Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers
Abstract
Simple Summary
Abstract
1. Introduction
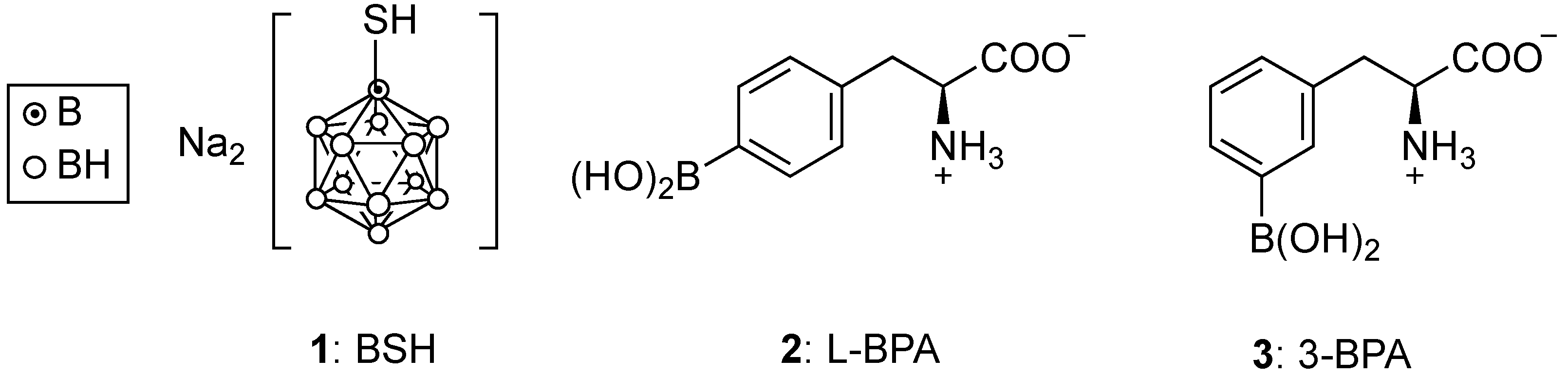
2. Clinically Approved Agents for BNCT
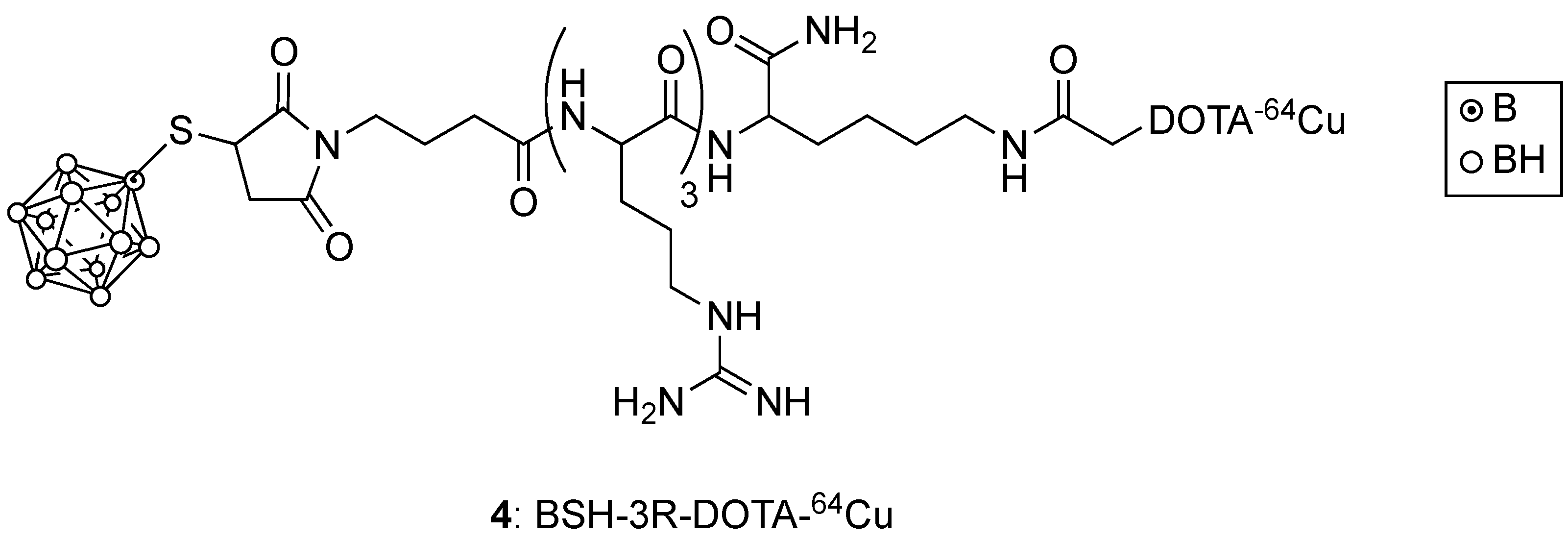
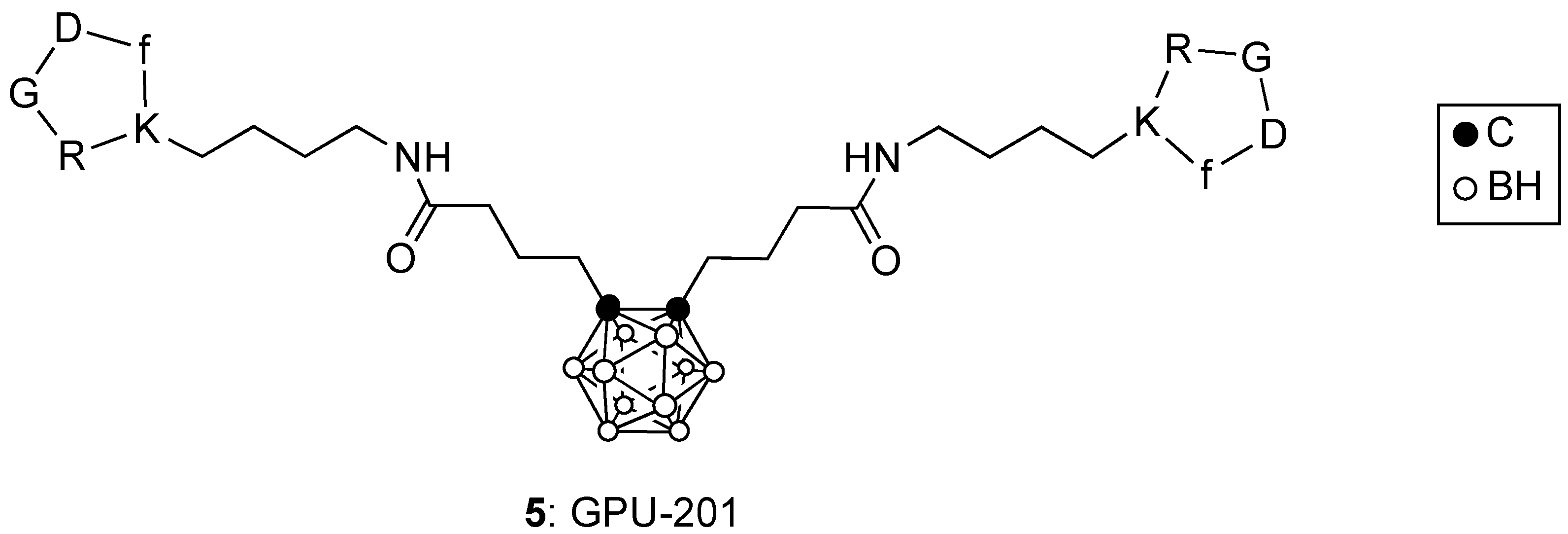
3. Boronated Peptides and Monoclonal Antibodies
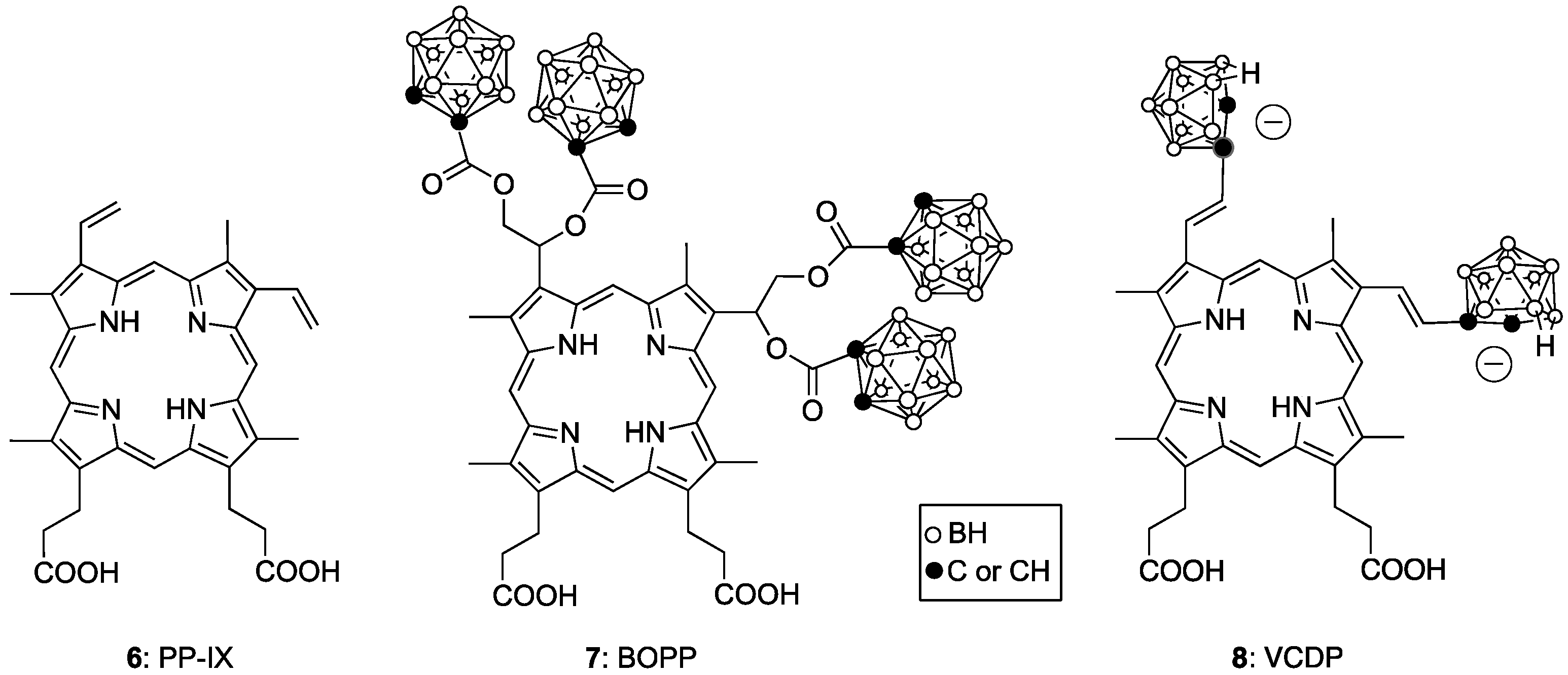
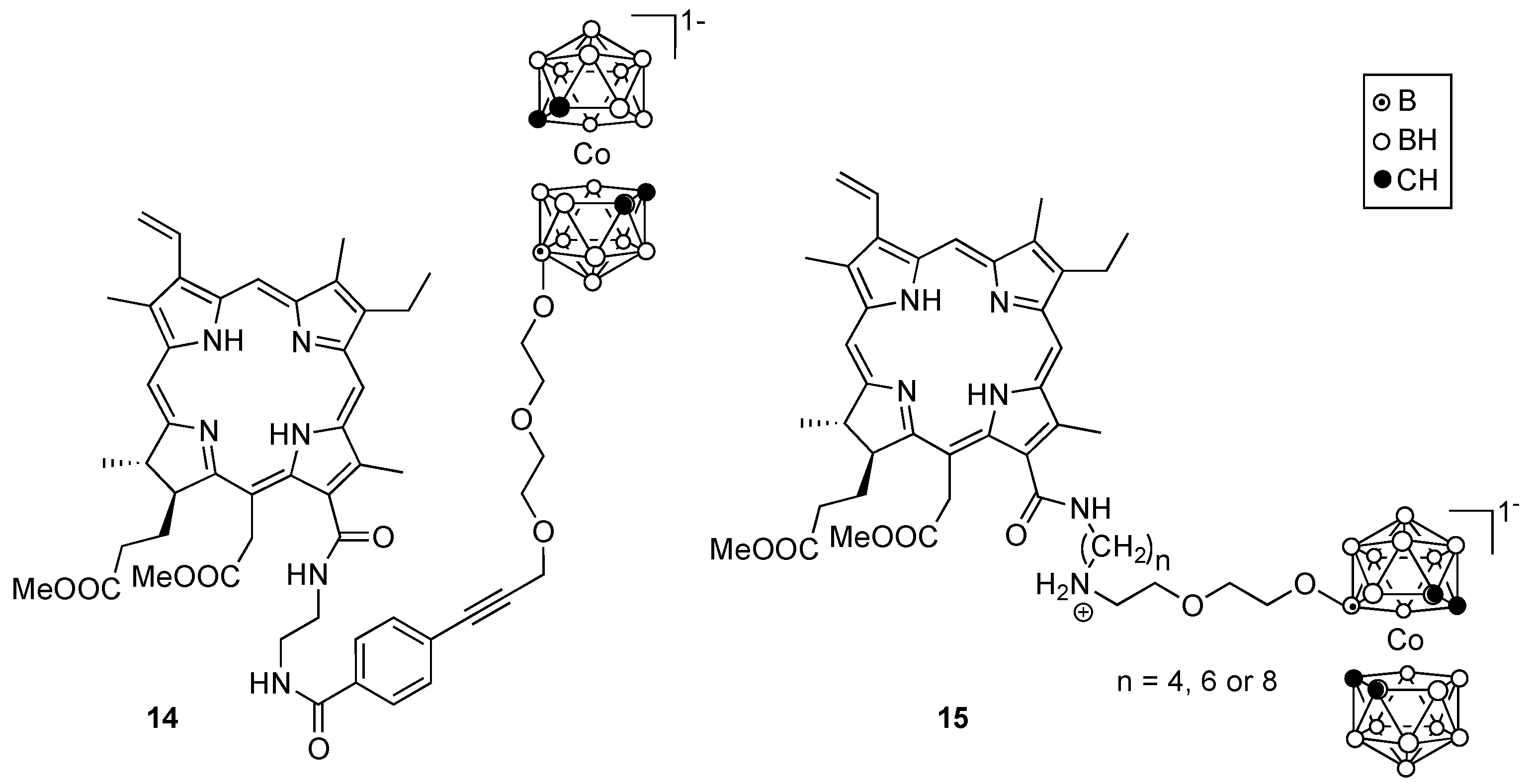
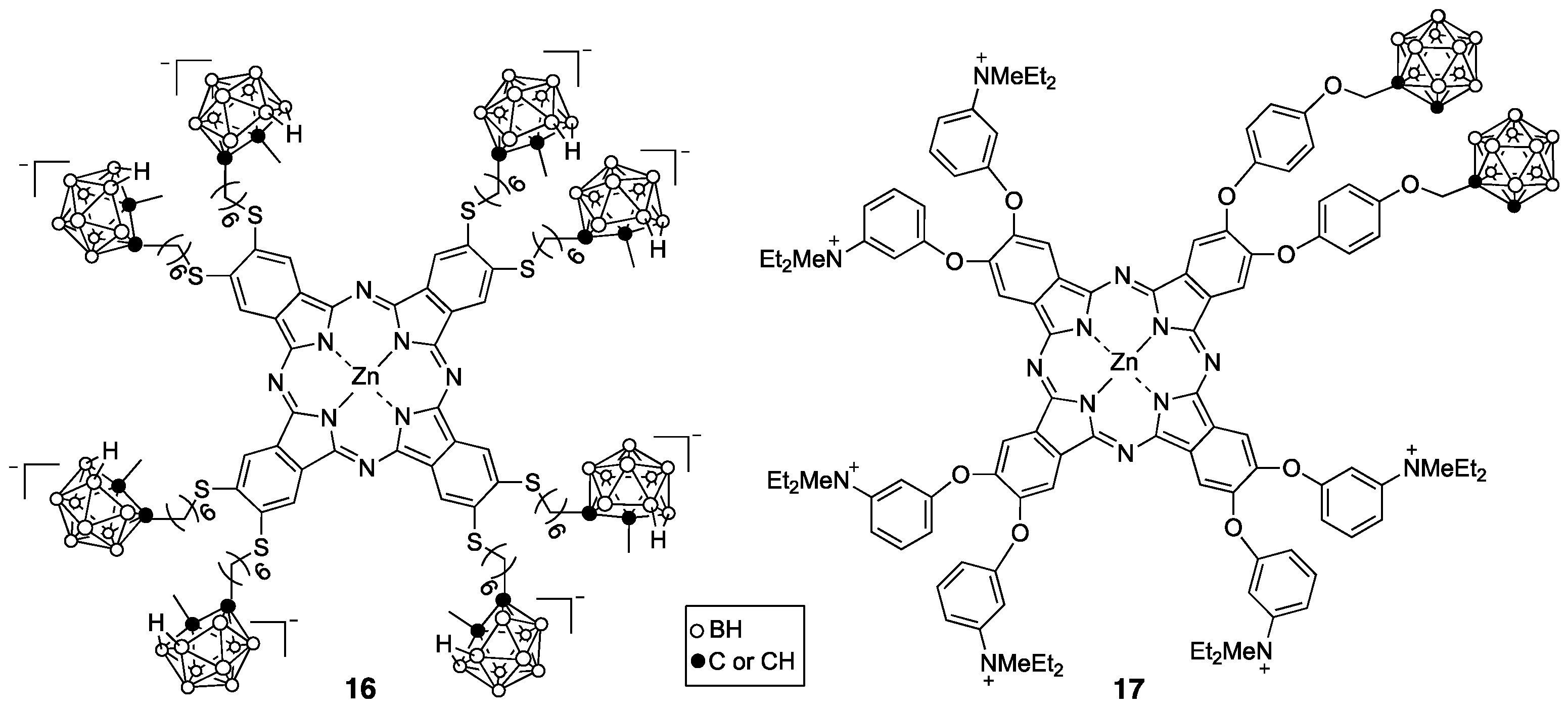
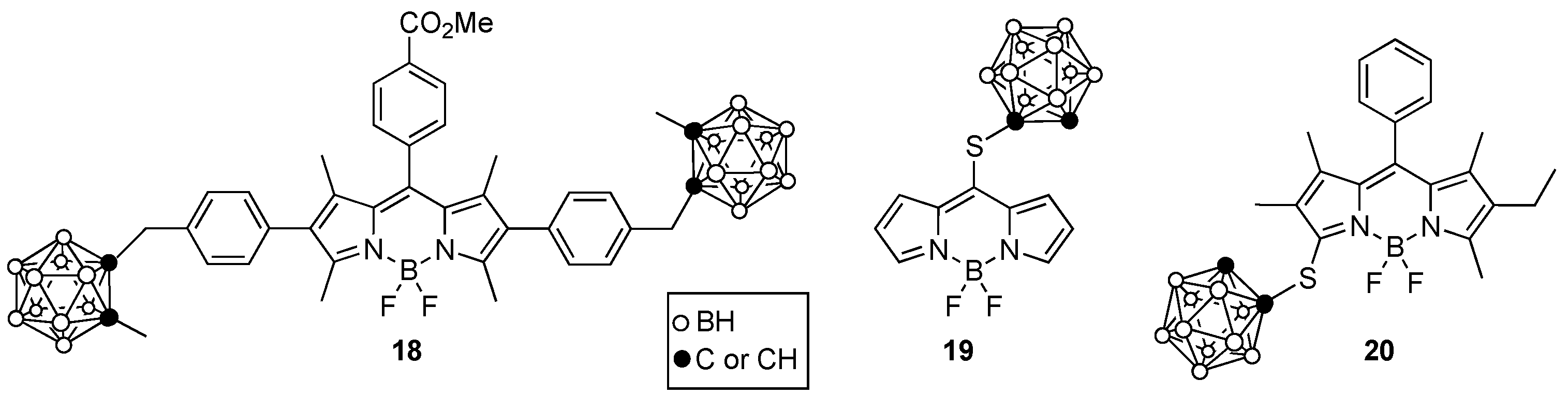
4. Porphyrin Derivatives for BNCT
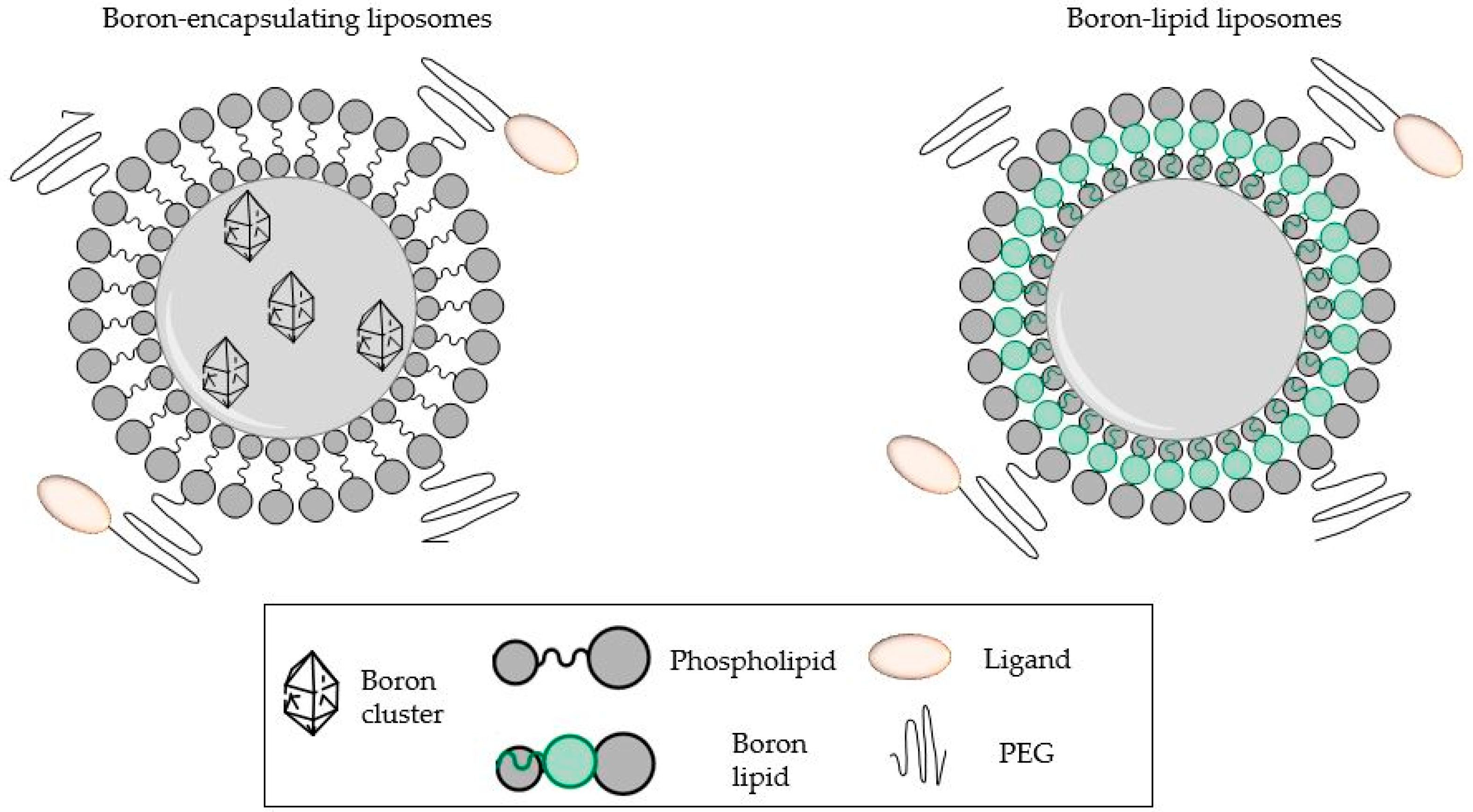
5. Boron-Containing Liposomes
6. Boron-Containing Nanoparticles
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soloway, A.H.; Tjarks, W.; Barnum, B.A.; Rong, F.-G.; Barth, R.F.; Codogni, I.M.; Wilson, J.G. The Chemistry of Neutron Capture Therapy. Chem. Rev. 1998, 98, 2389–2390. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Vicente, M.H.; Harling, O.K.; Kiger, W.S., 3rd; Riley, K.J.; Binns, P.J.; Wagner, F.M.; Suzuki, M.; Aihara, T.; Kato, I.; et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Saarilahti, K.; Atula, T.; Collan, J.; Salli, E.; Kortesniemi, M.; Uusi-Simola, J.; Välimäki, P.; et al. Boron Neutron Capture Therapy in the Treatment of Locally Recurred Head-and-Neck Cancer: Final Analysis of a Phase I/II Trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e67–e75. [Google Scholar] [CrossRef]
- Hopewell, J.; Gorlia, T.; Pellettieri, L.; Giusti, V.; H-Stenstam, B.; Sköld, K. Boron neutron capture therapy for newly diagnosed glioblastoma multiforme: An assessment of clinical potential. Appl. Radiat. Isot. 2011, 69, 1737–1740. [Google Scholar] [CrossRef]
- Moss, R.L. Critical review, with an optimistic outlook, on Boron Neutron Capture Therapy (BNCT). Appl. Radiat. Isot. 2014, 88, 2–11. [Google Scholar] [CrossRef]
- Barth, R.F.; Zhang, Z.; Liu, T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun. 2018, 38, 36–37. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coord. Chem. Rev. 2020, 405, 213139. [Google Scholar] [CrossRef]
- Sibrian, V.; Vicente, M.G.H. Boron Tumor-Delivery for BNCT: Recent developments and perspectives. In Boron Science: New Technologies & Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 203–232. ISBN 978-1-4398-2662-1. [Google Scholar]
- Nakamura, H.; Kirihata, M. Boron Compounds: New Candidates for Boron Carriers in BNCT. In Neutron Capture Therapy; Sauerwein, W., Wittig, A., Moss, R., Nakagawa, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 99–116. ISBN 978-3-642-31333-2. [Google Scholar]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef]
- Xuan, S.; Vicente, M.G.H. Recent development of Boron Delivery Agents for Boron Neutron Capture Therapy. In Medicinal Chemistry of Boron Compounds; Vinas, C., Hey-Hawkins, Eds.; Wiley Publishers: Hoboken, NJ, USA, 2019; pp. 298–342. [Google Scholar]
- Ali, F.; Hosmane, N.S.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef]
- Couto, M.; Alamón, C.; Nievas, S.; Perona, M.; Dagrosa, M.A.; Teixidor, F.; Cabral, P.; Viñas, C.; Cerecetto, H. Bimodal Therapeutic Agents Against Glioblastoma, One of the Most Lethal Forms of Cancer. Chem. A Eur. J. 2020, 26, 14335–14340. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.G.; Marques, F.; Robalo, M.P.; Fontrodona, X.; Garcia, M.H.; Crich, S.G.; Viñas, C.; Valente, A. Ruthenium carboranyl complexes with 2,2′-bipyridine derivatives for potential bimodal therapy application. RSC Adv. 2020, 10, 16266–16276. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Hirano, F.; Temma, T. Evaluation of 3-Borono-l-Phenylalanine as a Water-Soluble Boron Neutron Capture Therapy Agent. Pharmaceutics 2022, 14, 1106. [Google Scholar] [CrossRef] [PubMed]
- Bonjoch, J.; Drew, M.G.B.; González, A.; Greco, F.; Jawaid, S.; Osborn, H.M.I.; Williams, N.A.O.; Yaqoob, P. Synthesis and Evaluation of Novel Boron-Containing Complexes of Potential Use for the Selective Treatment of Malignant Melanoma. J. Med. Chem. 2008, 51, 6604–6608. [Google Scholar] [CrossRef]
