Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies
Abstract
Simple Summary
Abstract
1. Introduction
2. Background
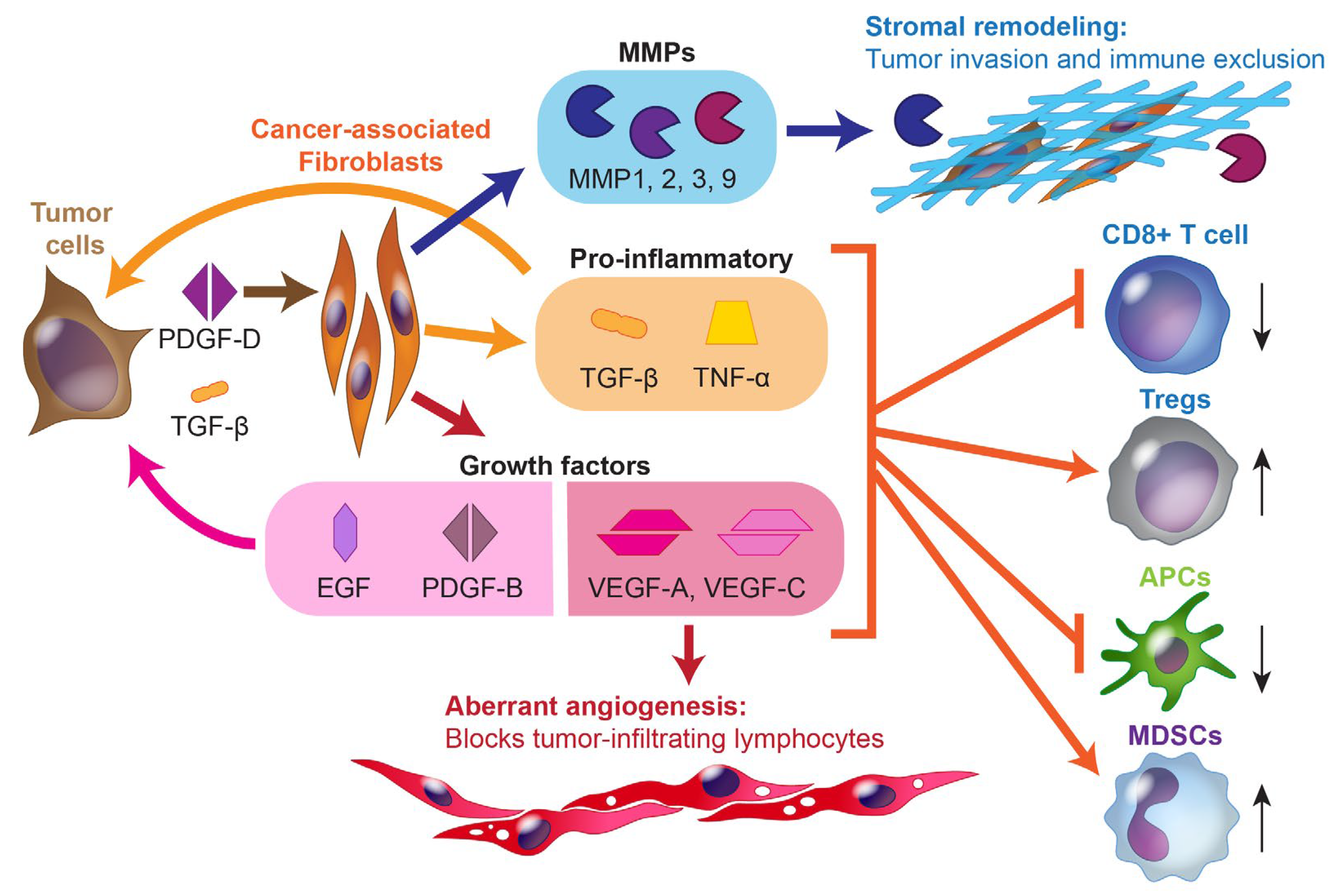
2.1. The Immune Microenvironment of Biliary Tract Cancers
| Oncogene | iCCA | eCCA | GBA | Targeted Agents | Combinations with IO in BTC Trials |
|---|---|---|---|---|---|
| IDH1/IDH2 | 4.9–36% | 0–7.4% | 1.5% | Ivosidenib * | Ivosidenib + nivolumab |
| FGFR1, 2, or 3 fusion/mut./amp. | 11–45% | - | 3% | Pemigatinib ** Futibatinib ** | Pemigatinib + pembrolizumab |
| MAP kinase pathway: KRAS mut. BRAF mut. | 8.6–24.2% 3–7.1% | 8.3–42% 3% | 4–13% 1–5.9% | - Dabrafenib | Mutation agnostic: Cobimetinib + atezolizumab Cobimetinib + atezolizumab + varlilumab |
| ERBB2 or 3 (HER2 or 3) amp. | 7% | 11–17% | 9.8–19% | Trastuzumab Pertuzumab | Trastuzumab + nivolumab + chemo |
2.2. Landscape of Chemotherapy in Advanced Biliary Tract Cancers
3. Immunotherapy in Advanced Biliary Tract Cancers
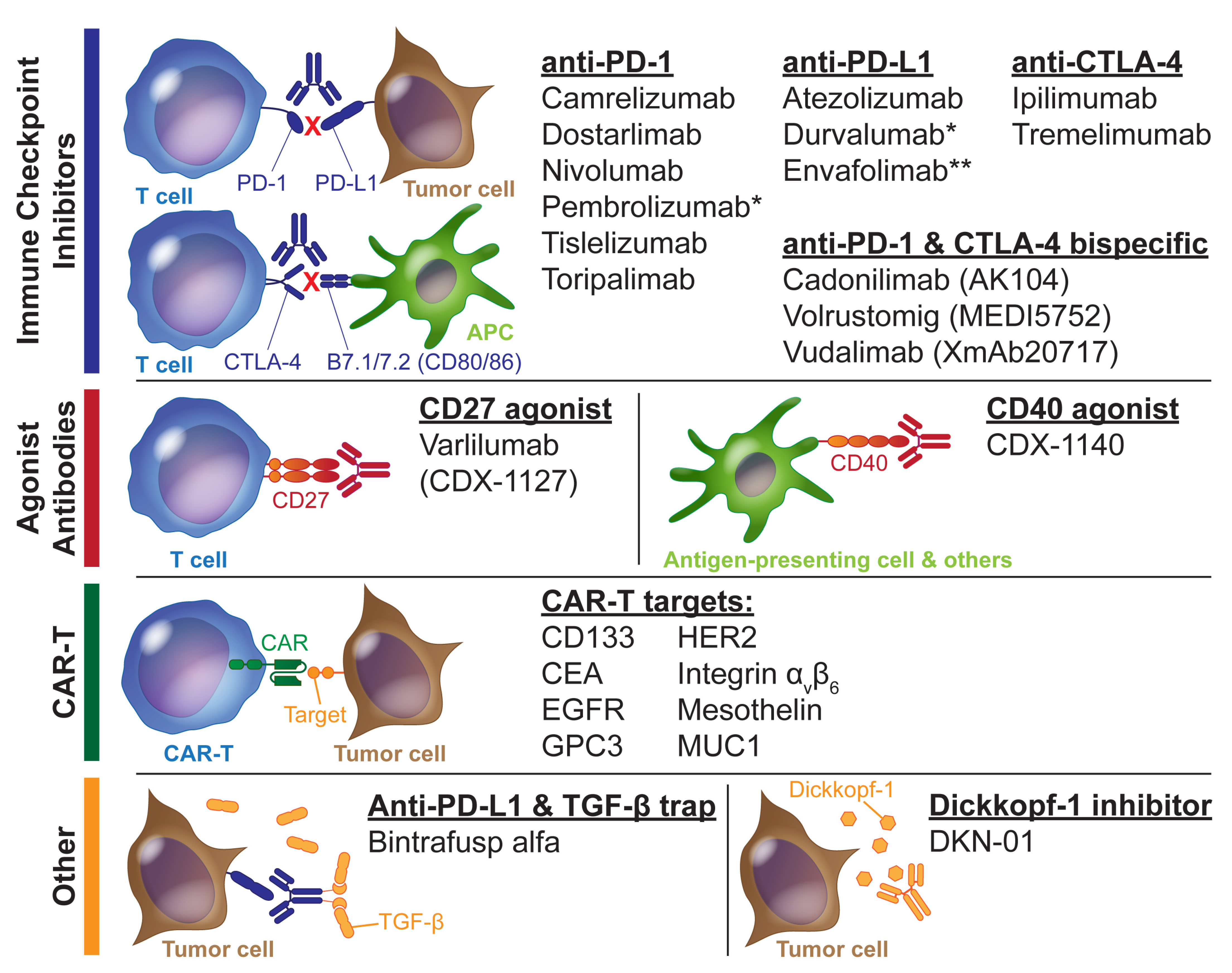
3.1. Immune Checkpoint Inhibitors as Monotherapy or Doublet Therapy
3.2. ICIs in Combination with Chemotherapy
| NCT Number | Trial Name | Ph. | Treatment | Ln. | Prim. Endpoint | Met Endpt. | ORR | PFS (mo.) | OS (mo.) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Immune-checkpoint-inhibitor-only monotherapy and combination therapy | ||||||||||
| NCT02054806 | KEYNOTE-028 | I | Pembrolizumab | 2nd | ORR | - | 13.0% | 1.8 | 5.7 | [37] |
| NCT02628067 | KEYNOTE-158 | II | Pembrolizumab | 2nd | ORR | - | 5.8% | 2.0 | 7.4 | [37] |
| NCT02829918 | - | II | Nivolumab | 2nd | ORR | - | 10.9% | 3.7 | 14.2 | [44] |
| NCT02923934 | CA209-538 | II | Ipilimumab/nivolumab | 2nd | DCR | 23.1% | 2.9 | 5.7 | [38] | |
| NCT01938612 | - | I | Durvalumab Durvalumab/tremelimumab | 2nd | DLT/AE | - | 4.8% 10.8% | 1.5 1.6 | 8.1 10.1 | [45] |
| NCT03704480 | IMMUNO-BIL/ PRODIGE 57 | II | Durvalumab/tremelimumab (interim) | 2nd | OS (6 mo) | - | 9.7% | 2.5 | 8.0 | [40] |
| Immune checkpoint inhibitors plus chemotherapy | ||||||||||
| NCT03875235 | TOPAZ-1 | III | Gem/cis + durvalumab Gem/cis | 1st | OS | Yes | 26.7% 18.7% | 7.2 5.7 | 12.8 11.5 | [6] |
| NCT04003636 | KEYNOTE-966 | III | Gem/cis + pembrolizumab Gem/cis | 1st | OS | Yes | 28.7% 28.5% | 6.5 5.6 | 12.7 10.9 | [46] |
| NCT03101566 | BilT-01 | II | Gem/cis + nivolumab Ipilimumab/nivolumab | 1st | PFS | No | 22.9% 3.0% | 6.6 3.9 | 10.6 8.2 | [43] |
| NCT03046862 | - | II | Gem/cis + durvalumab + tremelimumab Gem/cis + durvalumab Gem/cis, then gem/cis + durvalumab + tremelimumab | 1st | ORR | Yes | 70.2% 72.3% 50.0% | 12.3 11.8 12.8 | 18.7 20.2 15.0 | [47] |
| Immune checkpoint inhibitors plus molecularly targeted therapy | ||||||||||
| NCT02393248 | FIGHT-101 | I/II | Pemigatinib + pembrolizumab | 2nd | AEs, ORR | - | No BTC-only analysis | [48] | ||
| NCT03201458 | - | II | Cobimetinib + atezolizumab Atezolizumab | 2nd | PFS | Yes | 3.3% 2.8% | 3.7 1.9 | - | [49] |
