Myelodysplastic Syndromes/Myeloproliferative Overlap Neoplasms and Differential Diagnosis in the WHO and ICC 2022 Era: A Focused Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Chronic Myelomonocytic Leukemia
2.1. Cytogenetics and Molecular Genetics
2.2. Flow Cytometry Immunophenotyping
2.3. Diagnosis and Differential Diagnosis of CMML
3. Clonal Monocytosis of Undetermined Significance and Clonal Cytopenia with Monocytosis of Undetermined Significance
4. MDS/MPN with Neutrophilia (Also Known as aCML)
5. MDS/MPN with SF3B1 Mutation and Thrombocytosis and MDS/MPN with Ring Sideroblasts and Thrombocytosis
6. Myelodysplastic/Myeloproliferative Neoplasm, Not Otherwise Specified
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Orazi, A.; Germing, U. The myelodysplastic/myeloproliferative neoplasms: Myeloproliferative diseases with dysplastic features. Leukemia 2008, 22, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Tefferi, A. Atypical chronic myeloid leukemia and myelodysplastic/myeloproliferative neoplasm, not otherwise specified: 2023 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2023, 98, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, M.M.; Lasho, T. Myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes: A focused review. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Elena, C.; Gallì, A.; Such, E.; Meggendorfer, M.; Germing, U.; Rizzo, E.; Cervera, J.; Molteni, E.; Fasan, A.; Schuler, E.; et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood 2016, 128, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Itzykson, R.; Fenaux, P.; Bowen, D.; Cross, N.C.P.; Cortes, J.; De Witte, T.; Germing, U.; Onida, F.; Padron, E.; Platzbecker, U.; et al. Diagnosis and Treatment of Chronic Myelomonocytic Leukemias in Adults: Recommendations From the European Hematology Association and the European LeukemiaNet. Hemasphere 2018, 2, e150. [Google Scholar] [CrossRef]
- Mason, C.C.; Khorashad, J.S.; Tantravahi, S.K.; Kelley, T.W.; Zabriskie, M.S.; Yan, D.; Pomicter, A.D.; Reynolds, K.R.; Eiring, A.M.; Kronenberg, Z.; et al. Age-related mutations and chronic myelomonocytic leukemia. Leukemia 2016, 30, 906–913. [Google Scholar] [CrossRef]
- Palomo, L.; Ibáñez, M.; Abáigar, M.; Vázquez, I.; Álvarez, S.; Cabezón, M.; Tazón-Vega, B.; Rapado, I.; Fuster-Tormo, F.; Cervera, J.; et al. Spanish Guidelines for the use of targeted deep sequencing in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br. J. Haematol. 2020, 188, 605–622. [Google Scholar] [CrossRef]
- Woo, J.; Choi, D.R.; Storer, B.E.; Yeung, C.; Halpern, A.B.; Salit, R.B.; Sorror, M.L.; Woolston, D.W.; Monahan, T.; Scott, B.L.; et al. Impact of clinical, cytogenetic, and molecular profiles on long-term survival after transplantation in patients with chronic myelomonocytic leukemia. Haematologica 2020, 105, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Merlevede, J.; Droin, N.; Qin, T.; Meldi, K.; Yoshida, K.; Morabito, M.; Chautard, E.; Auboeuf, D.; Fenaux, P.; Braun, T.; et al. Mutation allele burden remains unchanged in chronic myelomonocytic leukaemia responding to hypomethylating agents. Nat. Commun. 2016, 7, 10767. [Google Scholar] [CrossRef] [PubMed]
