| Mean age, years (IQR) | 69 (12) | Dissected lymph nodes, n | Total | 290 | |
| SLNs | 179 (62%) | ||||
| Smoking habit, n | Yes | 37 (77%) | nSLNs | 87 (30%) | |
| No | 11 (23%) | eSLNs | 24 (8%) | ||
| Histoype, n | Adenocarcinoma | 34 (71%) | Metastatic lymph nodes, n | Total | 44 |
| Squamous cells | 14 (29%) | SLNs | 36 (82%) | ||
| Neoadjuvant treatment, n | Yes | 10 (21%) | nSLNs | 8 (18%) | |
| No | 38 (79%) | eSLNs | 0 (0%) | ||
| Clinical T (cT) staging, n | cT1 | 14 (29%) | Pathological T (pT) staging, n | pT1 | 15 (31%) |
| cT2 | 16 (33%) | pT2 | 18 (38%) | ||
| cT3 | 15 (31%) | pT3 | 9 (19%) | ||
| cT4 | 3 (6%) | pT4 | 6 (12%) | ||
| Clinical N (cN) staging, n | cN0 | 25 (52%) | Pathological N (pN) staging, n | pN0 | 26 (54%) |
| cN1 | 10 (21%) | pN1 | 9 (19%) | ||
| cN2 | 13 (27%) | pN2 | 13 (27%) | ||
| Patient # | Primary Localization | Histotype | cTNM, yTNM | Positive SLN Stations | Positive nSLN Stations | Negative SLN Stations |
|---|---|---|---|---|---|---|
| 31 | Left lung | Squamous cells | cT2N2 | Within the left lung | Subaortic | Left hilus, subcarinal, paraesophageal |
| 37 | Left upper lobe | Adenocarcinoma | cT2N1 | Subaortic | Para-aortic, left hilus, subcarinal, paraesophageal | Within the left upper lobe |
| 47 | Right upper lobe | Adenocarcinoma | cT3N2, yT2N2 | Within the right upper lobe, upper/lower tracheal right, subcarinal | Anterior mediastinum, upper tracheal right, upper tracheal corner | Lower tracheal left |
| Nodal Staging | Number of Patients | Number of Metastases in SLNs | Number of Metastases in nSLNs | Skip Metastases |
|---|---|---|---|---|
| cN0 | 25 | 5 | None | 1 * |
| cN1 | 10 | 5 | 4 | None |
| cN2 | 13 | 26 | 4 | 1 * |
| Parameter | OR | Significance | LB | UB |
|---|---|---|---|---|
| Sex (male) | 8.273 | 0.065 | 0.875 | 78.204 |
| Neoadjuvant chemotherapy | 12.882 | 0.078 | 0.753 | 220.279 |
| cN0 | Reference | |||
| cN1 | 9.665 | 0.083 | 0.746 | 125.195 |
| cN2 | 17.506 | 0.021 | 1.548 | 198.031 |
| Parameter | OR | Significance | LB | UB |
|---|---|---|---|---|
| Neoadjuvant chemotherapy | 0.17 | 0.159 | 0.015 | 1.996 |
| Uptake time (>median) | 0.227 | 0.029 | 0.06 | 0.862 |
| cN0 | Reference | |||
| cN1 | 0.737 | 0.783 | 0.084 | 6.439 |
| cN2 | 0.653 | 0.645 | 0.107 | 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pini, C.; Bottoni, E.; Fiz, F.; Giudici, V.M.; Alloisio, M.; Testori, A.; Rodari, M.; Sollini, M.; Chiti, A.; Cariboni, U.; et al. Radioisotope-Guided Excision of Mediastinal Lymph Nodes in Patients with Non-Small Cell Lung Carcinoma: Feasibility and Clinical Impact. Cancers 2023, 15, 3320. https://doi.org/10.3390/cancers15133320
Pini C, Bottoni E, Fiz F, Giudici VM, Alloisio M, Testori A, Rodari M, Sollini M, Chiti A, Cariboni U, et al. Radioisotope-Guided Excision of Mediastinal Lymph Nodes in Patients with Non-Small Cell Lung Carcinoma: Feasibility and Clinical Impact. Cancers. 2023; 15(13):3320. https://doi.org/10.3390/cancers15133320
Chicago/Turabian StylePini, Cristiano, Edoardo Bottoni, Francesco Fiz, Veronica Maria Giudici, Marco Alloisio, Alberto Testori, Marcello Rodari, Martina Sollini, Arturo Chiti, Umberto Cariboni, and et al. 2023. "Radioisotope-Guided Excision of Mediastinal Lymph Nodes in Patients with Non-Small Cell Lung Carcinoma: Feasibility and Clinical Impact" Cancers 15, no. 13: 3320. https://doi.org/10.3390/cancers15133320
APA StylePini, C., Bottoni, E., Fiz, F., Giudici, V. M., Alloisio, M., Testori, A., Rodari, M., Sollini, M., Chiti, A., Cariboni, U., & Antunovic, L. (2023). Radioisotope-Guided Excision of Mediastinal Lymph Nodes in Patients with Non-Small Cell Lung Carcinoma: Feasibility and Clinical Impact. Cancers, 15(13), 3320. https://doi.org/10.3390/cancers15133320







