Relevance of the Updated Recursive Partitioning Analysis (U-RPA) Classification in the Contemporary Care of Patients with Brain Metastases
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Updated-RPA Prognostic Index
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, F.G.; Dolecek, T.A.; McCarthy, B.J.; Villano, J.L. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro-Oncology 2012, 14, 1171–1177. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Wright, C.H.; Barnholtz-Sloan, J.S. Brain metastases: Epidemiology. Handb. Clin. Neurol. 2018, 149, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Lamba, N.; Wen, P.Y.; Aizer, A.A. Epidemiology of brain metastases and leptomeningeal disease. Neuro-Oncology 2021, 23, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Koekkoek, J.A.F.; van den Bent, M.J.; Bulbeck, H.J.; Fleming, J.; Grant, R.; Golla, H.; Henriksson, R.; Kerrigan, S.; Marosi, C.; et al. Determining medical decision-making capacity in brain tumor patients: Why and how? Neuro-Oncol. Pract. 2020, 7, 599–612. [Google Scholar] [CrossRef]
- Kim, A.E.; Wang, G.M.; Waite, K.A.; Elder, S.; Fine, A.; Ahluwalia, M.S.; Brat, D.; Mehta, M.P.; Page, R.; Dunbar, E.; et al. Cross-sectional survey of patients, caregivers, and physicians on diagnosis and treatment of brain metastases. Neuro-Oncol. Pract. 2021, 8, 662–673. [Google Scholar] [CrossRef]
- Chow, E.; Davis, L.; Panzarella, T.; Hayter, C.; Szumacher, E.; Loblaw, A.; Wong, R.; Danjoux, C. Accuracy of survival prediction by palliative radiation oncologists. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; Bauman, G.; Palma, D.; Louie, A.V.; Mocanu, J.; Senan, S.; Lagerwaard, F. Systematic review of brain metastases prognostic indices. Pract. Radiat. Oncol. 2013, 3, 101–106. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Mesko, S.; Li, J.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Survival in Patients with Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3773–3784. [Google Scholar] [CrossRef]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 745–751. [Google Scholar] [CrossRef]