| NCT03639935 | BilT-02 | II | Rucaparib + nivolumab maintenance after platinum-based chemotherapy | Mnt. | PFS4 | No | 6.4% | 4.6 * | 15.9 * | [50,51] |
| Immune checkpoint inhibitors plus antiangiogenics | ||||||||||
| NCT03895970 | - | II | Lenvatinib + pembrolizumab | 2nd | ORR, DCR, PFS | - | 25.0% | 4.9 | 11.0 | [52] |
| NCT03797326 | LEAP-005 | II | Lenvatinib + pembrolizumab | 2nd | ORR | - | 9.7% | 6.1 | 8.6 | [53] |
| NCT03475953 | REGOMUNE | II | Regorafenib + avelumab | 2nd | ORR | - | 13.8% | 2.5 | 11.9 | [54] |
| NCT02443324 | JVDF | I | Ramucirumab + pembrolizumab | 2nd | DLT | - | 3.8% | 1.6 | 6.4 | [55] |
| NCT04677504 | IMBrave-151 | II | Gem/cis + atezolizumab + bevacizumab Gem/cis + atezolizumab | 1st | PFS | 24.1% 25.3% | 8.4 7.9 | - | [56] | |
| NCT03951597 | - | II | Gem/ox + lenvatinib + toripalimab | 1st | ORR | Yes | 80.0% | 10.2 | 22.5 | [57,58] |
| Other | ||||||||||
| NCT02699515 | - | I | Bintrafusp alfa | 2nd | ORR | - | 20.0% | 2.5 | 12.7 | [59] |
| NCT03833661 | INTR@PID BTC 047 | II | Bintrafusp alfa | 2nd | ORR | No | 10.7% | 1.8 | 7.6 | [60] |
| NCT02375880 | - | I | Gem/cis + DKN-01 | 1st | ORR | - | 21.3% | 8.7 | 12.4 | [61] |
| NCT01869166 | - | I | EGFR CAR-T | 2nd | AEs | - | 5.9% | - | - | [62] |
| NCT03358849 | - | I/II | Allogeneic NK cells + pembrolizumab | 2nd | DLT | - | 17.4% | - | - | [63] |
| - | - | I | WT1 peptide vaccine + gemcitabine | Safety | - | - | - | 9.5 | [64] | |
| - | - | I | MUC1 peptide vaccine | Safety | - | 0.0% | - | - | [65] | |
| NCT/Name | Ph. | Treatment | Line | Prim. Endpoint | Status | Est. Comp. |
|---|---|---|---|---|---|---|
| Immune-checkpoint-inhibitor-only monotherapy and combination therapy | ||||||
| NCT05297903 | II | XmAb20717 (vudalimab) | 2nd | ORR | Recruiting | 12/2024 |
| Immune checkpoint inhibitors plus chemotherapy | ||||||
| NCT03260712 (ABC-09/EORTC-1607) | II | Gem/cis + pembrolizumab | 1st | PFS6 | Active, not rec. | 6/2023 |
| NCT04172402 | II | Gem/S-1 + nivolumab | 1st | ORR | Active, not rec. | 12/2024 |
| NCT04191343 | II | Gem/ox + toripalimab | 1st | ORR | Recruiting | 6/2024 |
| NCT03478488 | III | Gem/ox + envafolimab (KN035) Gem/ox | 1st | OS | Recruiting | 7/2024 |
| NCT03785873 | I/II | 5-fluorouracil/nal-irinotecan + nivolumab | 2nd | DLT (I), PFS (II) | Active, not rec. | 5/2023 |
| NCT04308174 (DEBATE) | II | Gem/cis + durvalumab (NA) + durvalumab (A) Gem/cis (NA) + durvalumab (A) | NA + A | R0 resection | Active, not rec. | 12/2023 |
| NCT04333927 (ACCORD) | II | Camrelizumab + capecitabine + radiation Observation | A | OS | Active, not rec. | 6/2024 |
| NCT05239169 | II | Capecitabine + durvalumab + tremelimumab Durvalumab + tremelimumab | A | RFS12 | Recruiting | 12/2024 |
| Immune checkpoint inhibitors plus molecularly targeted therapy | ||||||