- Cargo, C.; Cullen, M.; Taylor, J.; Short, M.; Glover, P.; Van Hoppe, S.; Smith, A.; Evans, P.; Crouch, S. The use of targeted sequencing and flow cytometry to identify patients with a clinically significant monocytosis. Blood 2019, 133, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Palomo, L.; Meggendorfer, M.; Hutter, S.; Twardziok, S.; Ademà, V.; Fuhrmann, I.; Fuster-Tormo, F.; Xicoy, B.; Zamora, L.; Acha, P.; et al. Molecular landscape and clonal architecture of adult myelodysplastic/myeloproliferative neoplasms. Blood 2020, 136, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Vallapureddy, R.; Lasho, T.L.; Hoversten, K.; Finke, C.M.; Ketterling, R.; Hanson, C.; Gangat, N.; Tefferi, A.; Patnaik, M.M. Nucleophosmin 1 (NPM1) mutations in chronic myelomonocytic leukemia and their prognostic relevance. Am. J. Hematol. 2017, 92, E614–E618. [Google Scholar] [CrossRef]
- Ball, M.; List, A.F.; Padron, E. When clinical heterogeneity exceeds genetic heterogeneity: Thinking outside the genomic box in chronic myelomonocytic leukemia. Blood 2016, 128, 2381–2387. [Google Scholar] [CrossRef]
- Meggendorfer, M.; Roller, A.; Haferlach, T.; Eder, C.; Dicker, F.; Grossmann, V.; Kohlmann, A.; Alpermann, T.; Yoshida, K.; Ogawa, S.; et al. SRSF2 mutations in 275 cases with chronic myelomonocytic leukemia (CMML). Blood 2012, 120, 3080–3088. [Google Scholar] [CrossRef]
- Itzykson, R.; Kosmider, O.; Renneville, A.; Gelsi-Boyer, V.; Meggendorfer, M.; Morabito, M.; Berthon, C.; Adès, L.; Fenaux, P.; Beyne-Rauzy, O.; et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J. Clin. Oncol. 2013, 31, 2428–2436. [Google Scholar] [CrossRef]
- Malcovati, L.; Papaemmanuil, E.; Ambaglio, I.; Elena, C.; Gallì, A.; Della Porta, M.G.; Travaglino, E.; Pietra, D.; Pascutto, C.; Ubezio, M.; et al. Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia. Blood 2014, 124, 1513–1521. [Google Scholar] [CrossRef]
- Ricci, C.; Fermo, E.; Corti, S.; Molteni, M.; Faricciotti, A.; Cortelezzi, A.; Lambertenghi Deliliers, G.; Beran, M.; Onida, F. RAS mutations contribute to evolution of chronic myelomonocytic leukemia to the proliferative variant. Clin. Cancer Res. 2010, 16, 2246–2256. [Google Scholar] [CrossRef]
- Kohlmann, A.; Grossmann, V.; Klein, H.U.; Schindela, S.; Weiss, T.; Kazak, B.; Dicker, F.; Schnittger, S.; Dugas, M.; Kern, W.; et al. Next-generation sequencing technology reveals a characteristic pattern of molecular mutations in 72.8% of chronic myelomonocytic leukemia by detecting frequent alterations in TET2, CBL, RAS, and RUNX1. J. Clin. Oncol. 2010, 28, 3858–3865. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Lasho, T.L.; Vijayvargiya, P.; Finke, C.M.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Tefferi, A. Prognostic interaction between ASXL1 and TET2 mutations in chronic myelomonocytic leukemia. Blood Cancer J. 2016, 6, e385. [Google Scholar] [CrossRef]
- Steensma, D.P.; Dewald, G.W.; Lasho, T.L.; Powell, H.L.; McClure, R.F.; Levine, R.L.; Gilliland, D.G.; Tefferi, A. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood 2005, 106, 1207–1209. [Google Scholar] [CrossRef]
- Itzykson, R.; Kosmider, O.; Renneville, A.; Morabito, M.; Preudhomme, C.; Berthon, C.; Adès, L.; Fenaux, P.; Platzbecker, U.; Gagey, O.; et al. Clonal architecture of chronic myelomonocytic leukemias. Blood 2013, 121, 2186–2198. [Google Scholar] [CrossRef]