- Venur, V.A.; Ahluwalia, M.S. Prognostic scores for brain metastasis patients: Use in clinical practice and trial design. Chin. Clin. Oncol. 2015, 4, 18. [Google Scholar] [CrossRef]
- Kondziolka, D.; Parry, P.V.; Lunsford, L.D.; Kano, H.; Flickinger, J.C.; Rakfal, S.; Arai, Y.; Loeffler, J.S.; Rush, S.; Knisely, J.P.; et al. The accuracy of predicting survival in individual patients with cancer. J. Neurosurg. 2014, 120, 24–30. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, Y.; Serizawa, T.; Kawabe, T.; Higuchi, Y.; Nagano, O.; Barfod, B.E.; Ono, J.; Kasuya, H.; Urakawa, Y. Subclassification of recursive partitioning analysis Class II patients with brain metastases treated radiosurgically. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.; van Timmeren, J.E.; Frei, S.; Mayinger, M.; Borsky, K.; Kirchner, C.; Stark, L.S.; Tanadini-Lang, S.; Wolpert, F.; Weller, M.; et al. Comprehensive summary and retrospective evaluation of prognostic scores for patients with newly diagnosed brain metastases treated with upfront radiosurgery in a modern patient collective. Radiother. Oncol. 2022, 172, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Komosinska, K.; Kepka, L.; Niwinska, A.; Pietrzak, L.; Wierzchowski, M.; Tyc-Szczepaniak, D.; Kaczmarczyk, A.; Bujko, K. Prospective evaluation of the palliative effect of whole-brain radiotherapy in patients with brain metastases and poor performance status. Acta Oncol. 2010, 49, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Tsakonas, G.; Hellman, F.; Gubanski, M.; Friesland, S.; Tendler, S.; Lewensohn, R.; Ekman, S.; de Petris, L. Prognostic factors affecting survival after whole brain radiotherapy in patients with brain metastasized lung cancer. Acta Oncol. 2018, 57, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance during Whole-Brain Radiotherapy Plus Memantine for Patients with Brain Metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Mulvenna, P.; Nankivell, M.; Barton, R.; Faivre-Finn, C.; Wilson, P.; McColl, E.; Moore, B.; Brisbane, I.; Ardron, D.; Holt, T.; et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet 2016, 388, 2004–2014. [Google Scholar] [CrossRef]
