Diffusion of Minimally Invasive Approach for Lung Cancer Surgery in France: A Nationwide, Population-Based Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Patient Characteristics
2.3. Region and Hospital Characteristics
2.4. Statistical Analysis
3. Results
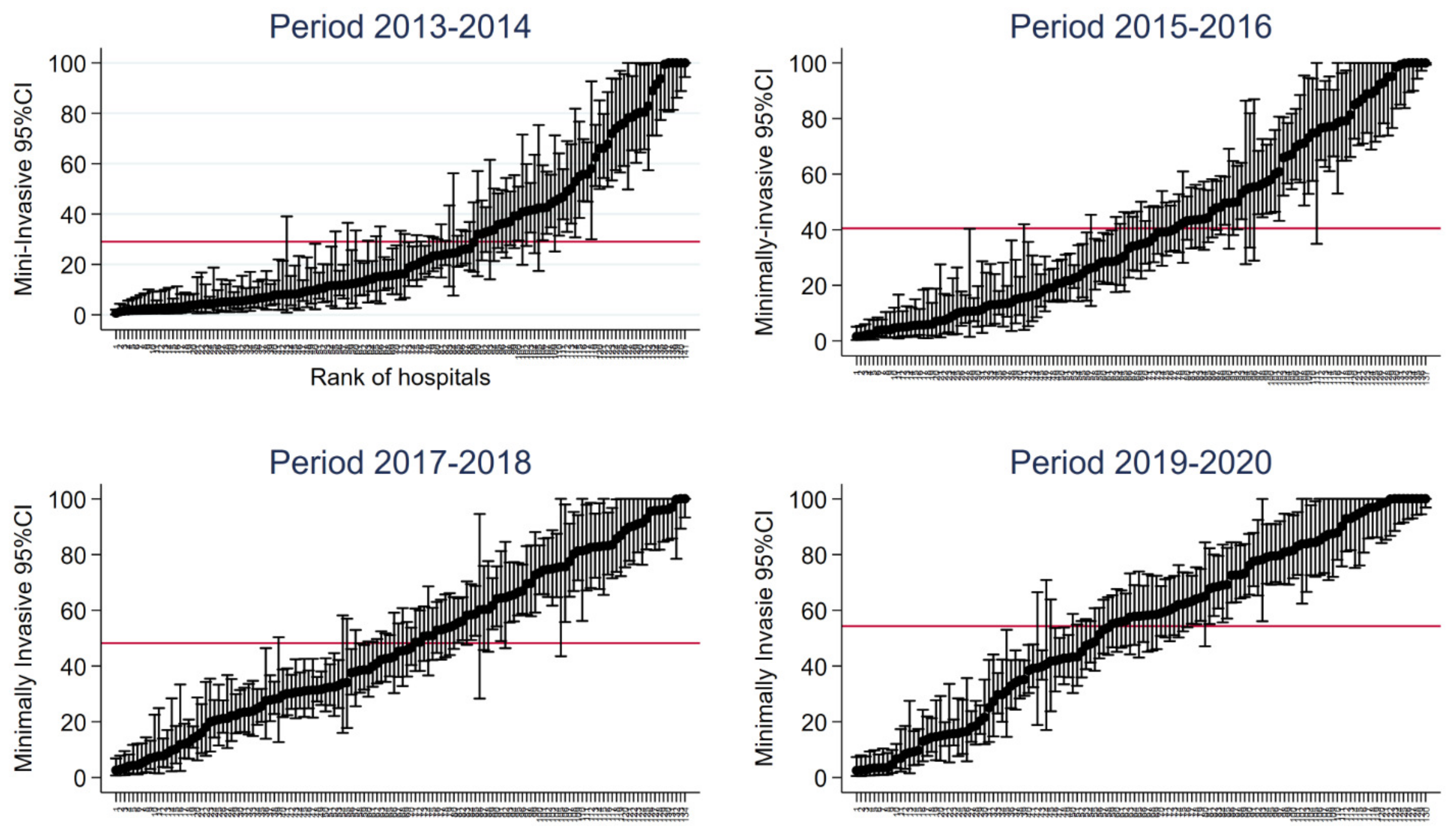
3.1. Variations in the Use of the Minimally Invasive Approach in Hospitals over Time
3.2. Between-Hospital Variations in Adjusted Minimally Invasive Approach Rates in Each Region of France
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institut National Du Cancer. Available online: https://www.e-cancer.fr/ (accessed on 1 March 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Soria, J.C.; Peters, S. ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer: An update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann. Oncol. 2021, 32, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Rocco, G.; Internullo, E.; Cassivi, S.D.; Van Raemdonck, D.; Ferguson, M.K. The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac. Surg. Clin. 2008, 18, 235–247. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Crinò, L.; Dooms, C.; Douillard, J.Y.; Faivre-Finn, C.; Lim, E.; Rocco, G.; Senan, S.; van Schil, P.; Veronesi, G.; et al. 2nd ESMO Consensus Conference on Lung Cancer: Early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol. 2014, 25, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.; Osasona, A.; Shan, Y.; Goodwin, J.S.; Okereke, I.C. Trends and Outcomes of Thoracoscopic Lobectomy or Segmentectomy: A National Surgical Quality Improvement Project Analysis. Semin. Thorac Cardiovasc. Surg. 2018, 30, 350–359. [Google Scholar] [CrossRef]
- Gómez Hernández, M.T.; Valentín, N.N.; Rodríguez Alvarado, I.; Fuentes Gago, M.; Varela Simó, G.; Jiménez López, M.F. Changes in the Risk of Mortality and Morbidity After Lung Resection in the Last 20 Years. Arch. Bronconeumol. 2020, 56, 23–27. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision. Available online: http://apps.who.int/classifications/icd10/browse/2016/en (accessed on 1 March 2016).
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Normand, S.L.T.; Shahian, D.M. Statistical and Clinical Aspects of Hospital Outcomes Profiling. Stat. Sci. 2007, 22, 206–226. [Google Scholar] [CrossRef]
- Broderick, S.R.; Grau-Sepulveda, M.; Kosinski, A.S.; Kurlansky, P.A.; Shahian, D.M.; Jacobs, J.P.; Becker, S.; DeCamp, M.M.; Seder, C.W.; Grogan, E.L.; et al. The Society of Thoracic Surgeons Composite Score Rating for Pulmonary Resection for Lung Cancer. Ann. Thorac. Surg. 2020, 109, 848–855. [Google Scholar] [CrossRef]