- Beran, M.; Shen, Y.; Onida, F.; Wen, S.; Kantarjian, H.; Estey, E. Prognostic significance of monocytosis in patients with myeloproliferative disorders. Leuk Lymphoma 2006, 47, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Meggendorfer, M.; Jeromin, S.; Haferlach, C.; Kern, W.; Haferlach, T. The mutational landscape of 18 investigated genes clearly separates four subtypes of myelodysplastic/myeloproliferative neoplasms. Haematologica 2018, 103, e192–e195. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zuo, Z.; Fu, B.; Oki, Y.; Tang, G.; Goswami, M.; Priyanka, P.; Muzzafar, T.; Medeiros, L.J.; Luthra, R.; et al. Chronic myelomonocytic leukemia with nucleophosmin (NPM1) mutation. Eur. J. Haematol. 2016, 96, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ernst, T.; Chase, A.; Zoi, K.; Waghorn, K.; Hidalgo-Curtis, C.; Score, J.; Jones, A.; Grand, F.; Reiter, A.; Hochhaus, A.; et al. Transcription factor mutations in myelodysplastic/myeloproliferative neoplasms. Haematologica 2010, 95, 1473–1480. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Selimoglu-Buet, D.; Wagner-Ballon, O.; Saada, V.; Bardet, V.; Itzykson, R.; Bencheikh, L.; Morabito, M.; Met, E.; Debord, C.; Benayoun, E.; et al. Characteristic repartition of monocyte subsets as a diagnostic signature of chronic myelomonocytic leukemia. Blood 2015, 125, 3618–3626. [Google Scholar] [CrossRef]
- Barraco, D.; Cerquozzi, S.; Gangat, N.; Patnaik, M.M.; Lasho, T.; Finke, C.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; Tefferi, A. Monocytosis in polycythemia vera: Clinical and molecular correlates. Am. J. Hematol. 2017, 92, 640–645. [Google Scholar] [CrossRef]
- Tefferi, A.; Shah, S.; Mudireddy, M.; Lasho, T.L.; Barraco, D.; Hanson, C.A.; Ketterling, R.P.; Elliott, M.A.; Patnaik, M.S.; Pardanani, A.; et al. Monocytosis is a powerful and independent predictor of inferior survival in primary myelofibrosis. Br. J. Haematol. 2018, 183, 835–838. [Google Scholar] [CrossRef]
- Patnaik, M.M. How I diagnose and treat chronic myelomonocytic leukemia. Haematologica 2022, 107, 1503–1517. [Google Scholar] [CrossRef]
- Wan, Z.; Han, B. Comparison and Implications of Mutational Profiles of Myelodysplastic Syndromes, Myeloproliferative Neoplasms, and Myelodysplastic/Myeloproliferative Neoplasms: A Meta-Analysis. Front. Oncol. 2020, 10, 579221. [Google Scholar] [CrossRef]
- Valent, P.; Orazi, A.; Savona, M.R.; Patnaik, M.M.; Onida, F.; van de Loosdrecht, A.A.; Haase, D.; Haferlach, T.; Elena, C.; Pleyer, L.; et al. Proposed diagnostic criteria for classical chronic myelomonocytic leukemia (CMML), CMML variants and pre-CMML conditions. Haematologica 2019, 104, 1935–1949. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, M.M.; Barraco, D.; Lasho, T.L.; Finke, C.M.; Reichard, K.; Hoversten, K.P.; Ketterling, R.P.; Gangat, N.; Tefferi, A. Targeted next generation sequencing and identification of risk factors in World Health Organization defined atypical chronic myeloid leukemia. Am. J. Hematol. 2017, 92, 542–548. [Google Scholar] [CrossRef]
- Vardiman, J.W.; Bain, B.; Inbert, M.; Brunning, R.D.; Pierre, R.V.; Flandrin, G. Atypical chronic myeloid leukemia. In WHO Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues; Jaffe, E., Harris, N.L., Stein, H., Vardiman, J., Eds.; IARC Press: Lyon, France, 2001; pp. 53–57. [Google Scholar]
- Breccia, M.; Biondo, F.; Latagliata, R.; Carmosino, I.; Mandelli, F.; Alimena, G. Identification of risk factors in atypical chronic myeloid leukemia. Haematologica 2006, 91, 1566–1568. [Google Scholar] [PubMed]
- Oscier, D. Atypical chronic myeloid leukemias. Pathol. Biol. 1997, 45, 587–593. [Google Scholar]