- Barth, R.F.; Kabalka, G.W.; Yang, W.; Huo, T.; Nakkula, R.J.; Shaikh, A.L.; Haider, S.A.; Chandra, S. Evaluation of unnatural cyclic amino acids as boron delivery agents for treatment of melanomas and gliomas. Appl. Radiat. Isot. 2014, 88, 38–42. [Google Scholar] [CrossRef]
- Iguchi, Y.; Michiue, H.; Kitamatsu, M.; Hayashi, Y.; Takenaka, F.; Nishiki, T.-I.; Matsui, H. Tumor-specific delivery of BSH-3R for boron neutron capture therapy and positron emission tomography imaging in a mouse brain tumor model. Biomaterials 2015, 56, 10–17. [Google Scholar] [CrossRef]
- Michiue, H.; Sakurai, Y.; Kondo, N.; Kitamatsu, M.; Bin, F.; Nakajima, K.; Hirota, Y.; Kawabata, S.; Nishiki, T.-I.; Ohmori, I.; et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials 2014, 35, 3396–3405. [Google Scholar] [CrossRef]
- Nakase, I.; Katayama, M.; Hattori, Y.; Ishimura, M.; Inaura, S.; Fujiwara, D.; Takatani-Nakase, T.; Fujii, I.; Futaki, S.; Kirihata, M. Intracellular target delivery of cell-penetrating peptide-conjugated dodecaborate for boron neutron capture therapy (BNCT). Chem. Commun. 2019, 55, 13955–13958. [Google Scholar] [CrossRef]
- Worm, D.J.; Els-Heindl, S.; Beck-Sickinger, A.G. Targeting of peptide-binding receptors on cancer cells with peptide-drug conjugates. Pept. Sci. 2020, 112, e24171. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies. Front. Chem. 2020, 8, 571. [Google Scholar] [CrossRef]
- Kimura, S.; Masunaga, S.-I.; Harada, T.; Kawamura, Y.; Ueda, S.; Okuda, K.; Nagasawa, H. Synthesis and evaluation of cyclic RGD-boron cluster conjugates to develop tumor-selective boron carriers for boron neutron capture therapy. Bioorganic Med. Chem. 2011, 19, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Masunaga, S.-I.; Kimura, S.; Harada, T.; Okuda, K.; Sakurai, Y.; Tanaka, H.; Suzuki, M.; Kondo, N.; Maruhashi, A.; Nagasawa, H.; et al. Evaluating the Usefulness of a Novel 10B-Carrier Conjugated With Cyclic RGD Peptide in Boron Neutron Capture Therapy. World J. Oncol. 2012, 3, 103. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Nishimura, K.; Okada, S.; Sato, S.; Suzuki, M.; Takata, T.; Nakamura, H. Cyclic RGD-Functionalized closo-Dodecaborate Albumin Conjugates as Integrin Targeting Boron Carriers for Neutron Capture Therapy. Mol. Pharm. 2020, 17, 3740–3747. [Google Scholar] [CrossRef] [PubMed]
- Worm, D.J.; Hoppenz, P.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Köbberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. Selective Neuropeptide Y Conjugates with Maximized Carborane Loading as Promising Boron Delivery Agents for Boron Neutron Capture Therapy. J. Med. Chem. 2019, 63, 2358–2371. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Lerchen, H.-G.; Köbberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. A Selective Carborane-Functionalized Gastrin-Releasing Peptide Receptor Agonist as Boron Delivery Agent for Boron Neutron Capture Therapy. J. Org. Chem. 2019, 85, 1446–1457. [Google Scholar] [CrossRef]
- Yang, W.; Wu, G.; Barth, R.F.; Swindall, M.R.; Bandyopadhyaya, A.K.; Tjarks, W.; Tordoff, K.; Moeschberger, M.; Sferra, T.J.; Binns, P.J.; et al. Molecular Targeting and Treatment of Composite EGFR and EGFRvIII-Positive Gliomas Using Boronated Monoclonal Antibodies. Clin. Cancer Res. 2008, 14, 883–891. [Google Scholar] [CrossRef]
- Yang, W.; Barth, R.; Wu, G.; Tjarks, W.; Binns, P.; Riley, K. Boron neutron capture therapy of EGFR or EGFRvIII positive gliomas using either boronated monoclonal antibodies or epidermal growth factor as molecular targeting agents. Appl. Radiat. Isot. 2009, 67, S328–S331. [Google Scholar] [CrossRef]
- Pandey Ravindra, K.; Zheng, G. Porphyrins as Photosensitizers in Photodynamic Therapy. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 157–230. [Google Scholar]
- Usuda, J.; Kato, H.; Okunaka, T.; Furukawa, K.; Tsutsui, H.; Yamada, K.; Suga, Y.; Honda, H.; Nagatsuka, Y.; Ohira, T.; et al. Photodynamic Therapy (PDT) for Lung Cancers. J. Thorac. Oncol. 2006, 1, 489–493. [Google Scholar] [CrossRef]