- Mazzella, A.; Mohamed, S.; Maisonneuve, P.; Sedda, G.; Cara, A.; Casiraghi, M.; Petrella, F.; Donghi, S.M.; Lo Iacono, G.; Spaggiari, L. Learning Curve of Robotic Lobectomy for the Treatment of Lung Cancer: How Does It Impact on the Autonomic Nervous System of the Surgeon? J. Pers. Med. 2023, 13, 193. [Google Scholar] [CrossRef]
- Wilson-Smith, A.R.; Anning, N.; Muston, B.; Eranki, A.; Williams, M.L.; Wilson-Smith, C.J.; Rivas, D.G.; Yan, T.D. The learning curve of the robotic-assisted lobectomy-a systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2023, 12, 1–8. [Google Scholar] [CrossRef]
- Yang, H.X.; Woo, K.M.; Sima, C.S.; Bains, M.S.; Adusumilli, P.S.; Huang, J.; Finley, D.J.; Rizk, N.P.; Rush, V.W.; Jones, D.R.; et al. Long-term Survival Based on the Surgical Approach to Lobectomy for Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann. Surg. 2017, 265, 431–437. [Google Scholar] [CrossRef]
- Liang, H.; Liang, W.; Zhao, L.; Chen, D.; Zhang, J.; Zhang, Y.; Tang, S.; He, J. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann. Surg. 2018, 268, 254–259. [Google Scholar] [CrossRef]
- O’Sullivan, K.E.; Kreaden, U.S.; Hebert, A.E.; Eaton, D.; Redmond, K.C. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc. Thorac Surg. 2019, 28, 526–534. [Google Scholar] [CrossRef]
- Kamel, M.K.; Lee, B.; Harrison, S.; Port, J.L.; Pua, B.; Altorki, N.K.; Stiles, B.M. Do the surgical results in the National Lung Screening Trial reflect modern thoracic surgical practice? J. Thorac. Cardiovasc. Surg. 2019, 157, 2038–2046.e1. [Google Scholar] [CrossRef]
- Lim, E.; Batchelor, T.J.P.; Dunning, J.; Shackcloth, M.; Anikin, V.; Naidu, B.; Belcher, E.; Loubani, M.; Zamvar, V.; Harris, R.A.; et al. Video-Assisted Thoracoscopic or Open Lobectomy in Early-Stage Lung Cancer. NEJM Evid. 2022, 1, EVIDoa2100016. [Google Scholar] [CrossRef]
- Bendixen, M.; Kronborg, C.; Jørgensen, O.D.; Andersen, C.; Licht, P.B. Cost-utility analysis of minimally invasive surgery for lung cancer: A randomized controlled trial. Eur. J. Cardiothorac. Surg. 2019, 56, 754–761. [Google Scholar] [CrossRef]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Wei, S.; Chen, M.; Chen, N.; Liu, L. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2017, 15, 98. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, S. Robot-assisted thoracic surgery versus open thoracic surgery for lung cancer: A system review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 17804–17810. [Google Scholar]