- Cazzola, M.; Malcovati, L.; Invernizzi, R. Myelodysplastic/myeloproliferative neoplasms. Hematol. Am. Soc. Hematol. Educ. Program 2011, 1, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.; Villaescusa, T.; Arefi, M.; López, L.; Garcia, J. Atypical Chronic Myeloid Leukemia (aCML). Atlas Genet. Cytogenet. Oncol. Haematol. 2009, 13, 432–433. [Google Scholar] [CrossRef]
- Zhang, H.; Wilmot, B.; Bottomly, D.; Dao, K.T.; Stevens, E.; Eide, C.A.; Khanna, V.; Rofelty, A.; Savage, S.; Reister Schultz, A.; et al. Genomic landscape of neutrophilic leukemias of ambiguous diagnosis. Blood 2019, 134, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.; Ramazzotti, D.; Aroldi, A.; Redaelli, S.; Magistroni, V.; Pirola, A.; Niro, A.; Massimino, L.; Mastini, C.; Brambilla, V.; et al. Integrated Genomic, Functional, and Prognostic Characterization of Atypical Chronic Myeloid Leukemia. Hemasphere 2020, 4, e497. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Pathak, R.; Martin, M.G.; Bhatt, V.R. Characteristics and survival of BCR/ABL negative chronic myeloid leukemia: A retrospective analysis of the Surveillance, Epidemiology and End Results database. Ther. Adv. Hematol. 2015, 6, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Orazi, A.; Bennet, J.M.; Bain, B.J.; Brunning, R.D.; Thiele, J. Atypical chronic myeloid leukaemia. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Eds.; IARC Press: Lyon, France, 2017; pp. 87–89. [Google Scholar]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellström-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef]
- Crisà, E.; Nicolosi, M.; Ferri, V.; Favini, C.; Gaidano, G.; Patriarca, A. Atypical Chronic Myeloid Leukemia: Where Are We Now? Int. J. Mol. Sci. 2020, 21, 6862. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.; Gambacorti-Passerini, C.; Piazza, R. Molecular Pathogenesis of BCR-ABL-Negative Atypical Chronic Myeloid Leukemia. Front. Oncol. 2021, 11, 756348. [Google Scholar] [CrossRef]
- Kurzrock, R.; Bueso-Ramos, C.E.; Kantarjian, H.; Freireich, E.; Tucker, S.L.; Siciliano, M.; Pilat, S.; Talpaz, M. BCR rearrangement-negative chronic myelogenous leukemia revisited. J. Clin. Oncol. 2001, 19, 2915–2926. [Google Scholar] [CrossRef]
- Gotlib, J.; Maxson, J.E.; George, T.I.; Tyner, J.W. The new genetics of chronic neutrophilic leukemia and atypical CML: Implications for diagnosis and treatment. Blood 2013, 122, 1707–1711. [Google Scholar] [CrossRef]
- Maxson, J.E.; Tyner, J.W. Genomics of chronic neutrophilic leukemia. Blood 2017, 129, 715–722. [Google Scholar] [CrossRef]
- Dao, K.H.; Tyner, J.W. What’s different about atypical CML and chronic neutrophilic leukemia? Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 264–271. [Google Scholar] [CrossRef]
- Maxson, J.E.; Gotlib, J.; Pollyea, D.A.; Fleischman, A.G.; Agarwal, A.; Eide, C.A.; Bottomly, D.; Wilmot, B.; McWeeney, S.K.; Tognon, C.E.; et al. Oncogenic CSF3R Mutations in Chronic Neutrophilic Leukemia and Atypical CML. N. Engl. J. Med. 2013, 368, 1781–1790. [Google Scholar] [CrossRef]
- Schwartz, L.C.; Mascarenhas, J. Current and evolving understanding of atypical chronic myeloid leukemia. Blood Rev. 2019, 33, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Carreño-Tarragona, G.; Alvarez-Larran, A.; Harrison, C.N.; Martínez-Ávila, J.C.; Hernandez-Boluda, J.C.; Ferrer-Marin, F.; Radia, D.H.; Mora Casterá, E.; Francis, S.; González-Martínez, T.; et al. CNL and aCML should be considered as single entity based on molecular profiles and outcomes. Blood Adv. 2022, 9, 1672–1681. [Google Scholar] [CrossRef]