| NCT04941287 (ETCTN 10476) | II | Atezolizumab + varlilumab + cobimetinib Atezolizumab + varlilumab | 2nd | ORR, PFS | Active, not rec. | 9/2023 |
| NCT04056910 | II | Ivosidenib + nivolumab | 2nd | ORR, PFS6 | Recruiting | 5/2026 |
| NCT05749900 (HERBOT) | I/II | Trastuzumab + nivolumab + gem/cis | 1st | Dosing (I), ORR (II) | Not yet rec. | 2/2027 |
| NCT04306367 | II | Olaparib + pembrolizumab | 2nd | ORR | Active, not rec. | 12/2023 |
| NCT03991832 | II | Olaparib + durvalumab | 2nd | ORR, DCR | Recruiting | 3/2025 |
| NCT04298008 | II | AZD6738 (ceralasertib) + durvalumab | 2nd | DCR | Recruiting | 12/2024 |
| Immune checkpoint inhibitors plus antiangiogenics | ||||||
| NCT04506281 | II | Gem/ox+ toripalimab + lenvatinib (NA) + capecitabine (A) Capecitabine (A) only | NA + A | EFS | Recruiting | 8/2023 |
| NCT05254847 | II | Capecitabine + lenvatinib + tislelizumab | A | DFS12 | Recruiting | 12/2024 |
| NCT05342194 | III | Gem/ox or gem/cis + toripalimab + Lenvatinib Gem/ox or gem/cis + toripalimab Gem/ox or gem/cis | 1st | OS | Not yet rec. | 5/2027 |
| NCT05742750 | I/II | Gem/cis + camrelizumab + apatinib | 1st | DLT (I), ORR (II) | Not yet rec. | 12/2024 |
| NCT05820906 | II | Gem/cis + cadonilimab + regorafenib | 1st | ORR | Not yet rec. | 9/2025 |
| NCT05775159 (GEMINI- Hepatobiliary) | II | MEDI5752 (volrustomig) MEDI5752 + lenvatinib MEDI5752 + bevacizumab | Any | ORR | Recruiting | 11/2025 |
| NCT05052099 (COMBATBIL) | I/II | mFOLFOX6 + atezolizumab + bevacizumab | 2nd | ORR | Recruiting | 6/2024 |
| Other | ||||||
| NCT04057365 | II | DKN-01 + nivolumab | 2nd | ORR | Recruiting | 8/2024 |
| NCT05849480 | I/II | Capecitabine/oxaliplatin + pembrolizumab + CDX-1140 | 2nd | Dosing (I), ORR and PFS6 (II) | Not yet rec. | - |
| See Section 3.5 of the text for a list of ongoing CAR-T therapies | ||||||
3.3. ICIs in Combination with Molecularly Targeted Therapeutics
3.3.1. Current Landscape of Molecularly Targeted Therapeutics in BTCs
3.3.2. IDH1-Targeted Therapies Plus ICI
3.3.3. FGFR2-Targeted Therapies Plus ICI
3.3.4. MAP-Kinase-Pathway-Targeted Therapies Plus ICI
3.3.5. EGFR Blockade Plus ICI
3.3.6. HER2 Blockade Plus ICI
3.3.7. PARP Inhibitors Plus ICI
3.3.8. Summary of ICIs in Combination with Molecularly Targeted Therapeutics
3.4. ICIs in Combination with Anti-Angiogenic Therapeutics
3.5. Other Immunotherapies
4. Immunotherapy in the Adjuvant or Neoadjuvant Setting
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2022; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Fast Stats: An Interactive Tool for Access to SEER Cancer Statistics. Surveillance Research Program, National Cancer Institute. Available online: http://seer.cancer.gov/faststats (accessed on 23 May 2015).
- American Cancer Society Medical and Editorial Content Team. Survival Rates for Bile Duct Cancer. Available online: https://www.cancer.org/cancer/types/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html (accessed on 5 May 2023).