- Kent, M.S.; Hartwig, M.G.; Vallières, E.; Abbas, A.E.; Cerfolio, R.J.; Dylewski, M.R.; Fabian, T.; Herrera, L.J.; Jett, K.G.; Lazzaro, R.S.; et al. Pulmonary Open, Robotic, and Thoracoscopic Lobectomy (PORTaL) Study: An Analysis of 5721 Cases. Ann. Surg. 2023, 277, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.K.; Thibault, D.; O’Brien, S.M.; Jacobs, J.P.; Gaynor, J.W.; Romano, J.C.; Gaies, M.; Hill, K.D.; Jacobs, M.L.; Shahian, D.M.; et al. National Variation in Congenital Heart Surgery Outcomes. Circulation 2020, 142, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Giannini, A.; Seghieri, C.; Simoncini, T.; Vainieri, M. Regional practice variation in pelvic organ prolapse surgery in Tuscany, Italy: A retrospective cohort study on administrative health data. BMJ Open 2023, 13, e068145. [Google Scholar] [CrossRef] [PubMed]
- Piroth, L.; Cottenet, J.; Mariet, A.S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2021, 9, 251–259. [Google Scholar] [CrossRef]
- Magnin, J.; Bernard, A.; Cottenet, J.; Lequeu, J.B.; Ortega-Deballon, P.; Quantin, C.; Facy, O. Impact of hospital volume in liver surgery on postoperative mortality and morbidity: Nationwide study. Br. J. Surg. 2023, 110, 441–448. [Google Scholar] [CrossRef]
- Simon, E.; Bechraoui-Quantin, S.; Tapia, S.; Cottenet, J.; Mariet, A.S.; Cottin, Y.; Giroud, M.; Eicher, J.-C.; Thilaganathan, B.; Quantin, C. Time to onset of cardiovascular and cerebrovascular outcomes after hypertensive disorders of pregnancy: A nationwide, population-based retrospective cohort study. Am. J. Obstet. Gynecol. 2023, in press. [Google Scholar] [CrossRef]
- Maitre, T.; Cottenet, J.; Godet, C.; Roussot, A.; Abdoul Carime, N.; Ok, V.; Parrot, A.; Bonniaud, P.; Quantin, C.; Cadranel, C. Chronic pulmonary aspergillosis: Prevalence, favouring pulmonary diseases and prognosis. Eur. Respir. J. 2021, 58, 2003345. [Google Scholar] [CrossRef]
- Chauvet-Gelinier, J.C.; Cottenet, J.; Guillaume, M.; Endomba, F.T.; Jollant, F.; Quantin, C. Risk of hospitalization for self-harm among adults hospitalized with SARS-CoV-2 in France: A nationwide retrospective cohort study. Psychiatry Res. 2023, 324, 115214. [Google Scholar] [CrossRef]
- Mariet, A.S.; Giroud, M.; Benzenine, E.; Cottenet, J.; Roussot, A.; Aho-Glélé, L.S.; Tubert-Bitter, P.; Béjot, Y.; Quantin, C. Hospitalizations for stroke in France during the COVID-19 pandemic before, during and after the national lockdown. Stroke 2021, 52, 1362–1369. [Google Scholar] [CrossRef]
- Bernard, A.; Cottenet, J.; Mariet, A.S.; Quantin, C.; Pagès, P.B. Is an activity volume threshold really realistic for lung cancer resection? J. Thorac. Dis. 2018, 10, 5685–5694. [Google Scholar] [CrossRef]
- Pagès, P.B.; Cottenet, J.; Mariet, A.S.; Bernard, A.; Quantin, C. In-hospital mortality following lung cancer resection: Nationwide administrative database. Eur. Respir. J. 2016, 47, 1809–1817. [Google Scholar] [CrossRef]