- Thomopoulos, T.P.; Symeonidis, A.; Kourakli, A.; Papageorgiou, S.G.; Pappa, V. Chronic Neutrophilic Leukemia: A Comprehensive Review of Clinical Characteristics, Genetic Landscape and Management. Front. Oncol. 2022, 12, 891961. [Google Scholar] [CrossRef] [PubMed]
- Thakral, B.; Anastasi, J.; Wang, S.A. 17-Myeloproliferative and “Overlap” Myelodysplastic/Myeloproliferative Neoplasms. Found. Diagn. Pathol. 2018, 488–538.e484. [Google Scholar] [CrossRef]
- Wang, S.A.; Hasserjian, R.P.; Fox, P.S.; Rogers, H.J.; Geyer, J.T.; Chabot-Richards, D.; Weinzierl, E.; Hatem, J.; Jaso, J.; Kanagal-Shamanna, R.; et al. Atypical chronic myeloid leukemia is clinically distinct from unclassifiable myelodysplastic/myeloproliferative neoplasms. Blood 2014, 123, 2645–2651. [Google Scholar] [CrossRef]
- Wang, S.A.; Hasserjian, R.P.; Tam, W.; Tsai, A.G.; Geyer, J.T.; George, T.I.; Foucar, K.; Rogers, H.J.; Hsi, E.D.; Rea, B.A.; et al. Bone marrow morphology is a strong discriminator between chronic eosinophilic leukemia, not otherwise specified and reactive idiopathic hypereosinophilic syndrome. Haematologica 2017, 102, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Fend, F.; Horn, T.; Koch, I.; Vela, T.; Orazi, A. Atypical chronic myeloid leukemia as defined in the WHO classification is a JAK2 V617F negative neoplasm. Leuk. Res. 2008, 32, 1931–1935. [Google Scholar] [CrossRef]
- Gambacorti-Passerini, C.B.; Donadoni, C.; Parmiani, A.; Pirola, A.; Redaelli, S.; Signore, G.; Piazza, V.; Malcovati, L.; Fontana, D.; Spinelli, R.; et al. Recurrent ETNK1 mutations in atypical chronic myeloid leukemia. Blood 2015, 125, 499–503. [Google Scholar] [CrossRef]
- Fontana, D.; Mauri, M.; Renso, R.; Docci, M.; Crespiatico, I.; Røst, L.M.; Jang, M.; Niro, A.; D’Aliberti, D.; Massimino, L.; et al. ETNK1 mutations induce a mutator phenotype that can be reverted with phosphoethanolamine. Nat. Commun. 2020, 11, 5938. [Google Scholar] [CrossRef]
- Fontana, D.; Gambacorti-Passerini, C.; Piazza, R. Impact of ETNK1 somatic mutations on phosphoethanolamine synthesis, ROS production and DNA damage. Mol. Cell. Oncol. 2021, 8, 1877598. [Google Scholar] [CrossRef]
- Piazza, R.; Valletta, S.; Winkelmann, N.; Redaelli, S.; Spinelli, R.; Pirola, A.; Antolini, L.; Mologni, L.; Donadoni, C.; Papaemmanuil, E.; et al. Recurrent SETBP1 mutations in atypical chronic myeloid leukemia. Nat. Genet. 2013, 45, 18–24. [Google Scholar] [CrossRef]
- Piazza, R.; Magistroni, V.; Redaelli, S.; Mauri, M.; Massimino, L.; Sessa, A.; Peronaci, M.; Lalowski, M.; Soliymani, R.; Mezzatesta, C.; et al. SETBP1 induces transcription of a network of development genes by acting as an epigenetic hub. Nat. Commun. 2018, 9, 2192. [Google Scholar] [CrossRef]
- Meggendorfer, M.; Bacher, U.; Alpermann, T.; Haferlach, C.; Kern, W.; Gambacorti-Passerini, C.; Haferlach, T.; Schnittger, S. SETBP1 mutations occur in 9% of MDS/MPN and in 4% of MPN cases and are strongly associated with atypical CML, monosomy 7, isochromosome i(17)(q10), ASXL1 and CBL mutations. Leukemia 2013, 27, 1852–1860. [Google Scholar] [CrossRef]
- Dhakal, P.; Gundabolu, K.; Amador, C.; Rayamajhi, S.; Bhatt, V.R. Atypical chronic myeloid leukemia: A rare entity with management challenges. Future Oncol. 2018, 14, 177–185. [Google Scholar] [CrossRef]