- El-Zaria, M.E.; Ban, H.S.; Nakamura, H. Boron-Containing Protoporphyrin IX Derivatives and Their Modification for Boron Neutron Capture Therapy: Synthesis, Characterization, and Comparative In Vitro Toxicity Evaluation. Chem. A Eur. J. 2010, 16, 1543–1552. [Google Scholar] [CrossRef]
- Ozawa, T.; Afzal, J.; Lamborn, K.R.; Bollen, A.W.; Bauer, W.; Koo, M.-S.; Kahl, S.B.; Deen, D.F. Toxicity, biodistribution, and convection-enhanced delivery of the boronated porphyrin BOPP in the 9L intracerebral rat glioma model. Int. J. Radiat. Oncol. 2005, 63, 247–252. [Google Scholar] [CrossRef]
- Miura, M.; Gabel, D.; Oenbrink, G.; Fairchild, R.G. Preparation of carboranyl porphyrins for boron neutron capture therapy. Tetrahedron Lett. 1990, 31, 2247–2250. [Google Scholar] [CrossRef]
- Triesscheijn, M.; Ruevekamp, M.; Aalders, M.; Baas, P.; Stewart, F.A. Outcome of mTHPC Mediated Photodynamic Therapy is Primarily Determined by the Vascular Response. Photochem. Photobiol. 2005, 81, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, H.M.; Slatkin, D.N.; Micca, P.L.; Miura, M. Microlocalization of lipophilic porphyrins: Non-toxic enhancers of boron neutron-capture therapy. Int. J. Radiat. Biol. 2013, 89, 611–617. [Google Scholar] [CrossRef]
- Fabris, C.; Vicente, M.H.; Hao, E.; Friso, E.; Borsetto, L.; Jori, G.; Miotto, G.; Colautti, P.; Moro, D.; Esposito, J.; et al. Tumour-localizing and -photosensitising properties of meso-tetra(4-nido-carboranylphenyl)porphyrin (H2TCP). J. Photochem. Photobiol. B: Biol. 2007, 89, 131–138. [Google Scholar] [CrossRef]
- Jori, G.; Soncin, M.; Friso, E.; Vicente, M.; Hao, E.; Miotto, G.; Colautti, P.; Moro, D.; Esposito, J.; Rosi, G.; et al. A novel boronated-porphyrin as a radio-sensitizing agent for boron neutron capture therapy of tumours: In vitro and in vivo studies. Appl. Radiat. Isot. 2009, 67, S321–S324. [Google Scholar] [CrossRef]
- Gottumukkala, V.; Luguya, R.; Fronczek, F.R.; Vicente, M.G.H. Synthesis and cellular studies of an octa-anionic 5,10,15,20-tetra[3,5-(nido-carboranylmethyl)phenyl]porphyrin (H2OCP) for application in BNCT. Bioorganic Med. Chem. 2005, 13, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Sibrian-Vazquez, M.; Serem, W.; Garno, J.C.; Fronczek, F.R.; Vicente, M.G.H. Synthesis, Aggregation and Cellular Investigations of Porphyrin–Cobaltacarborane Conjugates. Chem. A Eur. J. 2007, 13, 9035–9042. [Google Scholar] [CrossRef]
- Bhupathiraju, N.D.K.; Gottumukkala, V.; Hao, E.; Hu, X.; Fronczek, F.R.; Baker, D.G.; Wakamatsu, N.; Vicente, M.G.H. Synthesis and toxicity of cobaltabisdicarbollide-containing porphyrins of high boron content. J. Porphyrins Phthalocyanines 2011, 15, 973–983. [Google Scholar] [CrossRef]
- Sibrian-Vazquez, M.; Hao, E.; Jensen, T.J.; Vicente, M.G.H. Enhanced Cellular Uptake with a Cobaltacarborane−Porphyrin−HIV-1 Tat 48−60 Conjugate. Bioconjugate Chem. 2006, 17, 928–934. [Google Scholar] [CrossRef]
- Bhupathiraju, N.V.S.D.K.; Hu, X.; Zhou, Z.; Fronczek, F.R.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Vicente, M.G.H. Synthesis and in Vitro Evaluation of BBB Permeability, Tumor Cell Uptake, and Cytotoxicity of a Series of Carboranylporphyrin Conjugates. J. Med. Chem. 2014, 57, 6718–6728. [Google Scholar] [CrossRef]
- Hiramatsu, R.; Kawabata, S.; Tanaka, H.; Sakurai, Y.; Suzuki, M.; Ono, K.; Miyatake, S.-I.; Kuroiwa, T.; Hao, E.; Vicente, M.G.H. Tetrakis(p-Carboranylthio-Tetrafluorophenyl)Chlorin (TPFC): Application for Photodynamic Therapy and Boron Neutron Capture Therapy. J. Pharm. Sci. 2015, 104, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Pushpan, S.K.; Venkatraman, S.; Anand, V.G.; Sankar, J.; Parmeswaran, D.; Ganesan, S.; Chandrashekar, T.K. Porphyrins in Photodynamic Therapy—A Search for Ideal Photosensitizers. Curr. Med. Chem. Agents 2002, 2, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Loewen, G.M.; Pandey, R.; Bellnier, D.; Henderson, B.; Dougherty, T. Endobronchial photodynamic therapy for lung cancer. Lasers Surg. Med. 2006, 38, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, Z.; Luck, D.; Beckers, J.; Brun, P.-H.; Wilson, B.C.; Scherz, A.; Salomon, Y.; Hetzel, F.W. Preclinical Studies in Normal Canine Prostate of a Novel Palladium-Bacteriopheophorbide (WST09) Photosensitizer for Photodynamic Therapy of Prostate Cancer. Photochem. Photobiol. 2007, 76, 438–445. [Google Scholar] [CrossRef]