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Évid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Fabris, L.; Sato, K.; Alpini, G.; Strazzabosco, M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology 2021, 73 (Suppl. 1), 75–85. [Google Scholar] [CrossRef]
- Micke, P.; Strell, C.; Mattsson, J.; Martín-Bernabé, A.; Brunnström, H.; Huvila, J.; Sund, M.; Wärnberg, F.; Ponten, F.; Glimelius, B.; et al. The prognostic impact of the tumour stroma fraction: A machine learning-based analysis in 16 human solid tumour types. Ebiomedicine 2021, 65, 103269. [Google Scholar] [CrossRef]
- Job, S.; Rapoud, D.; Dos Santos, A.; Gonzalez, P.; Desterke, C.; Pascal, G.; Elarouci, N.; Ayadi, M.; Adam, R.; Azoulay, D.; et al. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 72, 965–981. [Google Scholar] [CrossRef]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef]
- Friedrich, M.; Sankowski, R.; Bunse, L.; Kilian, M.; Green, E.; Guevara, C.R.; Pusch, S.; Poschet, G.; Sanghvi, K.; Hahn, M.; et al. Tryptophan metabolism drives dynamic immunosuppressive myeloid states in IDH-mutant gliomas. Nat. Cancer 2021, 2, 723–740. [Google Scholar] [CrossRef]
- Bunse, L.; Pusch, S.; Bunse, T.; Sahm, F.; Sanghvi, K.; Friedrich, M.; Alansary, D.; Sonner, J.K.; Green, E.; Deumelandt, K.; et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat. Med. 2018, 24, 1192–1203. [Google Scholar] [CrossRef]
- Wu, M.-J.; Shi, L.; Dubrot, J.; Merritt, J.; Vijay, V.; Wei, T.-Y.; Kessler, E.; Olander, K.E.; Adil, R.; Pankaj, A.; et al. Mutant IDH Inhibits IFNγ–TET2 Signaling to Promote Immunoevasion and Tumor Maintenance in Cholangiocarcinoma. Cancer Discov. 2022, 12, 812–835. [Google Scholar] [CrossRef]
- Ruan, R.; Li, L.; Li, X.; Huang, C.; Zhang, Z.; Zhong, H.; Zeng, S.; Shi, Q.; Xia, Y.; Zeng, Q.; et al. Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumor microenvironment. Mol. Cancer 2023, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Palakurthi, S.; Kuraguchi, M.; Zacharek, S.J.; Zudaire, E.; Huang, W.; Bonal, D.M.; Liu, J.; Dhaneshwar, A.; DePeaux, K.; Gowaski, M.R.; et al. The Combined Effect of FGFR Inhibition and PD-1 Blockade Promotes Tumor-Intrinsic Induction of Antitumor Immunity. Cancer Immunol. Res. 2019, 7, 1457–1471. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Shapiro, B.; Vucic, E.A.; Vogt, S.; Bar-Sagi, D. Tumor Cell–Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res 2020, 80, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.B.; Cheng, N.; Markosyan, N.; Sor, R.; Kim, I.-K.; Hallin, J.; Shoush, J.; Quinones, L.; Brown, N.V.; Bassett, J.B.; et al. Efficacy of a Small-Molecule Inhibitor of KrasG12D in Immunocompetent Models of Pancreatic Cancer. Cancer Discov. 2023, 13, 298–311. [Google Scholar] [CrossRef]
- Park, S.; Jiang, Z.; Mortenson, E.D.; Deng, L.; Radkevich-Brown, O.; Yang, X.; Sattar, H.; Wang, Y.; Brown, N.K.; Greene, M.; et al. The Therapeutic Effect of Anti-HER2/neu Antibody Depends on Both Innate and Adaptive Immunity. Cancer Cell 2010, 18, 160–170. [Google Scholar] [CrossRef]
- Stagg, J.; Loi, S.; Divisekera, U.; Ngiow, S.F.; Duret, H.; Yagita, H.; Teng, M.W.; Smyth, M.J. Anti–ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti–PD-1 or anti-CD137 mAb therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 7142–7147. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef]
- Cadamuro, M.; Nardo, G.; Indraccolo, S.; Dall’Olmo, L.; Sambado, L.; Moserle, L.; Franceschet, I.; Colledan, M.; Massani, M.; Stecca, T.; et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2013, 58, 1042–1053. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef]
- Li, Y.-L.; Zhao, H.; Ren, X.-B. Relationship of VEGF/VEGFR with immune and cancer cells: Staggering or forward? Cancer Biol. Med. 2016, 13, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Tamma, R.; Annese, T.; Ruggieri, S.; Brunetti, O.; Longo, V.; Cascardi, E.; Mastropasqua, M.G.; Maiorano, E.; Silvestris, N.; Ribatti, D. Inflammatory cells infiltrate and angiogenesis in locally advanced and metastatic cholangiocarcinoma. Eur. J. Clin. Investig. 2019, 49, e13087. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, A.R.; Villena-Vargas, J.; Adusumilli, P.S. Cisplatin-Induced Antitumor Immunomodulation: A Review of Preclinical and Clinical Evidence. Clin. Cancer Res. 2014, 20, 5384–5391. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, Y.; Zhang, P.; Chu, Q. Acquired Resistance to Immune Checkpoint Blockades: The Underlying Mechanisms and Potential Strategies. Front. Immunol. 2021, 12, 693609. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef]
- Shroff, R.T.; Guthrie, K.A.; Scott, A.J.; Borad, M.J.; Goff, L.W.; Matin, K.; Mahipal, A.; Kalyan, A.; Javle, M.M.; Aghajanian, C.; et al. SWOG 1815: A phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J. Clin. Oncol. 2023, 41, LBA490. [Google Scholar] [CrossRef]
- Phelip, J.M.; Desrame, J.; Edeline, J.; Barbier, E.; Terrebonne, E.; Michel, P.; Perrier, H.; Dahan, L.; Bourgeois, V.; Akouz, F.K.; et al. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients With Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J. Clin. Oncol. 2022, 40, 262–271. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, K.-P.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): A multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Hyung, J.; Kim, I.; Kim, K.-P.; Ryoo, B.-Y.; Jeong, J.H.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; et al. Treatment With Liposomal Irinotecan Plus Fluorouracil and Leucovorin for Patients With Previously Treated Metastatic Biliary Tract Cancer: The Phase 2b NIFTY Randomized Clinical Trial. JAMA Oncol. 2023, 9, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, B.A.; Xiu, J.; Lindberg, M.R.; Shields, A.F.; Hwang, J.J.; Poorman, K.; Salem, M.E.; Pishvaian, M.J.; Holcombe, R.F.; Marshall, J.L.; et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J. Gastrointest. Oncol. 2019, 10, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Oh, D.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE -028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Nagrial, A.; Markman, B.; Underhill, C.; Michael, M.; Jackett, L.; Lum, C.; Behren, A.; Palmer, J.; et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients With Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1405–1409. [Google Scholar] [CrossRef]