- Gotlib, J. How I treat atypical chronic myeloid leukemia. Blood 2017, 129, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Carulli, G.; Fabiani, O.; Azzara, A. The syndrome of abnormal chromatin clumping in leukocytes. Haematologica 1997, 82, 635–636. [Google Scholar] [PubMed]
- Tefferi, A.; Elliot, M.A.; Pardanani, A. Atypical myeloproliferative disorders: Diagnosis and management. Mayo Clin. Proc. 2006, 81, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.V.; Kreil, S.; Zoi, K.; Waghorn, K.; Curtis, C.; Zhang, L.Y.; Score, J.; Seear, R.; Chase, A.J.; Grand, F.H.; et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood 2005, 106, 2162–2168. [Google Scholar] [CrossRef]
- Mughal, T.I.; Cross, N.C.; Padron, E.; Tiu, R.V.; Savona, M.; Malcovati, L.; Tibes, R.; Komrokji, R.S.; Kiladjian, J.J.; Garcia-Manero, G.; et al. An International MDS/MPN Working Group’s perspective and recommendations on molecular pathogenesis, diagnosis and clinical characterization of myelodysplastic/myeloproliferative neoplasms. Haematologica 2015, 100, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Tiu, R.V.; Sekeres, M.A. Making sense of the myelodysplastic/myeloproliferative neoplasms overlap syndromes. Curr. Opin. Hematol. 2014, 21, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Zoi, K.; Cross, N.C. Molecular pathogenesis of atypical CML, CMML and MDS/MPN-unclassifiable. Int. J. Hematol. 2015, 101, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Meggendorfer, M.; Haferlach, T.; Alpermann, T.; Jeromin, S.; Haferlach, C.; Kern, W.; Schnittger, S. Specific molecular mutation patterns delineate chronic neutrophilic leukemia, atypical chronic myeloid leukemia, and chronic myelomonocytic leukemia. Haematologica 2014, 99, e244–e246. [Google Scholar] [CrossRef]
- Makishima, H.; Yoshida, K.; Nguyen, N.; Przychodzen, B.; Sanada, M.; Okuno, Y.; Ng, K.P.; Gudmundsson, K.O.; Vishwakarma, B.A.; Jerez, A.; et al. Somatic SETBP1 mutations in myeloid malignancies. Nat. Genet. 2013, 45, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, O.; Mullally, A.; Hedvat, C.; Garcia-Manero, G.; Patel, J.; Wadleigh, M.; Malinge, S.; Yao, J.; Kilpivaara, O.; Bhat, R.; et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood 2009, 114, 144–147. [Google Scholar] [CrossRef]

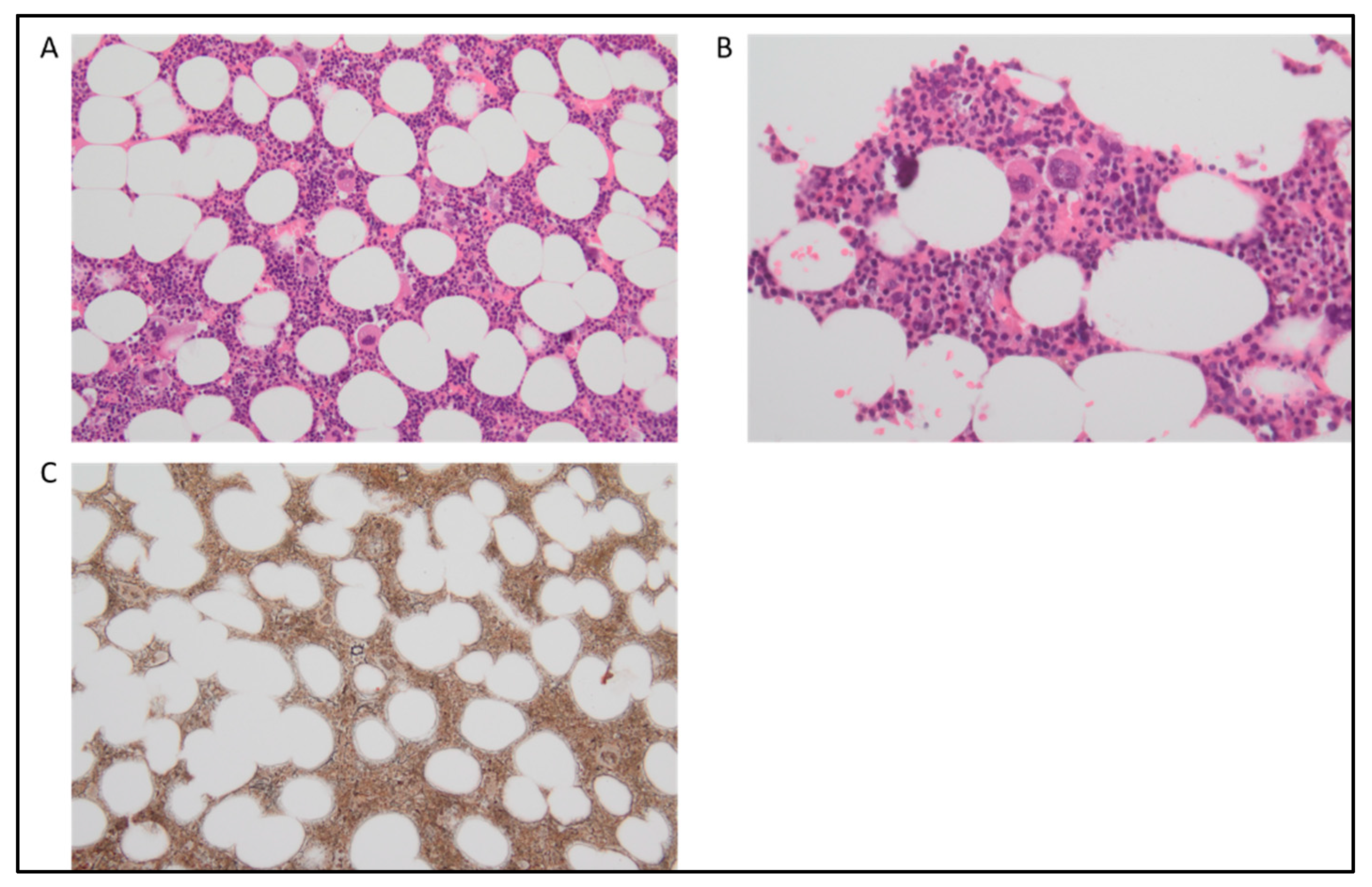
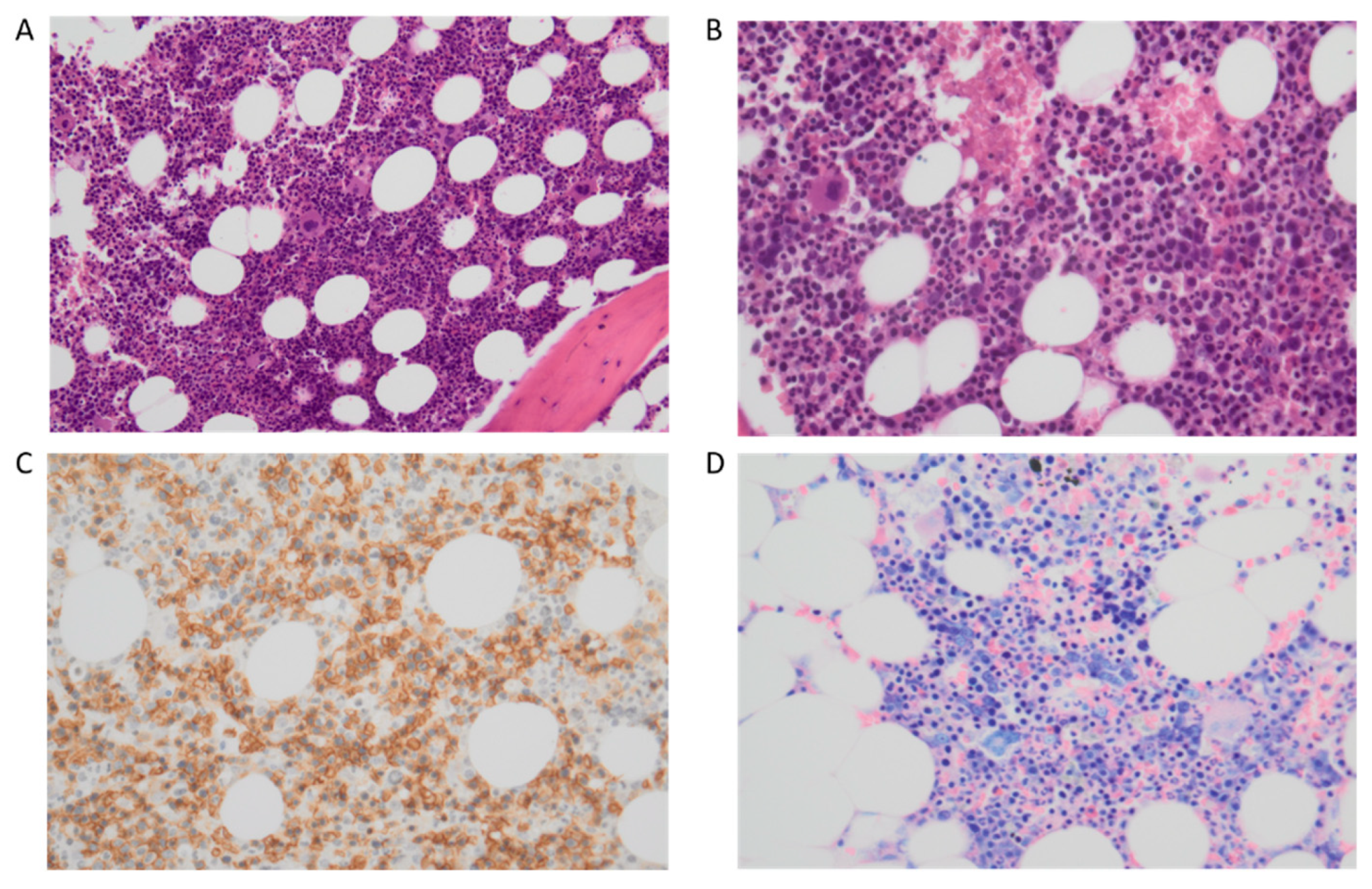
| WHO 2016 Classification | WHO 2022 Classification | ICC 2022 Classification |
|---|---|---|
| Chronic myelomonocytic leukemia | Chronic myelomonocytic leukemia | Chronic myelomonocytic leukemia |
| Clonal cytopenia with monocytosis of undetermined significance Clonal monocytosis of undetermined significance | ||
| Atypical chronic myeloid leukemia (aCML), BCR-ABL1− | Myelodysplastic/myeloproliferative neoplasm with neutrophilia | Atypical chronic myeloid leukemia |
| Juvenile myelomonocytic leukemia (JMML) | ||
| MDS/MPN with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T) | Myelodysplastic/myeloproliferative neoplasm with SF3B1 mutation and thrombocytosis | Myelodysplastic/myeloproliferative neoplasm with thrombocytosis and SF3B1 mutation |
| Myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, not otherwise specified | ||
| MDS/MPN, unclassifiable | Myelodysplastic/myeloproliferative neoplasm, not otherwise specified | Myelodysplastic/myeloproliferative neoplasm, not otherwise specified |
| Prerequisite Criteria |
|---|
| 1. Persistent absolute (≥0.5 × 109/L) and relative (≥10%) peripheral blood monocytosis |