- Mironov, A.F. Chemical Transformations of Chlorophyll a and Possible Areas for Application of Its Derivatives. Russ. J. Gen. Chem. 2019, 89, 1952–1983. [Google Scholar] [CrossRef]
- Golovina, V.G.; Rychkov, N.G.; Ol’shevskaya, A.V.; Zaitsev, V.A.; Kalinin, N.V.; Kuzmin, A.V.; Shtil, A.A. Differential Binding Preference of Methylpheophorbide a and its Diboronated derivatives to Albumin and low density Lipoproteins. Anti Cancer Agents Med. Chem. 2013, 13, 639–646. [Google Scholar] [CrossRef]
- Grin, M.A.; Titeev, R.A.; Brittal, D.I.; Chestnova, A.V.; Feofanov, A.; Lobanova, I.A.; Sivaev, I.B.; Bregadze, V.I.; Mironov, A.F. Synthesis of cobalt bis(dicarbollide) conjugates with natural chlorins by the Sonogashira reaction. Russ. Chem. Bull. 2010, 59, 219–224. [Google Scholar] [CrossRef]
- Volovetsky, A.B.; Sukhov, V.S.; Balalaeva, I.V.; Dudenkova, V.V.; Shilyagina, N.Y.; Feofanov, V.; Efremenko, A.V.; Grin, M.A.; Mironov, A.F.; Sivaev, I.B.; et al. Pharmacokinetics of Chlorin e6-Cobalt Bis(Dicarbollide) Conjugate in Balb/c Mice with Engrafted Carcinoma. Int. J. Mol. Sci. 2017, 18, 2556. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Nar, I.; Bortolussi, S.; Postuma, I.; Atsay, A.; Berksun, E.; Viola, E.; Ferrari, C.; Cansolino, L.; Ricciardi, G.; Donzello, M.P.; et al. A Phthalocyanine-ortho-Carborane Conjugate for Boron Neutron Capture Therapy: Synthesis, Physicochemical Properties, and in vitro Tests. Chempluschem 2019, 84, 345–351. [Google Scholar] [CrossRef]
- Pietrangeli, D.; Rosa, A.; Pepe, A.; Altieri, S.; Bortolussi, S.; Postuma, I.; Protti, N.; Ferrari, C.; Cansolino, L.; Clerici, A.M.; et al. Water-soluble carboranyl-phthalocyanines for BNCT. Synthesis, characterization, and in vitro tests of the Zn(ii)-nido-carboranyl-hexylthiophthalocyanine. Dalton Trans. 2015, 44, 11021–11028. [Google Scholar] [CrossRef] [PubMed]
- Pietrangeli, D.; Ricciardi, G. Neutral and polyanionic carboranylporphyrazines: Synthesis and physico-chemical properties. Appl. Radiat. Isot. 2009, 67, S97–S100. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, T.-P.; Li, D.; Leamer, L.; Shan, H.; Li, Z.; Gabbaï, F.P.; Conti, P.S. Lewis Acid-Assisted Isotopic 18F-19F Exchange in BODIPY Dyes: Facile Generation of Positron Emission Tomography/Fluorescence Dual Modality Agents for Tumor Imaging. Theranostics 2013, 3, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.H.; Wang, H.; Bhupathiraju, N.D.K.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Synthesis and properties of a series of carboranyl-BODIPYs. J. Organomet. Chem. 2015, 798, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.; Zhao, N.; Zhou, Z.; Fronczek, F.R.; Vicente, M.G.H. Synthesis and in Vitro Studies of a Series of Carborane-Containing Boron Dipyrromethenes (BODIPYs). J. Med. Chem. 2016, 59, 2109–2117. [Google Scholar] [CrossRef]
- Eloy, J.O.; de Souza, M.C.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.J.; Marchetti, J.M. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surf. B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes: From a Clinically Established Drug Delivery System to a Nanoparticle Platform for Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef]
- Olusanya, T.O.; Calabrese, G.; Fatouros, D.G.; Tsibouklis, J.; Smith, J.R. Liposome formulations of o-carborane for the boron neutron capture therapy of cancer. Biophys. Chem. 2019, 247, 25–33. [Google Scholar] [CrossRef]
- Nakamura, H. Liposomal Boron Delivery for Neutron Capture Therapy. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 465, pp. 179–208. ISBN 978-0-12-381379-4. [Google Scholar]