- Ioka, T.; Ueno, M.; Oh, D.-Y.; Fujiwara, Y.; Chen, J.-S.; Doki, Y.; Mizuno, N.; Park, K.; Asagi, A.; Hayama, M.; et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC). J. Clin. Oncol. 2019, 37, 387. [Google Scholar] [CrossRef]
- Delaye, M.; Assenat, E.; Dahan, L.; Blanc, J.-F.; Tougeron, D.; Metges, J.-P.; Lievre, A.; Turpin, A.; Fares, N.; De La Fouchardiere, C.; et al. Durvalumab (D) plus tremelimumab (T) immunotherapy in patients (Pts) with advanced biliary tract carcinoma (BTC) after failure of platinum-based chemotherapy (CTx): Interim results of the IMMUNOBIL GERCOR D18-1 PRODIGE-57 study. J. Clin. Oncol. 2022, 40, 4108. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Évid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Li, J.; Jiang, N.; Chen, L.; Tang, L.; Mao, Y.; Li, W.; Zhou, G.; Li, Y.; et al. The immune modulation effects of gemcitabine plus cisplatin induction chemotherapy in nasopharyngeal carcinoma. Cancer Med. 2022, 11, 3437–3444. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Beg, M.S.; Shaib, W.L.; Mahalingam, D.; Zhen, D.B.; Deming, D.A.; Zalupski, M.M. A randomized phase 2 trial of nivolumab, gemcitabine, and cisplatin or nivolumab and ipilimumab in previously untreated advanced biliary cancer: BilT-01. Cancer 2022, 128, 3523–3530. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Doki, Y.; Ueno, M.; Hsu, C.; Oh, D.; Park, K.; Yamamoto, N.; Ioka, T.; Hara, H.; Hayama, M.; Nii, M.; et al. Tolerability and efficacy of durvalumab, either as monotherapy or in combination with tremelimumab, in patients from Asia with advanced biliary tract, esophageal, or head-and-neck cancer. Cancer Med. 2022, 11, 2550–2560. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.-J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Yoon, J.; Kim, T.-Y.; Bang, J.-H.; Nam, A.-R.; Oh, K.-S.; Kim, J.-M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Subbiah, V.; Nemunaitis, J.J.; Mettu, N.B.; Papadopoulos, K.P.; Barve, M.A.; Féliz, L.; Lihou, C.F.; Tian, C.; Ji, T.; et al. Safety and efficacy of pemigatinib plus pembrolizumab combination therapy in patients (pts) with advanced malignancies: Results from FIGHT-101, an open-label phase I/II study. J. Clin. Oncol. 2020, 38, 3606. [Google Scholar] [CrossRef]
- Yarchoan, M.; Cope, L.; Ruggieri, A.N.; Anders, R.A.; Noonan, A.M.; Goff, L.W.; Goyal, L.; Lacy, J.; Li, D.; Patel, A.K.; et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J. Clin. Investig. 2021, 131, e152670. [Google Scholar] [CrossRef] [PubMed]
- Sahai, V.; Tran, N.H.; Griffith, K.A.; Zalupski, M. A multicenter phase II trial of rucaparib in combination with nivolumab as maintenance therapy for patients with advanced biliary tract cancer. J. Clin. Oncol. 2019, 37, TPS4153. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Goff, L.W.; Crysler, O.V.; Enzler, T.; Zalupski, M. Phase 2 multicenter trial of rucaparib and nivolumab as maintenance therapy following first-line platinum-based chemotherapy in patients with advanced biliary tract cancer (BTC): BilT-02. J. Clin. Oncol. 2023, 41, 4113. [Google Scholar] [CrossRef]
- Lin, J.; Yang, X.; Long, J.; Zhao, S.; Mao, J.; Wang, D.; Bai, Y.; Bian, J.; Zhang, L.; Yang, X.; et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg. Nutr. 2020, 9, 414–424. [Google Scholar] [CrossRef]
- Villanueva, L.; Lwin, Z.; Chung, H.C.; Gomez-Roca, C.; Longo, F.; Yanez, E.; Senellart, H.; Doherty, M.; García-Corbacho, J.; Hendifar, A.E.; et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study. J. Clin. Oncol. 2021, 39, 321. [Google Scholar] [CrossRef]
- Cousin, S.; Cantarel, C.; Guegan, J.-P.; Mazard, T.; Gomez-Roca, C.; Metges, J.-P.; Bellera, C.; Adenis, A.; Korakis, I.; Poureau, P.-G.; et al. Regorafenib–avelumab combination in patients with biliary tract cancer (REGOMUNE): A single-arm, open-label, phase II trial. Eur. J. Cancer 2022, 162, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Arkenau, H.-T.; Martin-Liberal, J.; Calvo, E.; Penel, N.; Krebs, M.G.; Herbst, R.S.; Walgren, R.A.; Widau, R.C.; Mi, G.; Jin, J.; et al. Ramucirumab Plus Pembrolizumab in Patients with Previously Treated Advanced or Metastatic Biliary Tract Cancer: Nonrandomized, Open-Label, Phase I Trial (JVDF). Oncologist 2018, 23, 1407-e136. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Ren, Z.; Chon, H.; Park, J.O.; Kim, J.W.; Pressiani, T.; Li, D.; Zhukova, L.; Chen, M.-H.; Hack, S.P.; et al. IMbrave151: A phase 2, randomized, double-blind, placebo-controlled study of atezolizumab with or without bevacizumab in combination with cisplatin plus gemcitabine in patients with untreated, advanced biliary tract cancer. J. Clin. Oncol. 2023, 41, 491. [Google Scholar] [CrossRef]
- Jian, Z.; Fan, J.; Shi, G.-M.; Huang, X.-Y.; Wu, D.; Yang, G.-H.; Ji, Y.; Chen, Y.; Liang, F.; Lu, J.-C.; et al. Gemox chemotherapy in combination with anti-PD1 antibody toripalimab and lenvatinib as first-line treatment for advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial. J. Clin. Oncol. 2021, 39, 4094. [Google Scholar] [CrossRef]
- Shi, G.-M.; Huang, X.-Y.; Wu, D.; Sun, H.-C.; Liang, F.; Ji, Y.; Chen, Y.; Yang, G.-H.; Lu, J.-C.; Meng, X.-L.; et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: A single-center, single-arm, phase 2 study. Signal Transduct. Target. Ther. 2023, 8, 106. [Google Scholar] [CrossRef]