| 2. Blasts constitute < 20% of the cells in the peripheral blood and bone marrow a |
| 3. Not meeting diagnostic criteria of chronic myeloid leukemia or other myeloproliferative neoplasms b |
| 4. Not meeting diagnostic criteria of myeloid/lymphoid neoplasms with tyrosine kinase fusions c |
| Supporting criteria |
| 1. Dysplasia involving ≥1 myeloid lineages d |
| 2. Acquired clonal cytogenetic or molecular abnormality |
| 3. Abnormal partitioning of peripheral blood monocyte subsets e |
| Requirements for diagnosis |
| - Pre-requisite criteria must be present in all cases |
| - If monocytosis is ≥1 × 109/L: one or more supporting criteria must be met |
| - If monocytosis is ≥0.5 and <1 × 109/L: supporting criteria 1 and 2 must be met |
| Subtyping criteria |
| - Myelodysplastic CMML (MD-CMML): WBC < 13 × 109/L |
| - Myeloproliferative CMML (MP-CMML): WBC ≥ 13 × 109/L |
| Subgrouping criteria (based on percentage of blasts and promonocytes) |
| CMML-1: <5% in peripheral blood and <10% in bone marrow |
| CMML-2: 5–19% in peripheral blood and 10–19% in bone marrow |
| Diagnostic Criteria |
|---|
| Persistent monocytosis defined as monocytes > 0.5 × 109/L and >10% of the WBC |
| Absence or presence of cytopenia (thresholds same as for MDS) a |
| Presence of at least one myeloid neoplasm-associated mutation of appropriate allele frequency (i.e., ≥2%) b |
| No significant dysplasia, increased blasts (including promonocytes), or morphologic findings of CMML on bone marrow examination c |
| No criteria for a myeloid or other hematopoietic neoplasm are fulfilled |
| No reactive condition that would explain a monocytosis is detected |
| Criteria | ICC 2022 Classification | WHO 2022 Classification |
|---|---|---|
| Nomenclature | Atypical chronic myeloid leukemia | Myelodysplastic/myeloproliferative neoplasm with neutrophilia |
| White blood cell count | ≥13 × 109/L with immature a myeloid cells constituting ≥ 10% of WBC | ≥13 × 109/L with neutrophilia, with immature a myeloid cells constituting ≥10% of WBC |
| Cytopenia | MDS b -qualifying thresholds | Not specifically mentioned in the WHO criteria |
| Peripheral blood and bone marrow blasts | <20% | <20% |
| Dysplasia | Dysgranulopoiesis; hyposegmented or hypersegmented neutrophils, with or without abnormal chromatin clumping | Circulating immature a myeloid cells constituting ≥ 10% of WBC, with neutrophilic dysplasia |
| Eosinophils | <10% | Not specifically mentioned |
| Monocytes | <10% | <10% |
| Bone marrow cellularity and hematopoiesis | Hypercellular with granulocytic hyperplasia and granulocytic dysplasia, with or without involvement of other lineages | Hypercellular with granulocytic hyperplasia and granulocytic dysplasia, with or without involvement of other lineages |
| Molecular exclusionary criteria | BCR::ABL1 or tyrosine kinase fusions associated with myeloid/lymphoid neoplasms with eosinophilia. JAK2, MPL, and CALR mutations | BCR::ABL1 or tyrosine kinase fusions associated with myeloid/lymphoid neoplasms with eosinophilia. JAK2, MPL, and CALR mutations. CSF3R mutations MDS/MPN-RS-T with SF3B1 mutations |
| Next generation sequencing data c | Desirable to document the presence of ASXL1 and SETBP1 mutations. | Desirable to document the presence of SETBP1 and/or ETNK1 mutations |
| Diagnostic Criteria |
|---|
| Thrombocytosis, with platelet count ≥ 450 × 109/L |