- Takeuchi, I.; Kanno, Y.; Uchiro, H.; Makino, K. Polyborane-encapsulated PEGylated Liposomes Prepared Using Post-insertion Technique for Boron Neutron Capture Therapy. J. Oleo Sci. 2019, 68, 1261–1270. [Google Scholar] [CrossRef]
- Xie, Y.; Ye, L.; Zhang, X.; Cui, W.; Lou, J.; Nagai, T.; Hou, X. Transport of nerve growth factor encapsulated into liposomes across the blood–brain barrier: In vitro and in vivo studies. J. Control. Release 2005, 105, 106–119. [Google Scholar] [CrossRef]
- Liu, D.; Guo, P.; McCarthy, C.; Wang, B.; Tao, Y.; Auguste, D. Peptide density targets and impedes triple negative breast cancer metastasis. Nat. Commun. 2018, 9, 2612. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Sarkar, S.; Ahn, H.; Kim, J.Y.; Lee, Y.J.; Chang, Y.; Yoo, J. PEGylated liposome encapsulating nido-carborane showed significant tumor suppression in boron neutron capture therapy (BNCT). Biochem. Biophys. Res. Commun. 2020, 522, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Khan, M.A.; Burgess, D.J. Predicting hydrophilic drug encapsulation inside unilamellar liposomes. Int. J. Pharm. 2012, 423, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Guan, L.; Kane, R.R.; Kasamatsu, H.; Hawthorne, M.F. Toward a cancer therapy with boron-rich oligomeric phosphate diesters that target the cell nucleus. Proc. Natl. Acad. Sci. USA 1999, 96, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Yanagie, H.; Yanagawa, M.; Morishita, Y.; Shinohara, A.; Dewi, N.; Nonaka, Y.; Furuya, Y.; Mizumachi, R.; Murata, Y.; Nakamura, H.; et al. Suppression of Tumor Growth in a Rabbit Hepatic Cancer Model by Boron Neutron Capture Therapy With Liposomal Boron Delivery Systems. In Vivo 2021, 35, 3125–3135. [Google Scholar] [CrossRef]
- Ishida, O.; Maruyama, K.; Tanahashi, H.; Iwatsuru, M.; Sasaki, K.; Eriguchi, M.; Yanagie, H. Liposomes Bearing Polyethyleneglycol-Coupled Transferrin with Intracellular Targeting Property to the Solid Tumors In Vivo. Pharm. Res. 2001, 18, 1042–1048. [Google Scholar] [CrossRef]
- Kanygin, V.; Zaboronok, A.; Taskaeva, I.; Zavjalov, E.; Mukhamadiyarov, R.; Kichigin, A.; Kasatova, A.; Razumov, I.; Sibirtsev, R.; Mathis, B.J. In Vitro and In Vivo Evaluation of Fluorescently Labeled Borocaptate-Containing Liposomes. J. Fluoresc. 2021, 31, 73–83. [Google Scholar] [CrossRef]
- Lozano, N.; Al-Ahmady, Z.S.; Beziere, N.S.; Ntziachristos, V.; Kostarelos, K. Monoclonal antibody-targeted PEGylated liposome-ICG encapsulating doxorubicin as a potential theranostic agent. Int. J. Pharm. 2015, 482, 2–10. [Google Scholar] [CrossRef]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 2014, 192, 67–81. [Google Scholar] [CrossRef]
- Zavjalov, E.; Zaboronok, A.; Kanygin, V.; Kasatova, A.; Kichigin, A.; Mukhamadiyarov, R.; Razumov, I.; Sycheva, T.; Mathis, B.J.; Maezono, S.E.B.; et al. Accelerator-based boron neutron capture therapy for malignant glioma: A pilot neutron irradiation study using boron phenylalanine, sodium borocaptate and liposomal borocaptate with a heterotopic U87 glioblastoma model in SCID mice. Int. J. Radiat. Biol. 2020, 96, 868–878. [Google Scholar] [CrossRef]
- Shimizu, K.; Oku, N. Liposomes Conjugated with a Pilot Molecule. In Cancer Drug Delivery Systems Based on the Tumor Microenvironment; Matsumura, Y., Tarin, D., Eds.; Springer: Tokyo, Japan, 2019; pp. 187–216. ISBN 978-4-431-56878-0. [Google Scholar]
- Kullberg, E.B.; Wei, Q.; Capala, J.; Giusti, V.; Malmström, P.-U.; Gedda, L. EGF-receptor targeted liposomes with boronated acridine: Growth inhibition of cultured glioma cells after neutron irradiation. Int. J. Radiat. Biol. 2005, 81, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Kullberg, E.B.; Gedda, L. Trastuzumab-conjugated boron-containing liposomes for tumor-cell targeting; development and cellular studies. Int. J. Oncol. 2003, 23, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Koltover, I.; Salditt, T.; Rädler, J.O.; Safinya, C.R. An Inverted Hexagonal Phase of Cationic Liposome-DNA Complexes Related to DNA Release and Delivery. Science 1998, 281, 78–81. [Google Scholar] [CrossRef]