- Pagès, P.B.; Mariet, A.S.; Pforr, A.; Cottenet, J.; Madelaine, L.; Abou-Hanna, H.; Bernard, A.; Quantin, C. Does age over 80 years have to be a contraindication for lung cancer surgery-a nationwide database study. J. Thorac. Dis. 2018, 10, 4764–4773. [Google Scholar] [CrossRef]
- Bernard, A.; Falcoz, P.E.; Thomas, P.A.; Rivera, C.; Brouchet, L.; Baste, J.M.; Puyraveau, M.; Quantin, C.; Benoit Pages, P.; Dahan, M. Comparison of Epithor clinical national database and medico-administrative database to identify the influence of case-mix on the estimation of hospital outliers. PLoS ONE 2019, 14, e0219672. [Google Scholar] [CrossRef]
- Pages, P.-B.; Cottenet, J.; Bonniaud, P.; Tubert-Bitter, P.; Piroth, L.; Cadranel, J.; Bernard, A.; Quantin, C. Impact of the SARS-CoV-2 Epidemic on Lung Cancer Surgery in France: A Nationwide Study. Cancers 2021, 13, 6277. [Google Scholar] [CrossRef]
- Bernard, A.; Cottenet, J.; Pages, P.B.; Quantin, C. Is there variation between hospitals within each region in postoperative mortality for lung cancer surgery in France? A nationwide study from 2013 to 2020. Front. Med. 2023, 10, 1110977. [Google Scholar] [CrossRef]


| Period | |||||
|---|---|---|---|---|---|
| 2013–2014 | 2015–2016 | 2017–2018 | 2019–2020 | p-Value * | |
| N | 16,933 | 18,778 | 20,903 | 21,351 | |
| Age | |||||
| 38–56 | 3203 (18.92%) | 3397 (18.09%) | 3336 (15.96%) | 3135 (14.68%) | |
| 57–61 | 2908 (17.17%) | 3029 (16.13%) | 3219 (15.40%) | 2969 (13.91%) | |
| 62–65 | 2798 (16.52%) | 2972 (15.83%) | 3134 (14.99%) | 3302 (15.47%) | 0.00001 |
| 66–69 | 2850 (16.83%) | 3361 (17.90%) | 3836 (18.35%) | 3898 (18.26%) | |
| 70–74 | 2461 (14.53%) | 2934 (15.62%) | 3814 (18.25%) | 4222 (19.77%) | |
| ≥75 | 2713 (16.02%) | 3085 (16.43%) | 3564 (17.05%) | 3825 (17.91%) | |
| Female | 5460 (32.24%) | 6528 (34.76%) | 7683 (36.76%) | 8407 (39.38%) | 0.00001 |
| Modified CCI | |||||
| 0 | 5829 (34.42%) | 6448 (34.34%) | 7300 (34.92%) | 12,451 (58.32%) | 0.00001 |
| 1 | 1762 (10.41%) | 1992 (10.61%) | 2387 (11.42%) | 1750 (8.20%) | |
| 2 | 1785 (10.54%) | 2129 (11.34%) | 2533 (12.12%) | 1918 (8.98%) | |
| ≥3 | 7557 (44.63%) | 8209 (43.72%) | 8683 (41.54%) | 5232 (24.50%) | |
| VATS/RATS | 3841 (22.68%) | 6489 (34.56%) | 9194 (43.98%) | 10,734 (50.27%) | 0.00001 |
| Limited | 3085 (18.22%) | 3368 (17.94%) | 3673 (17.57%) | 3583 (16.78%) | 0.0012 |
| Lobectomy | 13,848 (81.78%) | 15,410 (82.06%) | 17,230 (82.43%) | 17,768 (83.22%) | |
| Period | ||||
|---|---|---|---|---|
| 2013–2014 | 2015–2016 | 2017–2018 | 2019–2020 | |
| Number of hospitals | 141 | 137 | 134 | 130 |
| Type of hospitals | ||||
| Non academic | 31 | 32 | 31 | 30 |