- Yoo, C.; Oh, D.-Y.; Choi, H.J.; Kudo, M.; Ueno, M.; Kondo, S.; Chen, L.-T.; Osada, M.; Helwig, C.; Dussault, I.; et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J. Immunother. Cancer 2019, 8, e000564. [Google Scholar] [CrossRef]
- Yoo, C.; Javle, M.M.; Mata, H.V.; de Braud, F.; Trojan, J.; Raoul, J.-L.; Kim, J.W.; Ueno, M.; Lee, C.-K.; Hijioka, S.; et al. Phase 2 trial of bintrafusp alfa as second-line therapy for patients with locally advanced/metastatic biliary tract cancers. Hepatology 2023. [Google Scholar] [CrossRef]
- Goyal, L.; Sirard, C.; Schrag, M.; Kagey, M.H.; Eads, J.R.; Stein, S.; El-Khoueiry, A.B.; Manji, G.A.; Abrams, T.A.; Khorana, A.A.; et al. Phase I and Biomarker Study of the Wnt Pathway Modulator DKN-01 in Combination with Gemcitabine/Cisplatin in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2020, 26, 6158–6167. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.-C.; Liu, Y.; Wu, Z.; Dai, H.; Yang, Q.-M.; Wang, Y.; Jia, H.; Han, W. Phase I Study of Chimeric Antigen Receptor–Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin. Cancer Res. 2018, 24, 1277–1286. [Google Scholar] [CrossRef]
- Leem, G.; Jang, S.-I.; Cho, J.-H.; Jo, J.H.; Lee, H.S.; Chung, M.J.; Park, J.Y.; Bang, S.; Yoo, D.-K.; Cheon, H.-C.; et al. Safety and Efficacy of Allogeneic Natural Killer Cells in Combination with Pembrolizumab in Patients with Chemotherapy-Refractory Biliary Tract Cancer: A Multicenter Open-Label Phase 1/2a Trial. Cancers 2022, 14, 4229. [Google Scholar] [CrossRef]
- Kaida, M.; Morita-Hoshi, Y.; Soeda, A.; Wakeda, T.; Yamaki, Y.; Kojima, Y.; Ueno, H.; Kondo, S.; Morizane, C.; Ikeda, M.; et al. Phase 1 Trial of Wilms Tumor 1 (WT1) Peptide Vaccine and Gemcitabine Combination Therapy in Patients With Advanced Pancreatic or Biliary Tract Cancer. J. Immunother. 2011, 34, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ueno, T.; Kawaoka, T.; Hazama, S.; Fukui, M.; Suehiro, Y.; Hamanaka, Y.; Ikematsu, Y.; Imai, K.; Oka, M.; et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer. Res. 2005, 25, 3575–3579. [Google Scholar] [PubMed]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Kim, T.Y.; Bang, J.-H.; Nam, A.-R.; Lee, Y.; Zhang, Q.; Rebelatto, M.; Li, W.; et al. Phase II study assessing tolerability, efficacy, and biomarkers for durvalumab (D) ± tremelimumab (T) and gemcitabine/cisplatin (GemCis) in chemo-naïve advanced biliary tract cancer (aBTC). J. Clin. Oncol. 2020, 38, 4520. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.; Lee, T.; Lee, M.; Kitano, M.; et al. 78P Updated overall survival (OS) from the phase III TOPAZ-1 study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+ GC) in patients (pts) with advanced biliary tract cancer (BTC). Ann. Oncol. 2022, 33, S1462–S1463. [Google Scholar] [CrossRef]
- FDA Oncology Center of Excellence. FDA Approves Durvalumab for Locally Advanced or Metastatic Biliary Tract Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-locally-advanced-or-metastatic-biliary-tract-cancer (accessed on 3 May 2023).
- Papadopoulos, K.P.; Harb, W.; Peer, C.J.; Hua, Q.; Xu, S.; Lu, H.; Lu, N.; He, Y.; Xu, T.; Dong, R.; et al. First-in-Human Phase I Study of Envafolimab, a Novel Subcutaneous Single-Domain Anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors. Oncologist 2021, 26, e1514–e1525. [Google Scholar] [CrossRef]
- Lecona, E.; Fernandez-Capetillo, O. Targeting ATR in cancer. Nat. Rev. Cancer 2018, 18, 586–595. [Google Scholar] [CrossRef]
- Kim, R.; Kwon, M.; An, M.; Kim, S.; Smith, S.; Loembé, A.; Mortimer, P.; Armenia, J.; Lukashchuk, N.; Shah, N.; et al. Phase II study of ceralasertib (AZD6738) in combination with durvalumab in patients with advanced/metastatic melanoma who have failed prior anti-PD-1 therapy. Ann. Oncol. 2021, 33, 193–203. [Google Scholar] [CrossRef]
- Pellino, A.; Loupakis, F.; Cadamuro, M.; Dadduzio, V.; Fassan, M.; Guido, M.; Cillo, U.; Indraccolo, S.; Fabris, L. Precision medicine in cholangiocarcinoma. Transl. Gastroenterol. Hepatol. 2018, 3, 40. [Google Scholar] [CrossRef]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.-T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.; Wasan, H.; et al. FIGHT-302: First-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Futur. Oncol. 2020, 16, 2385–2399. [Google Scholar] [CrossRef]
- Brandi, G.; Rizzo, A. IDH Inhibitors and Immunotherapy for Biliary Tract Cancer: A Marriage of Convenience? Int. J. Mol. Sci. 2022, 23, 10869. [Google Scholar] [CrossRef]
- Dushyanthen, S.; Teo, Z.L.; Caramia, F.; Savas, P.; Mintoff, C.P.; Virassamy, B.; Henderson, M.A.; Luen, S.J.; Mansour, M.; Kershaw, M.H.; et al. Agonist immunotherapy restores T cell function following MEK inhibition improving efficacy in breast cancer. Nat. Commun. 2017, 8, 606. [Google Scholar] [CrossRef]
- Loi, S.; Dushyanthen, S.; Beavis, P.A.; Salgado, R.; Denkert, C.; Savas, P.; Combs, S.; Rimm, D.L.; Giltnane, J.M.; Estrada, M.V.; et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin. Cancer Res. 2016, 22, 1499–1509. [Google Scholar] [CrossRef]
- Ebert, P.J.; Cheung, J.; Yang, Y.; McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S.E.; Maecker, H.; Irving, B.A.; Kim, J.M.; et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016, 44, 609–621. [Google Scholar] [CrossRef]
- Dennison, L.; Ruggieri, A.; Mohan, A.; Leatherman, J.; Cruz, K.; Woolman, S.; Azad, N.; Lesinski, G.B.; Jaffee, E.M.; Yarchoan, M. Context-Dependent Immunomodulatory Effects of MEK Inhibition Are Enhanced with T-cell Agonist Therapy. Cancer Immunol. Res. 2021, 9, 1187–1201. [Google Scholar] [CrossRef]