- Palmer, J.D.; Klamer, B.G.; Ballman, K.V.; Brown, P.D.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; et al. Association of Long-term Outcomes with Stereotactic Radiosurgery vs Whole-Brain Radiotherapy for Resected Brain Metastasis: A Secondary Analysis of The N107C/CEC.3 (Alliance for Clinical Trials in Oncology/Canadian Cancer Trials Group) Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1809–1815. [Google Scholar] [CrossRef]
- Wang, B.; Fu, S.; Huang, Y.; Liu, L.; Liang, Y.; An, W.; Fan, Y.; Zhao, Y. The Effect of Hippocampal Avoidance Whole Brain Radiotherapy on the Preservation of Long-Term Neurocognitive Function in Non-Small Cell Lung Cancer Patients with Brain Metastasis. Technol. Cancer Res. Treat. 2021, 20, 15330338211034269. [Google Scholar] [CrossRef]
- Loh, D.; Hogg, F.; Edwards, P.; MacColl, J.; Brogna, C.; Bhangoo, R.; Ashkan, K.; Vergani, F. Two-year experience of multi-disciplinary team (MDT) outcomes for brain metastases in a tertiary neuro-oncology centre. Br. J. Neurosurg. 2017, 32, 53–60. [Google Scholar] [CrossRef]
- Moss, N.S.; El Ahmadieh, T.Y.; Brown, S.; Chen, J.; Imber, B.S.; Pike, L.; Reiner, A.S.; Panageas, K.S.; Brennan, C.; Tabar, V.; et al. Integrated Multidisciplinary Brain Metastasis Care Reduces Patient Visits and Shortens Time to Adjuvant Irradiation. JCO Oncol. Pract. 2022, 18, e1732–e1738. [Google Scholar] [CrossRef]
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R.; et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef]
- Schiff, D.; Messersmith, H.; Brastianos, P.K.; Brown, P.D.; Burri, S.; Dunn, I.F.; Gaspar, L.E.; Gondi, V.; Jordan, J.T.; Maues, J.; et al. Radiation Therapy for Brain Metastases: ASCO Guideline Endorsement of ASTRO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 2271–2276. [Google Scholar] [CrossRef] [PubMed]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef]