| Academic | 27 | 27 | 26 | 26 |
| Private non-profit | 13 | 10 | 11 | 9 |
| Private for profit | 70 | 68 | 66 | 65 |
| Hospital volume * | ||||
| Non academic | 48 [30–74] | 62 [48–84] | 68 [48–114] | 77 [55–129] |
| Academic | 183 [147–386] | 208 [125–380] | 270 [155–443] | 284 [161–426] |
| Private non-profit | 125 [35–366] | 246 [85–428] | 225 [31–464] | 258 [188–501] |
| Private for profit | 60 [30–103] | 70 [37–124] | 93 [43–153] | 92 [49–158] |
| Observed rate of minimally invasive approach ** | ||||
| Non academic | 0.042 [0–0.25] | 0.16 [0.04–0.41] | 0.23 [0.05–0.61] | 0.33 [0.03–0.64] |
| Academic | 0.11 [0.04–0.31] | 0.29 [0.13–0.57] | 0.46 [0.25–0.66] | 0.56 [0.41–0.68] |
| Private non-profit | 0.28 [0.17–0.4] | 0.52 [0.38–0.57] | 0.6 [0.49–0.77] | 0.65 [0.5–0.7] |
| Private for profit | 0.11 [0.05–0.31] | 0.23 [0.08- 0.45] | 0.34 [0.18–0.55] | 0.43 [0.17–0.66] |
| Overall | 0.12 [0.04–0.31] | 0.26 [0.08–0.5] | 0.37 [0.21–0.61] | 0.47 [0.24–0.68] |
| Adjusted rate of minimally invasive approach ** | ||||
| Non academic | 0.06 [0.04–0.33] | 0.22 [0.06–0.55] | 0.32 [0.08–0.75] | 0.42 [0.07–0.78] |
| Academic | 0.15 [0.06–0.47] | 0.42 [0.18–0.7] | 0.57 [0.31–0.75] | 0.68 [0.53–0.85] |
| Private non-profit | 0.41 [0.24–0.55] | 0.65 [0.5–0.79] | 0.65 [0.58–0.82] | 0.77 [0.63–0.8] |
| Private for profit | 0.16 [0.08–0.42] | 0.3 [0.12–0.59] | 0.38 [0.23–0.6] | 0.56 [0.22–0.8] |
| Overall | 0.12 [0.06–0.43] | 0.35 [0.13–0.66] | 0.45 [0.25–0.74] | 0.58 [0.3–0.8] |
| 2013–2014 | 2015–2016 | 2017–2018 | 2019–2020 | |||||
|---|---|---|---|---|---|---|---|---|
| Median [IQR] | Extreme Ratio Interquartile Ratio | Median [IQR] | Extreme Ratio Interquartile Ratio | Median [IQR] | Extreme Ratio Interquartile Ratio | Median [IQR] | Extreme Ratio Interquartile Ratio | |
| ARA | 0.43 | 32 | 0.57 | 37 | 0.75 | 15 | 0.8 | 22 |
| [0.14–0.74] | 5.29 | [0.35–0.8] | 2.29 | [0.56–0.9] | 1.61 | [0.54–1] | 1.85 | |
| BFR | 0.15 | 15 | 0.13 | 15 | 0.31 | 6 | 0.09 | 19 |
| [0.05–0.32] | 6.40 | [0.09–0.35] | 3.89 | [0.28–0.32] | 1.14 | [0.08–0.15] | 1.88 | |
| BRE | 0.28 | 20 | 0.67 | 20 | 0.66 | 2 | 0.71 | 2 |
| [0.09–0.65] | 7.22 | [0.27–0.8] | 2.96 | [0.48–0.83] | 1.73 | [0.58–0.84] | 1.45 | |
| CVL | 0.1 | 8 | 0.194 | 10 | 0.31 | 6 | 0.3 | 16 |
| [0.08–0.2] | 2.50 | [0.08–0.33] | 4.13 | [0.3–0.37] | 1.23 | [0.14–0.56] | 4.00 | |
| GE | 0.075 | 61 | 0.302 | 41 | 0.24 | 25 | 0.39 | 7 |
| [0.023–0.21] | 9.13 | [0.11–0.42] | 3.82 | [0.12–0.4] | 3.33 | [0.16–0.64] | 4.00 | |
| HdF | 0.12 | 37 | 0.192 | 37 | 0.34 | 27 | 0.51 | 36 |
| [0.04–0.33] | 8.25 | [0.11–0.35] | 3.18 | [0.22–0.5] | 2.27 | [0.38–0.6] | 1.58 | |
| IdF | 0.24 | 53 | 0.41 | 19 | 0.58 | 24 | 0.63 | 11 |