- Ristori, S.; Oberdisse, J.; Grillo, I.; Donati, A.; Spalla, O. Structural Characterization of Cationic Liposomes Loaded with Sugar-Based Carboranes. Biophys. J. 2005, 88, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Altieri, S.; Balzi, M.; Bortolussi, S.; Bruschi, P.; Ciani, L.; Clerici, A.M.; Faraoni, P.; Ferrari, C.; Gadan, M.A.; Panza, L.; et al. Carborane Derivatives Loaded into Liposomes as Efficient Delivery Systems for Boron Neutron Capture Therapy. J. Med. Chem. 2009, 52, 7829–7835. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Q.; Lu, C.; Xiao, H.; Guo, Z.; Duan, D.; Zhang, Z.; Liu, T.; Liu, Z. Boron encapsulated in a liposome can be used for combinational neutron capture therapy. Nat. Commun. 2022, 13, 2143. [Google Scholar] [CrossRef]
- Ailuno, G.; Balboni, A.; Caviglioli, G.; Lai, F.; Barbieri, F.; Dellacasagrande, I.; Florio, T.; Baldassari, S. Boron Vehiculating Nanosystems for Neutron Capture Therapy in Cancer Treatment. Cells 2022, 11, 4029. [Google Scholar] [CrossRef]
- Sumitani, S.; Oishi, M.; Nagasaki, Y. Carborane confined nanoparticles for boron neutron capture therapy: Improved stability, blood circulation time and tumor accumulation. React. Funct. Polym. 2011, 71, 684–693. [Google Scholar] [CrossRef]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef]
- Uspenskii, S.A.; Khaptakhanova, P.A.; Zaboronok, A.A.; Kurkin, T.S.; Volkova, O.Y.; Mechetina, L.V.; Taranin, A.V.; Kanygin, V.V.; Akira, M.; Taskaev, S.Y. Elemental Boron Nanoparticles: Production by Ultrasonication in Aqueous Medium and Application in Boron Neutron Capture Therapy. Dokl. Chem. 2020, 491, 45–48. [Google Scholar] [CrossRef]
- Zaboronok, A.; Khaptakhanova, P.; Uspenskii, S.; Bekarevich, R.; Mechetina, L.; Volkova, O.; Mathis, B.J.; Kanygin, V.; Ishikawa, E.; Kasatova, A.; et al. Polymer-Stabilized Elemental Boron Nanoparticles for Boron Neutron Capture Therapy: Initial Irradiation Experiments. Pharmaceutics 2022, 14, 761. [Google Scholar] [CrossRef] [PubMed]
- Kaniowski, D.; Ebenryter-Olbińska, K.; Kulik, K.; Suwara, J.; Cypryk, W.; Jakóbik-Kolon, A.; Leśnikowski, Z.; Nawrot, B. Composites of Nucleic Acids and Boron Clusters (C2B10H12) as Functional Nanoparticles for Downregulation of EGFR Oncogene in Cancer Cells. Int. J. Mol. Sci. 2021, 22, 4863. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanoparticle Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed]
- Cioran, A.M.; Teixidor, F.; Krpetić, Ž.; Brust, M.; Viñas, C. Preparation and characterization of Au nanoparticles capped with mercaptocarboranyl clusters. Dalton Trans. 2014, 43, 5054–5061. [Google Scholar] [CrossRef]
- Sawant, R.R.; Torchilin, V.P. Multifunctional nanocarriers and intracellular drug delivery. Curr. Opin. Solid State Mater. Sci. 2012, 16, 269–275. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lin, J.-J.; Chang, W.-Y.; Hsieh, C.-Y.; Wu, C.-C.; Chen, H.-S.; Hsu, H.-J.; Yang, A.-S.; Hsu, M.-H.; Kuo, W.-Y. Development of theranostic active-targeting boron-containing gold nanoparticles for boron neutron capture therapy (BNCT). Colloids Surf. B Biointerfaces 2019, 183, 110387. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Ye, J.; Li, Z.; Jiang, H.; Yan, H.; Stogniy, M.Y.; Sivaev, I.B.; Bregadze, V.I.; Wang, X. Carborane Derivative Conjugated with Gold Nanoclusters for Targeted Cancer Cell Imaging. Biomacromolecules 2017, 18, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.V.; Pyshnaya, I.A.; Zakharova, O.D.; Akulov, A.E.; Shevelev, O.B.; Poletaeva, J.; Zavjalov, E.L.; Silnikov, V.N.; Ryabchikova, E.I.; Godovikova, T.S. Rational Design of Albumin Theranostic Conjugates for Gold Nanoparticles Anticancer Drugs: Where the Seed Meets the Soil? Biomedicines 2021, 9, 74. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Hsieh, H.-H.; Chang, T.-Y.; Lin, J.-J.; Wu, C.-C.; Hsu, M.-H.; Lin, M.-C.; Peng, S.-L. Development of MRI-Detectable Boron-Containing Gold Nanoparticle-Encapsulated Biodegradable Polymeric Matrix for Boron Neutron Capture Therapy (BNCT). Int. J. Mol. Sci. 2021, 22, 8050. [Google Scholar] [CrossRef]