- Habibi, A.; Wu, S.P.; Gorovets, D.; Sansosti, A.; Kryger, M.; Beaudreault, C.; Chung, W.Y.; Shelton, G.; Silverman, J.; Lowy, J.; et al. Early Palliative Care for Patients with Brain Metastases Decreases Inpatient Admissions and Need for Imaging Studies. Am. J. Hosp. Palliat. Care 2018, 35, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Baine, M.; Meza, J.; Lin, C. The impact of treatment facility type on the survival of brain metastases patients regardless of the primary cancer type. BMC Cancer 2021, 21, 387. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Trifiletti, D.M.; Gondi, V.; Chan, M.; Minniti, G.; Rusthoven, C.G.; Schild, S.E.; Mishra, M.V.; Bovi, J.; Williams, N.; et al. Multidisciplinary patient-centered management of brain metastases and future directions. Neuro-Oncol. Adv. 2020, 2, vdaa034. [Google Scholar] [CrossRef]
- Moravan, M.J.; Fecci, P.E.; Anders, C.K.; Clarke, J.M.; Salama, A.K.S.; Adamson, J.D.; Floyd, S.R.; Torok, J.A.; Salama, J.K.; Sampson, J.H.; et al. Current multidisciplinary management of brain metastases. Cancer 2020, 126, 1390–1406. [Google Scholar] [CrossRef]
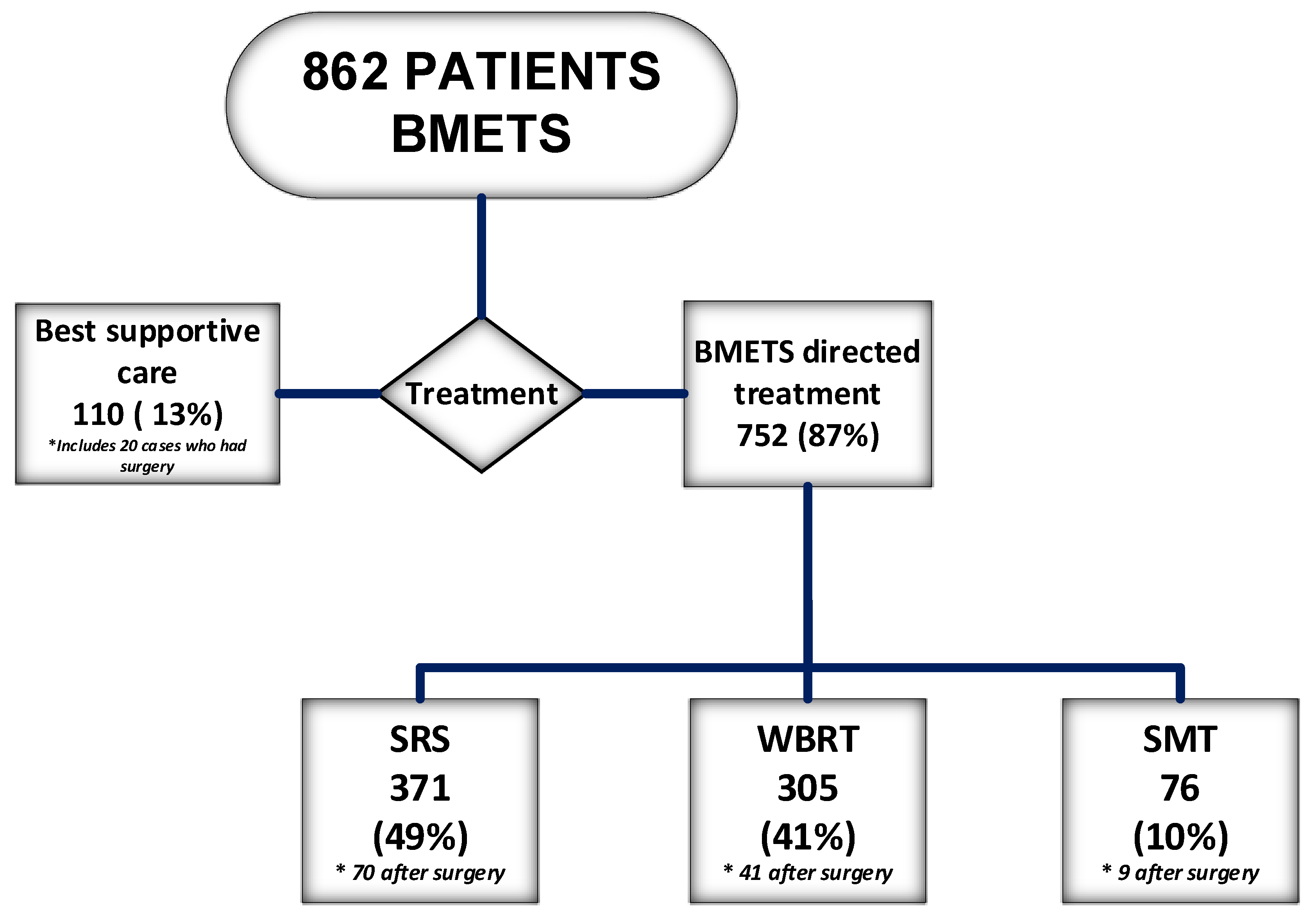

| Variable | Total Number (862 Patients) | |
|---|---|---|
| Median age years (range) | 65 (23–93) | |
| Median KPS (range) | 80 (20–100) | |
| Sex | ||
| Female (%) | 420 (49%) | |
| Male (%) | 442 (51%) | |
| Primary Tumor type | ||
| NSCLC (%) | 393 (46%) | |
| Melanoma (%) | 96 (11%) | |
| Breast (%) | 94 (11%) | |
| SCLC (%) | 63 (7%) | |
| Renal (%) | 53 (6%) | |
| Others (%) | 163 (19%) | |
| RPA | ||
| I (%) | 84 (10%) | |
| II (%) | 570 (66%) | |
| III (%) | 208 (24%) | |
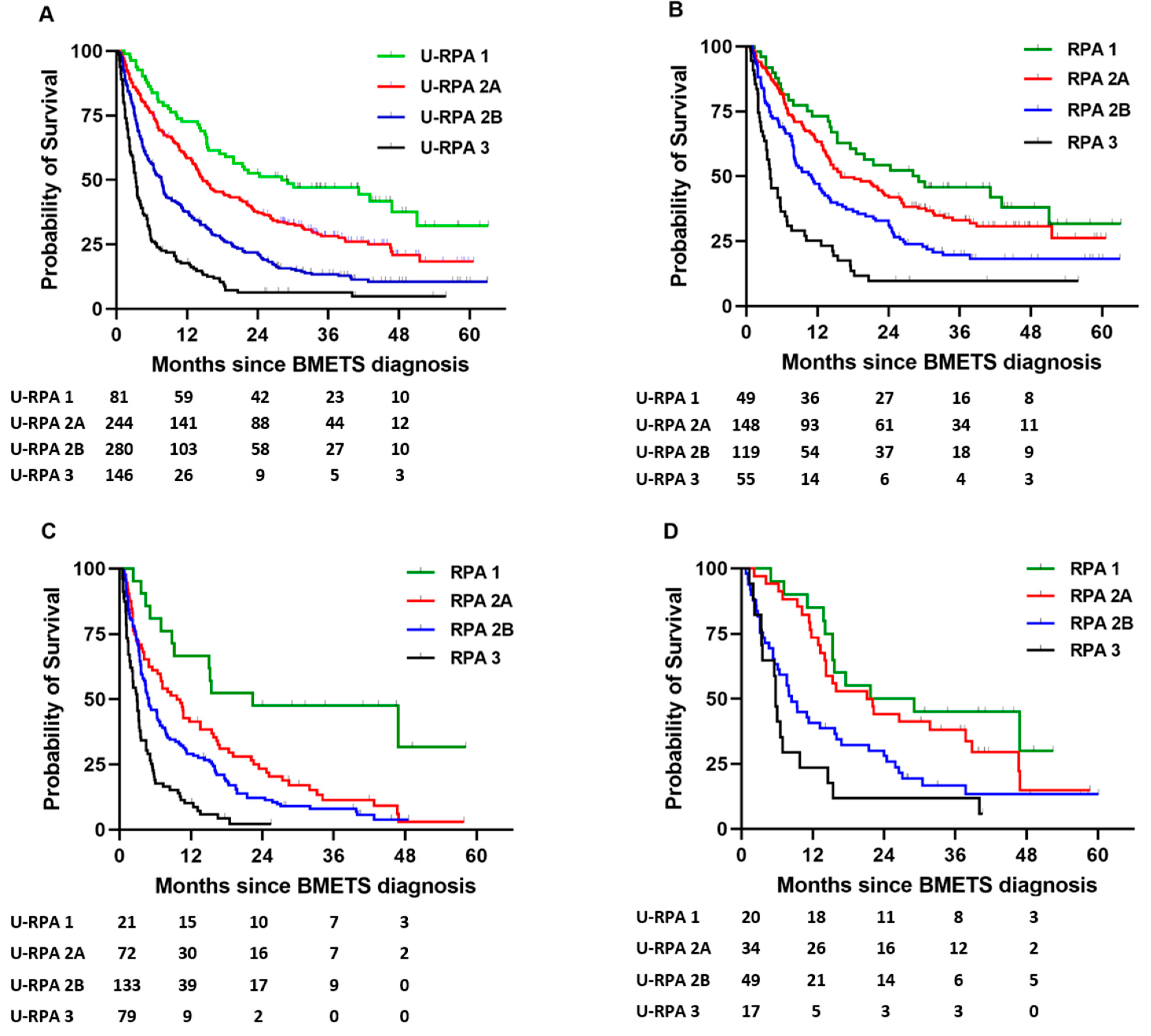
| U-RPA | ||
| 1 (%) | 84 (10%) | |
| 2A (%) | 259(30%) | |
| 2B (%) | 311 (36%) | |
| 3 (%) | 208 (24%) | |
| Treatment | ||
| SRS (70 after surgery) | 371 (43%) | |
| WBRT (41 after surgery) | 305 (35%) | |
| SMT (9 after surgery) | 76 (9%) | |
| BSC (20 had surgery) | 110 (13%) | |
| Class | RPA * | RPA | U-RPA |
|---|---|---|---|
| 1979–1993 | 2017–2019 | 2017–2019 | |
| n = 1200 | n = 752 | n = 752 | |
| 1 | 7.4 | 28.1 | 28.1 |
| 2A | 4.2 * | 10.9 * | 14.7 |
| 2B | 7.6 | ||
| 3 | 2.3 | 3.3 | 3.3 |
| HR | 95.0% CI | p-Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| U-RPA 1 | 0.001 | |||