| [0.05–0.7] | 14.00 | [0.13–0.8] | 6.15 | [0.26–0.84] | 3.23 | [0.2–0.93] | 4.65 | |
| NA | 0.1 | 35 | 0.132 | 26 | 0.232 | 24 | 0.41 | 32 |
| [0.04–0.16] | 4.00 | [0.06–0.27] | 4.50 | [0.08–0.33] | 4.13 | [0.07–0.6] | 8.57 | |
| NOR | 0.21 | 143 | 0.435 | 8 | 0.46 | 5 | 0.59 | 6 |
| [0.11–0.6] | 5.45 | [0.2–0.66] | 3.30 | [0.32–0.72] | 2.25 | [0.35–0.9] | 2.57 | |
| OC | 0.24 | 58 | 0.51 | 25 | 0.462 | 10 | 0.67 | 4 |
| [0.12–0.41] | 3.42 | [0.24–0.6] | 2.50 | [0.33–0.73] | 2.21 | [0.57–0.9] | 1.58 | |
| PACA | 0.41 | 33 | 0.48 | 6 | 0.624 | 4 | 0.67 | 3 |
| [0.15–0.49] | 3.27 | [0.3–0.85] | 2.83 | [0.43–0.76] | 1.65 | [0.53–0.9] | 1.70 | |
| PdL | 0.36 | 26 | 0.49 | 18 | 0.6 | 7 | 0.6 | 10 |
| [0.11–0.43] | 3.91 | [0.24–0.7] | 2.92 | [0.22–0.78] | 3.55 | [0.18–0.83] | 4.61 | |
| Coefficient | p-Value | [95% Confidence Interval] | ||
|---|---|---|---|---|
| Period | ||||
| 2013–2014 | 0 | |||
| 2015–2016 | 0.12 | 0.001 | 0.06 | 0.18 |
| 2017–2018 | 0.18 | 0.001 | 0.12 | 0.24 |
| 2019–2020 | 0.25 | 0.001 | 0.19 | 0.31 |
| Logarithm hospital volume | 0.06 | 0.0001 | 0.03 | 0.09 |
| Type of Hospital | ||||
| Non-academic | 0 | |||
| Academic | 0.06 | 0.1 | −0.014 | 0.15 |
| Private non-profit | 0.17 | 0.001 | 0.07 | 0.27 |
| Private for-profit | 0.03 | 0.4 | −0.035 | 0.08 |
| Variance between regions | 0.015 | |||
| Variance within regions | 0.067 | |||
| Intraclass correlation coefficient | 0.18 | |||
| Explained variation within regions | 0.23 | |||
| Explained variation between regions | 0.14 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernard, A.; Cottenet, J.; Pages, P.-B.; Quantin, C. Diffusion of Minimally Invasive Approach for Lung Cancer Surgery in France: A Nationwide, Population-Based Retrospective Cohort Study. Cancers 2023, 15, 3283. https://doi.org/10.3390/cancers15133283
Bernard A, Cottenet J, Pages P-B, Quantin C. Diffusion of Minimally Invasive Approach for Lung Cancer Surgery in France: A Nationwide, Population-Based Retrospective Cohort Study. Cancers. 2023; 15(13):3283. https://doi.org/10.3390/cancers15133283
Chicago/Turabian StyleBernard, Alain, Jonathan Cottenet, Pierre-Benoit Pages, and Catherine Quantin. 2023. "Diffusion of Minimally Invasive Approach for Lung Cancer Surgery in France: A Nationwide, Population-Based Retrospective Cohort Study" Cancers 15, no. 13: 3283. https://doi.org/10.3390/cancers15133283
APA StyleBernard, A., Cottenet, J., Pages, P.-B., & Quantin, C. (2023). Diffusion of Minimally Invasive Approach for Lung Cancer Surgery in France: A Nationwide, Population-Based Retrospective Cohort Study. Cancers, 15(13), 3283. https://doi.org/10.3390/cancers15133283