- Ruggieri, A.N.; Yarchoan, M.; Goyal, S.; Liu, Y.; Sharon, E.; Chen, H.X.; Olson, B.M.; Paulos, C.M.; El-Rayes, B.F.; Maithel, S.K.; et al. Combined MEK/PD-L1 Inhibition Alters Peripheral Cytokines and Lymphocyte Populations Correlating with Improved Clinical Outcomes in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2022, 28, 4336–4345. [Google Scholar] [CrossRef]
- Heumann, T.R.; Yarchoan, M.; Murray, J.; Wang, H.; Wright, J.J.; Sharon, E.; Lesinski, G.B.; Azad, N.S. ETCTN 10476: A randomized phase 2 study of combination atezolizumab and varlilumab (CDX-1127) with or without addition of cobimetinib in previously treated unresectable biliary tract cancer. J. Clin. Oncol. 2023, 41, TPS639. [Google Scholar] [CrossRef]
- Lee, C.; Pirdas, A. Epidermal Growth Factor Receptor Immunoreactivity in Gallbladder and Extrahepatic Biliary Tract Tumours. Pathol.-Res. Pract. 1995, 191, 1087–1091. [Google Scholar] [CrossRef]
- Vogel, A.; Kasper, S.; Bitzer, M.; Block, A.; Sinn, M.; Schulze-Bergkamen, H.; Moehler, M.; Pfarr, N.; Endris, V.; Goeppert, B.; et al. PICCA study: Panitumumab in combination with cisplatin/gemcitabine chemotherapy in KRAS wild-type patients with biliary cancer—A randomised biomarker-driven clinical phase II AIO study. Eur. J. Cancer 2018, 92, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Malka, D.; Cervera, P.; Foulon, S.; Trarbach, T.; de la Fouchardière, C.; Boucher, E.; Fartoux, L.; Faivre, S.; Blanc, J.-F.; Viret, F.; et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): A randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014, 15, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, X.; Jiang, T.; Zhao, S.; Zhao, C.; Zhang, L.; Liu, X.; Shi, J.; Qiao, M.; Luo, J.; et al. EGFR-targeted therapy alters the tumor microenvironment in EGFR-driven lung tumors: Implications for combination therapies. Int. J. Cancer 2019, 145, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Wiest, N.; Majeed, U.; Seegobin, K.; Zhao, Y.; Lou, Y.; Manochakian, R. Role of Immune Checkpoint Inhibitor Therapy in Advanced EGFR-Mutant Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 751209. [Google Scholar] [CrossRef]
- Goff, L.W.; Cardin, D.B.; Whisenant, J.G.; Du, L.; Koyama, T.; Dahlman, K.B.; Salaria, S.N.; Young, R.T.; Ciombor, K.K.; Gilbert, J.; et al. A phase I trial investigating pulsatile erlotinib in combination with gemcitabine and oxaliplatin in advanced biliary tract cancers. Investig. New Drugs 2016, 35, 95–104. [Google Scholar] [CrossRef]
- Park, H.; Bekaii-Saab, T.S.; Kim, S.S.; Kamath, S.D.; Pishvaian, M.J.; Chen, C.; Zhen, D.B.; Mayor, J.G.; Tan, Q.; Strickler, J.H. Phase 1b/2, open-label, dose-escalation and expansion trial of tucatinib in combination with trastuzumab with and without oxaliplatin-based chemotherapy or pembrolizumab in patients with unresectable or metastatic HER2+ gastrointestinal cancers (trial in progress). J. Clin. Oncol. 2022, 40, TPS376. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Yang, X.; Pan, B.; Xu, Y.; Lu, X.; Zhao, H.-T. Clinical response to adding pyrotinib to pembrolizumab and lenvatinib for HER2-positive advanced intrahepatic cholangiocarcinoma: A case report. World J. Surg. Oncol. 2023, 21, 108. [Google Scholar] [CrossRef]
- Jiao, S.; Xia, W.; Yamaguchi, H.; Wei, Y.; Chen, M.K.; Hsu, J.M.; Hsu, J.L.; Yu, W.H.; Du, Y.; Lee, H.H.; et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. 2017, 23, 3711–3720. [Google Scholar] [CrossRef]
- Yin, C.; Armstrong, S.A.; Agarwal, S.; Wang, H.; Noel, M.S.; Weinberg, B.A.; Marshall, J.; He, A.R. Phase II study of combination pembrolizumab and olaparib in patients with advanced cholangiocarcinoma: Interim results. J. Clin. Oncol. 2022, 40, 452. [Google Scholar] [CrossRef]
- Ricci, A.D.; Rizzo, A.; Bonucci, C.; Tober, N.; Palloni, A.; Mollica, V.; Maggio, I.; Deserti, M.; Tavolari, S.; Brandi, G. PARP Inhibitors in Biliary Tract Cancer: A New Kid on the Block? Medicines 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Mahn, R.; Möhring, C.; Sadeghlar, F.; Meyer, C.; Toma, M.; Kreppel, B.; Essler, M.; Glowka, T.; Matthaei, H.; et al. Case Report: Sustained complete remission on combination therapy with olaparib and pembrolizumab in BRCA2-mutated and PD-L1-positive metastatic cholangiocarcinoma after platinum derivate. Front. Oncol. 2022, 12, 933943. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Hodi, F.S. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin. Cancer Res. 2019, 25, 5449–5457. [Google Scholar] [CrossRef]
- Parker, M.W.; Xu, P.; Guo, H.-F.; Kooi, C.W.V. Mechanism of Selective VEGF-A Binding by Neuropilin-1 Reveals a Basis for Specific Ligand Inhibition. PLoS ONE 2012, 7, e49177. [Google Scholar] [CrossRef]
- Lee, S.; Shroff, R.T.; Makawita, S.; Xiao, L.; De Armas, A.D.; Bhosale, P.; Reddy, K.; Shalaby, A.; Raghav, K.; Pant, S.; et al. Phase II Study of Ramucirumab in Advanced Biliary Tract Cancer Previously Treated By Gemcitabine-Based Chemotherapy. Clin. Cancer Res. 2022, 28, 2229–2236. [Google Scholar] [CrossRef]
- Valle, J.W.; Vogel, A.; Denlinger, C.S.; He, A.R.; Bai, L.-Y.; Orlova, R.; Van Cutsem, E.; Adeva, J.; Chen, L.-T.; Obermannova, R.; et al. Addition of ramucirumab or merestinib to standard first-line chemotherapy for locally advanced or metastatic biliary tract cancer: A randomised, double-blind, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 1468–1482. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Braud, F.d.; Bridgewater, J.; Furuse, J.; Hsu, C.-H.; Ikeda, M.; Lee, S.; Moehler, M.; Park, J.O.; Shen, L.; et al. Phase 2/3 study of bintrafusp alfa with gemcitabine plus cisplatin as first-line treatment of biliary tract cancer. Ann. Oncol. 2021, 32, S333. [Google Scholar] [CrossRef]
- Merck KGaA. Merck KGaA, Darmstadt, Germany Statement on Phase II Study of Bintrafusp Alfa in First-Line Treatment of Biliary Tract Cancer. Available online: https://www.emdgroup.com/en/news/bintrafusp-alfa-update-23-08-2021.html (accessed on 1 May 2023).