| U-RPA 2A | 1.63 | 1.16 | 2.31 | 0.005 |
| U-RPA 2B | 1.81 | 1.27 | 2.58 | <0.001 |
| U-RPA 3 | 2.5 | 1.5 | 4.16 | <0.001 |
| Age | 1.01 | 1 | 1.02 | 0.006 |
| SRS | 0.85 | 0.7 | 1.02 | 0.078 |
| WBRT | 0.81 | 0.72 | 0.92 | 0.002 |
| KPS | 0.99 | 0.97 | 1 | 0.013 |
| Surgery | 1.11 | 0.99 | 1.24 | 0.073 |
| Primary | No. | U-RPA CLASS | Log-Rank | |||
|---|---|---|---|---|---|---|
| 1 | 2A | 2B | 3 | p Value | ||
| NSCLC | 345 | 43.1 | 15.8 | 8.1 | 3.5 | <0.0001 |
| NSCLC * | 283 | 43.1 | 14.0 | 7.7 | 3.5 | <0.0001 |
| Melanoma | 86 | 47.6 | 19.3 | 4.3 | 2.3 | <0.0001 |
| Breast | 83 | 46.8 | 28.5 | 8.4 | 3.2 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadul, C.E.; Sarai, G.; Bovi, J.A.; Thomas, A.A.; Novicoff, W.; Anderson, R.; Amidon, R.F.; Schuetz, S.; Singh, R.; Chang, A.; et al. Relevance of the Updated Recursive Partitioning Analysis (U-RPA) Classification in the Contemporary Care of Patients with Brain Metastases. Cancers 2023, 15, 3255. https://doi.org/10.3390/cancers15123255
Fadul CE, Sarai G, Bovi JA, Thomas AA, Novicoff W, Anderson R, Amidon RF, Schuetz S, Singh R, Chang A, et al. Relevance of the Updated Recursive Partitioning Analysis (U-RPA) Classification in the Contemporary Care of Patients with Brain Metastases. Cancers. 2023; 15(12):3255. https://doi.org/10.3390/cancers15123255
Chicago/Turabian StyleFadul, Camilo E., Guneet Sarai, Joseph A. Bovi, Alissa A. Thomas, Wendy Novicoff, Roger Anderson, Ryan F. Amidon, Samantha Schuetz, Rohit Singh, Amy Chang, and et al. 2023. "Relevance of the Updated Recursive Partitioning Analysis (U-RPA) Classification in the Contemporary Care of Patients with Brain Metastases" Cancers 15, no. 12: 3255. https://doi.org/10.3390/cancers15123255
APA StyleFadul, C. E., Sarai, G., Bovi, J. A., Thomas, A. A., Novicoff, W., Anderson, R., Amidon, R. F., Schuetz, S., Singh, R., Chang, A., Gentzler, R. D., Gaughan, E. M., & Sheehan, J. P. (2023). Relevance of the Updated Recursive Partitioning Analysis (U-RPA) Classification in the Contemporary Care of Patients with Brain Metastases. Cancers, 15(12), 3255. https://doi.org/10.3390/cancers15123255