| Anemia (threshold same as for MDS) |
| Blasts < 1% in blood and <5% in bone marrow |
| Presence of SF3B1 mutation (VAF > 10%), isolated or associated with abnormal cytogenetics and/or other myeloid neoplasm-associated mutations |
| No history of recent cytotoxic or growth factor therapy that could explain the myelodysplastic/myeloproliferative features |
| No BCR::ABL1 or genetic abnormalities of myeloid/lymphoid neoplasms with eosinophilia and tyrosine kinase gene fusions; no t(3;3)(q21.3;q26.2), inv(3)(q21.3q26.2), or del(5q) * |
| No history of MPN, MDS, or other myelodysplastic/myeloproliferative neoplasm |
| Diagnostic Criteria |
|---|
| Thrombocytosis, with platelet count ≥ 450 × 109/L |
| Anemia associated with erythroid-lineage dysplasia, with or without multilineage dysplasia, and ≥15% ring sideroblasts |
| Blasts < 1% in blood and <5% in bone marrow |
| Presence of clonality: demonstration of a clonal cytogenetic abnormality and/or somatic mutation(s). In their absence, no history of recent cytotoxic or growth factor therapy that could explain the myelodysplastic/myeloproliferative features. |
| Absence of SF3B1 mutation; no BCR::ABL1 or genetic abnormalities of myeloid/lymphoid neoplasms with eosinophilia and tyrosine kinase gene fusions; no t(3;3)(q21.3;q26.2), inv(3) (q21.3q26.2), or del(5q) * |
| No history of MPN, MDS, or other MDS/MPN |
| Diagnostic Criteria |
|---|
| Myeloid neoplasm with mixed myeloproliferative and myelodysplastic features, not meeting the WHO criteria for any other myelodysplastic/myeloproliferative neoplasm, myelodysplastic syndrome, myeloproliferative neoplasm a |
| Cytopenia (thresholds same as for MDS) |
| Blasts <20% of the cells in blood and bone marrow |
| A platelet count of ≥450 × 109/L and/or a white blood cell count of ≥13 × 109/L |
| Presence of clonality: demonstration of a clonal cytogenetic abnormality and/or somatic mutation(s). If clonality cannot be determined, the findings have persisted and all other causes (e.g., history of cytotoxic or growth factor therapy or other primary cause that could explain the myelodysplastic/myeloproliferative features) have been excluded. |
| No BCR::ABL1 or genetic abnormalities of myeloid/lymphoid neoplasms with eosinophilia and tyrosine kinase gene fusions; no t(3;3)(q21.3;q26.2), inv(3)(q21.3q26.2), b or del(5q) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, D.; Elli, E.M.; Pagni, F.; Piazza, R. Myelodysplastic Syndromes/Myeloproliferative Overlap Neoplasms and Differential Diagnosis in the WHO and ICC 2022 Era: A Focused Review. Cancers 2023, 15, 3175. https://doi.org/10.3390/cancers15123175
Fontana D, Elli EM, Pagni F, Piazza R. Myelodysplastic Syndromes/Myeloproliferative Overlap Neoplasms and Differential Diagnosis in the WHO and ICC 2022 Era: A Focused Review. Cancers. 2023; 15(12):3175. https://doi.org/10.3390/cancers15123175
Chicago/Turabian StyleFontana, Diletta, Elena M. Elli, Fabio Pagni, and Rocco Piazza. 2023. "Myelodysplastic Syndromes/Myeloproliferative Overlap Neoplasms and Differential Diagnosis in the WHO and ICC 2022 Era: A Focused Review" Cancers 15, no. 12: 3175. https://doi.org/10.3390/cancers15123175
APA StyleFontana, D., Elli, E. M., Pagni, F., & Piazza, R. (2023). Myelodysplastic Syndromes/Myeloproliferative Overlap Neoplasms and Differential Diagnosis in the WHO and ICC 2022 Era: A Focused Review. Cancers, 15(12), 3175. https://doi.org/10.3390/cancers15123175