- Pulagam, K.R.; Gona, K.B.; Gómez-Vallejo, V.; Meijer, J.; Zilberfain, C.; Estrela-Lopis, I.; Baz, Z.; Cossío, U.; Llop, J. Gold Nanoparticles as Boron Carriers for Boron Neutron Capture Therapy: Synthesis, Radiolabelling and In Vivo Evaluation. Molecules 2019, 24, 3609. [Google Scholar] [CrossRef]
- Pulagam, K.R.; Henriksen-Lacey, M.; Uribe, K.B.; Renero-Lecuna, C.; Kumar, J.; Charalampopoulou, A.; Facoetti, A.; Protti, N.; Gómez-Vallejo, V.; Baz, Z.; et al. In Vivo Evaluation of Multifunctional Gold Nanorods for Boron Neutron Capture and Photothermal Therapies. ACS Appl. Mater. Interfaces 2021, 13, 49589–49601. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, W.A.G.; Sancey, L.; Hey-Hawkins, E.; Kellert, M.; Panza, L.; Imperio, D.; Balcerzyk, M.; Rizzo, G.; Scalco, E.; Herrmann, K.; et al. Theranostics in Boron Neutron Capture Therapy. Life 2021, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.; Kulkarni, S.; Nacev, A.; Muro, S.; Stepanov, P.Y.; Weinberg, I.N. Open challenges in magnetic drug targeting. WIREs Nanomed. Nanobiotechnology 2015, 7, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Clemons, T.D.; Kerr, R.H.; Joos, A. Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications. In Comprehensive Nanoscience and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–210. ISBN 978-0-12-812296-9. [Google Scholar]
- Torresan, V.; Guadagnini, A.; Badocco, D.; Pastore, P.; Medina, G.A.M.; van Raap, M.B.F.; Postuma, I.; Bortolussi, S.; Bekić, M.; Čolić, M.; et al. Biocompatible Iron–Boron Nanoparticles Designed for Neutron Capture Therapy Guided by Magnetic Resonance Imaging. Adv. Health Mater. 2021, 10, e2001632. [Google Scholar] [CrossRef]
- Malik, A.; Butt, T.T.; Zahid, S.; Zahid, F.; Waquar, S.; Rasool, M.; Qazi, M.H.; Qazi, A.M. Use of Magnetic Nanoparticles as Targeted Therapy: Theranostic Approach to Treat and Diagnose Cancer. J. Nanotechnol. 2017, 2017, 1098765. [Google Scholar] [CrossRef]
- Yang, Z.; Cui, B.; Bu, Y.; Wang, Y. Preparation of flower-dewdrops Fe3O4/carbon-SiO2 microsphere for microwave-triggered drug delivery. J. Alloys Compd. 2019, 775, 826–835. [Google Scholar] [CrossRef]
- Dutta, B.; Shetake, N.G.; Gawali, S.L.; Barick, B.; Barick, K.; Babu, P.; Pandey, B.; Priyadarsini, K.; Hassan, P. PEG mediated shape-selective synthesis of cubic Fe3O4 nanoparticles for cancer therapeutics. J. Alloys Compd. 2018, 737, 347–355. [Google Scholar] [CrossRef]
- Korolkov, I.; Ludzik, K.; Kozlovskiy, A.; Fadeev, M.; Shumskaya, A.; Gorin, Y.; Marciniak, B.; Jazdzewska, M.; Chudoba, D.; Kontek, R.; et al. Carboranes immobilization on Fe3O4 nanocomposites for targeted delivery. Mater. Today Commun. 2020, 24, 101247. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Yang, J.; Qiu, X.; Li, N.; Zhu, Y.; Yan, L.; Li, W.; Huang, X.; Liang, K.; et al. Carborane based mesoporous nanoparticles as a potential agent for BNCT. Mater. Chem. Front. 2021, 5, 2771–2776. [Google Scholar] [CrossRef]
- Vares, G.; Jallet, V.; Matsumoto, Y.; Rentier, C.; Takayama, K.; Sasaki, T.; Hayashi, Y.; Kumada, H.; Sugawara, H. Functionalized mesoporous silica nanoparticles for innovative boron-neutron capture therapy of resistant cancers. Nanomed. Nanotechnol. Biol. Med. 2020, 27, 102195. [Google Scholar] [CrossRef]
- Kuthala, N.; Vankayala, R.; Li, Y.; Chiang, C.; Hwang, K.C. Engineering Novel Targeted Boron-10-Enriched Theranostic Nanomedicine to Combat against Murine Brain Tumors via MR Imaging-Guided Boron Neutron Capture Therapy. Adv. Mater. 2017, 29, 1700850. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Strano, M.S.; Ajayan, P.M. Potential Applications of Carbon Nanotubes. In Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties, and Applications; Topics in applied physics; Jorio, A., Dresselhaus, G., Dresselhaus, M.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-72864-1. [Google Scholar]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhu, Y.; Hosmane, N. Nanostructured Boron Compounds for Boron Neutron Capture Therapy (BNCT) in cancer treatment. In Boron-Based Compounds; Hey-Hawkins, E., Teixidor, C.V., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 371–388. ISBN 978-1-119-27560-2. [Google Scholar]