- Metropulos, A.E.; Munshi, H.G.; Principe, D.R. The difficulty in translating the preclinical success of combined TGFβ and immune checkpoint inhibition to clinical trial. Ebiomedicine 2022, 86, 104380. [Google Scholar] [CrossRef]
- Vitale, L.A.; Thomas, L.J.; He, L.-Z.; O’neill, T.; Widger, J.; Crocker, A.; Sundarapandiyan, K.; Storey, J.R.; Forsberg, E.M.; Weidlick, J.; et al. Development of CDX-1140, an agonist CD40 antibody for cancer immunotherapy. Cancer Immunol. Immunother. 2019, 68, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, S.Y.; Wang, W.-W.; Bin Tan, Y.; Sim, R.H.Z.; Cheong, R.; Tan, C.; Hopkins, R.; Connolly, J.; Shuen, W.H.; et al. A Perspective on Cell Therapy and Cancer Vaccine in Biliary Tract Cancers (BTCs). Cancers 2020, 12, 3404. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Sangsuwannukul, T.; Supimon, K.; Sujjitjoon, J.; Phanthaphol, N.; Chieochansin, T.; Poungvarin, N.; Wongkham, S.; Junking, M.; Yenchitsomanus, P.-T. Anti-tumour effect of the fourth-generation chimeric antigen receptor T cells targeting CD133 against cholangiocarcinoma cells. Int. Immunopharmacol. 2020, 89, 107069. [Google Scholar] [CrossRef] [PubMed]
- Supimon, K.; Sangsuwannukul, T.; Sujjitjoon, J.; Phanthaphol, N.; Chieochansin, T.; Poungvarin, N.; Wongkham, S.; Junking, M.; Yenchitsomanus, P.-T. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci. Rep. 2021, 11, 6276. [Google Scholar] [CrossRef]
- Phanthaphol, N.; Somboonpatarakun, C.; Suwanchiwasiri, K.; Chieochansin, T.; Sujjitjoon, J.; Wongkham, S.; Maher, J.; Junking, M.; Yenchitsomanus, P.-T. Chimeric Antigen Receptor T Cells Targeting Integrin αvβ6 Expressed on Cholangiocarcinoma Cells. Front. Oncol. 2021, 11, 657868. [Google Scholar] [CrossRef]
- Kefas, J.; Bridgewater, J.; Vogel, A.; Stein, A.; Primrose, J. Adjuvant therapy of biliary tract cancers. Ther. Adv. Med. Oncol. 2023, 15, 17588359231163785. [Google Scholar] [CrossRef]
- Bridgewater, J.; Fletcher, P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Long-Term Outcomes and Exploratory Analyses of the Randomized Phase III BILCAP Study. J. Clin. Oncol. 2022, 40, 2048–2057. [Google Scholar] [CrossRef]
- Ebata, T.; Hirano, S.; Konishi, M.; Uesaka, K.; Tsuchiya, Y.; Ohtsuka, M.; Kaneoka, Y.; Yamamoto, M.; Ambo, Y.; Shimizu, Y.; et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br. J. Surg. 2018, 105, 192–202. [Google Scholar] [CrossRef]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.-P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.-L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Tan, E.K.; Taner, T.; Heimbach, J.K.; Gores, G.J.; Rosen, C.B. Liver Transplantation for Peri-hilar Cholangiocarcinoma. J. Gastrointest. Surg. 2020, 24, 2679–2685. [Google Scholar] [CrossRef]
- Maithel, S.K.; Hong, S.C.; Ethun, C.G.; Ferrone, C.R.; Rocha, F.G.; Staley, C.A.; O’Dwyer, P.J. Optimal perioperative therapy for incidental gallbladder cancer (OPT-IN): A randomized phase II/III trial—ECOG-ACRIN EA2197. J. Clin. Oncol. 2023, 41, TPS620. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Yong, M.C.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xue, R.; Zhu, Z.; Farrukh, H.; Song, W.; Li, T.; Zheng, L.; Pan, C.-X. Increasing cure rates of solid tumors by immune checkpoint inhibitors. Exp. Hematol. Oncol. 2023, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.-L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Futur. Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat. Res. Commun. 2021, 27, 100354. [Google Scholar] [CrossRef]
- Qiao, Z.-Y.; Zhang, Z.-J.; Lv, Z.-C.; Tong, H.; Xi, Z.-F.; Wu, H.-X.; Chen, X.-S.; Xia, L.; Feng, H.; Zhang, J.-J.; et al. Neoadjuvant Programmed Cell Death 1 (PD-1) Inhibitor Treatment in Patients With Hepatocellular Carcinoma Before Liver Transplant: A Cohort Study and Literature Review. Front. Immunol. 2021, 12, 653437. [Google Scholar] [CrossRef] [PubMed]
- Pelizzaro, F.; Gambato, M.; Gringeri, E.; Vitale, A.; Cillo, U.; Farinati, F.; Burra, P.; Russo, F.P. Management of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers 2021, 13, 4882. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, J.H.; Agarwal, R.; Goff, L.W.; Heumann, T.R. Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies. Cancers 2023, 15, 3312. https://doi.org/10.3390/cancers15133312
Lo JH, Agarwal R, Goff LW, Heumann TR. Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies. Cancers. 2023; 15(13):3312. https://doi.org/10.3390/cancers15133312
Chicago/Turabian StyleLo, Justin H., Rajiv Agarwal, Laura W. Goff, and Thatcher R. Heumann. 2023. "Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies" Cancers 15, no. 13: 3312. https://doi.org/10.3390/cancers15133312
APA StyleLo, J. H., Agarwal, R., Goff, L. W., & Heumann, T. R. (2023). Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies. Cancers, 15(13), 3312. https://doi.org/10.3390/cancers15133312