- Golberg, D.; Bando, Y.; Tang, C.C.; Zhi, C.Y. Boron Nitride Nanotubes. Adv. Mater. 2007, 19, 2413–2432. [Google Scholar] [CrossRef]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Folate Functionalized Boron Nitride Nanotubes and their Selective Uptake by Glioblastoma Multiforme Cells: Implications for their Use as Boron Carriers in Clinical Boron Neutron Capture Therapy. Nanoscale Res. Lett. 2009, 4, 113–121. [Google Scholar] [CrossRef]
- Ciofani, G.; Del Turco, S.; Genchi, G.G.; D’alessandro, D.; Basta, G.; Mattoli, V. Transferrin-conjugated boron nitride nanotubes: Protein grafting, characterization, and interaction with human endothelial cells. Int. J. Pharm. 2012, 436, 444–453. [Google Scholar] [CrossRef]
- Nakamura, H.; Koganei, H.; Miyoshi, T.; Sakurai, Y.; Ono, K.; Suzuki, M. Antitumor effect of boron nitride nanotubes in combination with thermal neutron irradiation on BNCT. Bioorganic Med. Chem. Lett. 2015, 25, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Achilli, C.; Grandi, S.; Ciana, A.; Guidetti, G.F.; Malara, A.; Abbonante, V.; Cansolino, L.; Tomasi, C.; Balduini, A.; Fagnoni, M.; et al. Biocompatibility of functionalized boron phosphate (BPO4) nanoparticles for boron neutron capture therapy (BNCT) application. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 589–597. [Google Scholar] [CrossRef]
- Kuthala, N.; Shanmugam, M.; Yao, C.-L.; Chiang, C.-S.; Hwang, K.C. One step synthesis of 10B-enriched 10BPO4 nanoparticles for effective boron neutron capture therapeutic treatment of recurrent head-and-neck tumor. Biomaterials 2022, 290, 121861. [Google Scholar] [CrossRef]
- Chiang, C.-W.; Chien, Y.-C.; Yu, W.-J.; Ho, C.-Y.; Wang, C.-Y.; Wang, T.-W.; Chiang, C.-S.; Keng, P.-Y. Polymer-Coated Nanoparticles for Therapeutic and Diagnostic Non-10B Enriched Polymer-Coated Boron Carbon Oxynitride (BCNO) Nanoparticles as Potent BNCT Drug. Nanomaterials 2021, 11, 2936. [Google Scholar] [CrossRef]
- Chien, Y.-C.; Hsu, Y.-T.; Chiang, C.-W.; Keng, P.Y.; Wang, T.-W. Investigating the electrostatic complexation of BCNO nanoparticles with a stimuli-responsive double hydrophilic graft copolymer. Giant 2023, 14, 100162. [Google Scholar] [CrossRef]
- Shao, M.; Lopes, D.; Lopes, J.; Yousefiasl, S.; Macário-Soares, A.; Peixoto, D.; Ferreira-Faria, I.; Veiga, F.; Conde, J.; Huang, Y.; et al. Exosome membrane-coated nanosystems: Exploring biomedical applications in cancer diagnosis and therapy. Matter 2023, 6, 761–799. [Google Scholar] [CrossRef]
- Li, J.; Kong, J.; Ma, S.; Li, J.; Mao, M.; Chen, K.; Chen, Z.; Zhang, J.; Chang, Y.; Yuan, H.; et al. Exosome-Coated 10 B Carbon Dots for Precise Boron Neutron Capture Therapy in a Mouse Model of Glioma In Situ. Adv. Funct. Mater. 2021, 31, 2100969. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zhang, Z.; Li, J.; Chen, K.; Liang, H.; Lv, L.; Chang, Y.; Liu, S.; Yang, W.; et al. Multifunctional high boron content MOFs nano-co-crystals for precise boron neutron capture therapy for brain glioma in situ. Nano Today 2022, 45, 101558. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oloo, S.O.; Smith, K.M.; Vicente, M.d.G.H. Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers. Cancers 2023, 15, 3277. https://doi.org/10.3390/cancers15133277
Oloo SO, Smith KM, Vicente MdGH. Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers. Cancers. 2023; 15(13):3277. https://doi.org/10.3390/cancers15133277
Chicago/Turabian StyleOloo, Sebastian O., Kevin M. Smith, and Maria da Graça H. Vicente. 2023. "Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers" Cancers 15, no. 13: 3277. https://doi.org/10.3390/cancers15133277
APA StyleOloo, S. O., Smith, K. M., & Vicente, M. d. G. H. (2023). Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers. Cancers, 15(13), 3277. https://doi.org/10.3390/cancers15133277





